Combined Ionizing Radiation Caused Cognition and Non-Cognition Behavior Benefits and Modulated Microglial Activity in Wild-Type and Alzheimer’s-like Transgenic Mice
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
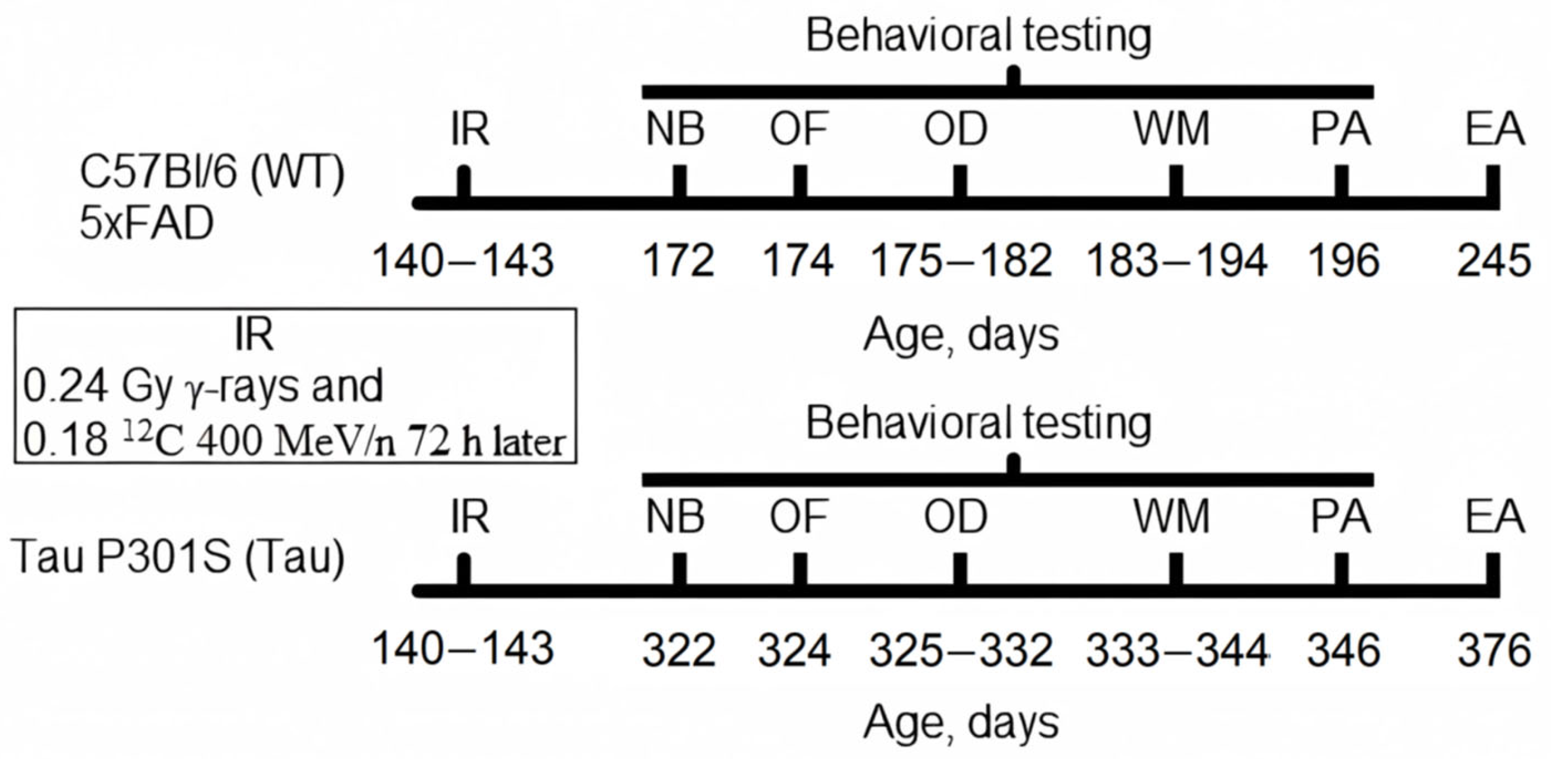
2.2. Experiment Timeline
2.3. Combined Irradiation
2.4. Nest Building
2.5. Open Field
2.6. Odor Discrimination
2.7. Water Maze
2.8. Step-Down-Type Passive Avoidance
2.9. Euthanasia and Tissue Preparation
2.10. Cytokines Multiplex Analysis
2.11. Data Analysis
3. Results
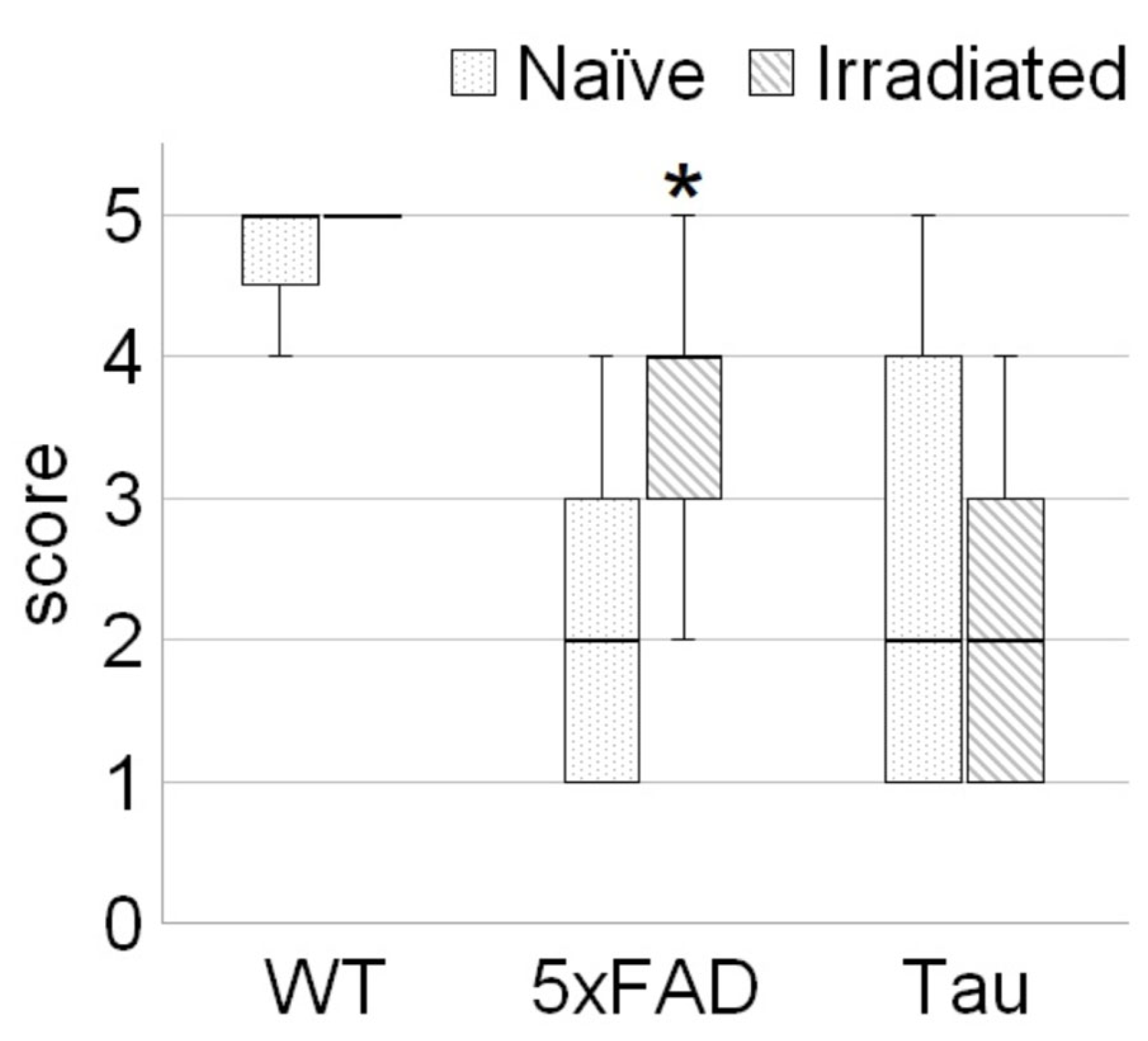
3.1. Irradiation Improved Welfare of 5xFAD Mice
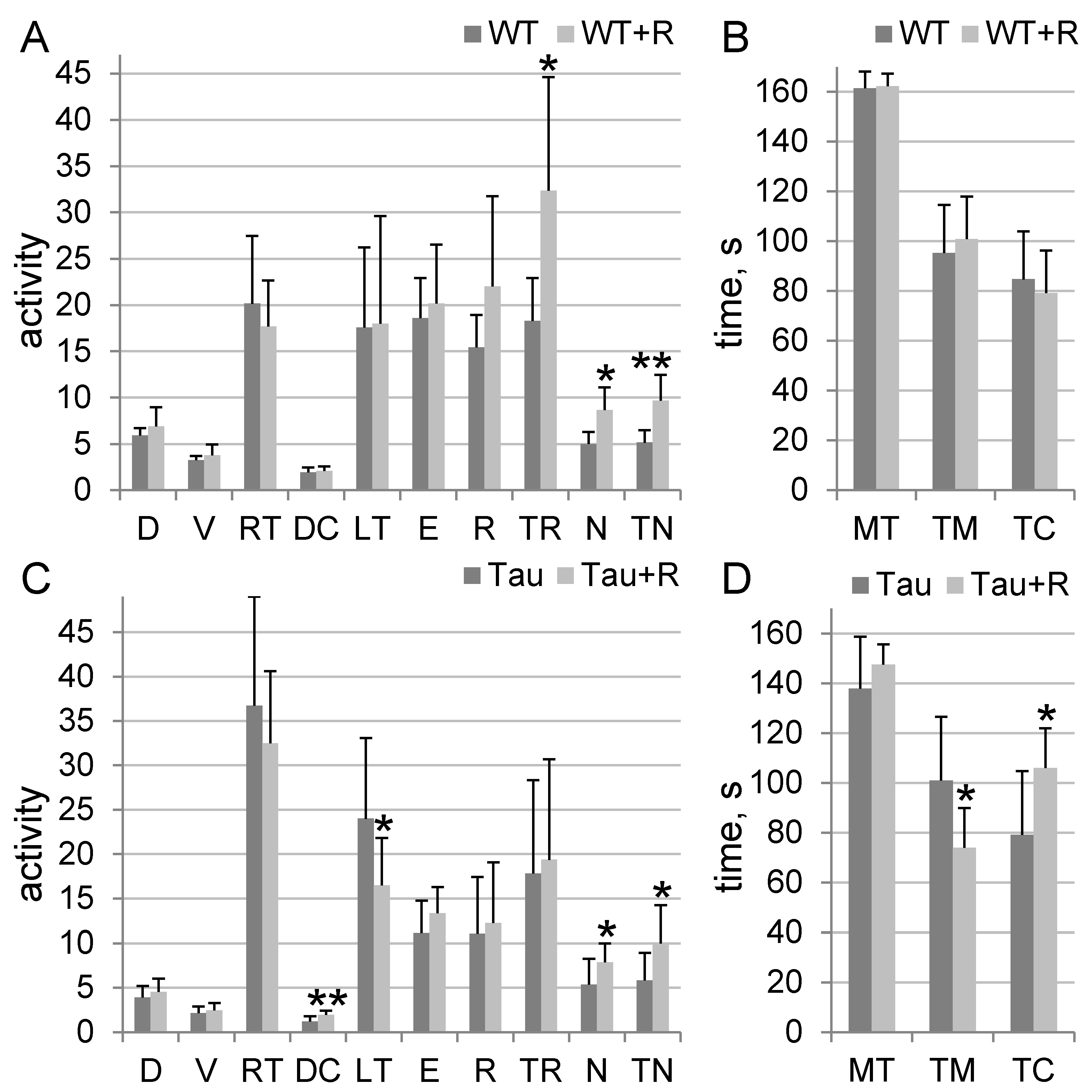
3.2. Irradiation Caused Anxiolytic Effects in Tau Mice and Stimulated Orientation and Exploratory Behavior in Tau and WT Mice
3.3. Irradiation Improved Ability to Discriminate Odors in 5xFAD Mice
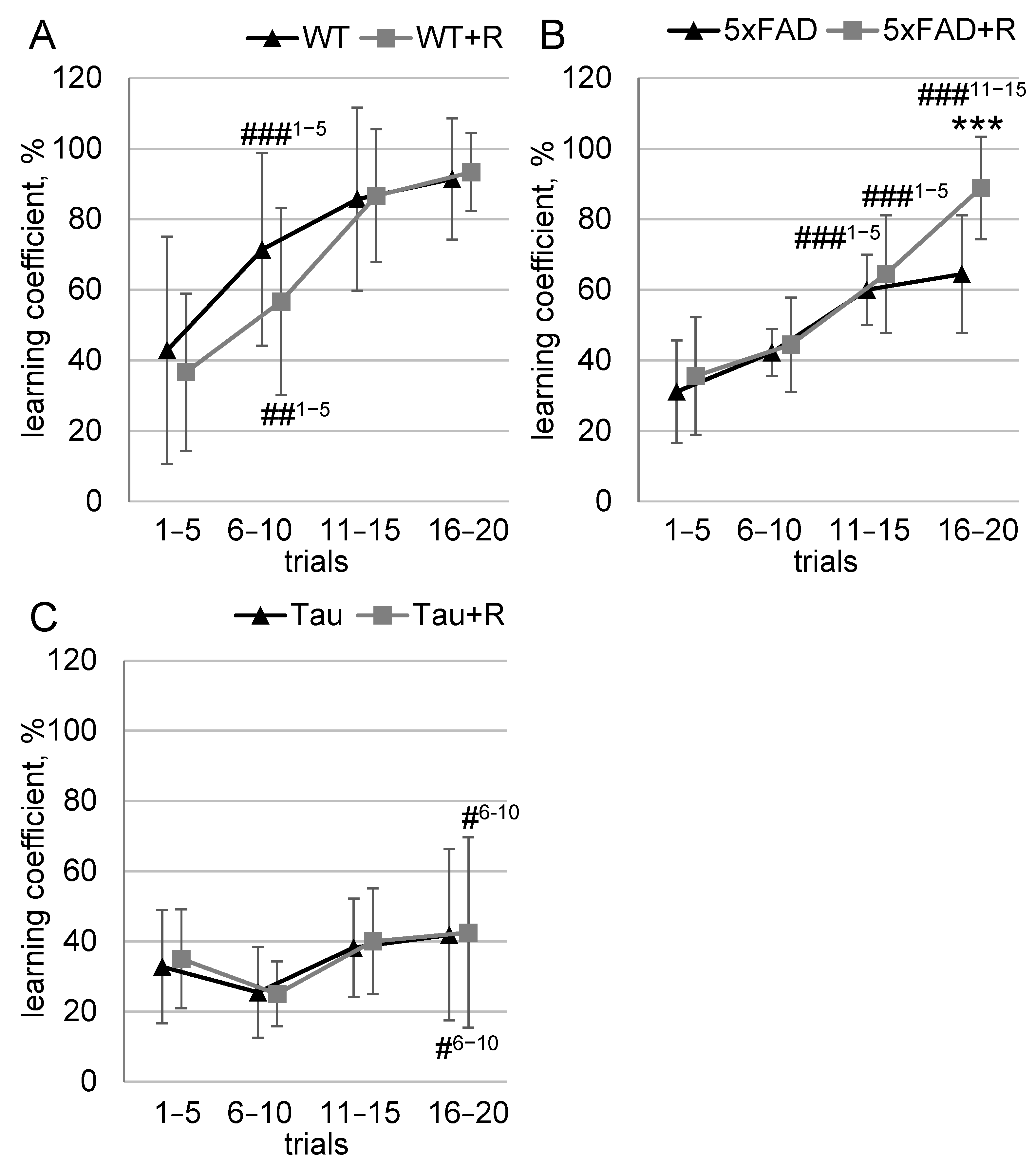
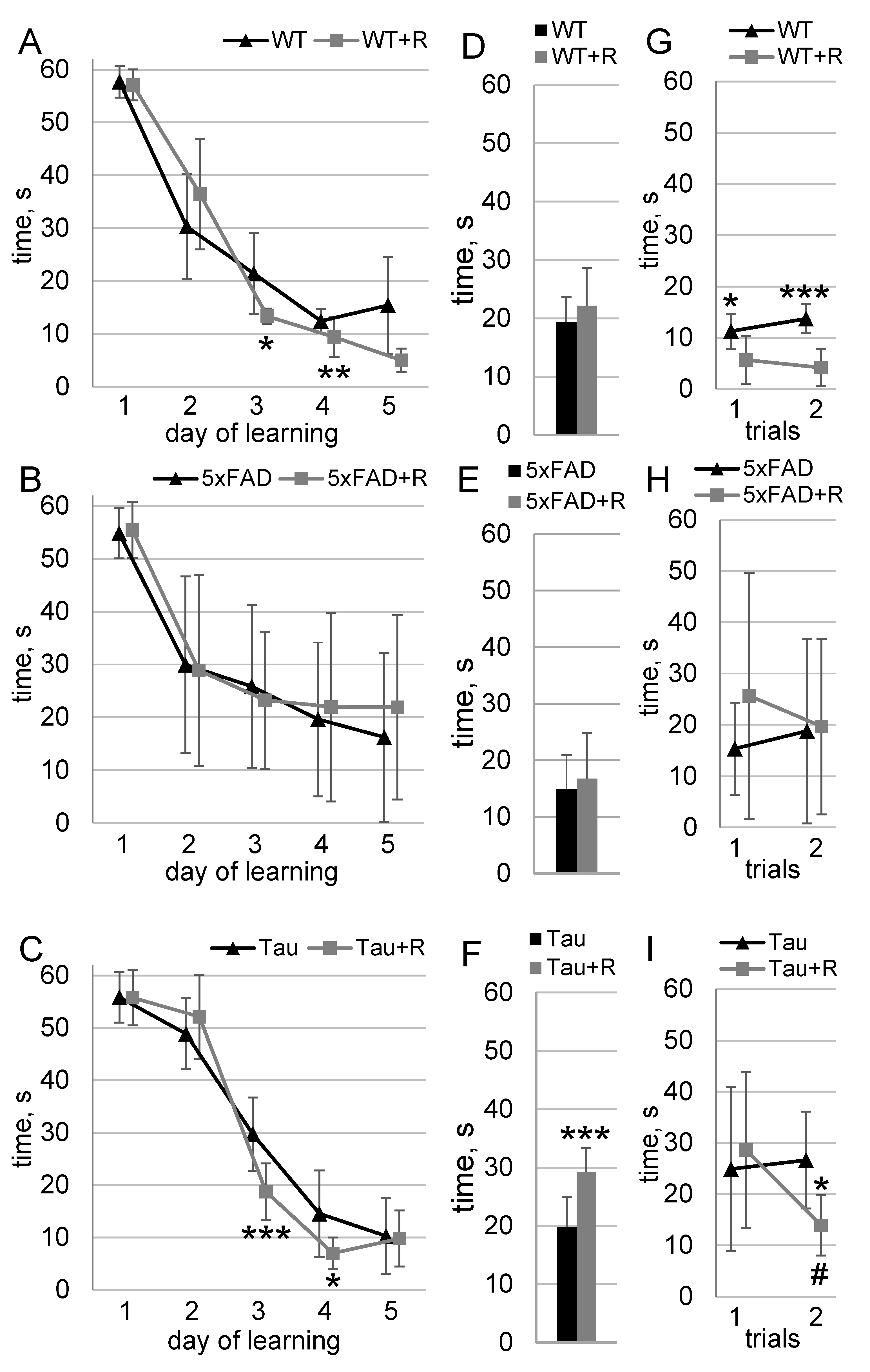
3.4. Irradiation Enhanced Spatial Learning and Long-Term Spatial Memory of WT and Tau Mice
3.5. Irradiation Had No Effect on Fear Memory
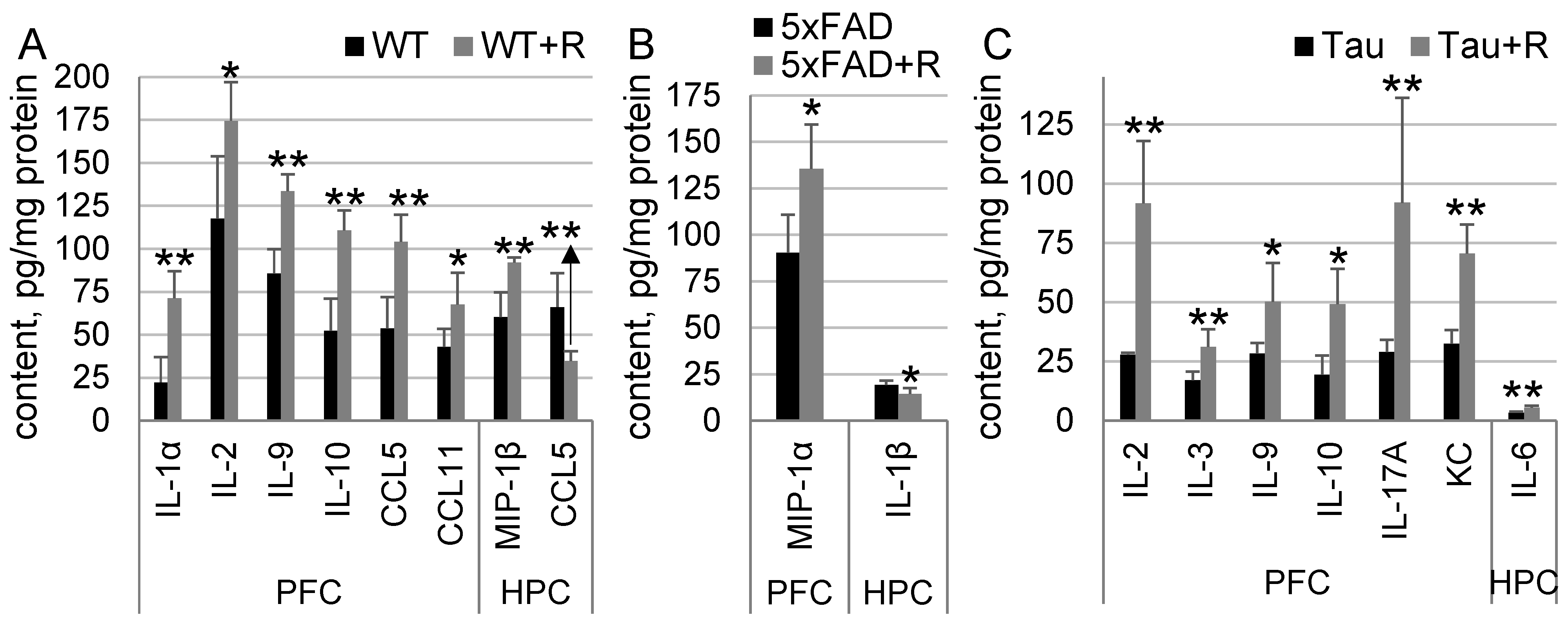
3.6. Irradiation Drastically Influenced Cytokine Content in WT and Tau Mice, and to a Much Lesser Extent in 5xFAD Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czapski, G.A.; Strosznajder, J.B. Glutamate and GABA in Microglia-Neuron Cross-Talk in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 11677. [Google Scholar] [CrossRef] [PubMed]
- Buccellato, F.R.; D’Anca, M.; Tartaglia, G.M.; Del Fabbro, M.; Scarpini, E.; Galimberti, D. Treatment of Alzheimer’s Disease: Beyond Symptomatic Therapies. Int. J. Mol. Sci. 2023, 24, 13900. [Google Scholar] [CrossRef] [PubMed]
- Melchiorri, D.; Merlo, S.; Micallef, B.; Borg, J.J.; Drafi, F. Alzheimer’s disease and neuroinflammation: Will new drugs in clinical trials pave the way to a multi-target therapy? Front. Pharmacol. 2023, 14, 1196413. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Cardoso, A.; Vale, N. Highlighting Immune System and Stress in Major Depressive Disorder, Parkinson’s, and Alzheimer’s Diseases, with a Connection with Serotonin. Int. J. Mol. Sci. 2021, 22, 8525. [Google Scholar] [CrossRef]
- Salani, F.; Sterbini, V.; Sacchinelli, E.; Garramone, M.; Bossu, P. Is Innate Memory a Double-Edge Sword in Alzheimer’s Disease? A Reappraisal of New Concepts and Old Data. Front. Immunol. 2019, 10, 1768. [Google Scholar] [CrossRef]
- Hoozemans, J.J.; Veerhuis, R.; Rozemuller, J.M.; Eikelenboom, P. Neuroinflammation and regeneration in the early stages of Alzheimer’s disease pathology. Int. J. Dev. Neurosci. 2006, 24, 157–165. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar]
- Li, T.; Lu, L.; Pember, E.; Li, X.; Zhang, B.; Zhu, Z. New Insights into Neuroinflammation Involved in Pathogenic Mechanism of Alzheimer’s Disease and Its Potential for Therapeutic Intervention. Cells 2022, 11, 1925. [Google Scholar] [CrossRef]
- Jin, X.; Yamashita, T. Microglia in central nervous system repair after injury. J. Biochem. 2016, 159, 491–496. [Google Scholar] [CrossRef]
- Parkhurst, C.N.; Yang, G.; Ninan, I.; Savas, J.N.; Yates, J.R., 3rd; Lafaille, J.J.; Hempstead, B.L.; Littman, D.R.; Gan, W.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 2013, 155, 1596–1609. [Google Scholar] [CrossRef] [PubMed]
