Intervention Potential of a Recombinant Tarim Red Deer HGF Protein in a Mouse Model of Alcoholic Liver Disease
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. RNA Extraction and cDNA Synthesis
2.3. Construction of Prokaryotic Expression Vector
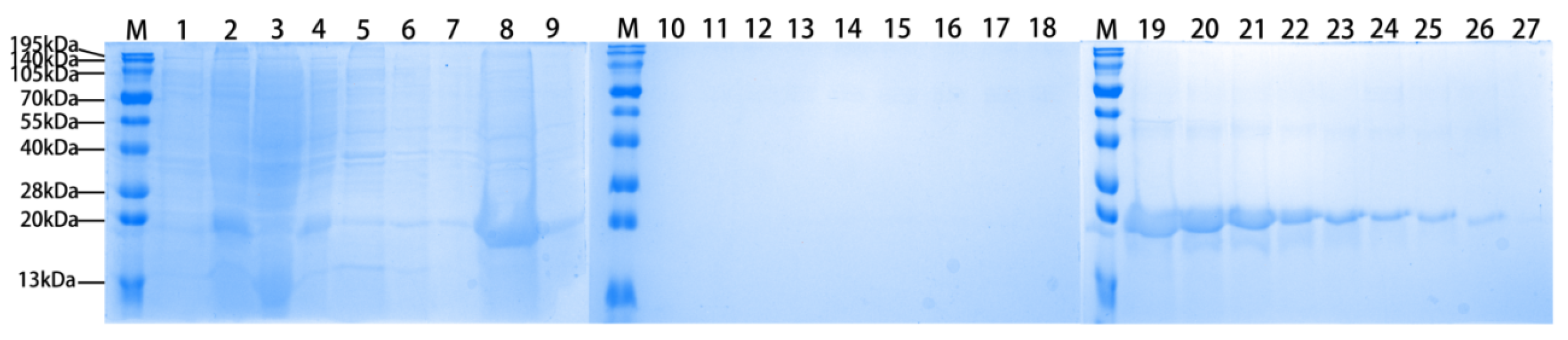
2.4. Expression and Purification of Recombinant Tarim Red Deer HGF
2.5. Animal Experiments
2.6. Histologic Examination
2.6.1. H&E Staining of Liver
2.6.2. Oil Red O Staining of Liver
2.6.3. Blood and Liver Biochemistry Tests
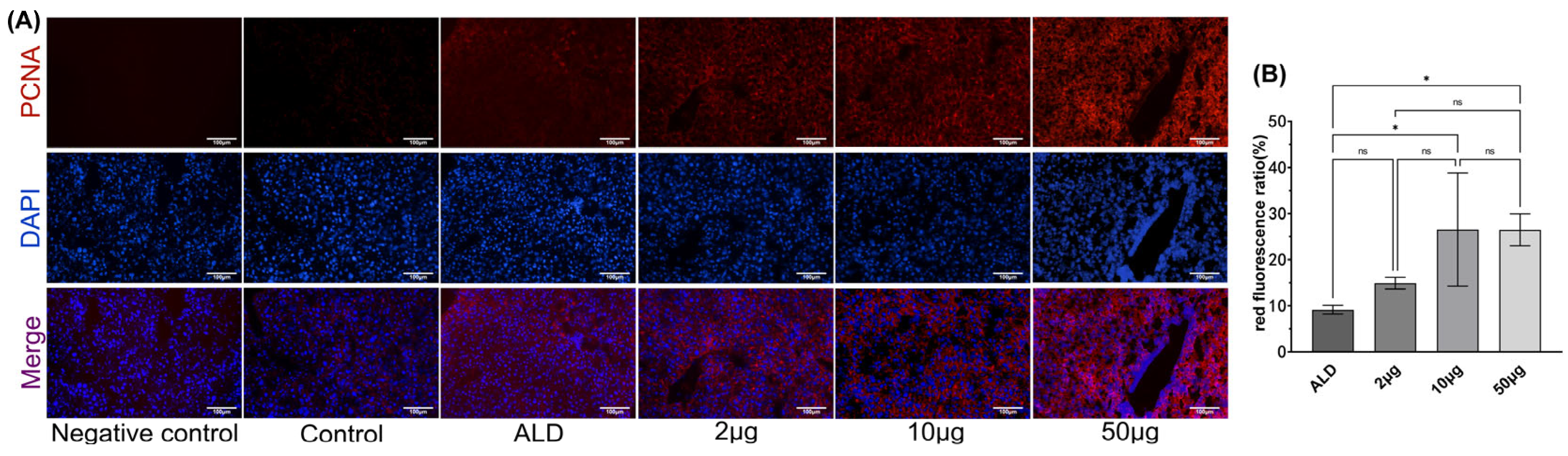
2.7. Immunofluorescence Detection of Cell Proliferation Marker (Proliferating Cell Nuclear Antigen, PCNA)
2.8. Statistical Method
3. Results
3.1. Expression and Purification of Recombinant Tarim Red Deer HGF
3.2. The Recombinant Agonist Treatment Can Alleviate Liver Injury in ALD Mice
3.3. The Recombinant Agonist Treatment Can Alleviate Liver Oxidative Stress in ALD Mice
3.4. The Recombinant Agonist Treatment Can Reduce Liver Fat Deposition in ALD Mice
3.5. The Recombinant Agonist Treatment Can Promote Liver Cell Proliferation in ALD Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nath, P.; Anand, A.C. Extrahepatic Manifestations in Alcoholic Liver Disease. J. Clin. Exp. Hepatol. 2022, 12, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Hu, W.; Tu, J.; Li, J.; Liang, Q.; Han, S. Pathogenic mechanisms and regulatory factors involved in alcoholic liver disease. J. Transl. Med. 2023, 21, 300. [Google Scholar] [CrossRef] [PubMed]
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.; Rehm, J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080. [Google Scholar] [CrossRef]
- Demkova, L.; Kucerova, L. Role of the HGF/c-MET tyrosine kinase inhibitors in metastasic melanoma. Mol. Cancer 2018, 17, 26. [Google Scholar] [CrossRef]
