Feeding Behavior and Ecological Significance of Craspedacusta sowerbii in a Freshwater Reservoir: Insights from Prey Composition and Trophic Interactions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of C. sowerbii and Sampling
2.2. Microscopic Observations and Photographing
2.3. Amplicon Sequencing and Analyzing
2.4. Measurement of the Water Physicochemical Variables
3. Results
3.1. Environmental Variables of the Sampling Point in the Danjiangkou Reservoir
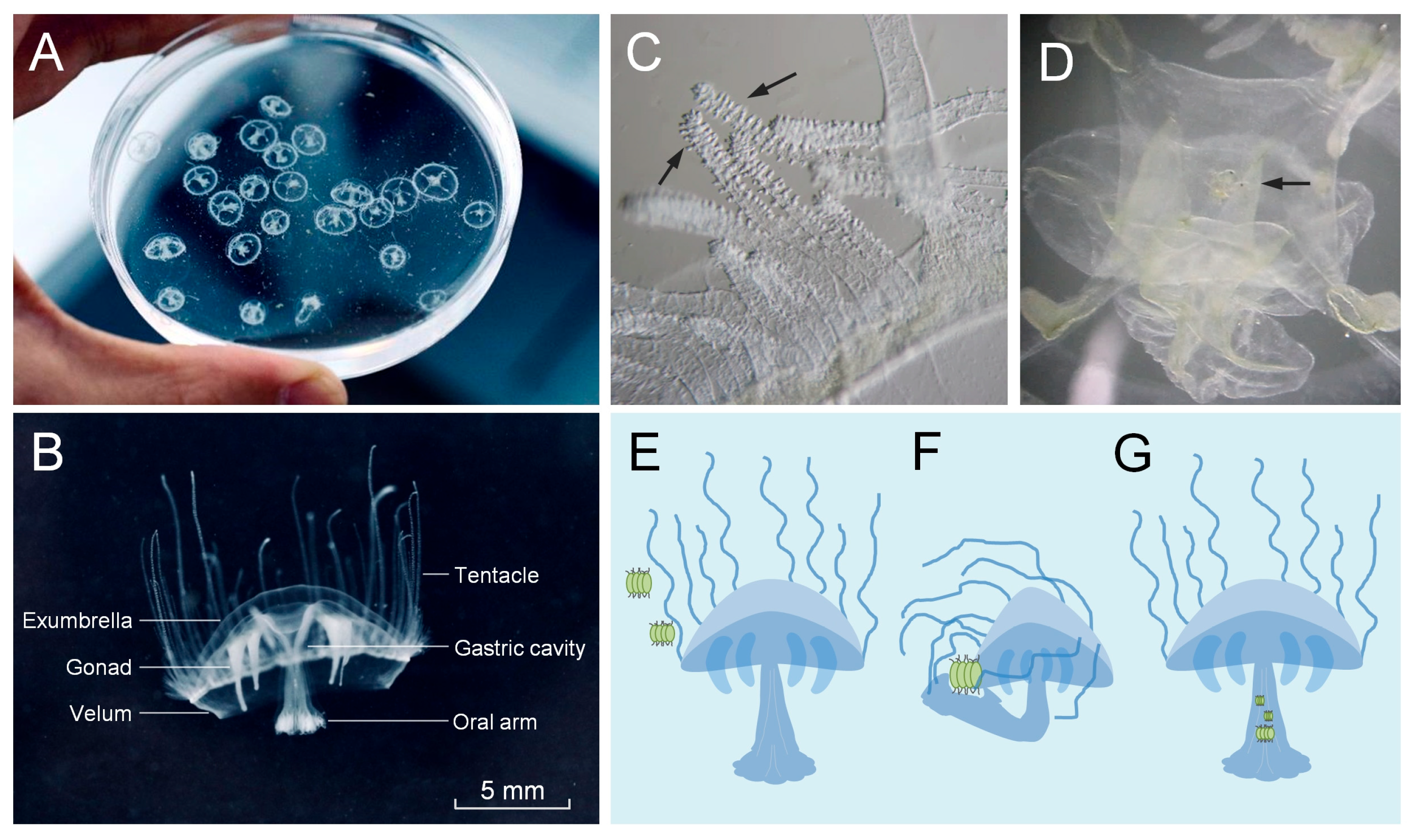
3.2. The Feeding Behavior and Anatomy of C. sowerbii
3.3. Microscopic Observations of the Environmental Species Community and the Food Composition of C. sowerbii
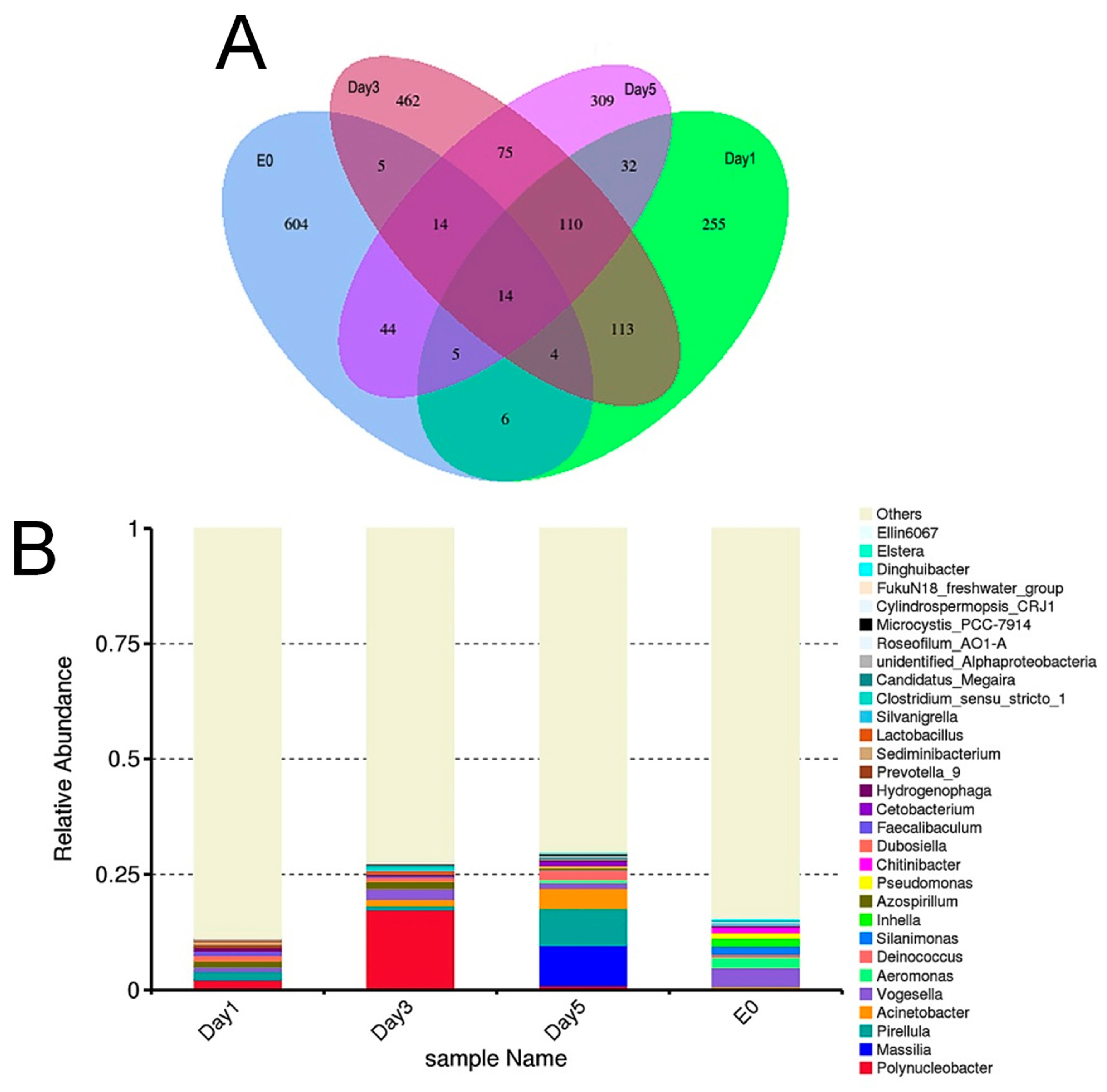
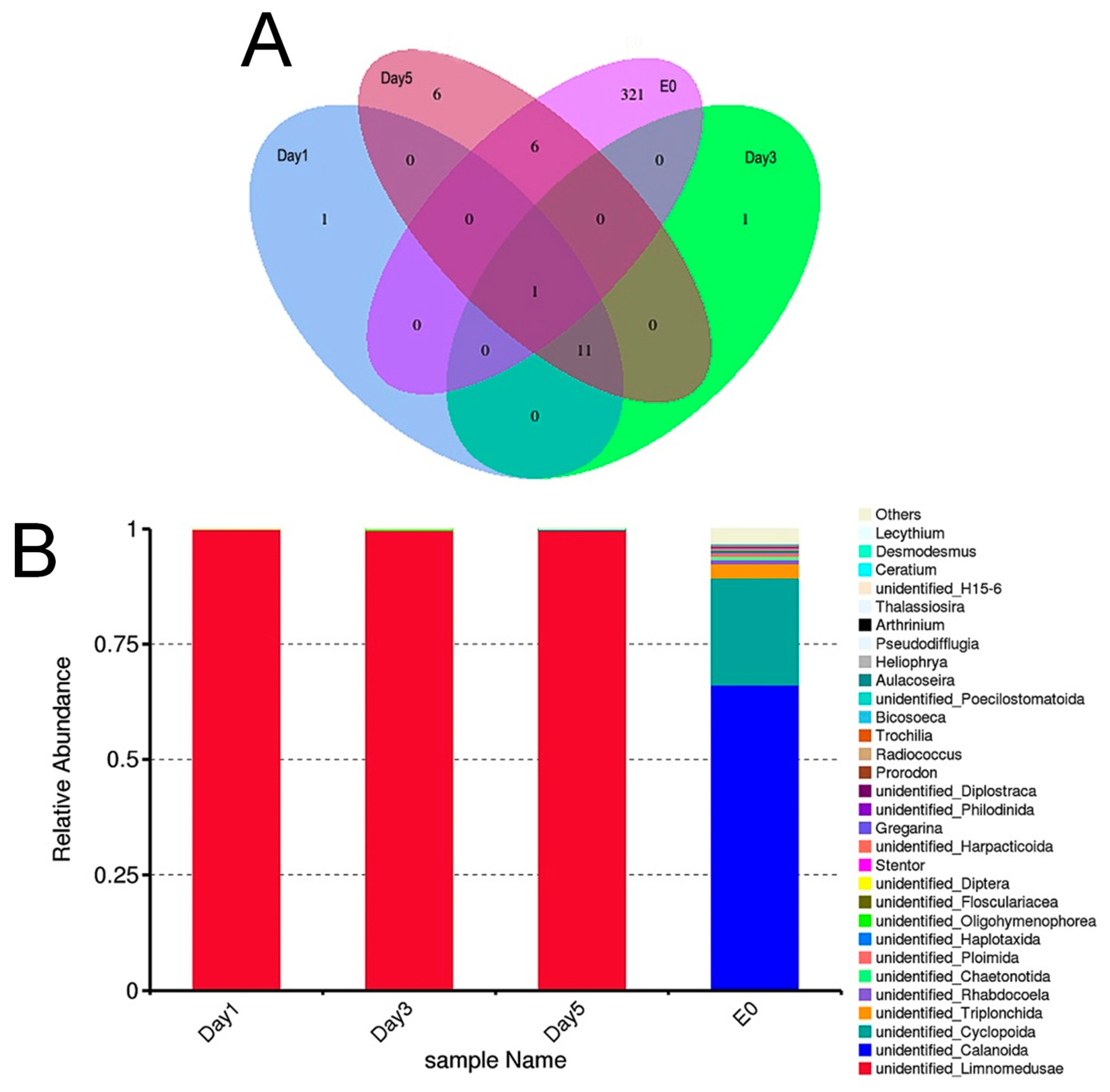
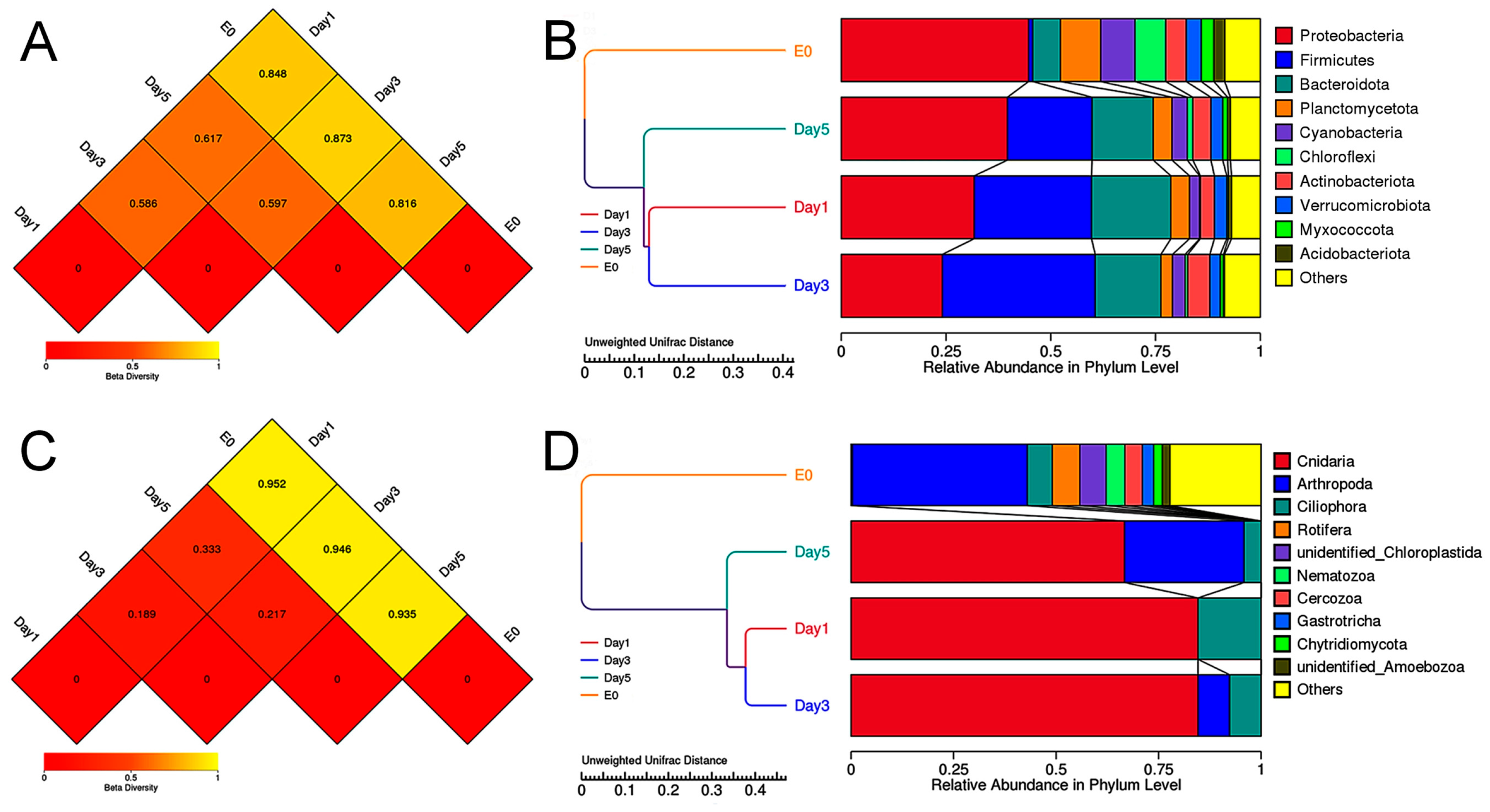
3.4. Amplicon Sequencing Analysis of the Food Composition of C. sowerbii
3.5. Beta Diversity Analysis of the Food Composition of C. sowerbii
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dang, Z.; Luo, Z.; Wang, S.; Liao, Y.; Jiang, Z.; Zhu, X.; Ji, G. Using hierarchical stable isotope to reveal microbial food web structure and trophic transfer efficiency differences during lake melt season. Sci. Total Environ. 2022, 842, 156893. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Li, C.; Liu, J.; Chai, B. Predation has a significant impact on the complexity and stability of microbial food webs in subalpine lakes. Microbiol. Spectr. 2023, 11, e0241123. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zeng, G.; Zhang, J.; Xu, P.; Chen, A.; Lu, L. Global Landscape of Total Organic Carbon, Nitrogen and Phosphorus in Lake Water. Sci. Rep. 2015, 5, 15043. [Google Scholar] [CrossRef] [PubMed]
- Domingues, C.D.; da Silva, L.H.S.; Rangel, L.M.; de Magalhães, L.; de Melo Rocha, A.; Lobão, L.M.; Paiva, R.; Roland, F.; Sarmento, H. Microbial Food-Web Drivers in Tropical Reservoirs. Microb. Ecol. 2017, 73, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Fan, P.; Li, Y.; Wang, H.; Wang, W.; Liu, H.; Messyasz, B.; Goldyn, R.; Li, B. Assessing the Ecosystem Health of Large Drinking-Water Reservoirs Based on the Phytoplankton Index of Biotic Integrity (P-IBI): A Case Study of Danjiangkou Reservoir. Sustainability 2023, 15, 5282. [Google Scholar] [CrossRef]
