High Variability in Human Sperm Membrane Potential over Time Can Limit Its Reliability as a Predictor in ART Outcomes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Sperm Culture Media
2.3. Human Sperm Preparation
2.4. Em Determination by Fluorometric Population Assay
2.5. Statistical Analysis
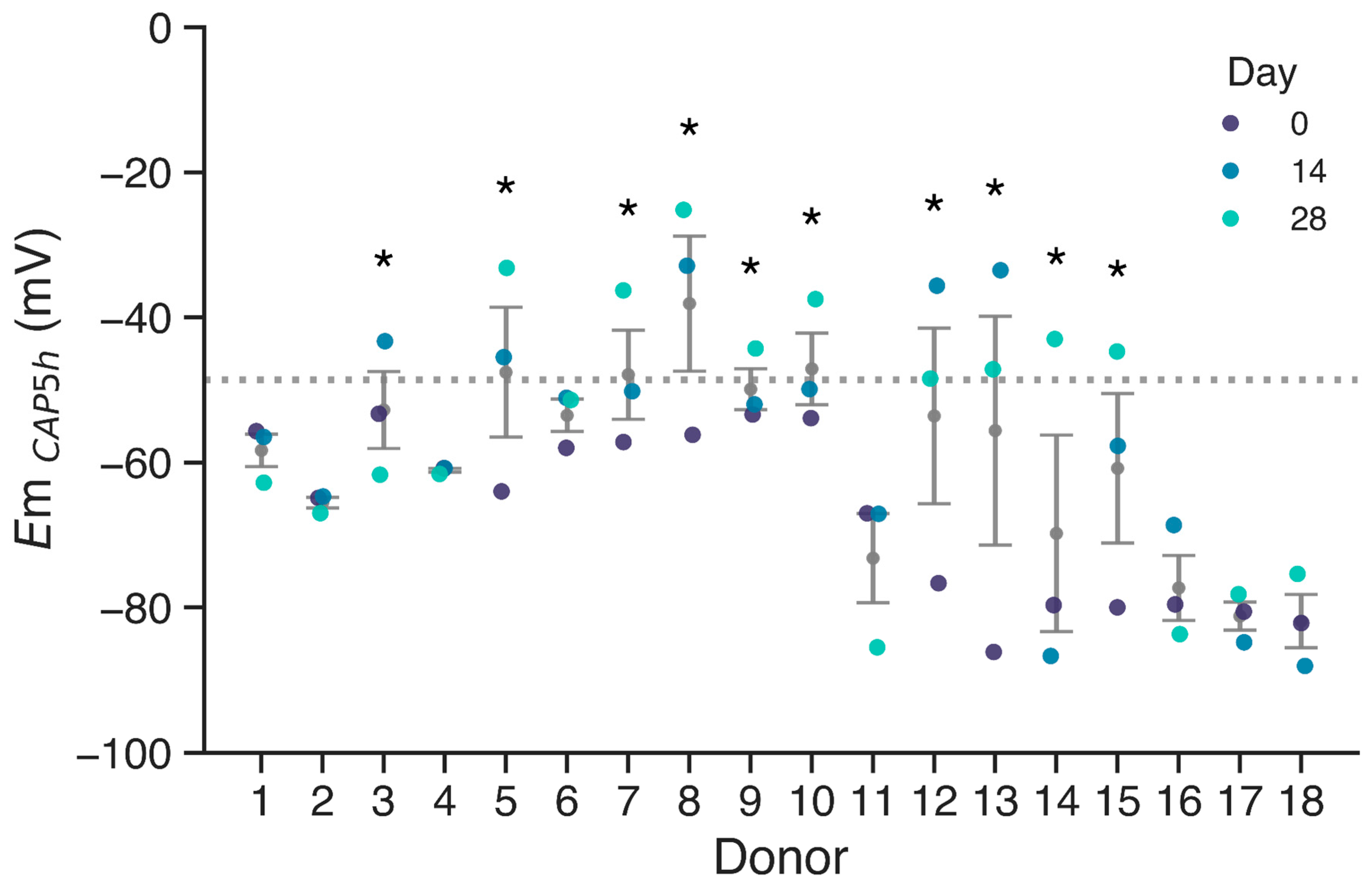
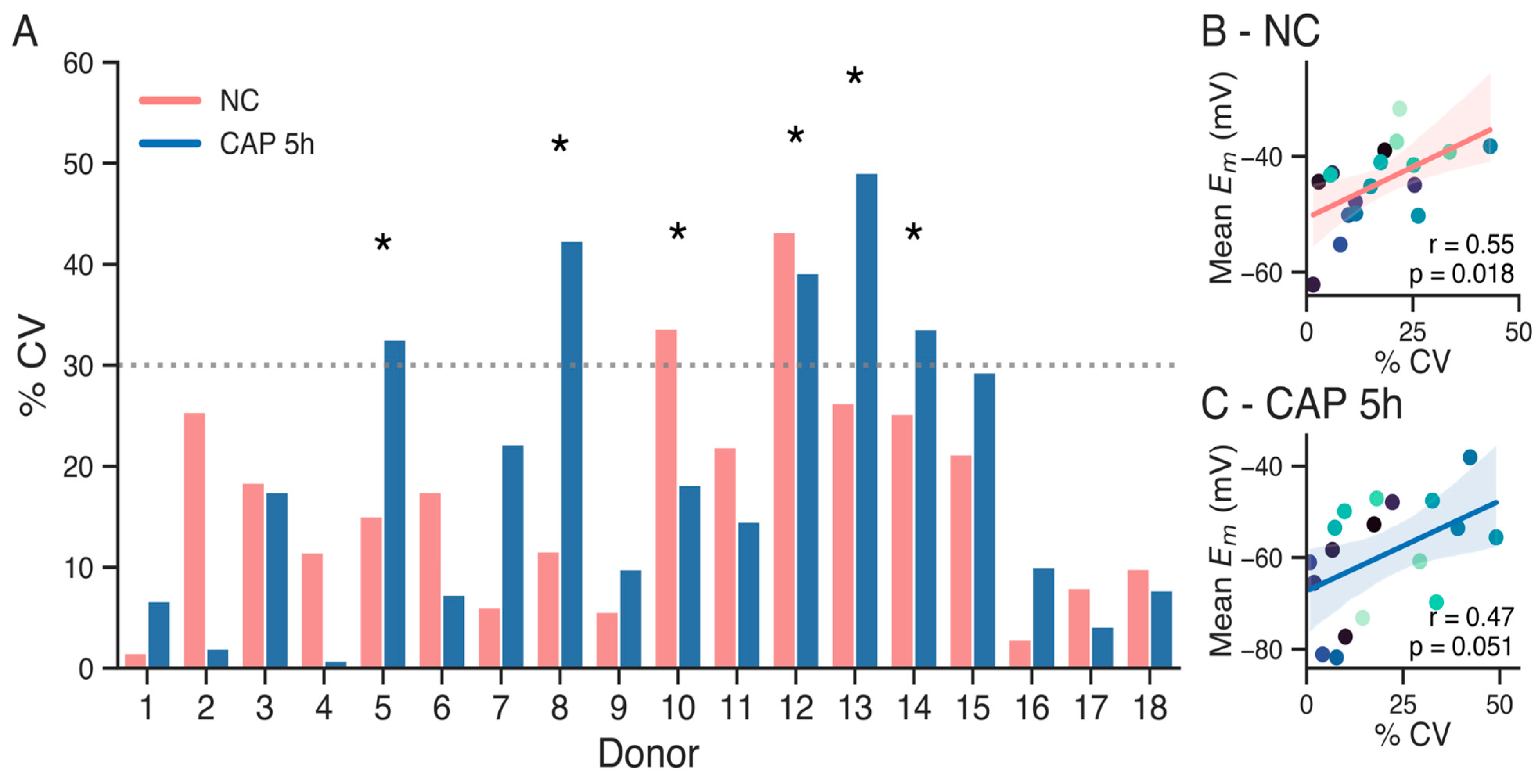
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | assisted reproductive treatments |
| Em | membrane potential |
| IVF | in vitro fertilization |
| NC | non-capacitating media |
| CAP | capacitating media |
References
- Slama, R.; Hansen, O.K.H.; Ducot, B.; Bohet, A.; Sorensen, D.; Giorgis Allemand, L.; Eijkemans, M.J.C.; Rosetta, L.; Thalabard, J.C.; Keiding, N.; et al. Estimation of the Frequency of Involuntary Infertility on a Nation-Wide Basis. Hum. Reprod. 2012, 27, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Datta, J.; Palmer, M.J.; Tanton, C.; Gibson, L.J.; Jones, K.G.; Macdowall, W.; Glasier, A.; Sonnenberg, P.; Field, N.; Mercer, C.H.; et al. Prevalence of Infertility and Help Seeking among 15,000 Women and Men. Hum. Reprod. 2016, 31, 2108–2118. [Google Scholar] [CrossRef]
- Human Fertilisation and Embryology Authority. Fertility Treatment in 2013: Trends and Figures; Human Fertilisation and Embryology Authority: London, UK, 2014. [Google Scholar]
- Barratt, C.L.R.; Björndahl, L.; De Jonge, C.J.; Lamb, D.J.; Osorio Martini, F.; McLachlan, R.; Oates, R.D.; Van Der Poel, S.; St John, B.; Sigman, M.; et al. The Diagnosis of Male Infertility: An Analysis of the Evidence to Support the Development of Global WHO Guidance—Challenges and Future Research Opportunities. Hum. Reprod. Update 2017, 23, 660–680. [Google Scholar] [CrossRef]