- Raj, S.; Kesari, K.K.; Kumar, A.; Rathi, B.; Sharma, A.; Gupta, P.K.; Jha, S.K.; Jha, N.K.; Slama, P.; Roychoudhury, S.; et al. Molecular mechanism(s) of regulation(s) of c-MET/HGF signaling in head and neck cancer. Mol. Cancer 2022, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lin, C.; Jia, X.; Fang, D.; Gao, Q.; Han, C. HGF/c-Met signaling promotes the migration and proliferation of deer antler MSCs. Sci. Rep. 2023, 13, 11121. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, W.; Wang, Y.; Chen, W. HGF/c-Met: A Key Promoter in Liver Regeneration. Front. Pharmacol. 2022, 13, 808855. [Google Scholar] [CrossRef]
- Choi, J.S.; Heang, O.S.; Kim, Y.M.; Lim, J.Y. Hyaluronic Acid/Alginate Hydrogel Containing Hepatocyte Growth Factor and Promotion of Vocal Fold Wound Healing. Tissue Eng. Regen. Med. 2020, 17, 651–658. [Google Scholar] [CrossRef]
- Wang, P.; Cui, Y.; Wang, J.; Liu, D.; Tian, Y.; Liu, K.; Wang, X.; Liu, L.; He, Y.; Pei, Y.; et al. Mesenchymal stem cells protect against acetaminophen hepatotoxicity by secreting regenerative cytokine hepatocyte growth factor. Stem Cell Res. Ther. 2022, 13, 94. [Google Scholar] [CrossRef]
- Li, N.; Dou, Z.; Liu, J.; Chai, B.; Li, Y.; An, X.; Chu, P.; Zhang, X. Therapeutic Effect of HGF on NASH Mice Through HGF/c-Met and JAK2-STAT3 Signalling Pathway. Ann. Hepatol. 2018, 17, 501–510. [Google Scholar] [CrossRef]
- de Nola, G.; Leclercq, B.; Mougel, A.; Taront, S.; Simonneau, C.; Forneris, F.; Adriaenssens, E.; Drobecq, H.; Iamele, L.; Dubuquoy, L.; et al. Dimerization of kringle 1 domain from hepatocyte growth factor/scatter factor provides a potent MET receptor agonist. Life Sci. Alliance 2022, 5, e202201424. [Google Scholar] [CrossRef] [PubMed]
- Bertola, A.; Mathews, S.; Ki, S.H.; Wang, H.; Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 2013, 8, 627–637. [Google Scholar] [CrossRef]
- Namachivayam, A.; Valsala, G.A. A review on molecular mechanism of alcoholic liver disease. Life Sci. 2021, 274, 119328. [Google Scholar] [CrossRef]