- Burlacot, A.; Peltier, G. Energy crosstalk between photosynthesis and the algal CO2-concentrating mechanisms. Trends Plant Sci. 2023, 28, 795–807. [Google Scholar] [CrossRef]
- Moore, M.V.; De Stasio, B.T., Jr.; Huizenga, K.N.; Silow, E.A. Trophic coupling of the microbial and the classical food web in Lake Baikal, Siberia. Freshw. Biol. 2019, 64, 138–151. [Google Scholar] [CrossRef]
- Gießler, S.; Strauss, T.; Schachtl, K.; Jankowski, T.; Klotz, R.; Stibor, H. Trophic Positions of Polyp and Medusa Stages of the Freshwater Jellyfish Craspedacusta sowerbii Based on Stable Isotope Analysis. Biology 2023, 12, 814. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; Qi, W.; Wang, D.; Lin, H.; Wu, X.; Wang, D.; Bai, Y.; Qu, J. Dam construction alters planktonic microbial predator-prey communities in the urban reaches of the Yangtze River. Water Res. 2023, 230, 119575. [Google Scholar] [CrossRef]
- Geist, J.; Hawkins, S.J. Habitat recovery and restoration in aquatic ecosystems: Current progress and future challenges. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 942–962. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Kumar, P.S.; Kabir, M.; Zuhara, F.T.; Mehjabin, A.; Tasannum, N.; Hoang, A.T.; Kabir, Z.; Mofijur, M. Threats, challenges and sustainable conservation strategies for freshwater biodiversity. Environ. Res. 2022, 214, 113808. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yin, J.; Feng, L. The Dynamic Changes in the Storage of the Danjiangkou Reservoir and the Influence of the South-North Water Transfer Project. Sci. Rep. 2018, 8, 8710. [Google Scholar] [CrossRef] [PubMed]
- Mai, S.; He, Y.; Li, W.; Zhao, T. Effects of environmental factors on vertical distribution of the eukaryotic plankton community in early summer in Danjiangkou Reservoir, China. Front. Ecol. Evol. 2023, 11, 1324932. [Google Scholar] [CrossRef]