- Mordelt, A.; de Witte, L.D. Microglia-mediated synaptic pruning as a key deficit in neurodevelopmental disorders: Hype or hope? Curr. Opin. Neurobiol. 2023, 79, 102674. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Sun, Y.; Peng, G. Neuroinflammation as a Potential Therapeutic Target in Alzheimer’s Disease. Clin. Interv. Aging 2022, 17, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Byrne, S.; Middleton, R.J.; Banati, R.B.; Liu, G.J. Control of Neuroinflammation through Radiation-Induced Microglial Changes. Cells 2021, 10, 2381. [Google Scholar] [CrossRef]
- Kempf, S.J.; Janik, D.; Barjaktarovic, Z.; Braga-Tanaka, I., 3rd; Tanaka, S.; Neff, F.; Saran, A.; Larsen, M.R.; Tapio, S. Chronic low-dose-rate ionising radiation affects the hippocampal phosphoproteome in the ApoE-/- Alzheimer’s mouse model. Oncotarget 2016, 7, 71817–71832. [Google Scholar] [CrossRef]
- Yang, E.J.; Kim, H.; Choi, Y.; Kim, H.J.; Kim, J.H.; Yoon, J.; Seo, Y.S.; Kim, H.S. Modulation of Neuroinflammation by Low-Dose Radiation Therapy in an Animal Model of Alzheimer’s Disease. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 658–670. [Google Scholar] [CrossRef]
- Marples, B.; McGee, M.; Callan, S.; Bowen, S.E.; Thibodeau, B.J.; Michael, D.B.; Wilson, G.D.; Maddens, M.E.; Fontanesi, J.; Martinez, A.A. Cranial irradiation significantly reduces beta amyloid plaques in the brain and improves cognition in a murine model of Alzheimer’s Disease (AD). Radiother. Oncol. 2016, 118, 43–51. [Google Scholar] [CrossRef]
- Wilson, G.D.; Wilson, T.G.; Hanna, A.; Fontanesi, G.; Kulchycki, J.; Buelow, K.; Pruetz, B.L.; Michael, D.B.; Chinnaiyan, P.; Maddens, M.E.; et al. Low Dose Brain Irradiation Reduces Amyloid-β and Tau in 3xTg-AD Mice. J. Alzheimer’s Dis. 2020, 75, 15–21. [Google Scholar] [CrossRef]
- Iacono, D.; Murphy, E.K.; Stimpson, C.D.; Perl, D.P.; Day, R.M. Low-dose brain radiation: Lowering hyperphosphorylated-tau without increasing DNA damage or oncogenic activation. Sci. Rep. 2023, 13, 21142. [Google Scholar] [CrossRef]
- Bevelacqua, J.J.; Mortazavi, S.M.J. Alzheimer ‘s Disease: Possible Mechanisms Behind Neurohormesis Induced by Exposure to Low Doses of Ionizing Radiation. J. Biomed. Phys. Eng. 2018, 8, 153–156. [Google Scholar] [CrossRef]
- Rola, R.; Fishman, K.; Baure, J.; Rosi, S.; Lamborn, K.R.; Obenaus, A.; Nelson, G.A.; Fike, J.R. Hippocampal neurogenesis and neuroinflammation after cranial irradiation with (56)Fe particles. Radiat. Res. 2008, 169, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Vlkolinsky, R.; Krucker, T.; Nelson, G.A.; Obenaus, A. (56)Fe-particle radiation reduces neuronal output and attenuates lipopolysaccharide-induced inhibition of long-term potentiation in the mouse hippocampus. Radiat. Res. 2008, 169, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tanaka, K.; Ji, B.; Ono, M.; Fang, Y.; Ninomiya, Y.; Maruyama, K.; Izumi-Nakajima, N.; Begum, N.; Higuchi, M.; et al. Low-dose total-body carbon-ion irradiations induce early transcriptional alteration without late Alzheimer’s disease-like pathogenesis and memory impairment in mice. J. Neurosci. Res. 2014, 92, 915–926. [Google Scholar] [CrossRef]
- Liu, B.; Hinshaw, R.G.; Le, K.X.; Park, M.A.; Wang, S.; Belanger, A.P.; Dubey, S.; Frost, J.L.; Shi, Q.; Holton, P.; et al. Space-like (56)Fe irradiation manifests mild, early sex-specific behavioral and neuropathological changes in wildtype and Alzheimer’s-like transgenic mice. Sci. Rep. 2019, 9, 12118. [Google Scholar] [CrossRef]