- Lin, C.; Wang, M.; Rui, X.; Chen, H.; Lv, H.; Huang, F.; Gao, Q.; Han, C. Hepatocyte growth factor (HGF) gene: Molecular characterisation of complete coding sequence and expression profile in Tarim red deer (Cervus hanglu yarkandensis) antlers. Anim. Prod. Sci. 2024, 64, AN2332. [Google Scholar] [CrossRef]
- Nakamura, T.; Mizuno, S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc. Jpn. Acad. Ser. B 2010, 86, 588–610. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sakai, K.; Nakamura, T.; Matsumoto, K. Hepatocyte growth factor twenty years on: Much more than a growth factor. J. Gastroenterol. Hepatol. 2011, 26 (Suppl. S1), 188–202. [Google Scholar] [CrossRef]
- Stolz, D.B.; Mars, W.M.; Petersen, B.E.; Kim, T.H.; Michalopoulos, G.K. Growth factor signal transduction immediately after two-thirds partial hepatectomy in the rat. Cancer Res. 1999, 59, 3954–3960. [Google Scholar] [PubMed]
- Lindroos, P.M.; Zarnegar, R.; Michalopoulos, G.K. Hepatocyte growth factor (hepatopoietin A) rapidly increases in plasma before DNA synthesis and liver regeneration stimulated by partial hepatectomy and carbon tetrachloride administration. Hepatology 1991, 13, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Botros, M.; Sikaris, K.A. The de ritis ratio: The test of time. Clin. Biochem. Rev. 2013, 34, 117–130. [Google Scholar]
- Han, J.; Lee, C.; Hur, J.; Jung, Y. Current Therapeutic Options and Potential of Mesenchymal Stem Cell Therapy for Alcoholic Liver Disease. Cells 2022, 12, 22. [Google Scholar] [CrossRef]
- Harjumaki, R.; Pridgeon, C.S.; Ingelman-Sundberg, M. CYP2E1 in Alcoholic and Non-Alcoholic Liver Injury. Roles of ROS, Reactive Intermediates and Lipid Overload. Int. J. Mol. Sci. 2021, 22, 8221. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; He, J.; Zhang, X.; Bian, Y.; Yang, L.; Xie, G.; Zhang, K.; Tang, W.; Stelter, A.A.; Wang, Q.; et al. Activation of the hedgehog pathway in human hepatocellular carcinomas. Carcinogenesis 2006, 27, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Cho, Y.E.; Hwang, S. Crosstalk between Oxidative Stress and Inflammatory Liver Injury in the Pathogenesis of Alcoholic Liver Disease. Int. J. Mol. Sci. 2022, 23, 774. [Google Scholar] [CrossRef]