- Wu, M.; Yan, H.; Fu, S.; Han, X.; Jia, M.; Dou, M.; Liu, H.; Fohrer, N.; Messyasz, B.; Li, Y. Seasonal Dynamics of Planktonic Algae in the Danjiangkou Reservoir: Nutrient Fluctuations and Ecological Implications. Sustainability 2025, 17, 406. [Google Scholar] [CrossRef]
- Chen, Z.; Yuan, J.; Sun, F.; Zhang, F.; Chen, Y.; Ding, C.; Shi, J.; Li, Y.; Yao, L. Planktonic fungal community structures and their relationship to water quality in the Danjiangkou Reservoir, China. Sci. Rep. 2018, 8, 10596. [Google Scholar] [CrossRef]
- Li, Y.; Khan, F.H.; Wu, J.; Zhang, Y.; Jiang, Y.; Chen, X.; Yao, Y.; Pan, Y.; Han, X. Drivers of Spatiotemporal Eukaryote Plankton Distribution in a Trans-Basin Water Transfer Canal in China. Front. Ecol. Evol. 2022, 10, 899993. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Wu, J. Assessing the impact of Danjiangkou reservoir on ecohydrological conditions in Hanjiang river, China. Ecol. Eng. 2015, 81, 41–52. [Google Scholar] [CrossRef]
- Chen, M.; Jin, X.; Liu, Y.; Guo, L.; Ma, Y.; Guo, C.; Wang, F.; Xu, J. Human activities induce potential aquatic threats of micropollutants in Danjiangkou Reservoir, the largest artificial freshwater lake in Asia. Sci. Total Environ. 2022, 850, 157843. [Google Scholar] [CrossRef]
- Dang, C.; Wang, J.; He, Y.; Yang, S.; Chen, Y.; Liu, T.; Fu, J.; Chen, Q.; Ni, J. Rare biosphere regulates the planktonic and sedimentary bacteria by disparate ecological processes in a large source water reservoir. Water Res. 2022, 216, 118296. [Google Scholar] [CrossRef]
- Wang, W.; Wang, R.; Li, Y.; Li, Y.; Zhang, P.; Gao, M.; Cao, Y.; Fohrer, N.; Zhang, Y.; Li, B.L. Cross-sectional-dependent microbial assembly and network stability: Bacteria sensitivity response was higher than eukaryotes and fungi in the Danjiangkou Reservoir. J. Environ. Manag. 2025, 379, 124851. [Google Scholar] [CrossRef]
