Stray Dogs as Reservoirs and Sources of Infectious and Parasitic Diseases in the Environment of the City of Uralsk in Western Kazakhstan
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Clinical Examination
2.1.1. Blood Collection
2.1.2. Urine Collection
2.2. Coprological Pathogen Detection
2.3. Serological Pathogen Detection
2.4. Molecular Pathogen Detection
3. Results
3.1. Results of Blood and Urine Testing for Infectious Diseases Using PCR
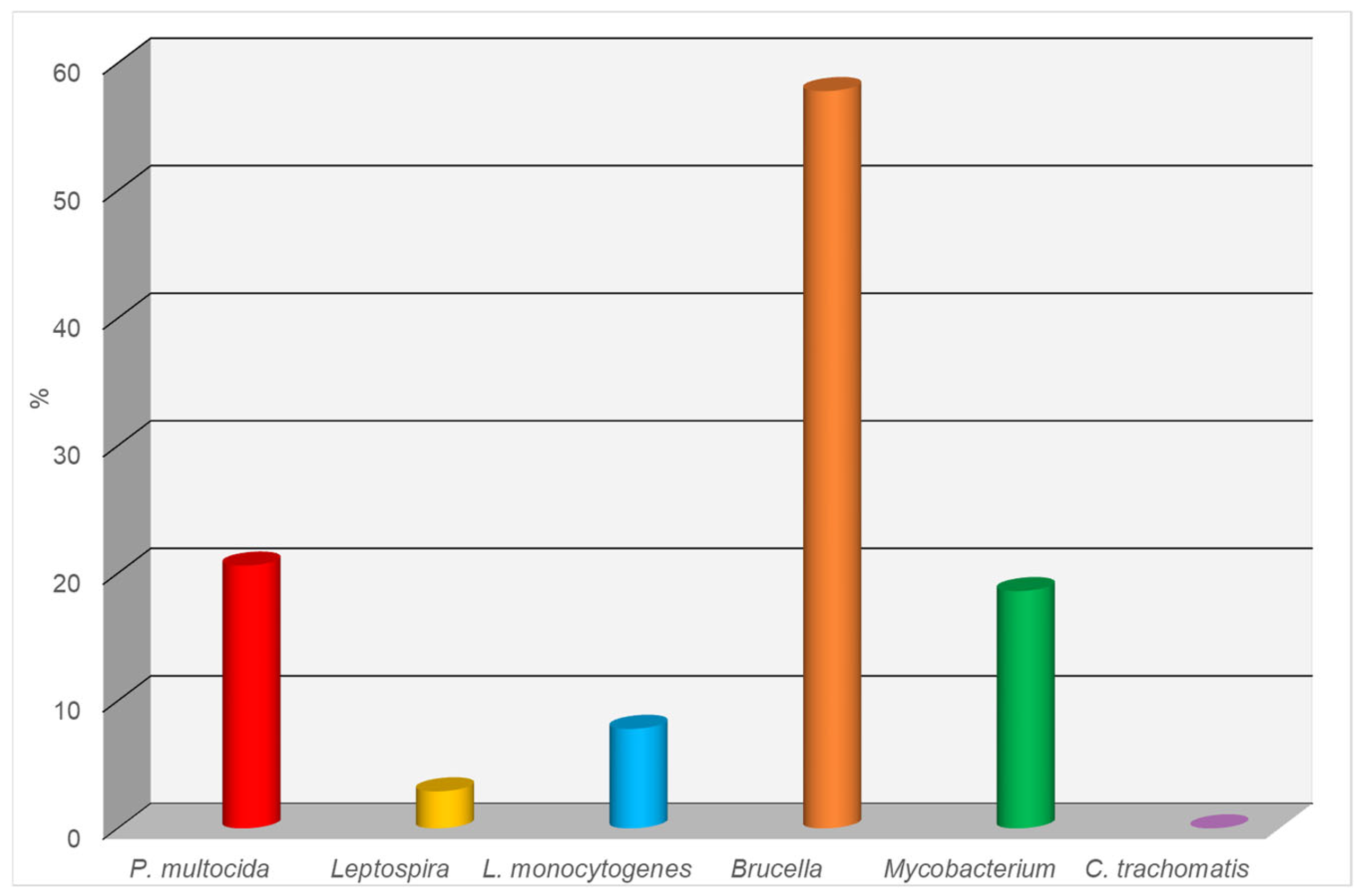
3.2. Results of ELISA Testing of Canine Serum Samples for Infectious Diseases
3.3. Results of Parasitological Examination of Fecal Samples from Stray Dogs Using the Fülleborn Flotation Method
3.4. Results of Dog Fecal Sample Analysis for Parasitic Diseases Using PCR Method
3.5. Results of Serum Analysis in Dogs for Parasitic Diseases Using ELISA
3.6. Analysis of Data Provided by the Veterinary Service of Uralsk and the West Kazakhstan Region
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCR | Polymerase Chain Reaction |
| EI | Extensity of Infection |
| II | Intensity of Infection |
References
- Mendoza, R.J.; Otranto, D. Zoonotic parasites associated with predation by dogs and cats. Par. Vect. 2023, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Kalambhe, D.; Wavhal, N. Neglected bacterial and parasitic zoonoses of tropical countries. In Current Topics in Zoonoses; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Zanzani, S.A.; Gazzonis, A.L.; Scarpa, P.; Berrilli, F.; Manfredi, M.T. Intestinal parasites of owned dogs and cats from metropolitan and micropolitan areas: Prevalence, zoonotic risks, and pet owner awareness in Northern Italy. BioMed Res. Int. 2014, 2014, 696508. [Google Scholar] [CrossRef] [PubMed]
- Abulude, O.A. Prevalence of intestinal helminth infections of stray dogs of public health significance in Lagos metropolis, Nigeria. Int. Ann. Sci. 2020, 9, 24–32. [Google Scholar] [CrossRef]