- Tomita, K.; Azuma, T.; Kitamura, N.; Nishida, J.; Tamiya, G.; Oka, A.; Inokuchi, S.; Nishimura, T.; Suematsu, M.; Ishii, H. Pioglitazone prevents alcohol-induced fatty liver in rats through up-regulation of c-Met. Gastroenterology 2004, 126, 873–885. [Google Scholar] [CrossRef]
- Jing, Y.; Sun, Q.; Xiong, X.; Meng, R.; Tang, S.; Cao, S.; Bi, Y.; Zhu, D. Hepatocyte growth factor alleviates hepatic insulin resistance and lipid accumulation in high-fat diet-fed mice. J. Diabetes Investig. 2019, 10, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Tan, X.; Kwon, Y.; Delgado, E.R.; Zarnegar, A.; DeFrances, M.C.; Duncan, A.W.; Zarnegar, R. A Novel Humanized Model of NASH and Its Treatment with META4, A Potent Agonist of MET. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sun, C.; Zhao, Y.; Ye, B.; Yu, G. Signaling pathways of liver regeneration: Biological mechanisms and implications. iScience 2024, 27, 108683. [Google Scholar] [CrossRef]
- Liu, M.L.; Mars, W.M.; Zarnegar, R.; Michalopoulos, G.K. Collagenase pretreatment and the mitogenic effects of hepatocyte growth factor and transforming growth factor-alpha in adult rat liver. Hepatology 1994, 19, 1521–1527. [Google Scholar] [CrossRef]
- Kaibori, M.; Inoue, T.; Oda, M.; Naka, D.; Kawaguchi, T.; Kitamura, N.; Miyazawa, K.; Kwon, A.H.; Kamiyama, Y.; Okumura, T. Exogenously administered HGF activator augments liver regeneration through the production of biologically active HGF. Biochem. Biophys. Res. Commun. 2002, 290, 475–481. [Google Scholar] [CrossRef]
- Scheving, L.A.; Stevenson, M.C.; Taylormoore, J.M.; Traxler, P.; Russell, W.E. Integral role of the EGF receptor in HGF-mediated hepatocyte proliferation. Biochem. Biophys. Res. Commun. 2002, 290, 197–203. [Google Scholar] [CrossRef]