- Xiong, M.; Li, R.; Zhang, T.; Liao, C.; Yu, G.; Yuan, J.; Liu, J.; Ye, S. Zooplankton Compositions in the Danjiangkou Reservoir, a Water Source for the South-to-North Water Diversion Project of China. Water 2022, 14, 3253. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Li, Y.; Lin, L.; Zheng, B.; Ji, M.; Li, B.L.; Han, X. The Seasonal Patterns, Ecological Function and Assembly Processes of Bacterioplankton Communities in the Danjiangkou Reservoir, China. Front. Microbiol. 2022, 13, 884765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, K.; Wang, X.; Zhang, J.a.; Duan, J.; Hu, Z.; Liu, Q. Food Web Structure and Ecosystem Functions of the Water Source in the Middle Route of China’s South-to-North Water Diversion Project. Fishes 2024, 9, 202. [Google Scholar] [CrossRef]
- Caputo, L.; Fuentes, R.; Woelfl, S.; Castañeda, L.E.; Cárdenas, L. Phenotypic plasticity of clonal populations of the freshwater jellyfish Craspedacusta sowerbii (Lankester, 1880) in Southern Hemisphere lakes (Chile) and the potential role of the zooplankton diet. Austral Ecol. 2021, 46, 1192–1197. [Google Scholar] [CrossRef]
- Fritz, G.B.; Pfannkuchen, M.; Reuner, A.; Schill, R.O.; BrÜMmer, F. Craspedacusta sowerbii, Lankester 1880—population dispersal analysis using COI and ITS sequences. J. Limnol. 2009, 68, 46–52. [Google Scholar] [CrossRef][Green Version]
- Marchessaux, G.; Gadreaud, J.; Belloni, B. The freshwater jellyfish Craspedacusta sowerbii Lankester, 1880: An overview of its distribution in France. Vie Milieu 2019, 69, 201–213. [Google Scholar]
- Jankowski, T. The freshwater medusae of the world—A taxonomic and systematic literature study with some remarks on other inland water jellyfish. Hydrobiologia 2001, 462, 91–113. [Google Scholar] [CrossRef]
- Winata, K.; Zhu, J.A.; Hanselman, K.M.; Zerbe, E.; Langguth, J.; Folino-Rorem, N.; Cartwright, P. Life Cycle Transitions in the Freshwater Jellyfish Craspedacusta sowerbii. Biology 2024, 13, 1069. [Google Scholar] [CrossRef]
- Folino-Rorem, N.C.; Reid, M.; Peard, T. Culturing the freshwater hydromedusa, Craspedacusta sowerbii under controlled laboratory conditions. Invertebr. Reprod. Dev. 2016, 60, 17–27. [Google Scholar] [CrossRef]