- Zurita, M.; Moscuzza, H.; Álvarez, G.; Acerbo, M.; Aranda, V.; Creixell, J.; Gutiérrez, B.; Tropeano, M.; Capitelli, G.; Perna, R. Relevamiento de parásitos zoonóticos y no zoonóticos en materia fecal canina en el Partido de San Pedro (Buenos Aires). Revista EXT. 2012, 4. Available online: https://revistas.unc.edu.ar/index.php/ext/article/view/1246 (accessed on 6 June 2025).
- Bruno, A.; De Salvo, M.N.; Beaudoin, J. Relevamiento de parasitosis zoonóticas realizado en siete plazas de la Ciudad Autónoma de Buenos Aires. Revista Arg. Parasit. 2015, 1, 22–23. [Google Scholar]
- Lucero, N.E.; Corazza, R.; Almuzara, M.N.; Reynes, E.; Escobar, G.I.; Boeri, E.; Ayala, S.M. Human Brucella canis outbreak linked to infection in dogs. Epidem. Inf. 2010, 138, 280–285. [Google Scholar] [CrossRef]
- Semenas, L.; Flores, V.; Viozzi, G.; Vázquez, G.; Pérez, A.; Ritossa, L. Helmintos zoonóticos en heces caninas de barrios de Bariloche (Río Negro, Patagonia, Argentina). Revista Arg. Parasit. 2014, 2, 22–27. [Google Scholar]
- Toews, E.; Musiani, M.; Smith, A.; Checkley, S.; Visscher, D.; Massolo, A. Risk factors for Echinococcus multilocularis intestinal infections in owned domestic dogs in a North American metropolis (Calgary, Alberta). Sci. Rep. 2024, 14, 5066. [Google Scholar] [CrossRef]
- Bonina, O.M.; Udaltzov, E.A.; Alekseenko, A.O.; Efremova, E.A. Aspects of Natural Focality of Opisthorchiasis in Novosibirsk Region. In Theory and Practice of Combating Parasitic Diseases, Proceedings of the Materials of the International Scientific Conference, Moscow, Russia, 21–24 November 2017; All-Russian Scientific Research Institute for Fundamental and Applied Parasitology of Animals and Plants—A branch of the Federal State Budget Scientific Institution “Federal Scientific Centre VIEV”: Moscow, Russia, 2017; Volume 18, pp. 78–80. Available online: https://cyberleninka.ru/article/n/nekotorye-aspekty-epidemicheskoy-i-epizooticheskoy-situatsii-po-trihinellezu-v-novosibirskoy-oblastii (accessed on 6 June 2025).
