Stomatal and Non-Stomatal Leaf Traits for Enhanced Water Use Efficiency in Rice
Simple Summary
Abstract
1. Introduction
2. Stomatal Leaf Traits and WUE in Rice
2.1. Stomatal Density, Size, and Arrangement
2.2. Stomatal Conductance and Aperture
2.3. Regulation of Transpiration and Carbon Dioxide Uptake
3. Non-Stomatal Leaf Traits and WUE in Rice
3.1. The Role of ΦPSII in Photosynthetic Efficiency
3.2. Leaf Anatomy
3.3. Leaf Cuticles and Epicuticular Wax
3.4. Metabolomic Changes in Leaves
3.5. Mesophyll Conductance (gm) and Intrinsic WUE
3.6. Leaf Canopy Architecture
3.7. Leaf Pubescence and Boundary Layer Resistance
3.8. Carbon Fixation Efficiency
4. Integrating Stomatal and Non-Stomatal Traits for WUE Improvement
4.1. Interactions and Trade-Offs Among Leaf Traits
4.2. Breeding Strategies for Optimising WUE Through Leaf Traits
4.3. Agronomic Practices and Environmental Factors Influencing WUE
4.4. WUE Optimization Strategies Between Traditional and Dryland Rice Farming Systems
5. Future Perspectives and Research Directions
5.1. Emerging Technologies and Approaches for Studying Leaf Traits and WUE
5.2. Challenges in Developing Water-Efficient Rice Cultivars
5.3. Interaction with Root Traits
6. Enhanced WUE in Rice
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WUE | water use efficiency |
| gs | stomatal conductance |
| Δ13C | carbon isotope discrimination |
| gm | mesophyll conductance |
References
- Food and Agriculture Organization of the United Nations (FAO). Water Scarcity—One of the Greatest Challenges of Our Time. Available online: https://www.fao.org/newsroom/story/Water-Scarcity-One-of-the-greatest-challenges-of-our-time/en (accessed on 20 February 2024).
- World Health Organization (WHO). 1 in 3 People Globally Do Not Have Access to Safe Drinking Water—UNICEF, WHO. Available online: https://www.who.int/news/item/18-06-2019-1-in-3-people-globally-do-not-have-access-to-safe-drinking-water-unicef-who (accessed on 27 December 2022).
- Pitaloka, M.K.; Caine, R.S.; Hepworth, C.; Harrison, E.L.; Sloan, J.; Chutteang, C.; Phunthong, C.; Nongngok, R.; Toojinda, T.; Ruengphayak, S.; et al. Induced Genetic Variations in Stomatal Density and Size of Rice Strongly Affects Water Use Efficiency and Responses to Drought Stresses. Front. Plant Sci. 2022, 13, 801706. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of Stomatal Density and Morphology on Water-Use Efficiency in a Changing World. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef]
- Chapagain, T.; Riseman, A.; Yamaji, E. Achieving more with less water: Alternate wet and dry irrigation (AWDI) as an alternative to the conventional water management practices in rice farming. J. Agric. Sci. 2011, 3, 3. [Google Scholar] [CrossRef]
- Mallareddy, M.; Thirumalaikumar, R.; Balasubramanian, P.; Naseeruddin, R.; Nithya, N.; Mariadoss, A.; Eazhilkrishna, N.; Choudhary, A.K.; Deiveegan, M.; Subramanian, E. Maximizing water use efficiency in rice farming: A comprehensive review of innovative irrigation management technologies. Water 2023, 15, 1802. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; Yang, J.; Zhang, J. Yield, grain quality and water use efficiency of rice under non-flooded mulching cultivation. Field Crops Res. 2008, 108, 71–81. [Google Scholar] [CrossRef]
- Borrell, A.; Garside, A.; Fukai, S. Improving efficiency of water use for irrigated rice in a semi-arid tropical environment. Field Crops Res. 1997, 52, 231–248. [Google Scholar] [CrossRef]
- Fukai, S.; Mitchell, J. Factors determining water use efficiency in aerobic rice. Crop Environ. 2022, 1, 24–40. [Google Scholar] [CrossRef]
- Gago, J.; Douthe, C.; Florez-Sarasa, I.; Escalona, J.M.; Galmes, J.; Fernie, A.R.; Medrano, H. Opportunities for improving leaf water use efficiency under climate change conditions. Plant Sci. 2014, 226, 108–119. [Google Scholar] [CrossRef]
- Gobu, R.; Dash, G.K.; Lal, J.P.; Swain, P.; Mahender, A.; Anandan, A.; Ali, J. Unlocking the Nexus between Leaf-Level Water Use Efficiency and Root Traits Together with Gas Exchange Measurements in Rice (Oryza sativa L.). Plants 2022, 11, 1270. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and Challenges in a Changing Climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Ramachandra, A.; Vijayaraghavareddy, P.; Purushothama, C.; Nagaraju, S.; Sreeman, S. Decoding stomatal characteristics regulating water use efficiency at leaf and plant scales in rice genotypes. Planta 2024, 260, 56. [Google Scholar] [CrossRef]
- Reddy, S.H.; Singhal, R.K.; DaCosta, M.V.J.; Kambalimath, S.K.; Rajanna, M.P.; Muthurajan, R.; Sevanthi, A.M.; Mohapatra, T.; Sarla, N.; Chinnusamy, V.; et al. Leaf mass area determines water use efficiency through its influence on carbon gain in rice mutants. Physiol. Plant. 2020, 169, 194–213. [Google Scholar] [CrossRef]
- Xiong, D.; Flexas, J. From one side to two sides: The effects of stomatal distribution on photosynthesis. New Phytol. 2020, 228, 1754–1766. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.; Rebetzke, G.; Farquhar, G. Improving intrinsic water-use efficiency and crop yield. Crop Sci. 2002, 42, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, S.O.; Habila, D.G.; Mamadou, F.; Abolanle, B.M.; Olatunbosun, A.N. Grain yield and leaf gas exchange in upland NERICA rice under repeated cycles of water deficit at reproductive growth stage. Agric. Water Manag. 2022, 264, 107507. [Google Scholar] [CrossRef]
- Centritto, M.; Lauteri, M.; Monteverdi, M.C.; Serraj, R. Leaf gas exchange, carbon isotope discrimination, and grain yield in contrasting rice genotypes subjected to water deficits during the reproductive stage. J. Exp. Bot. 2009, 60, 2325–2339. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Wang, Y.; Agathokleous, E.; Hamoud, Y.A.; Qiu, R.; Hong, C.; Tian, M.; Shaghaleh, H.; Guo, X. Relationships between stable isotope natural abundances (δ13C and δ15N) and water use efficiency in rice under alternate wetting and drying irrigation in soils with high clay contents. Front. Plant Sci. 2022, 13, 1077152. [Google Scholar] [CrossRef]
- Wang, X.; Huang, J.; Peng, S.; Xiong, D. Leaf rolling precedes stomatal closure in rice (Oryza sativa) under drought conditions. J. Exp. Bot. 2023, 74, 6650–6661. [Google Scholar] [CrossRef]
- Blankenagel, S.; Yang, Z.; Avramova, V.; Schön, C.-C.; Grill, E. Generating Plants with Improved Water Use Efficiency. Agronomy 2018, 8, 194. [Google Scholar] [CrossRef]
- Bramley, H.; Turner, N.C.; Siddique, K.H.M. Water Use Efficiency. In Genomics and Breeding for Climate-Resilient Crops: Vol. 2 Target Traits; Kole, C., Ed.; Springer: Berlin, Heidelberg, 2013; pp. 225–268. [Google Scholar] [CrossRef]
- Brendel, O. The relationship between plant growth and water consumption: A history from the classical four elements to modern stable isotopes. Ann. For. Sci. 2021, 78, 47. [Google Scholar] [CrossRef]
- Petrík, P.; Petek-Petrik, A.; Mukarram, M.; Schuldt, B.; Lamarque, L.J. Leaf physiological and morphological constraints of water-use efficiency in C3 plants. AoB Plants 2023, 15, plad047. [Google Scholar] [CrossRef] [PubMed]
- Pandya, P.; Parmar, S.; Vadalia, D.; Prajapati, G. Thermal Imaging and Its Application in Irrigation Water Management. AgriTech Today 2023, 1, 40. [Google Scholar]
