DoDELLA-GAI2 Integrates Gibberellin and Ethylene Signaling to Regulate Chinese Yam (Dioscorea opposita) Tuber Development
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants, Cultivation, and Treatments
2.2. Morphological, Physiological, and Histological Analyses
2.3. Key Gene Expression Patterns Analysis
2.4. Targeted Metabolomics Analysis
2.5. Isolation and Cloning of the DoDELLA-GAI2 Gene
2.6. Subcellular Localization and Functional Validation of the DoDELLA-GAI2 Gene
2.7. Yeast Two-Hybrid (Y2H) Assay
2.8. Bimolecular Fluorescence Complementation (BiFC) Assays
2.9. Statistical Analyses
3. Results
3.1. Exogenous GA Enhances Yam Tubers’ Growth
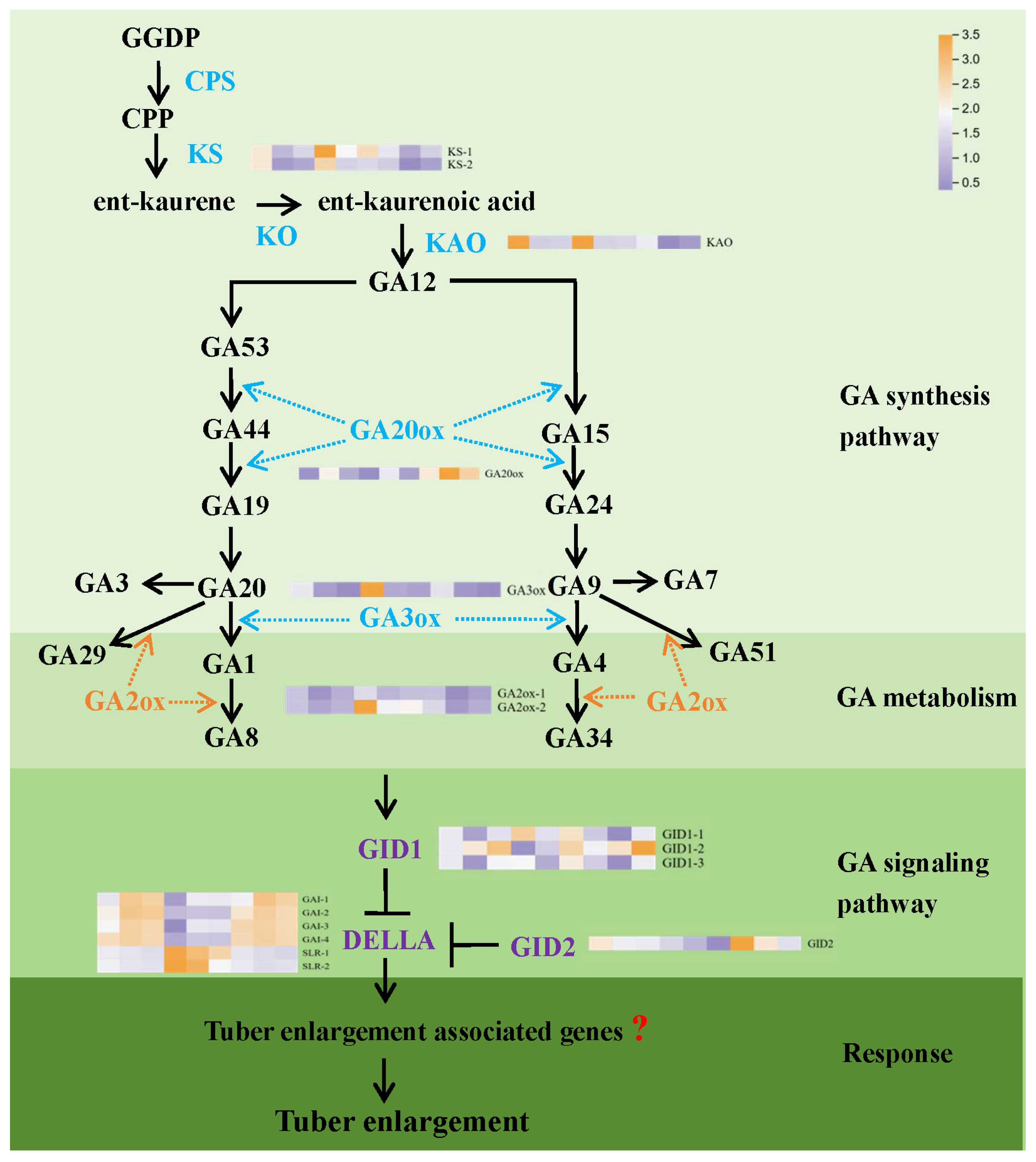
3.2. GA Modulates the Expression of Key Genes in GA Metabolism and Signal Transduction Pathways
3.3. Targeted Metabonomics Reveals Changes in Phytohormone Profiles During Tuber Development
3.4. Dynamic Changes in Phytohormone Signal Transduction Pathways During Tuber Development
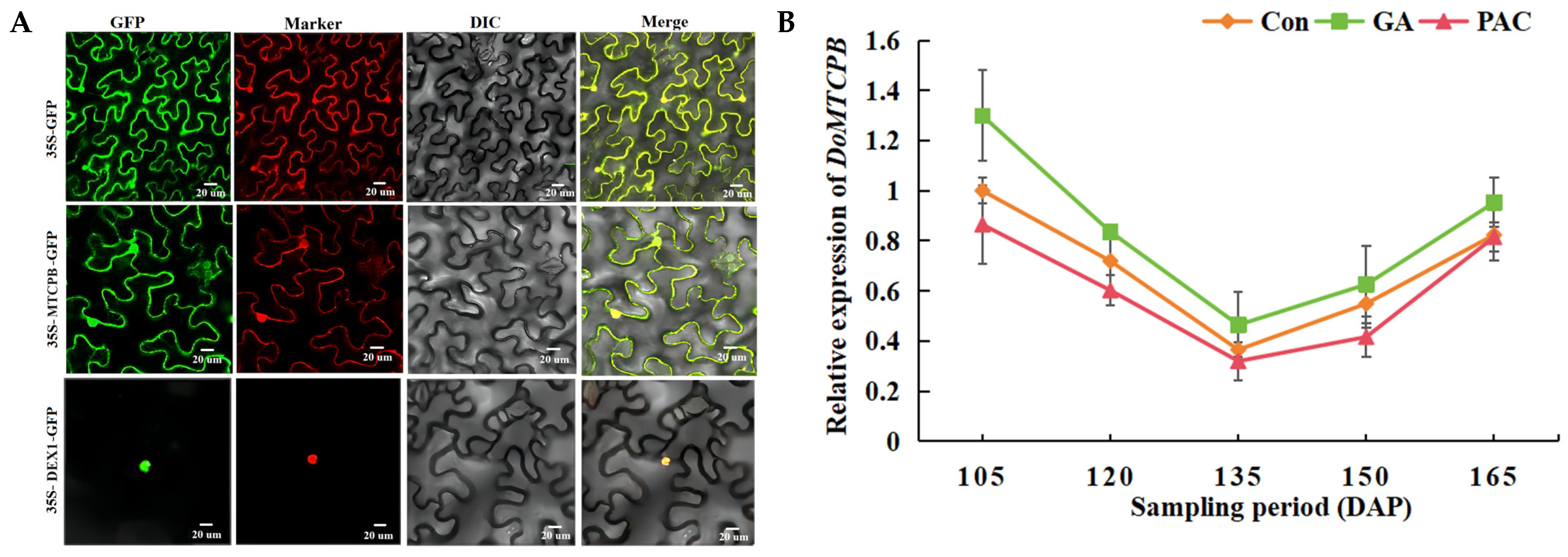
3.5. Isolation and Subcellular Localization of DoDELLA-GAI2
3.6. Overexpressed DoDELLA-GAI2 in Tobacco Affects Plant Growth and Anatomical Structure
3.7. GA Treatment Alters the Physiological Characteristics of Overexpressed DoDELLA-GAI2 Tobacco
3.8. Ethephon Treatment Alters Physiological Characteristics of Overexpressed DoDELLA-GAI2 Tobacco
3.9. Self-Activation Detection of Decoy Vector pGBKT7-DoDELLA-GAI2
3.10. Screening and Identification of DoDELLA-GAI2 Interacting Proteins
3.11. Yeast Two-Hybrid Point-to-Point Rotation Verification and Bimolecular Fluorescence Complementation Assays