- Karmaliev, R.S.; Sidikhov, B.M.; Zhubantaev, I.N. Opisthorchiasis of carnivores in the conditions of West Kazakhstan region. In Proceedings of the Theory and Practice of Combating Parasitic Diseases, Proceedings of the Materials of the International Scientific Conference, Amritapuri, India, 2–4 November 2023; Volume 24, pp. 204–208. [Google Scholar] [CrossRef]
- Shabdarbayeva, G.; Yalysheva, S. A retrospective analysis of the prevalence of echinococcosis in the Republic of Kazakhstan. Bull. Nat. Acad. Sci. Rep. Kaz. 2020, 6, 63–70. [Google Scholar] [CrossRef]
- Karmaliev, R.S.; Aituganov, B.E. Parasitic diseases of dogs in Uralsk city: Epizootiology and prevention. Bull. Kaz. Agrotec. Univer. S. Seifullin. 2013, 2, 9–14. Available online: https://rmebrk.kz/magazine/4540# (accessed on 6 June 2025).
- Karmaliev, R.S.; Kereev, Y.M. Prevalence of Opisthorchiasis in the West Kazakhstan Region. Vet. J. 2013, 3, 33–34. Available online: https://elibrary.ru/download/elibrary_18948889_47577035.pdf (accessed on 6 June 2025).
- Nurgaliyev, B.; Kadraliyeva, B.; Kushmukhanov, Z.; Taubaev, U.; Tuleuov, A.; Zhumabayev, A. Results of parasitological research on hydrobionts from water bodies in West Kazakhstan region. Int. J. Vet. Sci. 2024, 13, 85–93. [Google Scholar]
- Karmaliev, R.S.; Sidikhov, B.M.; Dushaeva, L.Z.; Sabyrzhanov, A.; Nametov, A.M. Epizootic monitoring and control measures of helminthiasis in dogs and cats in Oral city. Sci. Educ. J. 2023, 2-2, 177–185. [Google Scholar] [CrossRef]
- Uakhit, R.S.; Lider, L.A.; Smagulova, A.M.; Leontyev, S.V.; Abdrakhmanov, S.K.; Kiyan, V.S. First molecular identification of Dirofilaria repens found in a wolf heart in Kazakhstan. Adv. Anim. Vet. Sci. 2021, 9, 1342–1346. [Google Scholar] [CrossRef]
- Lai, C.H.; Ting, C.H.; Tung, K.C.; Wang, J.S. Variation in the prevalence of dirofilariasis in stray dogs from central Taiwan. J. Chin. Soci. Vet. Sci. 2001, 27, 69–73. [Google Scholar]
- Tsai, I.; Tung, K.C.; Lai, C.H.; Wu, J.-T.; Wang, J.-S.; Cheng, F.-P. Short communication: Epidemiological survey of dirofilariasis of stray dogs in Taichung area. Taiwan Vet. J. 2003, 29, 71–75. [Google Scholar]
- Wu, C.C.; Fan, P.C. Prevalence of canine dirofilariasis in Taiwan. J. Helm. 2003, 77, 83–88. [Google Scholar] [CrossRef]
- Arkhipov, I.A.; Bashankaev, V.A.; Arkhipova, D.R. Spread of dirofilariasis and pathogenic role of its agents for dogs, cats and humans. In Proceedings of the Materials of Scientific Conference “Theory and Practice of Combating Parasitic Diseases (Zoonoses)”, Proceedings of the Scientific Conference, Moscow, Russia, 18–22 June 2002; Volume 3, pp. 22–24. [Google Scholar]
- Arkhipova, D.R. Biology of Dirofilariae and Epizootiology of Canine Dirofilariasis in the Steppe Zone of Southern Russia; Candidate of Biological Sciences Dissertation Abstract; Volgograd State University: Volgograd, Russia, 2003; 25p. [Google Scholar]
- Yastreb, V.B.; Arkhipov, I.A. Recommendations for Diagnosis, Treatment and Prevention of Canine Dirofilariasis in the Moscow Region. Rus. Parasit. J. 2008, 4, 109–115. Available online: https://cyberleninka.ru/article/n/rekomendatsii-po-diagnostike-lecheniyu-i-profilak-tike-dirofilyarioza-sobak-v-moskovskom-regione (accessed on 6 June 2025).