- Marchessaux, G.; Bejean, M. From frustules to medusae: A new culture system for the study of the invasive hydrozoan Craspedacusta sowerbii in the laboratory. Invertebr. Biol. 2020, 139, e12308. [Google Scholar] [CrossRef]
- Duggan, I.C.; Eastwood, K.R. Detection and distribution of Craspedacusta sowerbii: Observations of medusae are not enough. Aquat. Invasions 2012, 7, 271–275. [Google Scholar] [CrossRef]
- Jankowski, T.; Strauss, T.; Ratte, H.T. Trophic interactions of the freshwater jellyfish Craspedacusta sowerbii. J. Plankton Res. 2005, 27, 811–823. [Google Scholar] [CrossRef]
- Caputo, L.; Huovinen, P.; Sommaruga, R.; Gómez, I. Water transparency affects the survival of the medusa stage of the invasive freshwater jellyfish Craspedacusta sowerbii. Hydrobiologia 2018, 817, 179–191. [Google Scholar] [CrossRef]
- Lüskow, F.; López-González, P.J.; Pakhomov, E.A. Freshwater jellyfish in northern temperate lakes: Craspedacusta sowerbii in British Columbia, Canada. Aquat. Biol. 2021, 30, 69–84. [Google Scholar] [CrossRef]
- Boothroyd, I.K.G.; Etheredge, M.K.; Green, J.D. Spatial distribution, size structure, and prey of Craspedacusta sowerbyi Lankester in a shallow New Zealand lake. Hydrobiologia 2002, 468, 23–32. [Google Scholar] [CrossRef]
- Smith, A.S.; Alexander, J.E., Jr. Potential effects of the freshwater jellyfish Craspedacusta sowerbii on zooplankton community abundance. J. Plankton Res. 2008, 30, 1323–1327. [Google Scholar] [CrossRef]
- Lüskow, F.; Boersma, M.; López-González, P.J.; Pakhomov, E.A. Genetic variability, biomass parameters, elemental composition and energy content of the non-indigenous hydromedusa Craspedacusta sowerbii in North America. J. Plankton Res. 2022, 45, 82–98. [Google Scholar] [CrossRef]
- Lüskow, F.; Pakhomov, E.A. Spatiotemporal distribution of the non-indigenous peach blossom jellyfish Craspedacusta sowerbii in British Columbia, Canada. Can. J. Zool. 2024, 102, 735–745. [Google Scholar] [CrossRef]