- Chicheva, M.M.; Mal’tsev, A.V.; Kokhan, V.S.; Bachurin, S.O. The Effect of Ionizing Radiation on Cognitive Functions in Mouse Models of Alzheimer’s Disease. Dokl. Biol. Sci./Transl. Russ. 2020, 494, 225–227. [Google Scholar] [CrossRef]
- Capilla-Gonzalez, V.; Guerrero-Cazares, H.; Bonsu, J.M.; Gonzalez-Perez, O.; Achanta, P.; Wong, J.; Garcia-Verdugo, J.M.; Quinones-Hinojosa, A. The subventricular zone is able to respond to a demyelinating lesion after localized radiation. Stem Cells 2014, 32, 59–69. [Google Scholar] [CrossRef]
- Mao, X.W.; Nishiyama, N.C.; Pecaut, M.J.; Campbell-Beachler, M.; Gifford, P.; Haynes, K.E.; Becronis, C.; Gridley, D.S. Simulated Microgravity and Low-Dose/Low-Dose-Rate Radiation Induces Oxidative Damage in the Mouse Brain. Radiat. Res. 2016, 185, 647–657. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Lebedeva-Georgievskaya, K.B.; Kudrin, V.S.; Bazyan, A.S.; Maltsev, A.V.; Shtemberg, A.S. An investigation of the single and combined effects of hypogravity and ionizing radiation on brain monoamine metabolism and rats’ behavior. Life Sci. Space Res. 2019, 20, 12–19. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Anokhin, P.K.; Belov, O.V.; Gulyaev, M.V. Cortical Glutamate/GABA Imbalance after Combined Radiation Exposure: Relevance to Human Deep-Space Missions. Neuroscience 2019, 416, 295–308. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Dobynde, M.I. The Effects of Galactic Cosmic Rays on the Central Nervous System: From Negative to Unexpectedly Positive Effects That Astronauts May Encounter. Biology 2023, 12, 400. [Google Scholar] [CrossRef]
- Berron, D.; Vogel, J.W.; Insel, P.S.; Pereira, J.B.; Xie, L.; Wisse, L.E.M.; Yushkevich, P.A.; Palmqvist, S.; Mattsson-Carlgren, N.; Stomrud, E.; et al. Early stages of tau pathology and its associations with functional connectivity, atrophy and memory. Brain J. Neurol. 2021, 144, 2771–2783. [Google Scholar] [CrossRef] [PubMed]
- Forner, S.; Kawauchi, S.; Balderrama-Gutierrez, G.; Kramar, E.A.; Matheos, D.P.; Phan, J.; Javonillo, D.I.; Tran, K.M.; Hingco, E.; da Cunha, C.; et al. Systematic phenotyping and characterization of the 5xFAD mouse model of Alzheimer’s disease. Sci. Data 2021, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.; Ingram, E.; Takao, M.; Smith, M.J.; Jakes, R.; Virdee, K.; Yoshida, H.; Holzer, M.; Craxton, M.; Emson, P.C.; et al. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 9340–9351. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Treatment allocation by minimisation. BMJ 2005, 330, 843. [Google Scholar] [CrossRef]
- Deacon, R.M. Assessing nest building in mice. Nat. Protoc. 2006, 1, 1117–1119. [Google Scholar] [CrossRef]
- Yue, E.L.; Cleland, T.A.; Pavlis, M.; Linster, C. Opposing effects of D1 and D2 receptor activation on odor discrimination learning. Behav. Neurosci. 2004, 118, 184–190. [Google Scholar] [CrossRef]
- Bush, A.I.; Takeuchi, H.; Iba, M.; Inoue, H.; Higuchi, M.; Takao, K.; Tsukita, K.; Karatsu, Y.; Iwamoto, Y.; Miyakawa, T.; et al. P301S Mutant Human Tau Transgenic Mice Manifest Early Symptoms of Human Tauopathies with Dementia and Altered Sensorimotor Gating. PLoS ONE 2011, 6, e21050. [Google Scholar] [CrossRef]