- Akram, H.; Ali, A.; Sattar, A.; Rehman, H.; Bibi, A. Impact of water deficit stress on various physiological and agronomic traits of three basmati rice (Oryza sativa L.) cultivars. J. Anim. Plant Sci. 2013, 23, 1415–1423. [Google Scholar]
- Yadav, N.; Sevanthi, A.C.; Pandey, R.; Chinnusamy, V.; Singh, A.K.; Singh, N.K. Physiological response and agronomic performance of drought tolerance mutants of Aus rice cultivar Nagina 22 (Oryza sativa L). Field Crops Res. 2023, 290, 108760. [Google Scholar] [CrossRef]
- Lang, P.L.; Erberich, J.M.; Lopez, L.; Weiß, C.L.; Amador, G.; Fung, H.F.; Latorre, S.M.; Lasky, J.R.; Burbano, H.A.; Expósito-Alonso, M. Century-long timelines of herbarium genomes predict plant stomatal response to climate change. Nat. Ecol. Evol. 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, U.; Caine, R.; Atkinson, J.; Harrison, E.; Wells, D.; Chater, C.; Gray, J.; Swarup, R.; Murchie, E. IRice plants overexpressing OsEPF1 show reduced stomatal density and increased root cortical aerenchyma formation. Sci. Rep. 2019, 9, 5584. [Google Scholar] [CrossRef]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress. Adv. Exp. Med. Biol. 2018, 1081, 215–232. [Google Scholar] [CrossRef]
- Laza, M.R.C.; Kondo, M.; Ideta, O.; Barlaan, E.; Imbe, T. Quantitative trait loci for stomatal density and size in lowland rice. Euphytica 2010, 172, 149–158. [Google Scholar] [CrossRef]
- Phunthong, C.; Pitaloka, M.K.; Chutteang, C.; Ruengphayak, S.; Arikit, S.; Vanavichit, A. Rice mutants, selected under severe drought stress, show reduced stomatal density and improved water use efficiency under restricted water conditions. Front. Plant Sci. 2024, 15, 1307653. [Google Scholar] [CrossRef]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef]
- Ramachandra, A.; PRABHU, B.; Sheshshayee, M. Relevance of Stomatal Traits in Determining the Water Use and Water Use Efficiency in Rice Genotypes Adapted to Different Cultivation Systems. Mysore J. Agric. Sci. 2023, 57, 33–42. [Google Scholar]
- Ohsumi, A.; Tomomi, K.; Koki, H.; Takeshi, H.; Shiraiwa, T. Genotypic Variation of Stomatal Conductance in Relation to Stomatal Density and Length in Rice (Oryza sativa L.). Plant Prod. Sci. 2007, 10, 322–328. [Google Scholar] [CrossRef]
- Kumar, S.; Tripathi, S.; Singh, S.P.; Prasad, A.; Akter, F.; Syed, M.A.; Badri, J.; Das, S.P.; Bhattarai, R.; Natividad, M.A.; et al. Rice breeding for yield under drought has selected for longer flag leaves and lower stomatal density. J. Exp. Bot. 2021, 72, 4981–4992. [Google Scholar] [CrossRef]
- Malini, M.; Karwa, S.; Priyadarsini, P.; Kumar, P.; Nagar, S.; Kumar, M.; Kumar, S.; Chinnusamy, V.; Pandey, R.; Pal, M. Abscisic-Acid-Modulated Stomatal Conductance Governs High-Temperature Stress Tolerance in Rice Accessions. Agriculture 2023, 13, 545. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Tang, Y.; Zhu, X.-G. Stomata conductance as a goalkeeper for increased photosynthetic efficiency. Curr. Opin. Plant Biol. 2022, 70, 102310. [Google Scholar] [CrossRef]
- Villalobos-López, M.A.; Arroyo-Becerra, A.; Quintero-Jiménez, A.; Iturriaga, G. Biotechnological advances to improve abiotic stress tolerance in crops. Int. J. Mol. Sci. 2022, 23, 12053. [Google Scholar] [CrossRef]
- Yari Kamrani, Y.; Shomali, A.; Aliniaeifard, S.; Lastochkina, O.; Moosavi-Nezhad, M.; Hajinajaf, N.; Talar, U. Regulatory role of circadian clocks on ABA production and signaling, stomatal responses, and water-use efficiency under water-deficit conditions. Cells 2022, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, T.C.; O’Toole, J.C.; Yambao, E.B.; Turner, N.C. Influence of osmotic adjustment on leaf rolling and tissue death in rice (Oryza sativa L.). Plant Physiol. 1984, 75, 338–341. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Su, Y.; Shen, H. Rice Responses to Abiotic Stress: Key Proteins and Molecular Mechanisms. Int. J. Mol. Sci. 2025, 26, 896. [Google Scholar] [CrossRef]
- Singh, S. Guttation: Quantification, Microbiology and Implications for Phytopathology. In Progress in Botany; Lüttge, U., Beyschlag, W., Cushman, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 75, pp. 187–214. [Google Scholar] [CrossRef]
- Aloni, R. Leaf Development and Vascular Differentiation. In Vascular Differentiation and Plant Hormones; Aloni, R., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 141–162. [Google Scholar] [CrossRef]
- Li, R.; He, Y.; Chen, J.; Zheng, S.; Zhuang, C. Research progress in improving photosynthetic efficiency. Int. J. Mol. Sci. 2023, 24, 9286. [Google Scholar] [CrossRef]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriqui, M.; Diaz-Espejo, A.; Douthe, C.; Dreyer, E.; Ferrio, J.P.; Gago, J.; et al. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 2012, 193–194, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Díaz-Espejo, A.; Conesa, M.; Coopman, R.; Douthe, C.; Gago, J.; Gallé, A.; Galmés, J.; Medrano, H.; Ribas-Carbo, M. Mesophyll conductance to CO2 and Rubisco as targets for improving intrinsic water use efficiency in C3 plants. Plant Cell Environ. 2016, 39, 965–982. [Google Scholar] [CrossRef]
- Ye, Z.-P.; Ling, Y.; Yu, Q.; Duan, H.-L.; Kang, H.-J.; Huang, G.-M.; Duan, S.-H.; Chen, X.-M.; Liu, Y.-G.; Zhou, S.-X. Quantifying light response of leaf-scale water-use efficiency and its interrelationships with photosynthesis and stomatal conductance in C3 and C4 species. Front. Plant Sci. 2020, 11, 374. [Google Scholar] [CrossRef]
- Haworth, M.; Marino, G.; Loreto, F.; Centritto, M. Integrating stomatal physiology and morphology: Evolution of stomatal control and development of future crops. Oecologia 2021, 197, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Terashima, I.; Hanba, Y.T.; Tholen, D.; Niinemets, Ü. Leaf Functional Anatomy in Relation to Photosynthesis. Plant Physiol. 2010, 155, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.; Zhang, N.; Tan, T.; Zhang, Z.; Wang, R.; Wu, L. Leaf microstructure and photosynthetic characteristics of a rice midvein-deficient mutant dl-14. Biol. Plant. 2022, 66, 172–177. [Google Scholar] [CrossRef]
- Chen, J.; Yue, K.; Shen, L.; Zheng, C.; Zhu, Y.; Han, K.; Kai, L. Aquaporins and CO2 diffusion across biological membrane. Front. Physiol. 2023, 14, 1205290. [Google Scholar] [CrossRef]
- Ding, L.; Li, Y.; Gao, L.; Lu, Z.; Wang, M.; Ling, N.; Shen, Q.; Guo, S. Aquaporin Expression and Water Transport Pathways inside Leaves Are Affected by Nitrogen Supply through Transpiration in Rice Plants. Int. J. Mol. Sci. 2018, 19, 256. [Google Scholar] [CrossRef]
- Raza, Q.; Rashid, M.A.R.; Waqas, M.; Ali, Z.; Rana, I.A.; Khan, S.H.; Khan, I.A.; Atif, R.M. Genomic diversity of aquaporins across genus Oryza provides a rich genetic resource for development of climate resilient rice cultivars. BMC Plant Biol. 2023, 23, 172. [Google Scholar] [CrossRef]
- Nguyen, M.X.; Moon, S.; Jung, K.-H. Genome-wide expression analysis of rice aquaporin genes and development of a functional gene network mediated by aquaporin expression in roots. Planta 2013, 238, 669–681. [Google Scholar] [CrossRef]
- Flexas, J.; Cano, F.J.; Carriquí, M.; Coopman, R.E.; Mizokami, Y.; Tholen, D.; Xiong, D. CO2 Diffusion Inside Photosynthetic Organs. In The Leaf: A Platform for Performing Photosynthesis; Adams Iii, W.W., Terashima, I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 163–208. [Google Scholar] [CrossRef]