- Barratt, C.L.R.; De Jonge, C.J.; Sharpe, R.M. ‘Man Up’: The Importance and Strategy for Placing Male Reproductive Health Centre Stage in the Political and Research Agenda. Hum. Reprod. 2018, 33, 541–545. [Google Scholar] [CrossRef]
- Austin, C.R. The ‘Capacitation’ of the Mammalian Sperm. Nature 1952, 170, 326. [Google Scholar] [CrossRef]
- Chang, M.C. Fertilizing Capacity of Spermatozoa Deposited into the Fallopian Tubes. Nature 1951, 168, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Krapf, D.; de la Vega-Beltrán, J.L.; Acevedo, J.J.; Darszon, A. Ion Channels, Phosphorylation and Mammalian Sperm Capacitation. Asian J. Androl. 2011, 13, 395–405. [Google Scholar] [CrossRef]
- Hernández-González, E.O.; Sosnik, J.; Edwards, J.; Acevedo, J.J.; Mendoza-Lujambio, I.; López-González, I.; Demarco, I.; Wertheimer, E.; Darszon, A.; Visconti, P.E. Sodium and Epithelial Sodium Channels Participate in the Regulation of the Capacitation-Associated Hyperpolarization in Mouse Sperm. J. Biol. Chem. 2006, 281, 5623–5633. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Clark, E.N.; Florman, H.M. Sperm Membrane Potential: Hyperpolarization during Capacitation Regulates Zona Pellucida-Dependent Acrosomal Secretion. Dev. Biol. 1995, 171, 554–563. [Google Scholar] [CrossRef]
- Moseley, F.L.C.; Jha, K.N.; Björndahl, L.; Brewis, I.A.; Publicover, S.J.; Barratt, C.L.R.; Lefièvre, L. Protein Tyrosine Phosphorylation, Hyperactivation and Progesterone-Induced Acrosome Reaction Are Enhanced in IVF Media: An Effect That Is Not Associated with an Increase in Protein Kinase a Activation. MHR Basic Sci. Reprod. Med. 2005, 11, 523–529. [Google Scholar] [CrossRef]
- Ren, D.; Navarro, B.; Perez, G.; Jackson, A.C.; Hsu, S.; Shi, Q.; Tilly, J.L.; Clapham, D.E. A Sperm Ion Channel Required for Sperm Motility and Male Fertility. Nature 2001, 413, 603–609. [Google Scholar] [CrossRef]