- Yastreb, V.B.; Arkhipov, I.A. Dirofilariasis of Dogs in Russia: Current State and Prevention Strategies. Rus. Parasit. J. 2006, 1, 45–50. [Google Scholar]
- Bittirov, A.M.; Elmurzayeva, D.A.; Nakova, L.V.; Khulamkhanova, M.M.; Balaeva, S.M.; Mirzoeva, N.M.; Begieva, S.A.; Grineva, L.G. New Data on Morphological Characteristics of Nematodes Dirofilaria Repens (Railliet & Henry, 1911) and Dirofilaria Immitis (Leidy, 1856) in Dogs in the North Caucasus Ecosystem. Bull. Moscow Soc. Natural. Biol. Sect. 2019, 3, 13–16. Available online: https://cyberleninka.ru/article/n/novye-dannye-o-morfologicheskih-harakteristikah-nematod-dirofilaria-repens-railliet-et-henry-1911-i-dirofilaria-immitis-leidy-1856-u-sobak (accessed on 6 June 2025).
- Otranto, D.; Dantas-Torres, F.; Mihalca, A.; Traub, R.; Lappin, M.; Baneth, G. Zoonotic parasites of sheltered and stray dogs in the era of the global economic and political crisis. Trends Parasit. 2017, 33, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Republic of Kazakhstan. Law on Responsible Treatment of Animals. 2021, No. 97-VII ZRK, 30 December 2021. Available online: https://adilet.zan.kz/rus/docs/Z2100000097 (accessed on 6 June 2025).
- Gracheva, O.A.; Pakhomov, G.A.; Eldashev, A.V. Methods of Blood Sampling in Various Species of Animals, Birds, and Fish [Educational-Methodical Manual]. Kazan Vet. 2008. Available online: https://kazanveterinary.ru/wp-content/uploads/2019/10/%D0%9C%D0%B5%D1%82%D0%BE%D0%B4%D1%8B-%D0%B2%D0%B7%D1%8F%D1%82%D0%B8%D1%8F-%D0%BA%D1%80%D0%BE%D0%B2%D0%B8-%D1%83-%D1%80%D0%B0%D0%B7%D0%BD%D1%8B%D1%85-%D0%B2%D0%B8%D0%B4%D0%BE%D0%B2-%D0%B6%D0%B8%D0%B2%D0%BE%D1%82%D0%BD%D1%8B%D1%85-%D0%BF%D1%82%D0%B8%D1%86-%D0%B8-%D1%80%D1%8B%D0%B1.pdf (accessed on 6 June 2025).
- Elliott, J.; Groer, H. Nephrology and Urology of Dogs and Cats (E. Makhiyanova, Trans.). Aquarium. 2014. Available online: https://www.labirint.ru/books/432822/ (accessed on 6 June 2025).
- Kotelnikov, G.A. Helminthological Studies of Animals and Environment: Handbook. Kolos. 1984. Available online: https://rusneb.ru/catalog/010003_000061_fbac14a5be6076b491caaca8ad5db2ef/ (accessed on 6 June 2025).