- Oguchi, R.; Onoda, Y.; Terashima, I.; Tholen, D. Leaf Anatomy and Function. In The Leaf: A Platform for Performing Photosynthesis; Adams Iii, W.W., Terashima, I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 97–139. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakazono, M. Cavity Tissue for the Internal Aeration in Plants. In Responses of Plants to Soil Flooding; Sakagami, J.-I., Nakazono, M., Eds.; Springer Nature: Singapore, 2024; pp. 105–117. [Google Scholar] [CrossRef]
- Muir, C.D. Making pore choices: Repeated regime shifts in stomatal ratio. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151498. [Google Scholar] [CrossRef]
- Wang, X.; Guan, Y.; Zhang, D.; Dong, X.; Tian, L.; Qu, L.Q. A β-Ketoacyl-CoA Synthase Is Involved in Rice Leaf Cuticular Wax Synthesis and Requires a CER2-LIKE Protein as a Cofactor. Plant Physiol. 2016, 173, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, Y.; Zhang, H.; Huang, J. A β-ketoacyl-CoA Synthase OsCUT1 Confers Increased Drought Tolerance in Rice. Rice Sci. 2022, 29, 353–362. [Google Scholar] [CrossRef]
- Qin, B.-X.; Tang, D.; Huang, J.; Li, M.; Wu, X.-R.; Lu, L.-L.; Wang, K.-J.; Yu, H.-X.; Chen, J.-M.; Gu, M.-H. Rice OsGL1-1 is involved in leaf cuticular wax and cuticle membrane. Mol. Plant 2011, 4, 985–995. [Google Scholar] [CrossRef]
- Bernaola, L.; Butterfield, T.S.; Tai, T.H.; Stout, M.J. Epicuticular Wax Rice Mutants Show Reduced Resistance to Rice Water Weevil (Coleoptera: Curculionidae) and Fall Armyworm (Lepidoptera: Noctuidae). Environ. Entomol. 2021, 50, 948–957. [Google Scholar] [CrossRef]
- Pradhan, A.K.; Shandilya, Z.M.; Lahkar, L.; Hasnu, S.; Kalita, J.; Borgohain, D.; Tanti, B. Chapter 26—Comparative Metabolomics Approach Towards Understanding Chemical Variation in Rice Under Abiotic Stress. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 537–550. [Google Scholar]
- Hassanein, A.; Ibrahim, E.; Ali, R.A.; Hashem, H. Differential metabolic responses associated with drought tolerance in egyptian rice. J. Appl. Biol. Biotechnol. 2021, 9, 37–46. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.-A.; Asaf, S.; Lubna; Waqas, M.; Park, J.-R.; Asif, S.; Kim, N.; Lee, I.-J.; Kim, K.-M. Drought and UV radiation stress tolerance in rice is improved by overaccumulation of non-enzymatic antioxidant flavonoids. Antioxidants 2022, 11, 917. [Google Scholar] [CrossRef] [PubMed]
- Pons, T.L.; Flexas, J.; von Caemmerer, S.; Evans, J.R.; Genty, B.; Ribas-Carbo, M.; Brugnoli, E. Estimating mesophyll conductance to CO2: Methodology, potential errors, and recommendations. J. Exp. Bot. 2009, 60, 2217–2234. [Google Scholar] [CrossRef]
- von Caemmerer, S.; Evans, J.R. Enhancing C3 photosynthesis. Plant Physiol. 2010, 154, 589–592. [Google Scholar] [CrossRef]
- Flexas, J.; Niinemets, Ü.; Gallé, A.; Barbour, M.M.; Centritto, M.; Diaz-Espejo, A.; Douthe, C.; Galmés, J.; Ribas-Carbo, M.; Rodriguez, P.L. Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynth. Res. 2013, 117, 45–59. [Google Scholar] [CrossRef]
- Ouyang, W.; Struik, P.C.; Yin, X.; Yang, J. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J. Exp. Bot. 2017, 68, 5191–5205. [Google Scholar] [CrossRef] [PubMed]
- Zait, Y.; Ferrero-Serrano, Á.; Assmann, S.M. The α subunit of the heterotrimeric G protein regulates mesophyll CO2 conductance and drought tolerance in rice. New Phytol. 2021, 232, 2324–2338. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Zhang, Z.; Huang, G.; Xiong, Z.; Peng, S.; Li, Y.; Buckley, T. High leaf mass per area Oryza genotypes invest more leaf mass to cell wall and show a low mesophyll conductance. AoB Plants 2020, 12, plaa028. [Google Scholar] [CrossRef] [PubMed]
- This, D.; Comstock, J.; Courtois, B.; Xu, Y.; Ahmadi, N.; Vonhof, W.M.; McCouch, S. Genetic analysis of water use efficiency in rice (Oryza sativa L.) at the leaf level. Rice Sci. 2010, 3, 72–86. [Google Scholar] [CrossRef]
- Baloch, A.; Soomro, A.; Javed, M.; Ahmed, M.; Bughio, H.; Bughio, M.; Mastoi, N. Optimum plant density for high yield in rice (Oryza sativa L.). Asian J. Plant Sci. 2002, 1, 25–27. [Google Scholar] [CrossRef]
- Hamaoka, N.; Yasui, H.; Yamagata, Y.; Inoue, Y.; Furuya, N.; Araki, T.; Ueno, O.; Yoshimura, A. A hairy-leaf gene, BLANKET LEAF, of wild Oryza nivara increases photosynthetic water use efficiency in rice. Rice 2017, 10, 20. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L.; Liu, X.; Liu, L.; Cao, W.; Zhu, Y. Modeling the leaf angle dynamics in rice plant. PLoS ONE 2017, 12, e0171890. [Google Scholar] [CrossRef]
- Luo, X.; Zheng, J.; Huang, R.; Huang, Y.; Wang, H.; Jiang, L.; Fang, X. Phytohormones signaling and crosstalk regulating leaf angle in rice. Plant Cell Rep. 2016, 35, 2423–2433. [Google Scholar] [CrossRef]
- Chang, T.-G.; Zhao, H.; Wang, N.; Song, Q.-F.; Xiao, Y.; Qu, M.; Zhu, X.-G. A three-dimensional canopy photosynthesis model in rice with a complete description of the canopy architecture, leaf physiology, and mechanical properties. J. Exp. Bot. 2019, 70, 2479–2490. [Google Scholar] [CrossRef]
- Burgess, A.J.; Retkute, R.; Herman, T.; Murchie, E.H. Exploring relationships between canopy architecture, light distribution, and photosynthesis in contrasting rice genotypes using 3D canopy reconstruction. Front. Plant Sci. 2017, 8, 734. [Google Scholar] [CrossRef]
- T, S.; Vijayalakshmi, D. Physiological and biotechnological approach to improve water use efficiency in rice (Oryza sativa L.): Review. J. Pharmacogn. Phytochem. 2020, 9, 1044–1048. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Breeding for high water-use efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.; Richards, R. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Funct. Plant Biol. 1984, 11, 539–552. [Google Scholar] [CrossRef]
- Impa, S.M.; Nadaradjan, S.; Boominathan, P.; Shashidhar, G.; Bindumadhava, H.; Sheshshayee, M.S. Carbon Isotope Discrimination Accurately Reflects Variability in WUE Measured at a Whole Plant Level in Rice. Crop Sci. 2005, 45, 2517–2522. [Google Scholar] [CrossRef]
- Qu, M.; Hamdani, S.; Li, W.; Wang, S.; Tang, J.; Chen, Z.; Song, Q.; Li, M.; Zhao, H.; Chang, T. Rapid stomatal response to fluctuating light: An under-explored mechanism to improve drought tolerance in rice. Funct. Plant Biol. 2016, 43, 727–738. [Google Scholar] [CrossRef]
- Wu, A.; Hammer, G.L.; Doherty, A.; von Caemmerer, S.; Farquhar, G.D. Quantifying impacts of enhancing photosynthesis on crop yield. Nat. Plants 2019, 5, 380–388. [Google Scholar] [CrossRef]
- Raju, B.R.; Mohankumar, M.V.; Sumanth, K.K.; Rajanna, M.P.; Udayakumar, M.; Prasad, T.G.; Sheshshayee, M.S. Discovery of QTLs for water mining and water use efficiency traits in rice under water-limited condition through association mapping. Mol. Breed. 2016, 36, 35. [Google Scholar] [CrossRef]
- Vinarao, R.; Proud, C.; Snell, P.; Fukai, S.; Mitchell, J. QTL Validation and Development of SNP-Based High Throughput Molecular Markers Targeting a Genomic Region Conferring Narrow Root Cone Angle in Aerobic Rice Production Systems. Plants 2021, 10, 2099. [Google Scholar] [CrossRef]