3.12. Subcellular Localization of Interacting Proteins DoMTCPB and DoDEX1, and the Analysis of the Expression Pattern of DoMTCPB
3.13. Integrated Model of GA and ETH Signaling During Tuber Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| ACC | 1-aminocyclopropane-1-carboxylic acid |
| BiFC | Bimolecular fluorescence complementation |
| Cb | Cork cambium |
| CTK | Cytokinin |
| Co | Cortex |
| DAP | Days after planting |
| DoMTCPB | 1,2-dihydroxy-3-keto-5-methylthiopentene dioxygenase |
| DAMs | Differentially accumulated metabolites |
| DEGs | Differentially expressed genes |
| ETH | Ethylene |
| GA | Gibberellin |
| Gt | Basic tissue |
| JA | Jasmonic acid |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| PAC | Gibberellin biosynthesis inhibitor |
| PCA | Principal components analysis |
| Pd | Phelloderm |
| Pg | Phellem layer |
| Pi | Pith |
| SA | Salicylic acid |
| SSS | Soluble starch synthase |
| ST | Sieve tube |
| Ste | Stele |
| VB | Vascular bundle |
| VES | Vessel |
References
- Epping, J.; Laibach, N. An underutilized orphan tuber crop—Chinese yam: A review. Planta 2020, 252, 58. [Google Scholar] [CrossRef] [PubMed]
- Khol, M.; Ma, F.; Lei, L.; Liu, W.; Liu, X. A frontier review of nutraceutical chinese yam. Foods 2024, 13, 1426. [Google Scholar] [CrossRef]
- Wu, Z.-G.; Jiang, W.; Nitin, M.; Bao, X.-Q.; Chen, S.-L.; Tao, Z.-M. Characterizing diversity based on nutritional and bioactive compositions of yam germplasm (Dioscorea spp.) commonly cultivated in china. J. Food Drug Anal. 2016, 24, 367–375. [Google Scholar] [CrossRef]