- Migacheva, L.D.; Kotelnikov, G.A. Methodical recommendations for using a device for counting helminth eggs. Bull. All-Union Instit. Helmint. 1987, 48, 81–83. [Google Scholar]
- Maksimova, N.E.; Mochulskaya, N.N.; Emelyanov, V.V. Fundamentals of Immunoassay [Textbook]. Ural Federal University Publishing. 2021. Available online: https://elar.urfu.ru/bitstream/10995/106083/1/978%E2%80%915%E2%80%917996-3295-3_2021.pdf (accessed on 6 June 2025).
- Pei, A.Y.; Oberdorf, W.E.; Nossa, C.W.; Agarwal, A.; Chokshi, P.; Gerz, E.A.; Jin, Z.; Lee, P.; Yang, L.; Poles, M.; et al. Diversity of 16S rRNA genes within individual prokaryotic genomes. Appl. Environ. Microb. 2010, 76, 3886–3897. [Google Scholar] [CrossRef]
- OIE. Manual of Standards for Diagnostic Tests and Vaccines, 6th ed.; OIE: Paris, France, 2008; pp. 524–530.
- Mohammed, M.E.A.; Saeed, A.M.; Khalid, K.E.; Abd El-Razig, S.A.; Mohammed, K.O. PCR detection of Pasteurella multocida by amplification of the kmt1 gene. J. Microb. Methods 2016, 7, 269. [Google Scholar]
- Palaniappan, R.U.; Chang, Y.F.; Chang, C.F.; Pan, M.J.; Yang, C.W.; Harpending, P.; McDonough, S.P.; Dubovi, E.; Divers, T.; Qu, J.; et al. Evaluation of lig-based conventional and real time PCR for the detection of pathogenic leptospires. Molec. Cell. Prob. 2005, 19, 111–117. [Google Scholar] [CrossRef]
- Kiguen, A.X.; Marramá, M.; Ruiz, S.; Estofan, P.; Venezuela, R.F.; Mosmann, J.P.; Monetti, M.S.; Rivero, V.; Cuffini, C.G. Prevalence, risk factors and molecular characterization of Chlamydia trachomatis in pregnant women from Córdoba, Argentina: A prospective study. PLoS ONE 2019, 14, e0217245. [Google Scholar] [CrossRef]
- Bricker, B.J.; Ewalt, D.R.; Olsen, S.C.; Jensen, A.E. Evaluation of the Brucella abortus species-specific polymerase chain reaction assay, an improved version of the Brucella AMOS polymerase chain reaction assay for cattle. J. Vet. Diagn. Investig. 2003, 15, 374–378. [Google Scholar] [CrossRef]
- Khademvatan, S.; Rahim, F.; Tavalla, M.; Abdizadeh, R.; Hashemitabar, M. PCR-based molecular characterization of Toxocara spp. using feces of stray cats: A study from Southwest Iran. PLoS ONE. 2013, 8, e65293. [Google Scholar] [CrossRef]
- Mirbadie, S.R.; Kamyabi, H.; Mohammadi, M.A.; Shamsaddini, S.; Harandi, M.F. Copro-PCR prevalence of Echinococcus granulosus infection in dogs in Kerman, south-eastern Iran. J. Helm. 2017, 92, 17–21. [Google Scholar] [CrossRef]
- Innis, M.A.; Gelfand, D.H.; Sninsky, J.J.; White, T.J. PCR Protocols: A Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990; Available online: https://api.pageplace.de/preview/DT0400.9780080886718_A23529424/preview-9780080886718_A23529424.pdf (accessed on 6 June 2025).