- Yue, B.; Xue, W.-Y.; Luo, L.-J.; Xing, Y.-Z. QTL Analysis for Flag Leaf Characteristics and Their Relationships with Yield and Yield Traits in Rice. Acta Genet. Sin. 2006, 33, 824–832. [Google Scholar] [CrossRef]
- Hoang, G.T.; Gantet, P.; Nguyen, K.H.; Phung, N.T.P.; Ha, L.T.; Nguyen, T.T.; Lebrun, M.; Courtois, B.; Pham, X.H. Genome-wide association mapping of leaf mass traits in a Vietnamese rice landrace panel. PLoS ONE 2019, 14, e0219274. [Google Scholar] [CrossRef]
- Phetluan, W.; Wanchana, S.; Aesomnuk, W.; Adams, J.; Pitaloka, M.K.; Ruanjaichon, V.; Vanavichit, A.; Toojinda, T.; Gray, J.E.; Arikit, S. Candidate genes affecting stomatal density in rice (Oryza sativa L.) identified by genome-wide association. Plant Sci. 2023, 330, 111624. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Guo, Z.; Huang, C.; Wang, K.; Jiang, N.; Feng, H.; Chen, G.; Liu, Q.; Xiong, L. Genome-wide association study of rice (Oryza sativa L.) leaf traits with a high-throughput leaf scorer. J. Exp. Bot. 2015, 66, 5605–5615. [Google Scholar] [CrossRef]
- Liu, C.; Sack, L.; Li, Y.; Zhang, J.; Yu, K.; Zhang, Q.; He, N.; Yu, G. Relationships of stomatal morphology to the environment across plant communities. Nat. Commun. 2023, 14, 6629. [Google Scholar] [CrossRef]
- Xu, S. Estimation of Heritability. In Quantitative Genetics; Springer International Publishing: Cham, Switzerland, 2022; pp. 147–175. [Google Scholar]
- Narawatthana, S.; Phansenee, Y.; Thammasamisorn, B.-O.; Vejchasarn, P. Multi-model genome-wide association studies of leaf anatomical traits and vein architecture in rice. Front. Plant Sci. 2023, 14, 1107718. [Google Scholar] [CrossRef]
- Pavithra, S.; Senthil, A.; Djanaguiraman, M.; Raveendran, M.; Pushpam, R.; Boopathi, N.M. Estimating Genetic Variability and Diversity for Vein Density, Photosynthesis and Yield in Rice Genotypes. Int. J. Plant Soil Sci. 2022, 34, 48–56. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, W.; Chang, Y.; Ma, X.; Tu, H.; Xiong, F.; Jiang, N.; Feng, H.; Huang, C.; Yang, P.; et al. Genome-Wide Association Studies of Image Traits Reveal Genetic Architecture of Drought Resistance in Rice. Mol. Plant 2018, 11, 789–805. [Google Scholar] [CrossRef]
- Acevedo-Siaca, L.G.; Coe, R.; Quick, W.P.; Long, S.P. Evaluating natural variation, heritability, and genetic advance of photosynthetic traits in rice (Oryza sativa). Plant Breed. 2021, 140, 745–757. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Q.; Peng, S.; Xing, D.; Qin, J.; Laza, R.C.; Punzalan, B.R. Water use efficiency and physiological response of rice cultivars under alternate wetting and drying conditions. Sci. World J. 2012, 2012, 287907. [Google Scholar] [CrossRef]
- Dunn, B. Delaying Permanent Water on Drill Sown Rice, 4th ed.; Primefact 1238; NSW Department of Primary Industries: Orange, NS, USA; Leeton, NSW, Australia, 2023. Available online: https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0007/438955/Delaying-permanent-water-on-drill-sown-rice.pdf (accessed on 10 January 2025).
- Haonan, Q.; Jie, W.; Shihong, Y.; Zewei, J.; Yi, X. Current status of global rice water use efficiency and water-saving irrigation technology recommendations. J. Agron. Crop Sci. 2023, 209, 734–746. [Google Scholar] [CrossRef]
- Farmonaut. Revolutionizing Australian Rice Farming: Sustainable Water-Saving Technologies for Enhanced Productivity. 2024. Available online: https://farmonaut.com/australia/sustainable-rice-farming-5-water-saving-techs-boosting-australia (accessed on 20 October 2024).
- Smarter Irrigation for Profit Phase 2. Smart Irrigation Control in Rice Growing Systems: Application in Bankless Channel Irrigation. Smarter Irrigation for Profit Phase 2. 2021. Available online: https://smarterirrigation.com.au/wp-content/uploads/2021/08/Smart-Irrigation-control-in-rice-growing-systems_Case-Study_July-2021.pdf (accessed on 20 October 2024).
- Authority, M.D.B. New Technology Helping Rice Growers Use Less Water. 27 June 2022. Available online: https://www.mdba.gov.au/news-and-events/newsroom/new-technology-helping-rice-growers-use-less-water (accessed on 20 October 2024).
- Thakur, A.; Roychowdhury, S.; Kundu, D.; Singh, R. Evaluation of planting methods in irrigated rice. Arch. Agron. Soil Sci. 2004, 50, 631–640. [Google Scholar] [CrossRef]
- Sangavi, S.; Porpavai, S. Impact of irrigation scheduling and weed management on water use efficiency and yield of direct dry seeded rice. Madras Agric. J. 2018, 105, 1. [Google Scholar] [CrossRef]
- Cabangon, R.J.; Tuong, T.P.; Castillo, E.G.; Bao, L.X.; Lu, G.; Wang, G.; Cui, Y.; Bouman, B.A.M.; Li, Y.; Chen, C.; et al. Effect of irrigation method and N-fertilizer management on rice yield, water productivity and nutrient-use efficiencies in typical lowland rice conditions in China. Paddy Water Environ. 2004, 2, 195–206. [Google Scholar] [CrossRef]
- He, Q.; Di, D.; Yang, R.; Yuan, W.; Xiao, J.; Yao, Y.; Chen, Q.; Shi, W. Released control of vapor pressure deficit on rainfed rice evapotranspiration responses to extreme droughts in the subtropical zone. Plant Soil 2024, 1–19. [Google Scholar] [CrossRef]
- Haque, M.M.; Mackill, D.J.; Ingram, K.T. Inheritance of Leaf Epicuticular Wax Content in Rice. Crop Sci. 1992, 32, 865–868. [Google Scholar] [CrossRef]
- Wang, C.; Fa, X.; Meng, Q.; Zhang, Y.; Wang, W.; Zhu, K.; Zhang, W.; Gu, J.; Liu, L.; Zhang, J.; et al. Comparison of Agronomic and Physiological Characteristics for Rice Varieties Differing in Water Use Efficiency under Alternate Wetting and Drying Irrigation. Agronomy 2024, 14, 1986. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Q.; Gong, D.; Liu, H.; Luo, L.; Cui, J.; Qi, H.; Ma, F.; He, W.; Mancl, K. Biodegradable film mulching reduces the climate cost of saving water without yield penalty in dryland rice production. Resour. Conserv. Recycl. 2023, 197, 107071. [Google Scholar] [CrossRef]
- Malumpong, C.; Ruensuk, N.; Rossopa, B.; Channu, C.; Intarasathit, W.; Wongboon, W.; Poathong, K.; Kunket, K. Alternate Wetting and Drying (AWD) in broadcast rice (Oryza sativa L.) management to maintain yield, conserve water, and reduce gas emissions in Thailand. Agric. Res. 2021, 10, 116–130. [Google Scholar] [CrossRef]
- Omasa, K.; Konishi, A.; Tamura, H.; Hosoi, F. 3D Confocal laser scanning microscopy for the analysis of chlorophyll fluorescence parameters of chloroplasts in intact leaf tissues. Plant Cell Physiol. 2009, 50, 90–105. [Google Scholar] [CrossRef]
- Luan, Y.; Xu, J.; Lv, Y.; Liu, X.; Wang, H.; Liu, S. Improving the performance in crop water deficit diagnosis with canopy temperature spatial distribution information measured by thermal imaging. Agric. Water Manag. 2021, 246, 106699. [Google Scholar] [CrossRef]