- Peterson, M.; Tan, K.C.; Lewis Ames, C.; Collins, A.; Kitano, S.; Kusuoka, Y.; Suzuki, T.; Migita, M.; Iesa, I.; Pirro, S.; et al. A description of a novel swimming behavior in a dioecious population of Craspedacusta sowerbii, the rediscovery of the elusive Astrohydra japonica and the first genetic analysis of freshwater jellyfish in Japan. Plankton Benthos Res. 2022, 17, 231–248. [Google Scholar] [CrossRef]
- Lucas, K.; Colin, S.P.; Costello, J.H.; Katija, K.; Klos, E. Fluid Interactions That Enable Stealth Predation by the Upstream-Foraging Hydromedusa Craspedacusta sowerbyi. Biol. Bull. 2013, 225, 60–70. [Google Scholar] [CrossRef]
- Wu, L.; Wen, C.; Qin, Y.; Yin, H.; Tu, Q.; Van Nostrand, J.D.; Yuan, T.; Yuan, M.; Deng, Y.; Zhou, J. Phasing amplicon sequencing on Illumina Miseq for robust environmental microbial community analysis. BMC Microbiol. 2015, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Morpurgo, M.; Marrone, F.; Ciutti, F.; Cappelletti, C.; Vorhauser, S.; Alber, R.; Dossena, M.; Salmaso, N.; Fontaneto, D.; Caputo, L.; et al. Distribution and Genetic Lineages of the Craspedacusta sowerbii Species Complex (Cnidaria, Olindiidae) in Italy. Biology 2024, 13, 202. [Google Scholar] [CrossRef]
- Jin, X.; Shi, Y.; Sun, Z.; Wang, Y.; Zhao, Z. Microbial diversity and biogeography across gastrointestinal tracts of Takifugu pufferfish revealed by full-length 16S amplicon sequencing. Water Biol. Secur. 2025, 4, 100314. [Google Scholar] [CrossRef]
- Hugerth, L.W.; Andersson, A.F. Analysing Microbial Community Composition through Amplicon Sequencing: From Sampling to Hypothesis Testing. Front. Microbiol. 2017, 8, 1561. [Google Scholar] [CrossRef] [PubMed]
- Kishore, D.; Birzu, G.; Hu, Z.; DeLisi, C.; Korolev, K.S.; Segrè, D. Inferring microbial co-occurrence networks from amplicon data: A systematic evaluation. mSystems 2023, 8, e0096122. [Google Scholar] [CrossRef]
- Deserti, M.; Esquius, K.; Escalante, A.; Acuña, F. Trophic ecology and diet of Hydra vulgaris (Cnidaria; Hydrozoa). Anim. Biol. 2017, 67, 287–300. [Google Scholar] [CrossRef]
- Xue, X.; Wang, L.; Xing, H.; Zhao, Y.; Li, X.; Wang, G.; Wang, Z. Characteristics of phytoplankton-zooplankton communities and the roles in the transmission of antibiotic resistance genes under the pressure of river contamination. Sci. Total Environ. 2021, 780, 146452. [Google Scholar] [CrossRef]