- Yoshiyama, Y.; Higuchi, M.; Zhang, B.; Huang, S.M.; Iwata, N.; Saido, T.C.; Maeda, J.; Suhara, T.; Trojanowski, J.Q.; Lee, V.M. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 2007, 53, 337–351. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal β-Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice with Five Familial Alzheimer’s Disease Mutations: Potential Factors in Amyloid Plaque Formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Combs, C.; Devi, L.; Ohno, M. Phospho-eIF2α Level Is Important for Determining Abilities of BACE1 Reduction to Rescue Cholinergic Neurodegeneration and Memory Defects in 5XFAD Mice. PLoS ONE 2010, 5, e12974. [Google Scholar] [CrossRef]
- Xiao, N.-A.; Zhang, J.; Zhou, M.; Wei, Z.; Wu, X.-L.; Dai, X.-M.; Zhu, Y.-G.; Chen, X.-C. Reduction of Glucose Metabolism in Olfactory Bulb is an Earlier Alzheimer’s Disease-related Biomarker in 5XFAD Mice. Chin. Med. J. 2015, 128, 2220–2227. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, T.P.; Robertson, A.; Chipman, P.H.; Rafuse, V.F.; Brown, R.E. Motor function deficits in the 12 month-old female 5xFAD mouse model of Alzheimer’s disease. Behav. Brain Res. 2018, 337, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Kokhan, V.S.; Pikalov, V.A.; Chaprov, K.; Gulyaev, M.V. Combined Ionizing Radiation Exposure by Gamma Rays and Carbon-12 Nuclei Increases Neurotrophic Factor Content and Prevents Age-Associated Decreases in the Volume of the Sensorimotor Cortex in Rats. Int. J. Mol. Sci. 2024, 25, 6725. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, I.A.; Budennay, N.N.; Severiukhin, Y.S.; Lyakhova, K.N.; Utina, D.M. Analysis of morphofunctional state of experimental animals brain fields under proton irradiation over the long period. J. New Med. Technol. 2018, 25, 177–181. [Google Scholar]
- Pecaut, M.J.; Haerich, P.; Miller, C.N.; Smith, A.L.; Zendejas, E.D.; Nelson, G.A. The effects of low-dose, high-LET radiation exposure on three models of behavior in C57BL/6 mice. Radiat. Res. 2004, 162, 148–156. [Google Scholar] [CrossRef]
- Cao, J.; Amakye, W.K.; Qi, C.; Liu, X.; Ma, J.; Ren, J. Bifidobacterium Lactis Probio-M8 regulates gut microbiota to alleviate Alzheimer’s disease in the APP/PS1 mouse model. Eur. J. Nutr. 2021, 60, 3757–3769. [Google Scholar] [CrossRef]
- Barkai, E. Dynamics of learning-induced cellular modifications in the cortex. Biol. Cybern. 2005, 92, 360–366. [Google Scholar] [CrossRef]
- Patel, R.C.; Larson, J. Impaired olfactory discrimination learning and decreased olfactory sensitivity in aged C57Bl/6 mice. Neurobiol. Aging 2009, 30, 829–837. [Google Scholar] [CrossRef]
- Aqrabawi, A.J.; Kim, J.C. Hippocampal projections to the anterior olfactory nucleus differentially convey spatiotemporal information during episodic odour memory. Nat. Commun. 2018, 9, 2735. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Z.; Weinstein, P.R.; Fike, J.R.; Liu, J. Environmental enrichment enhances neurogenesis and improves functional outcome after cranial irradiation. Eur. J. Neurosci. 2007, 25, 38–46. [Google Scholar] [CrossRef]