- Jones, H.G.; Serraj, R.; Loveys, B.R.; Xiong, L.; Wheaton, A.; Price, A.H. Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct. Plant Biol. 2009, 36, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Mathers, A.W.; Hepworth, C.; Baillie, A.L.; Sloan, J.; Jones, H.; Lundgren, M.; Fleming, A.J.; Mooney, S.J.; Sturrock, C.J. Investigating the microstructure of plant leaves in 3D with lab-based X-ray computed tomography. Plant Methods 2018, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Van As, H. Intact plant MRI for the study of cell water relations, membrane permeability, cell-to-cell and long distance water transport. J. Exp. Bot. 2007, 58, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Gotarkar, D.; Doran, L.; Burns, M.; Hinkle, A.; Kromdijk, J.; Burgess, S.J. High-Throughput Analysis of Non-Photochemical Quenching in Crops Using Pulse Amplitude Modulated Chlorophyll Fluorometry. J. Vis. Exp. 2022, 2022, e63485. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Thorp, K.R.; Thompson, A.L.; Harders, S.J.; French, A.N.; Ward, R.W. High-throughput phenotyping of crop water use efficiency via multispectral drone imagery and a daily soil water balance model. Remote Sens. 2018, 10, 1682. [Google Scholar] [CrossRef]
- Langstroff, A.; Heuermann, M.C.; Stahl, A.; Junker, A. Opportunities and limits of controlled-environment plant phenotyping for climate response traits. Theor. Appl. Genet. 2022, 135, 1–16. [Google Scholar] [CrossRef]
- Fullana-Pericàs, M.; Conesa, M.À.; Gago, J.; Ribas-Carbó, M.; Galmés, J. High-throughput phenotyping of a large tomato collection under water deficit: Combining UAVs’ remote sensing with conventional leaf-level physiologic and agronomic measurements. Agric. Water Manag. 2022, 260, 107283. [Google Scholar] [CrossRef]
- Berni, J.A.; Zarco-Tejada, P.J.; Suárez, L.; Fereres, E. Thermal and narrowband multispectral remote sensing for vegetation monitoring from an unmanned aerial vehicle. IEEE Trans. Geosci. Remote Sens. 2009, 47, 722–738. [Google Scholar] [CrossRef]
- Asaari, M.S.M.; Mertens, S.; Verbraeken, L.; Dhondt, S.; Inzé, D.; Bikram, K.; Scheunders, P. Non-destructive analysis of plant physiological traits using hyperspectral imaging: A case study on drought stress. Comput. Electron. Agric. 2022, 195, 106806. [Google Scholar] [CrossRef]
- Omasa, K.; Hosoi, F.; Konishi, A. 3D lidar imaging for detecting and understanding plant responses and canopy structure. J. Exp. Bot. 2007, 58, 881–898. [Google Scholar] [CrossRef]
- Zhang, M.; Li, C.; Liu, Y.; Zhang, Y.; Nie, J.; Shao, S.; Mei, H.; Rogers, K.M.; Zhang, W.; Yuan, Y. Effects of Water Isotope Composition on Stable Isotope Distribution and Fractionation of Rice and Plant Tissues. J. Agric. Food Chem. 2024, 72, 8955–8962. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ellsworth, P.Z.; Zhou, J.; Cousins, A.B.; Sankaran, S. Evaluation of water-use efficiency in foxtail millet (Setaria italica) using visible-near infrared and thermal spectral sensing techniques. Talanta 2016, 152, 531–539. [Google Scholar] [CrossRef]
- Pérez-Bueno, M.L.; Pineda, M.; Barón, M. Phenotyping plant responses to biotic stress by chlorophyll fluorescence imaging. Front. Plant Sci. 2019, 10, 477268. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Rathnasamy, S.A.; Kambale, R.; Elangovan, A.; Mohanavel, W.; Shanmugavel, P.; Ramasamy, G.; Alagarsamy, S.; Marimuthu, R.; Rajagopalan, V.R.; Manickam, S. Altering Stomatal Density for Manipulating Transpiration and Photosynthetic Traits in Rice through CRISPR/Cas9 Mutagenesis. Curr. Issues Mol. Biol. 2023, 45, 3801–3814. [Google Scholar] [CrossRef] [PubMed]
- Kosová, K.; Urban, M.O.; Vítámvás, P.; Prášil, I.T. Drought stress response in common wheat, durum wheat, and barley: Transcriptomics, proteomics, metabolomics, physiology, and breeding for an enhanced drought tolerance. In Drought Stress Tolerance in Plants, Vol 2: Molecular and Genetic Perspectives; Springer: Berlin/Heidelberg, Germany, 2016; pp. 277–314. [Google Scholar]
- Sharma, N.; Raman, H.; Wheeler, D.; Kalenahalli, Y.; Sharma, R. Data-driven approaches to improve water-use efficiency and drought resistance in crop plants. Plant Sci. 2023, 336, 111852. [Google Scholar] [CrossRef]
- Mo, X.; Liu, S.; Lin, Z.; Xu, Y.; Xiang, Y.; McVicar, T. Prediction of crop yield, water consumption and water use efficiency with a SVAT-crop growth model using remotely sensed data on the North China Plain. Ecol. Model. 2005, 183, 301–322. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, B.; Cao, Y.; Zhang, Y.; Zhan, X.; Shen, X.; Cheng, S.; Lou, X.; Cao, L. Detection of epistatic and gene-environment interactions underlying three quality traits in rice using high-throughput genome-wide data. BioMed Res. Int. 2015, 2015, 135782. [Google Scholar] [CrossRef]
- Bouman, B.A.M.; Barker, R.; Humphreys, E.; Tuong, T.P.; Atlin, G.; Bennett, J.; Dawe, D.; Dittert, K.; Dobermann, A.; Facon, T.; et al. Rice: Feeding the billions. In Water for Food, Water for Life: A Comprehensive Assessment of Water Management in Agriculture; Molden, D., Ed.; Earthscan: London, UK, 2007; pp. 515–550. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, F.; Zhang, F.; Wang, W.; Zhou, Y.; Fu, B.; Li, Z. Comparative transcriptome sequencing of tolerant rice introgression line and its parents in response to drought stress. BMC Genom. 2014, 15, 1026. [Google Scholar] [CrossRef] [PubMed]
- Lenka, S.K.; Katiyar, A.; Chinnusamy, V.; Bansal, K.C. Comparative analysis of drought-responsive transcriptome in Indica rice genotypes with contrasting drought tolerance. Plant Biotechnol. J. 2011, 9, 315–327. [Google Scholar] [CrossRef]
- Chung, P.J.; Jung, H.; Jeong, D.-H.; Ha, S.-H.; Choi, Y.D.; Kim, J.-K. Transcriptome profiling of drought responsive noncoding RNAs and their target genes in rice. BMC Genom. 2016, 17, 563. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gong, F.; Cao, D.; Hu, X.; Wang, W. Advances in crop proteomics: PTMs of proteins under abiotic stress. Proteomics 2016, 16, 847–865. [Google Scholar] [CrossRef]
- Lawas, L.M.F.; Li, X.; Erban, A.; Kopka, J.; Jagadish, S.V.K.; Zuther, E.; Hincha, D.K. Metabolic responses of rice cultivars with different tolerance to combined drought and heat stress under field conditions. GigaScience 2019, 8, giz050. [Google Scholar] [CrossRef]
- Liang, Y.; Tabien, R.E.; Tarpley, L.; Mohammed, A.R.; Septiningsih, E.M. Transcriptome profiling of two rice genotypes under mild field drought stress during grain-filling stage. AoB Plants 2021, 13, plab043. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S.M.; Mir, R.A.; Ebinezer, L.B.; Masi, A.; Hami, A.; Manzoor, M.; Salgotra, R.K.; Sofi, N.R.; Mushtaq, R.; Rohila, J.S. Physiological and multi-omics approaches for explaining drought stress tolerance and supporting sustainable production of rice. Front. Plant Sci. 2022, 12, 803603. [Google Scholar] [CrossRef]
- Roy, N.; Debnath, P.; Gaur, H.S. Adoption of Multi-omics Approaches to Address Drought Stress Tolerance in Rice and Mitigation Strategies for Sustainable Production. Mol. Biotechnol. 2025, 1–13. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Z.; Tao, F.; Zhang, L.; Luo, Y.; Zhang, J.; Han, J.; Xie, J. Integrating multi-source data for rice yield prediction across China using machine learning and deep learning approaches. Agric. For. Meteorol. 2021, 297, 108275. [Google Scholar] [CrossRef]
- Singh, N.; Patel, D.; Khalekar, G. Methanogenesis and methane emission in rice/paddy fields. In Sustainable Agriculture Reviews 33. Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2018; Volume 33, pp. 135–170. [Google Scholar] [CrossRef]
- International Rice Research Institute (IRRI). Rice to Zero Hunger. 2019. Available online: https://www.irri.org/world-food-day-2019-rice-zero-hunger (accessed on 12 October 2024).