- Wang, D.; King, S.M.; Quill, T.A.; Doolittle, L.K.; Garbers, D.L. A New Sperm-Specific Na+/H+ Exchanger Required for Sperm Motility and Fertility. Nat. Cell Biol. 2003, 5, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Mannowetz, N.; Naidoo, N.M.; Choo, S.-A.S.; Smith, J.F.; Lishko, P.V. Slo1 Is the Principal Potassium Channel of Human Spermatozoa. eLife 2013, 2, e01009. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.M.; Martínez-López, P.; de la Vega-Beltrán, J.L.; Butler, A.; Alisio, A.; Darszon, A.; Salkoff, L. The SLO3 Sperm-Specific Potassium Channel Plays a Vital Role in Male Fertility. FEBS Lett. 2010, 584, 1041–1046. [Google Scholar] [CrossRef]
- Lishko, P.V.; Kirichok, Y.; Ren, D.; Navarro, B.; Chung, J.-J.; Clapham, D.E. The Control of Male Fertility by Spermatozoan Ion Channels. Annu. Rev. Physiol. 2012, 74, 453–475. [Google Scholar] [CrossRef] [PubMed]
- Darszon, A.; Nishigaki, T.; Beltran, C.; Treviño, C.L. Calcium Channels in the Development, Maturation, and Function of Spermatozoa. Physiol. Rev. 2011, 91, 1305–1355. [Google Scholar] [CrossRef]
- De La Vega-Beltran, J.L.; Sánchez-Cárdenas, C.; Krapf, D.; Hernandez-González, E.O.; Wertheimer, E.; Treviño, C.L.; Visconti, P.E.; Darszon, A. Mouse Sperm Membrane Potential Hyperpolarization Is Necessary and Sufficient to Prepare Sperm for the Acrosome Reaction. J. Biol. Chem. 2012, 287, 44384. [Google Scholar] [CrossRef]
- Patrat, C.; Serres, C.; Jouannet, P. Progesterone Induces Hyperpolarization after a Transient Depolarization Phase in Human Spermatozoa1. Biol. Reprod. 2002, 66, 1775–1780. [Google Scholar] [CrossRef]
- Linares-Hernández, L.; Guzmán-Grenfell, A.M.; Hicks-Gomez, J.J.; González-Martínez, M.T. Voltage-Dependent Calcium Influx in Human Sperm Assessed by Simultaneous Optical Detection of Intracellular Calcium and Membrane Potential. Biochim. Biophys. Acta (BBA) Biomembr. 1998, 1372, 1–12. [Google Scholar] [CrossRef]