- Li, Y.; Meng, J.; Zhang, C.; Ji, S.; Kong, Q.; Wang, R.; Liu, J. Bottom-up and top-down effects on phytoplankton communities in two freshwater lakes. PLoS ONE 2020, 15, e0231357. [Google Scholar] [CrossRef]
- Paver, S.F.; Youngblut, N.D.; Whitaker, R.J.; Kent, A.D. Phytoplankton succession affects the composition of olynucleobacter subtypes in humic lakes. Environ. Microbiol. 2015, 17, 816–828. [Google Scholar] [CrossRef]
- Nishu, S.D.; Kang, Y.; Han, I.; Jung, T.Y.; Lee, T.K. Nutritional status regulates algicidal activity of Aeromonas sp. L23 against cyanobacteria and green algae. PLoS ONE 2019, 14, e0213370. [Google Scholar] [CrossRef]
- Ren, S.; Jin, Y.; Ma, J.; Zheng, N.; Zhang, J.; Peng, X.; Xie, B. Isolation and characterization of algicidal bacteria from freshwater aquatic environments in China. Front. Microbiol. 2023, 14, 1156291. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, Q.; Wang, Q.; Liu, W.; Wei, Q.; Ren, H.; Ming, C.; Min, M.; Chen, P.; Ruan, R. Isolation of a bacterial strain, Acinetobacter sp. from centrate wastewater and study of its cooperation with algae in nutrients removal. Bioresour. Technol. 2017, 235, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Davison, H.R.; Hurst, G.D.D.; Siozios, S. ‘Candidatus Megaira’ are diverse symbionts of algae and ciliates with the potential for defensive symbiosis. Microb Genom 2023, 9, mgen000950. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Okazaki, Y.; Yoshida, M.; Matsuura, H.; Kai, A.; Shiratori, T.; Ishida, K.; Nakano, S.; Watanabe, M.M. A novel alphaproteobacterial ectosymbiont promotes the growth of the hydrocarbon-rich green alga Botryococcus braunii. Sci. Rep. 2015, 5, 10467. [Google Scholar] [CrossRef]
- Peng, H.; de-Bashan, L.E.; Higgins, B.T. Azospirillum brasilense reduces oxidative stress in the green microalgae Chlorella sorokiniana under different stressors. J. Biotechnol. 2021, 325, 179–185. [Google Scholar] [CrossRef]