- Siopongco, J.D.; Sekiya, K.; Yamauchi, A.; Egdane, J.; Ismail, A.M.; Wade, L.J. Stomatal responses in rainfed lowland rice to partial soil drying; evidence for root signals. Plant Prod. Sci. 2008, 11, 28–41. [Google Scholar] [CrossRef]
- International Rice Research Institute (IRRI), SNP-Seek Database. Available online: https://snp-seek.irri.org/ (accessed on 25 January 2024).
- Yu, H.; Murchie, E.H.; González-Carranza, Z.H.; Pyke, K.A.; Roberts, J.A. Decreased photosynthesis in the erect panicle 3 (ep3) mutant of rice is associated with reduced stomatal conductance and attenuated guard cell development. J. Exp. Bot. 2015, 66, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Yoon, J.; Kim, H.Y.; Min, M.K.; Kim, J.-A.; Choi, E.-H.; Lan, W.; Bae, Y.-M.; Luan, S.; Cho, H.; et al. Unique Features of Two Potassium Channels, OsKAT2 and OsKAT3, Expressed in Rice Guard Cells. PLoS ONE 2013, 8, e72541. [Google Scholar] [CrossRef]
- Moon, S.-J.; Kim, H.Y.; Hwang, H.; Kim, J.-A.; Lee, Y.; Min, M.K.; Yoon, I.S.; Kwon, T.-R.; Kim, B.-G. A Dominant Negative OsKAT2 Mutant Delays Light-Induced Stomatal Opening and Improves Drought Tolerance without Yield Penalty in Rice. Front. Plant Sci. 2017, 8, 772. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, L.; Yu, Q.; Zhou, W.; Gou, X.; Li, J.; Hou, S. Multiple transcriptional factors control stomata development in rice. New Phytol. 2019, 223, 220–232. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, X.; Zhai, L.; Shao, K.; Jiang, K.; Shen, C.; Chen, K.; Wang, S.; Wang, Y.; Xu, J. Genetic Bases of the Stomata-Related Traits Revealed by a Genome-Wide Association Analysis in Rice (Oryza sativa L.). Front. Genet. 2020, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.N.; Lee, S.B.; Suh, M.C.; An, G.; Jung, K.-H. OsABCG9 Is an Important ABC Transporter of Cuticular Wax Deposition in Rice. Front. Plant Sci. 2018, 9, 960. [Google Scholar] [CrossRef]
- Islam, M.A.; Du, H.; Ning, J.; Ye, H.; Xiong, L. Characterization of Glossy1-homologous genes in rice involved in leaf wax accumulation and drought resistance. Plant Mol. Biol. 2009, 70, 443–456. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, L.; Zhang, L.; Zhang, Z.; Zhang, H.; Quan, R.; Zhou, S.; Huang, R. An ethylene response factor OsWR1 responsive to drought stress transcriptionally activates wax synthesis related genes and increases wax production in rice. Plant Mol. Biol. 2011, 78, 275–288. [Google Scholar] [CrossRef]
- Sheng, Z.; Lv, Y.; Li, W.; Luo, R.; Wei, X.; Xie, L.; Jiao, G.; Shao, G.; Wang, J.; Tang, S.; et al. Yellow-Leaf 1 encodes a magnesium-protoporphyrin IX monomethyl ester cyclase, involved in chlorophyll biosynthesis in rice (Oryza sativa L.). PLoS ONE 2017, 12, e0177989. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B. MicroRNAs in Control of Plant Development. J. Cell. Physiol. 2015, 231, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, Y.; Guo, Z.; Ding, Y.; Ding, C. RICE CENTRORADIALIS 1, a TFL1-like Gene, Responses to Drought Stress and Regulates Rice Flowering Transition. Rice 2020, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, D.; Wang, K.; Wu, X.; Lu, L.; Yu, H.; Gu, M.; Yan, C.; Cheng, Z. Mutations in the F-box gene LARGER PANICLE improve the panicle architecture and enhance the grain yield in rice. Plant Biotechnol. J. 2011, 9, 1002–1013. [Google Scholar] [CrossRef]
- Sakamoto, T. Phytohormones and rice crop yield: Strategies and opportunities for genetic improvement. Transgenic Res. 2006, 15, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, A.; Ozawa, M.; Nagasaki, H.; Kato, M.; Noda, Y.; Yamaguchi, T.; Nosaka, M.; Shimizu-Sato, S.; Nagasaki, A.; Maekawa, M.; et al. Two WUSCHEL-related homeobox Genes, narrow leaf2 and narrow leaf3, Control Leaf Width in Rice. Plant Cell Physiol. 2013, 54, 779–792. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, L.; Zeng, D.; Gao, Z.; Guo, L.; Fang, Y.; Zhang, G.; Dong, G.; Yan, M.; Liu, J.; et al. Identification and characterization of NARROW AND ROLLED LEAF 1, a novel gene regulating leaf morphology and plant architecture in rice. Plant Mol. Biol. 2010, 73, 283–292. [Google Scholar] [CrossRef]