- Balentova, S.; Adamkov, M. Molecular, Cellular and Functional Effects of Radiation-Induced Brain Injury: A Review. Int. J. Mol. Sci. 2015, 16, 27796–27815. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Yamazaki, J.; Torres, E.R.S.; Kirchoff, N.; Stagaman, K.; Sharpton, T.; Turker, M.S.; Kronenberg, A. Combined Effects of Three High-Energy Charged Particle Beams Important for Space Flight on Brain, Behavioral and Cognitive Endpoints in B6D2F1 Female and Male Mice. Front. Physiol. 2019, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef]
- Filiou, M.D.; Arefin, A.S.; Moscato, P.; Graeber, M.B. ‘Neuroinflammation’ differs categorically from inflammation: Transcriptomes of Alzheimer’s disease, Parkinson’s disease, schizophrenia and inflammatory diseases compared. Neurogenetics 2014, 15, 201–212. [Google Scholar] [CrossRef]
- Osman, A.M.; Sun, Y.; Burns, T.C.; He, L.; Kee, N.; Oliva-Vilarnau, N.; Alevyzaki, A.; Zhou, K.; Louhivuori, L.; Uhlén, P.; et al. Radiation Triggers a Dynamic Sequence of Transient Microglial Alterations in Juvenile Brain. Cell Rep. 2020, 31, 107699. [Google Scholar] [CrossRef]
- Rauf, A.; Badoni, H.; Abu-Izneid, T.; Olatunde, A.; Rahman, M.M.; Painuli, S.; Semwal, P.; Wilairatana, P.; Mubarak, M.S. Neuroinflammatory Markers: Key Indicators in the Pathology of Neurodegenerative Diseases. Molecules 2022, 27, 3194. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Rydbirk, R.; Elfving, B.; Folke, J.; Pakkenberg, B.; Winge, K.; Brudek, T.; Aznar, S. Increased prefrontal cortex interleukin-2 protein levels and shift in the peripheral T cell population in progressive supranuclear palsy patients. Sci. Rep. 2019, 9, 7781. [Google Scholar] [CrossRef]
- Wang, Z.-B.; Ma, Y.-H.; Sun, Y.; Tan, L.; Wang, H.-F.; Yu, J.-T. Interleukin-3 is associated with sTREM2 and mediates the correlation between amyloid-β and tau pathology in Alzheimer’s disease. J. Neuroinflamm. 2022, 19, 316. [Google Scholar] [CrossRef]
- Lyra, E.S.N.M.; Goncalves, R.A.; Pascoal, T.A.; Lima-Filho, R.A.S.; Resende, E.P.F.; Vieira, E.L.M.; Teixeira, A.L.; de Souza, L.C.; Peny, J.A.; Fortuna, J.T.S.; et al. Pro-inflammatory interleukin-6 signaling links cognitive impairments and peripheral metabolic alterations in Alzheimer’s disease. Transl. Psychiatry 2021, 11, 251. [Google Scholar] [CrossRef]
- Wharton, W.; Kollhoff, A.L.; Gangishetti, U.; Verble, D.D.; Upadhya, S.; Zetterberg, H.; Kumar, V.; Watts, K.D.; Kippels, A.J.; Gearing, M.; et al. Interleukin 9 alterations linked to alzheimer disease in african americans. Ann. Neurol. 2019, 86, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Guillot-Sestier, M.V.; Doty, K.R.; Gate, D.; Rodriguez, J., Jr.; Leung, B.P.; Rezai-Zadeh, K.; Town, T. Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron 2015, 85, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Brigas, H.C.; Ribeiro, M.; Coelho, J.E.; Gomes, R.; Gomez-Murcia, V.; Carvalho, K.; Faivre, E.; Costa-Pereira, S.; Darrigues, J.; de Almeida, A.A.; et al. IL-17 triggers the onset of cognitive and synaptic deficits in early stages of Alzheimer’s disease. Cell Rep. 2021, 36, 109574. [Google Scholar] [CrossRef]
- Korbecki, J.; Gąssowska-Dobrowolska, M.; Wójcik, J.; Szatkowska, I.; Barczak, K.; Chlubek, M.; Baranowska-Bosiacka, I. The Importance of CXCL1 in Physiology and Noncancerous Diseases of Bone, Bone Marrow, Muscle and the Nervous System. Int. J. Mol. Sci. 2022, 23, 4205. [Google Scholar] [CrossRef]