- Hays, G.C.; Doyle, T.K.; Houghton, J.D.R. A Paradigm Shift in the Trophic Importance of Jellyfish? Trends Ecol. Evol. 2018, 33, 874–884. [Google Scholar] [CrossRef]
- Marchessaux, G.; Bejean, M. Growth and ingestion rates of the freshwater jellyfish Craspedacusta sowerbii. J. Plankton Res. 2020, 42, 783–786. [Google Scholar] [CrossRef]


| Variables | Mean ± SD |
|---|---|
| WT (°C) | 27.85 ± 1.28 |
| pH | 8.15 ± 0.37 |
| DO (mg/L) | 9.49 ± 0.61 |
| ORP (mV) | 160.43 ± 46.55 |
| EC (μS/cm) | 602.72 ± 50.01 |
| TN (mg/L) | 1.963 ± 0.52 |
| TP (mg/L) | 0.025 ± 0.01 |
| CODMn (mg/L) | 2.39 ± 0.52 |
| Chl a (μg/L) | 3.55 ± 1.38 |
| SD (m) | 2.21 ± 0.73 |
| Air Pressure (kPa) | 99.37 ± 3.60 |
| Humidity (%) | 87.44 ± 8.34 |
| Wind (m/s) | 1.14 ± 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Wang, Y.; Wu, M.; Li, Y.; Wang, W.; Zhang, D.; Guo, J.; Fohrer, N.; Li, B.L. Feeding Behavior and Ecological Significance of Craspedacusta sowerbii in a Freshwater Reservoir: Insights from Prey Composition and Trophic Interactions. Biology 2025, 14, 665. https://doi.org/10.3390/biology14060665
Yan H, Wang Y, Wu M, Li Y, Wang W, Zhang D, Guo J, Fohrer N, Li BL. Feeding Behavior and Ecological Significance of Craspedacusta sowerbii in a Freshwater Reservoir: Insights from Prey Composition and Trophic Interactions. Biology. 2025; 14(6):665. https://doi.org/10.3390/biology14060665
Chicago/Turabian StyleYan, Hailong, Yu Wang, Mengyao Wu, Yuying Li, Wanping Wang, Dongliang Zhang, Jingjing Guo, Nicola Fohrer, and Bailian Larry Li. 2025. "Feeding Behavior and Ecological Significance of Craspedacusta sowerbii in a Freshwater Reservoir: Insights from Prey Composition and Trophic Interactions" Biology 14, no. 6: 665. https://doi.org/10.3390/biology14060665
APA StyleYan, H., Wang, Y., Wu, M., Li, Y., Wang, W., Zhang, D., Guo, J., Fohrer, N., & Li, B. L. (2025). Feeding Behavior and Ecological Significance of Craspedacusta sowerbii in a Freshwater Reservoir: Insights from Prey Composition and Trophic Interactions. Biology, 14(6), 665. https://doi.org/10.3390/biology14060665