- Karmaliev, R.S.; Kadraliyeva, B.T.; Nametov, A.M.; Murzabayev, K.E.; Dushayeva, L.Z.; Orynkhanov, K.A.; Gabdulllin, D.E. Monitoring the prevalence of infectious and parasitic diseases and aggressive behavior of dogs in Uralsk. Gylym Zhane Bilim. Sci. Educ. 2024, 1, 193–202. [Google Scholar] [CrossRef]
- Likholat, S.D.; Konyaev, S.V. Prevalence of Canine Brucellosis Caused by Brucella canis in Shelters in Novosibirsk and Novosibirsk Region. Rus. Vet. J. Small Domest. Wild Anim. 2015, 5, 25–27. Available online: https://elibrary.ru/item.asp?id=24277023 (accessed on 6 June 2025).
- Menshenina, V.S. Canine Brucellosis. Vet. Doctor. 2013. Available online: https://vetexpert-rf.ru/article/brucellosis (accessed on 6 June 2025).
- Kalmykov, V.M.; Naimanov, A.K.; Kalmykova, M.S. Diagnosis of tuberculosis in dogs and cats using polymerase chain reaction. In Molecular Diagnostics 2017, Proceedings of the IX All-Russian Scientific and Practical Conference with International Participation, Novokuznetsk, Russia, 14–16 December 2017; Ulis Publishing House: Ulis, Russia, 2017; Volume 2, pp. 406–407. Available online: https://kpfu.ru/staff_files/F1634184337/Tom_2_s_oblozhkoj.pdf (accessed on 6 June 2025).
- Shalmenov, M.S.; Yastreb, V.B.; Valieva, Z.M.; Khairulin, A.G. Experience in Combating Echinococcosis Based on One Farm in the West Kazakhstan Region. Rus. Parasit. J. 2012, 100–102. Available online: https://www.vniigis.ru/izdaniya/rossiyskiy-parazitologicheskiy-zhurnal/vipuski_2012/rossiyskiy-parazitologicheskiy-zhurnal-2012-vypusk-4-kvartala (accessed on 6 June 2025).
- Aunpromma, S.; Kanjampa, P.; Papirom, P.; Tangkawattana, S.; Tangkawattana, P.; Tesana, S.; Boonmars, T.; Suwannatrai, A.; Uopsai, S.; Sukon, P.; et al. Prevalence and risk factors for Opisthorchis viverrini infection among cats and dogs in six districts surrounding the Ubolratana Dam, an endemic area for human opisthorchiasis in Northeastern Thailand. Southeast Asian J. Trop. Med. Public Health 2016, 47, 1153–1159. Available online: https://pubmed.ncbi.nlm.nih.gov/29634175/ (accessed on 6 June 2025). [PubMed]
- Destoumieux-Garzón, D.; Mavingui, P.; Boetsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Voituron, Y. The One Health Concept: 10 Years Old and a Long Road Ahead. Front. Vet. Sci. 2018, 5, 14. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Jeggo, M. The One Health Approach—Why Is It So Important? Trop. Med. Inf. Dis. 2019, 4, 88. [Google Scholar] [CrossRef]
- FAO; OIE; WHO. Taking a Multisectoral, One Health Approach: A Tripartite Guide to Addressing Zoonotic Diseases in Countries; World Health Org: Geneva, Switzerland, 2019.