- Chen, W.; Abud, E.A.; Yeung, S.T.; Lakatos, A.; Nassi, T.; Wang, J.; Blum, D.; Buee, L.; Poon, W.W.; Blurton-Jones, M. Increased tauopathy drives microglia-mediated clearance of beta-amyloid. Acta Neuropathol. Commun. 2016, 4, 63. [Google Scholar] [CrossRef]
- Alves, S.; Churlaud, G.; Audrain, M.; Michaelsen-Preusse, K.; Fol, R.; Souchet, B.; Braudeau, J.; Korte, M.; Klatzmann, D.; Cartier, N. Interleukin-2 improves amyloid pathology, synaptic failure and memory in Alzheimer’s disease mice. Brain J. Neurol. 2017, 140, 826–842. [Google Scholar] [CrossRef]
- Petitto, J.M.; McNamara, R.K.; Gendreau, P.L.; Huang, Z.; Jackson, A.J. Impaired learning and memory and altered hippocampal neurodevelopment resulting from interleukin-2 gene deletion. J. Neurosci. Res. 1999, 56, 441–446. [Google Scholar] [CrossRef]
- McAlpine, C.S.; Park, J.; Griciuc, A.; Kim, E.; Choi, S.H.; Iwamoto, Y.; Kiss, M.G.; Christie, K.A.; Vinegoni, C.; Poller, W.C.; et al. Astrocytic interleukin-3 programs microglia and limits Alzheimer’s disease. Nature 2021, 595, 701–706. [Google Scholar] [CrossRef]
- Chakrabarty, P.; Jansen-West, K.; Beccard, A.; Ceballos-Diaz, C.; Levites, Y.; Verbeeck, C.; Zubair, A.C.; Dickson, D.; Golde, T.E.; Das, P. Massive gliosis induced by interleukin-6 suppresses Aβ deposition in vivo: Evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2009, 24, 548–559. [Google Scholar] [CrossRef]
- Yang, J.; Kou, J.; Lalonde, R.; Fukuchi, K.I. Intracranial IL-17A overexpression decreases cerebral amyloid angiopathy by upregulation of ABCA1 in an animal model of Alzheimer’s disease. Brain Behav. Immun. 2017, 65, 262–273. [Google Scholar] [CrossRef]
- Parachikova, A.; Nichol, K.E.; Cotman, C.W. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol. Dis. 2008, 30, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Takemiya, T.; Fumizawa, K.; Yamagata, K.; Iwakura, Y.; Kawakami, M. Brain Interleukin-1 Facilitates Learning of a Water Maze Spatial Memory Task in Young Mice. Front. Behav. Neurosci. 2017, 11, 202. [Google Scholar] [CrossRef] [PubMed]
- Goshen, I.; Kreisel, T.; Ounallah-Saad, H.; Renbaum, P.; Zalzstein, Y.; Ben-Hur, T.; Levy-Lahad, E.; Yirmiya, R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology 2007, 32, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Awatsuji, H.; Furukawa, Y.; Nakajima, M.; Furukawa, S.; Hayashi, K. Interleukin-2 as a neurotrophic factor for supporting the survival of neurons cultured from various regions of fetal rat brain. J. Neurosci. Res. 1993, 35, 305–311. [Google Scholar] [CrossRef]
- Luo, X.J.; Li, M.; Huang, L.; Nho, K.; Deng, M.; Chen, Q.; Weinberger, D.R.; Vasquez, A.A.; Rijpkema, M.; Mattay, V.S.; et al. The interleukin 3 gene (IL3) contributes to human brain volume variation by regulating proliferation and survival of neural progenitors. PLoS ONE 2012, 7, e50375. [Google Scholar] [CrossRef]
- Zambrano, A.; Otth, C.; Mujica, L.; Concha, I.I.; Maccioni, R.B. Interleukin-3 prevents neuronal death induced by amyloid peptide. BMC Neurosci. 2007, 8, 82. [Google Scholar] [CrossRef]
- Zambrano, A.; Otth, C.; Maccioni, R.B.; Concha, I.I. IL-3 controls tau modifications and protects cortical neurons from neurodegeneration. Curr. Alzheimer Res. 2010, 7, 615–624. [Google Scholar] [CrossRef]
- Leibinger, M.; Müller, A.; Gobrecht, P.; Diekmann, H.; Andreadaki, A.; Fischer, D. Interleukin-6 contributes to CNS axon regeneration upon inflammatory stimulation. Cell Death Dis. 2013, 4, e609. [Google Scholar] [CrossRef]