- Sonah, H.; Deshmukh, R.; Chand, S.; Srinivasprasad, M.; Rao, G.; Upreti, H.; Singh, A.; Singh, N.; Sharma, T. Molecular mapping of quantitative trait loci for flag leaf length and other agronomic traits in rice (Oryza sativa). Cereal Res. Commun. 2012, 40, 362–372. [Google Scholar] [CrossRef]
- Miura, K.; Ikeda, M.; Matsubara, A.; Song, X.-J.; Ito, M.; Asano, K.; Matsuoka, M.; Kitano, H.; Ashikari, M. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat. Genet. 2010, 42, 545–549. [Google Scholar] [CrossRef]
- Hu, L.; Chen, W.; Yang, W.; Li, X.; Zhang, C.; Zhang, X.; Zheng, L.; Zhu, X.; Yin, J.; Qin, P.; et al. OsSPL9 Regulates Grain Number and Grain Yield in Rice. Front. Plant Sci. 2021, 12, 682018. [Google Scholar] [CrossRef]
- Yu, D.; Ranathunge, K.; Huang, H.; Pei, Z.; Franke, R.; Schreiber, L.; He, C. Wax Crystal-Sparse Leaf1 encodes a β–ketoacyl CoA synthase involved in biosynthesis of cuticular waxes on rice leaf. Planta 2008, 228, 675–685. [Google Scholar] [CrossRef]
- Tang, Y.; Bao, X.; Zhi, Y.; Wu, Q.; Guo, Y.; Yin, X.; Zeng, L.; Li, J.; Zhang, J.; He, W.; et al. Overexpression of a MYB Family Gene, OsMYB6, Increases Drought and Salinity Stress Tolerance in Transgenic Rice. Front. Plant Sci. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, J.; Ishikawa, F.; Yamaguchi, T.; Uemura, M.; Maeshima, M. Identification of 33 Rice Aquaporin Genes and Analysis of Their Expression and Function. Plant Cell Physiol. 2005, 46, 1568–1577. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, W.; Liu, L.; Chen, T.; Zhou, F.; Lin, Y. Identification and functional characterization of a rice NAC gene involved in the regulation of leaf senescence. BMC Plant Biol. 2013, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Matsukura, S.; Mizoi, J.; Yoshida, T.; Todaka, D.; Ito, Y.; Maruyama, K.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Comprehensive analysis of rice DREB2-type genes that encode transcription factors involved in the expression of abiotic stress-responsive genes. Mol. Genet. Genom. 2010, 283, 185–196. [Google Scholar] [CrossRef]
- Xiang, Y.; Tang, N.; Du, H.; Ye, H.; Xiong, L. Characterization of OsbZIP23 as a Key Player of the Basic Leucine Zipper Transcription Factor Family for Conferring Abscisic Acid Sensitivity and Salinity and Drought Tolerance in Rice. Plant Physiol. 2008, 148, 1938–1952. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Li, S.; He, S.; Waßmann, F.; Yu, C.; Qin, G.; Schreiber, L.; Qu, L.-J.; Gu, H. CFL1, a WW Domain Protein, Regulates Cuticle Development by Modulating the Function of HDG1, a Class IV Homeodomain Transcription Factor, in Rice and Arabidopsis. Plant Cell 2011, 23, 3392–3411. [Google Scholar] [CrossRef]
- Guo, T.; Wang, D.; Fang, J.; Zhao, J.; Yuan, S.; Xiao, L.; Li, X. Mutations in the Rice OsCHR4 Gene, Encoding a CHD3 Family Chromatin Remodeler, Induce Narrow and Rolled Leaves with Increased Cuticular Wax. Int. J. Mol. Sci. 2019, 20, 2567. [Google Scholar] [CrossRef]
- Cai, S.; Jiang, G.; Ye, N.; Chu, Z.; Xu, X.; Zhang, J.; Zhu, G.; Nguyen, H.T. A Key ABA Catabolic Gene, OsABA8ox3, Is Involved in Drought Stress Resistance in Rice. PLoS ONE 2015, 10, e0116646. [Google Scholar] [CrossRef]
- Jardim-Messeder, D.; Caverzan, A.; Balbinott, N.; Menguer, P.K.; Paiva, A.L.S.; Lemos, M.; Cunha, J.R.; Gaeta, M.L.; Costa, M.; Zamocky, M.; et al. Stromal Ascorbate Peroxidase (OsAPX7) Modulates Drought Stress Tolerance in Rice (Oryza sativa). Antioxidants 2023, 12, 387. [Google Scholar] [CrossRef]
- Li, H.-W.; Zang, B.-S.; Deng, X.-W.; Wang, X.-P. Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta 2011, 234, 1007–1018. [Google Scholar] [CrossRef]
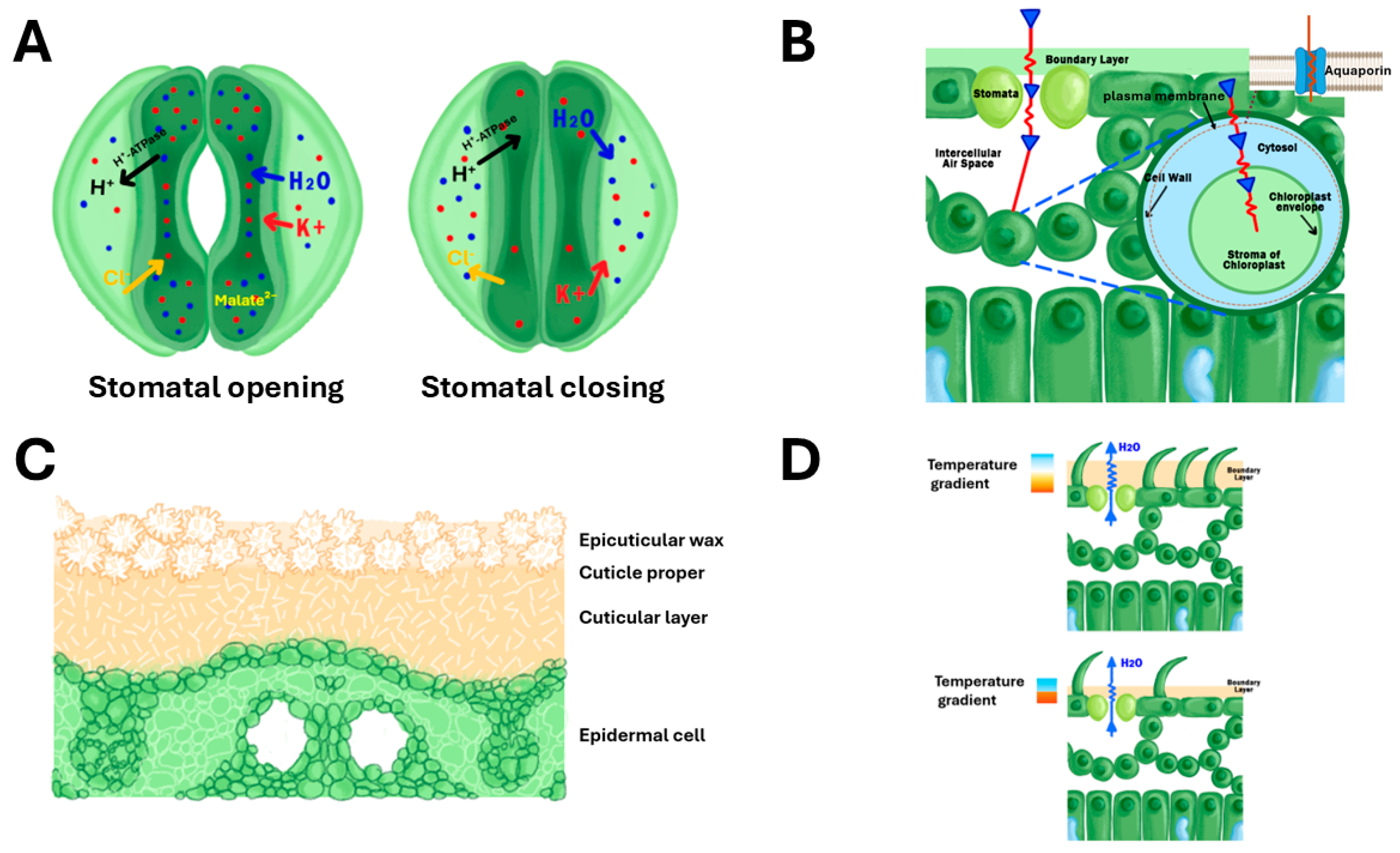
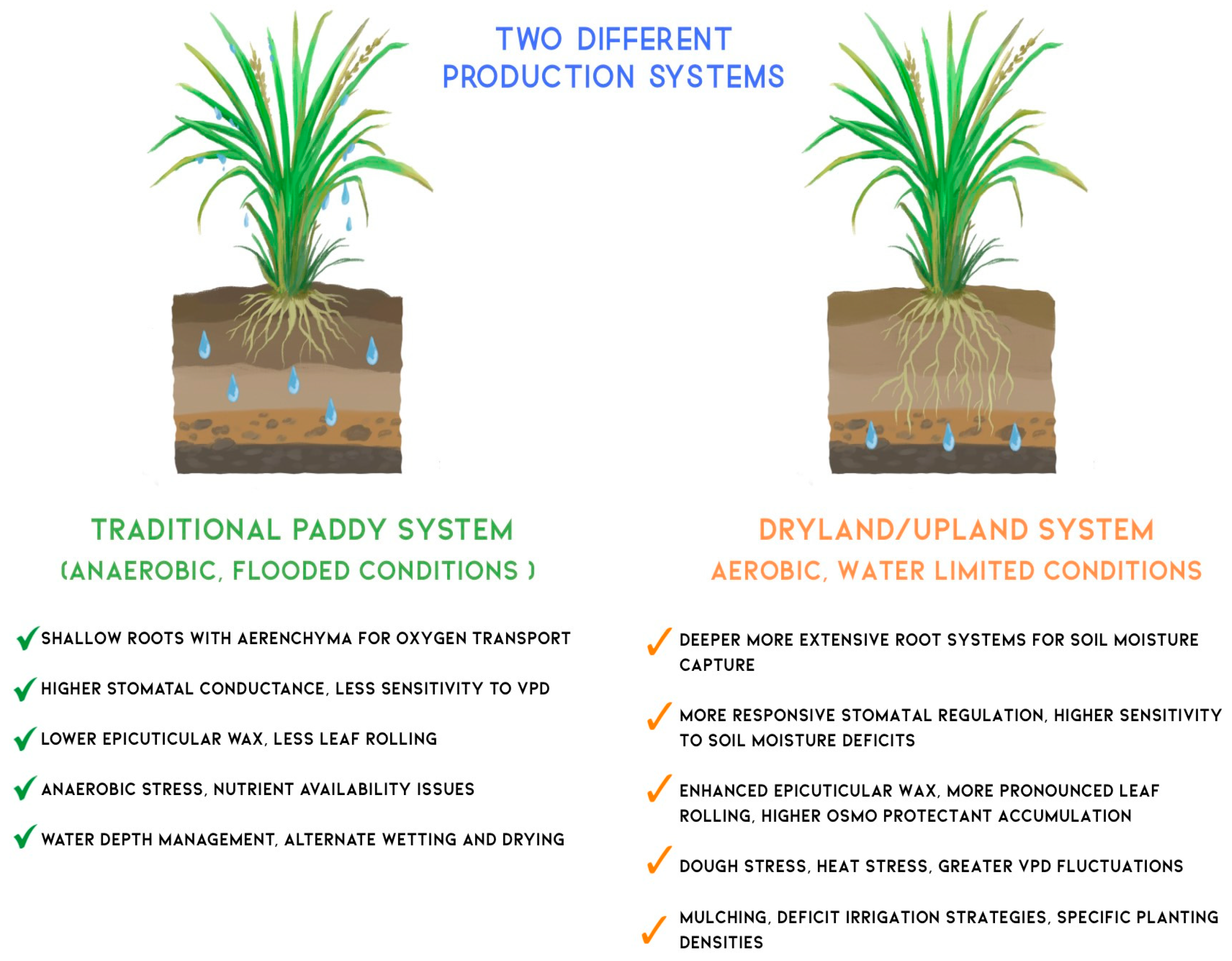
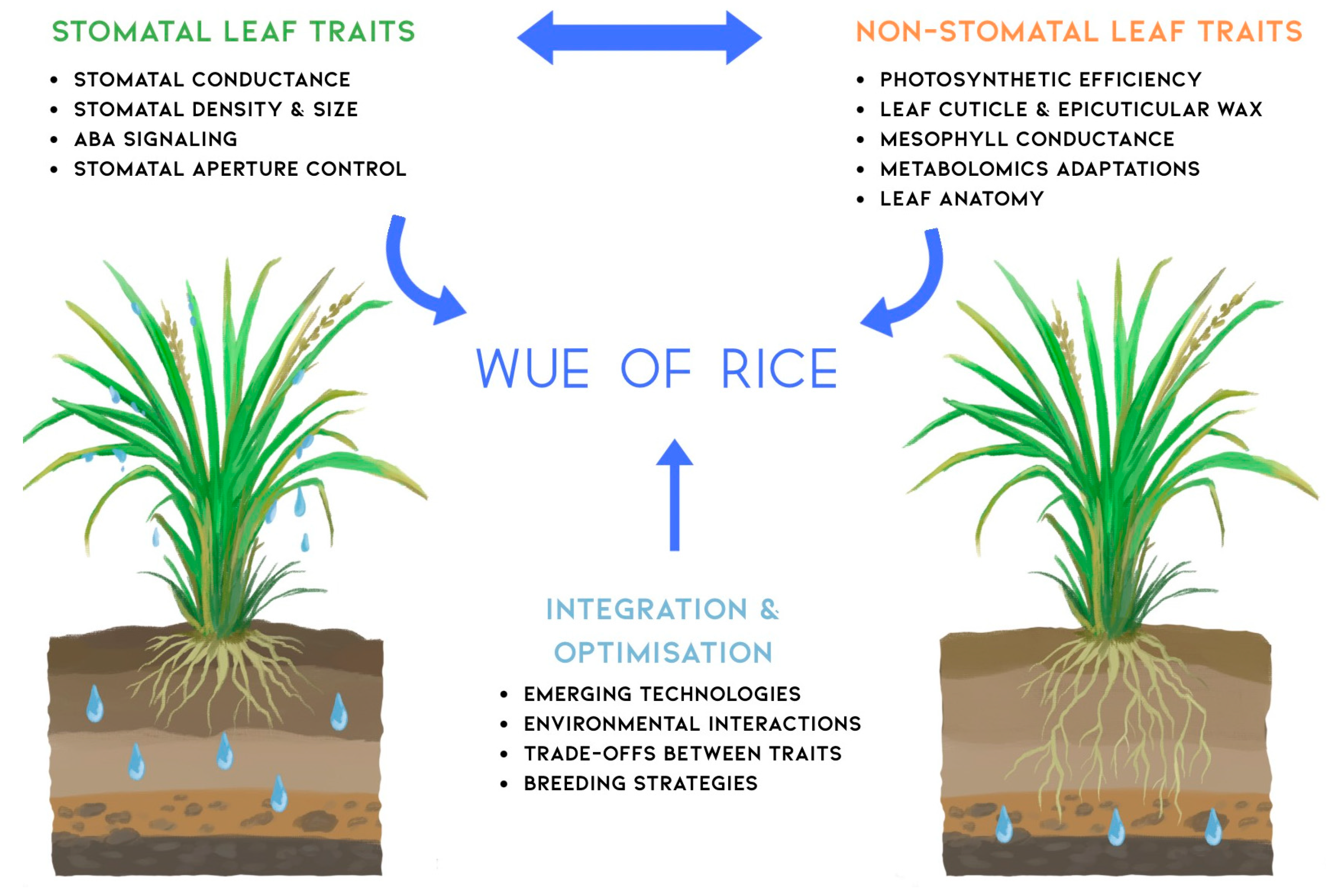
| Omics Level | Key Technologies | Applications to WUE | Notable Findings | Ref |
|---|---|---|---|---|