| No. | Pathogen | Method of Investigation | Biological Samples |
|---|---|---|---|
| Infectious Diseases | |||
| 1 | Pasteurella multocida | PCR, ELISA | Blood, urine, serum |
| 2 | Leptospira spp. | PCR, ELISA | Blood, urine, serum |
| 3 | Chlamydia trachomatis | PCR, ELISA | Blood, urine, serum |
| 4 | Brucella spp. | PCR, ELISA | Blood, urine, serum |
| 5 | Listeria monocytogenes | ELISA | Serum |
| 6 | Mycobacterium spp. | ELISA | Serum |
| Parasitic Diseases | |||
| 7 | Toxocara canis | PCR, ELISA | Feces, blood serum |
| 8 | Echinococcus granulosus | PCR, ELISA | Feces, blood serum |
| Disease Name | Number of Blood and Urine Samples Tested | Number of Positive Blood Samples (n/%) | Number of Positive Urine Samples (n/%) |
|---|---|---|---|
| Leptospirosis | 102 | 0 | 0 |
| Pasteurellosis | 102 | 0 | 0 |
| Brucellosis | 102 | 5/4.9 | 5/4.9 |
| Chlamydiosis | 102 | 0 | 0 |
| Helminth Species | Animals Examined (n) | Animals Infected (n) | EI (%) | II Eggs/Animal |
|---|---|---|---|---|
| Opisthorchis felineus | 102 | 32 | 29.6 | 18.4 |
| Family taeniidae | 102 | 16 | 14.8 | 15.6 |
| Dipylidium caninum | 102 | 35 | 34.3 | 9.6 |
| Toxascaris leonina | 102 | 30 | 29.4 | 18.5 |
| Toxocara canis | 102 | 33 | 32.3 | 14.2 |
| Ancylostoma caninum | 102 | 36 | 35.3 | 23.6 |
| Parasites | Number of Examined Specimens | Number of Positive Cases | % |
|---|---|---|---|
| Toxocara canis | 102 | 40 | 39.2 |
| Echinococcus granulosus | 102 | 17 | 16.6 |
| No. | Helminth Species | Examined (n) | Infected (n) | Infection Rate, % |
|---|---|---|---|---|
| Class Trematoda | ||||
| 1 | Opisthorchis felineus | 7368 | 2203 | 29.9 |
| Class Cestoda | ||||
| 2 | Echinococcus granulosus | 7368 | 1105 | 15.0 |
| 3 | Dipylidium caninum | 7368 | 4045 | 54.9 |
| Class Nematoda | ||||
| 4 | Toxascaris leonina | 7368 | 5150 | 69.9 |
| 5 | Toxocara canis | 7368 | 5305 | 72.0 |
| 6 | Ancylostoma caninum | 7368 | 5526 | 75.0 |
| 7 | Uncinaria stenocephala | 7368 | 7368 | 100.0 |
| 8 | Dirofilaria repens | 7368 | 2166 | 29.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nametov, A.; Karmaliyev, R.; Sidikhov, B.; Murzabayev, K.; Orynkhanov, K.; Kadraliyeva, B.; Yertleuova, B.; Gabdullin, D.; Abilova, Z.; Dushayeva, L. Stray Dogs as Reservoirs and Sources of Infectious and Parasitic Diseases in the Environment of the City of Uralsk in Western Kazakhstan. Biology 2025, 14, 683. https://doi.org/10.3390/biology14060683
Nametov A, Karmaliyev R, Sidikhov B, Murzabayev K, Orynkhanov K, Kadraliyeva B, Yertleuova B, Gabdullin D, Abilova Z, Dushayeva L. Stray Dogs as Reservoirs and Sources of Infectious and Parasitic Diseases in the Environment of the City of Uralsk in Western Kazakhstan. Biology. 2025; 14(6):683. https://doi.org/10.3390/biology14060683
Chicago/Turabian StyleNametov, Askar, Rashid Karmaliyev, Bekzhassar Sidikhov, Kenzhebek Murzabayev, Kanat Orynkhanov, Bakytkanym Kadraliyeva, Balaussa Yertleuova, Dosmukan Gabdullin, Zulkyya Abilova, and Laura Dushayeva. 2025. "Stray Dogs as Reservoirs and Sources of Infectious and Parasitic Diseases in the Environment of the City of Uralsk in Western Kazakhstan" Biology 14, no. 6: 683. https://doi.org/10.3390/biology14060683
APA StyleNametov, A., Karmaliyev, R., Sidikhov, B., Murzabayev, K., Orynkhanov, K., Kadraliyeva, B., Yertleuova, B., Gabdullin, D., Abilova, Z., & Dushayeva, L. (2025). Stray Dogs as Reservoirs and Sources of Infectious and Parasitic Diseases in the Environment of the City of Uralsk in Western Kazakhstan. Biology, 14(6), 683. https://doi.org/10.3390/biology14060683