- Merino, J.J.; Muneton-Gomez, V.; Muneton-Gomez, C.; Perez-Izquierdo, M.A.; Loscertales, M.; Toledano Gasca, A. Hippocampal CCR5/RANTES Elevations in a Rodent Model of Post-Traumatic Stress Disorder: Maraviroc (a CCR5 Antagonist) Increases Corticosterone Levels and Enhances Fear Memory Consolidation. Biomolecules 2020, 10, 212. [Google Scholar] [CrossRef]
- Ajoy, R.; Lo, Y.C.; Ho, M.H.; Chen, Y.Y.; Wang, Y.; Chen, Y.H.; Jing-Yuan, C.; Changou, C.A.; Hsiung, Y.C.; Chen, H.M.; et al. CCL5 promotion of bioenergy metabolism is crucial for hippocampal synapse complex and memory formation. Mol. Psychiatry 2021, 26, 6451–6468. [Google Scholar] [CrossRef]
- Ho, M.H.; Yen, C.H.; Hsieh, T.H.; Kao, T.J.; Chiu, J.Y.; Chiang, Y.H.; Hoffer, B.J.; Chang, W.C.; Chou, S.Y. CCL5 via GPX1 activation protects hippocampal memory function after mild traumatic brain injury. Redox Biol. 2021, 46, 102067. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, C.; Gobbel, G.T.; Lamborn, K.R.; Tada, E.; Fike, J.R. Apoptosis in the subependyma of young adult rats after single and fractionated doses of X-rays. Cancer Res. 1997, 57, 2694–2702. [Google Scholar]
- Mizumatsu, S.; Monje, M.L.; Morhardt, D.R.; Rola, R.; Palmer, T.D.; Fike, J.R. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003, 63, 4021–4027. [Google Scholar]
- Wang, F.; Baba, N.; Shen, Y.; Yamashita, T.; Tsuru, E.; Tsuda, M.; Maeda, N.; Sagara, Y. CCL11 promotes migration and proliferation of mouse neural progenitor cells. Stem Cell Res. Ther. 2017, 8, 26. [Google Scholar] [CrossRef]
- Jorda, A.; Cauli, O.; Santonja, J.M.; Aldasoro, M.; Aldasoro, C.; Obrador, E.; Vila, J.M.; Mauricio, M.D.; Iradi, A.; Guerra-Ojeda, S.; et al. Changes in Chemokines and Chemokine Receptors Expression in a Mouse Model of Alzheimer’s Disease. Int. J. Biol. Sci. 2019, 15, 453–463. [Google Scholar] [CrossRef]
- Donninelli, G.; Saraf-Sinik, I.; Mazziotti, V.; Capone, A.; Grasso, M.G.; Battistini, L.; Reynolds, R.; Magliozzi, R.; Volpe, E. Interleukin-9 regulates macrophage activation in the progressive multiple sclerosis brain. J. Neuroinflamm. 2020, 17, 149. [Google Scholar] [CrossRef]
- Fontaine, R.H.; Cases, O.; Lelievre, V.; Mesples, B.; Renauld, J.C.; Loron, G.; Degos, V.; Dournaud, P.; Baud, O.; Gressens, P. IL-9/IL-9 receptor signaling selectively protects cortical neurons against developmental apoptosis. Cell Death Differ. 2008, 15, 1542–1552. [Google Scholar] [CrossRef]
- Sharma, S.; Yang, B.; Xi, X.; Grotta, J.C.; Aronowski, J.; Savitz, S.I. IL-10 directly protects cortical neurons by activating PI-3 kinase and STAT-3 pathways. Brain Res. 2011, 1373, 189–194. [Google Scholar] [CrossRef]
- Weston, L.L.; Jiang, S.; Chisholm, D.; Jantzie, L.L.; Bhaskar, K. Interleukin-10 deficiency exacerbates inflammation-induced tau pathology. J. Neuroinflamm. 2021, 18, 161. [Google Scholar] [CrossRef]
- Castellani, R.J.; Lee, H.G.; Perry, G.; Smith, M.A. Antioxidant protection and neurodegenerative disease: The role of amyloid-beta and tau. Am. J. Alzheimer’s Dis. Other Dement. 2006, 21, 126–130. [Google Scholar] [CrossRef]