| Genomics | GWAS, QTL mapping, CRISPR-Cas9, transgenic overexpression | Identification of genomic regions associated with WUE; Targeted gene editing of stomatal regulators | Transgenic overexpression and CRISPR/Cas9-mediated editing of the OsEPF1 gene in rice have been shown to significantly reduce stomatal density, leading to improved drought tolerance and altered photosynthetic performance. These findings highlight OsEPF1 as a key regulator of WUE. | [33,91,130] |
| Transcriptomics | RNA-Seq, microarray analysis, RT-qPCR | Profiling gene expression networks under drought; Comparative transcriptome profiling of drought-tolerant and -sensitive rice genotypes; Identification of drought-responsive noncoding RNAs and their regulatory targets | Transcriptome analysis revealed hundreds of drought-responsive genes, including OsDREB2A and OsLEA3, which are key regulators in ABA-mediated drought response pathways; 66 miRNAs and 98 lncRNAs were differentially expressed under drought; miR171f-5p targeted Os03g0828701-00, suggesting a role in drought adaptation. | [137,138,139] |
| Proteomics | LC-MS/MS, iTRAQ, 2D electrophoresis | Identification of drought-responsive proteins; Analysis of PTMs facing water deficit | Over 2000 proteins were detected in rice leaves under drought; 42 showed significant changes. Key drought-responsive proteins included actin depolymerizing factor, S-like ribonuclease, and chloroplastic dehydroascorbate reductase. PTMs such as phosphorylation, ubiquitination, and glycosylation modulate protein function under drought, contributing to stress tolerance. | [139,140] |
| Metabolomics | GC-MS, LC-MS, NMR spectroscopy | Profiling of osmoprotectants and secondary metabolites under water stress, identifying antioxidant compounds that enhance WUE. | Flag leaves exhibited cultivar-specific increases in proline, sucrose, and malate under combined drought and heat stress. Overaccumulation of flavonoids, such as kaempferol and quercetin, enhances drought and UV tolerance by reducing oxidative damage, overexpressing flavanone 3-hydroxylase showed higher kaempferol and quercetin levels, lower levels of ROS and salicylic acid, and upregulated expression of DHN and UVR8 genes. | [66,141,142] |
| Phenomics | Thermal imaging, hyperspectral analysis, chlorophyll fluorescence, LiDAR | High-throughput field screening; Non-invasive measurement of physiological traits | Thermal imaging improves detection of crop water deficit by capturing spatial canopy temperature variations, enabling non-invasive and real-time assessments of plant responses to drought stress under field conditions; 3D LiDAR enables precise canopy structure analysis linked to stomatal function and transpiration. | [114,115,124,125] |
| Integrative Multi-Omics | Network analysis, systems biology, machine learning | Integration of genomic, transcriptomic, proteomic, and metabolomic data; Predictive modelling of WUE traits | Multi-omics integration revealed gene, protein, and metabolite interactions enhancing drought tolerance, highlighting the potential of big omics data to breed drought-resilient rice with improved WUE under climate change. Multi-omics integration provides a holistic view of biological responses to drought stress. Enables identification and manipulation of genes linked to drought tolerance. | [143,144,145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernando, Y.; Adams, M.; Kuhlmann, M.; Jr, V.B. Stomatal and Non-Stomatal Leaf Traits for Enhanced Water Use Efficiency in Rice. Biology 2025, 14, 843. https://doi.org/10.3390/biology14070843
Fernando Y, Adams M, Kuhlmann M, Jr VB. Stomatal and Non-Stomatal Leaf Traits for Enhanced Water Use Efficiency in Rice. Biology. 2025; 14(7):843. https://doi.org/10.3390/biology14070843
Chicago/Turabian StyleFernando, Yvonne, Mark Adams, Markus Kuhlmann, and Vito Butardo Jr. 2025. "Stomatal and Non-Stomatal Leaf Traits for Enhanced Water Use Efficiency in Rice" Biology 14, no. 7: 843. https://doi.org/10.3390/biology14070843
APA StyleFernando, Y., Adams, M., Kuhlmann, M., & Jr, V. B. (2025). Stomatal and Non-Stomatal Leaf Traits for Enhanced Water Use Efficiency in Rice. Biology, 14(7), 843. https://doi.org/10.3390/biology14070843