- Molina, L.C.P.; Gunderson, S.; Riley, J.; Lybaert, P.; Borrego-Alvarez, A.; Jungheim, E.S.; Santi, C.M. Membrane Potential Determined by Flow Cytometry Predicts Fertilizing Ability of Human Sperm. Front. Cell Dev. Biol. 2020, 7, 387. [Google Scholar] [CrossRef]
- Baro Graf, C.; Ritagliati, C.; Torres-Monserrat, V.; Stival, C.; Carizza, C.; Buffone, M.G.; Krapf, D. Membrane Potential Assessment by Fluorimetry as a Predictor Tool of Human Sperm Fertilizing Capacity. Front. Cell Dev. Biol. 2020, 7, 383. [Google Scholar] [CrossRef]
- Baro Graf, C.; Ritagliati, C.; Stival, C.; Balestrini, P.A.; Buffone, M.G.; Krapf, D. Determination of a Robust Assay for Human Sperm Membrane Potential Analysis. Front. Cell Dev. Biol. 2019, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Schwitzerland, 2010; ISBN 978-92-4-003078-7. [Google Scholar]
- Castilla, J.A.; Álvarez, C.; Aguilar, J.; González-Varea, C.; Gonzalvo, M.C.; Martínez, L. Influence of Analytical and Biological Variation on the Clinical Interpretation of Seminal Parameters. Hum. Reprod. 2006, 21, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.F.; Lynn, F.; Meade, B.D. Use of Coefficient of Variation in Assessing Variability of Quantitative Assays. Clin. Vaccine Immunol. 2002, 9, 1235–1239. [Google Scholar] [CrossRef]
- Baker, H.W.G.; Burger, H.G.; de Kretser, D.M.; Lording, D.W.; McGowan, P.; Rennie, G.G. Factors Affecting the Variability of Semen Analysis Results in Infertile Men. Int. J. Androl. 1981, 4, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Osadchuk, L.V.; Kleshchev, M.A.; Osadchuk, A.V. Steroid Hormones in Seminal Plasma: The Relationship with Sperm Quality. Bull. Exp. Biol. Med. 2023, 174, 318–321. [Google Scholar] [CrossRef]
- Maroto, M.; Torvisco, S.N.; García-Merino, C.; Fernández-González, R.; Pericuesta, E. Mechanisms of Hormonal, Genetic, and Temperature Regulation of Germ Cell Proliferation, Differentiation, and Death During Spermatogenesis. Biomolecules 2025, 15, 500. [Google Scholar] [CrossRef]