- Kontush, A. Amyloid-beta: An antioxidant that becomes a pro-oxidant and critically contributes to Alzheimer’s disease. Free Radic. Biol. Med. 2001, 31, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Belov, O.V.; Batmunkh, M.; Incerti, S.; Lkhagva, O. Radiation damage to neuronal cells: Simulating the energy deposition and water radiolysis in a small neural network. Phys. Medica PM Int. J. Devoted Appl. Phys. Med. Biol. Off. J. Ital. Assoc. Biomed. Phys. 2016, 32, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Oxidative Stress, Inflammation, and Disease; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2016; p. xiii. 389p. [Google Scholar]
- Guan, J.; Stewart, J.; Ware, J.H.; Zhou, Z.; Donahue, J.J.; Kennedy, A.R. Effects of dietary supplements on the space radiation-induced reduction in total antioxidant status in CBA mice. Radiat. Res. 2006, 165, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhong, L.; Han, X.; Xiong, G.; Xu, D.; Zhang, S.; Cheng, H.; Chiu, K.; Xu, Y. Brain and Retinal Abnormalities in the 5xFAD Mouse Model of Alzheimer’s Disease at Early Stages. Front. Neurosci. 2021, 15, 681831. [Google Scholar] [CrossRef]
- Kitazawa, M.; Cheng, D.; Tsukamoto, M.R.; Koike, M.A.; Wes, P.D.; Vasilevko, V.; Cribbs, D.H.; LaFerla, F.M. Blocking IL-1 Signaling Rescues Cognition, Attenuates Tau Pathology, and Restores Neuronal β-Catenin Pathway Function in an Alzheimer’s Disease Model. J. Immunol. 2011, 187, 6539–6549. [Google Scholar] [CrossRef]
- Azizi, G.; Khannazer, N.; Mirshafiey, A. The Potential Role of Chemokines in Alzheimer’s Disease Pathogenesis. Am. J. Alzheimer’s Dis. Other Dement. 2014, 29, 415–425. [Google Scholar] [CrossRef]
- Meraz-Rios, M.A.; Toral-Rios, D.; Franco-Bocanegra, D.; Villeda-Hernandez, J.; Campos-Pena, V. Inflammatory process in Alzheimer’s Disease. Front. Integr. Neurosci. 2013, 7, 59. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef]
- Roberto, M.; Patel, R.R.; Bajo, M. Ethanol and Cytokines in the Central Nervous System. In The Neuropharmacology of Alcohol; Grant, K., Lovinger, D., Eds.; Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2017; Volume 248, pp. 397–431. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokhan, V.S.; Levashova, A.I.; Nesterov, M.S.; Pikalov, V.A.; Chicheva, M.M. Combined Ionizing Radiation Caused Cognition and Non-Cognition Behavior Benefits and Modulated Microglial Activity in Wild-Type and Alzheimer’s-like Transgenic Mice. Biology 2025, 14, 682. https://doi.org/10.3390/biology14060682
Kokhan VS, Levashova AI, Nesterov MS, Pikalov VA, Chicheva MM. Combined Ionizing Radiation Caused Cognition and Non-Cognition Behavior Benefits and Modulated Microglial Activity in Wild-Type and Alzheimer’s-like Transgenic Mice. Biology. 2025; 14(6):682. https://doi.org/10.3390/biology14060682
Chicago/Turabian StyleKokhan, Viktor S., Anna I. Levashova, Maxim S. Nesterov, Vladimir A. Pikalov, and Maria M. Chicheva. 2025. "Combined Ionizing Radiation Caused Cognition and Non-Cognition Behavior Benefits and Modulated Microglial Activity in Wild-Type and Alzheimer’s-like Transgenic Mice" Biology 14, no. 6: 682. https://doi.org/10.3390/biology14060682
APA StyleKokhan, V. S., Levashova, A. I., Nesterov, M. S., Pikalov, V. A., & Chicheva, M. M. (2025). Combined Ionizing Radiation Caused Cognition and Non-Cognition Behavior Benefits and Modulated Microglial Activity in Wild-Type and Alzheimer’s-like Transgenic Mice. Biology, 14(6), 682. https://doi.org/10.3390/biology14060682