- Vallarola, A.; Tortarolo, M.; De Gioia, R.; Iamele, L.; de Jonge, H.; de Nola, G.; Bovio, E.; Pasetto, L.; Bonetto, V.; Freschi, M.; et al. A Novel HGF/SF Receptor (MET) Agonist Transiently Delays the Disease Progression in an Amyotrophic Lateral Sclerosis Mouse Model by Promoting Neuronal Survival and Dampening the Immune Dysregulation. Int. J. Mol. Sci. 2020, 21, 8542. [Google Scholar] [CrossRef] [PubMed]
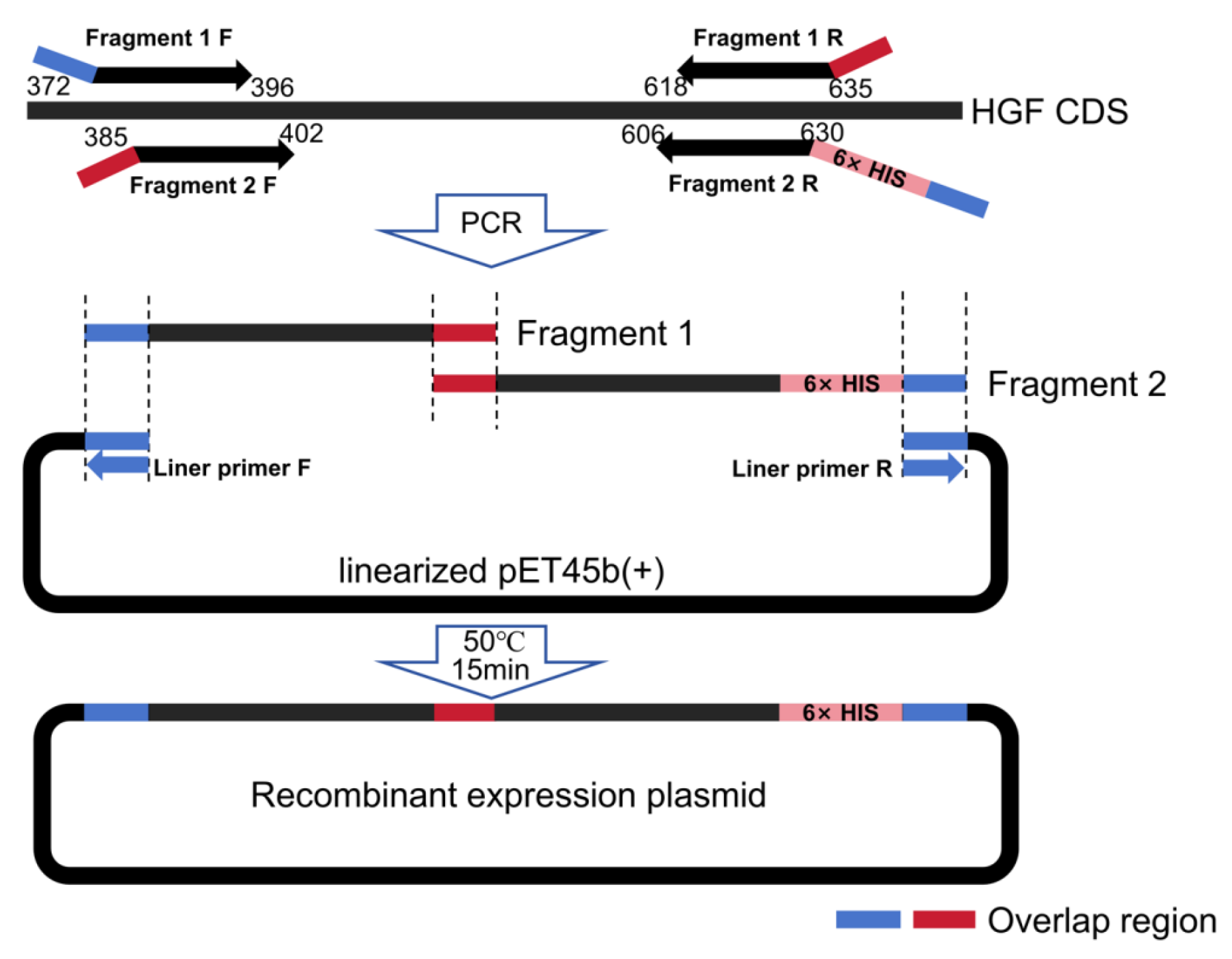



| Primer Sequence | Length | |
|---|---|---|
| Fragment 1 | F: AGAAGGAGATATACCATGGCCATTAGAAACTGTATCATTGGGAAA | 296 bp |
| R: CCTTTCCCAATGATGCATTCAACTTCTGAA | ||
| Fragment 2 | F: AAGTTGAATGCATCATTGGGAAAGGCGGT | 301 bp |
| R: CCAGACTCGAGTGCGGCCGCTCATCAATGATGATGATGATGATGTTCAACTTCTGAACACTGAGGAATG | ||
| Liner vector | F: GGCCATGGTATATCTCCTTCTT | 5152 bp |
| R: GCGGCCGCACTCGAGTCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Lin, C.; Xiang, X.; Yang, C.; Han, C.; Gao, Q. Intervention Potential of a Recombinant Tarim Red Deer HGF Protein in a Mouse Model of Alcoholic Liver Disease. Biology 2025, 14, 790. https://doi.org/10.3390/biology14070790
Chen H, Lin C, Xiang X, Yang C, Han C, Gao Q. Intervention Potential of a Recombinant Tarim Red Deer HGF Protein in a Mouse Model of Alcoholic Liver Disease. Biology. 2025; 14(7):790. https://doi.org/10.3390/biology14070790
Chicago/Turabian StyleChen, Hong, Chuan Lin, Xin Xiang, Chenchen Yang, Chunmei Han, and Qinghua Gao. 2025. "Intervention Potential of a Recombinant Tarim Red Deer HGF Protein in a Mouse Model of Alcoholic Liver Disease" Biology 14, no. 7: 790. https://doi.org/10.3390/biology14070790
APA StyleChen, H., Lin, C., Xiang, X., Yang, C., Han, C., & Gao, Q. (2025). Intervention Potential of a Recombinant Tarim Red Deer HGF Protein in a Mouse Model of Alcoholic Liver Disease. Biology, 14(7), 790. https://doi.org/10.3390/biology14070790