- Aitken, R.; Smith, T.; Jobling, M.; Baker, M.; De Iuliis, G. Oxidative Stress and Male Reproductive Health. Asian J. Androl. 2014, 16, 31. [Google Scholar] [CrossRef]
- Takalani, N.B.; Monageng, E.M.; Mohlala, K.; Monsees, T.K.; Henkel, R.; Opuwari, C.S. Role of Oxidative Stress in Male Infertility. Reprod. Fertil. 2023, 4, e230024. [Google Scholar] [CrossRef]
- Aten, S.; Ramirez-Plascencia, O.; Blake, C.; Holder, G.; Fishbein, E.; Vieth, A.; Zarghani-Shiraz, A.; Keister, E.; Howe, S.; Appo, A.; et al. A Time for Sex: Circadian Regulation of Mammalian Sexual and Reproductive Function. Front. Neurosci. 2025, 18, 1516767. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steeman, T.J.; Baro Graf, C.; Novero, A.G.; Buffone, M.G.; Krapf, D. High Variability in Human Sperm Membrane Potential over Time Can Limit Its Reliability as a Predictor in ART Outcomes. Biology 2025, 14, 851. https://doi.org/10.3390/biology14070851
Steeman TJ, Baro Graf C, Novero AG, Buffone MG, Krapf D. High Variability in Human Sperm Membrane Potential over Time Can Limit Its Reliability as a Predictor in ART Outcomes. Biology. 2025; 14(7):851. https://doi.org/10.3390/biology14070851
Chicago/Turabian StyleSteeman, Tomás J., Carolina Baro Graf, Analia G. Novero, Mariano G. Buffone, and Dario Krapf. 2025. "High Variability in Human Sperm Membrane Potential over Time Can Limit Its Reliability as a Predictor in ART Outcomes" Biology 14, no. 7: 851. https://doi.org/10.3390/biology14070851
APA StyleSteeman, T. J., Baro Graf, C., Novero, A. G., Buffone, M. G., & Krapf, D. (2025). High Variability in Human Sperm Membrane Potential over Time Can Limit Its Reliability as a Predictor in ART Outcomes. Biology, 14(7), 851. https://doi.org/10.3390/biology14070851