- Li, Y.; Ji, S.; Xu, T.; Zhong, Y.; Xu, M.; Liu, Y.; Li, M.; Fan, B.; Wang, F.; Xiao, J.; et al. Chinese yam (Dioscorea): Nutritional value, beneficial effects, and food and pharmaceutical applications. Trends Food Sci. Technol. 2023, 134, 29–40. [Google Scholar] [CrossRef]
- Datir, S.; Kumbhar, R.; Kumatkar, P. Understanding physiological and biochemical mechanisms associated with post-harvest storage of yam tuber (Dioscorea sp.). Technol. Hortic. 2024, 4, e004. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, S.; Hameed, S.; Xiao, D.; Zhan, J.; Wang, A.; He, L. Integrated mrna and mirna transcriptome analysis reveals a regulatory network for tuber expansion in chinese yam (Dioscorea opposita). BMC Genom. 2020, 21, 117. [Google Scholar] [CrossRef]
- Gong, M.; Luo, H.; Wang, A.; Zhou, Y.; Huang, W.; Zhu, P.; He, L. Phytohormone profiling during tuber development of chinese yam by ultra-high performance liquid chromatography–triple quadrupole tandem mass spectrometry. J. Plant Growth Regul. 2017, 36, 362–373. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Dong, Y.; Li, S.; Yuan, J.; Li, C.; Zhang, X.; Li, M. Transcriptome analysis reveals key pathways and hormone activities involved in early microtuber formation of Dioscorea opposita. BioMed Res. Int. 2020, 2020, 8057929. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A century of gibberellin research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef]
- Claeys, H.; De Bodt, S.; Inzé, D. Gibberellins and dellas: Central nodes in growth regulatory networks. Trends Plant Sci. 2014, 19, 231–239. [Google Scholar] [CrossRef]
- Guo, L.; Plunkert, M.; Luo, X.; Liu, Z. Developmental regulation of stolon and rhizome. Curr. Opin. Plant Biol. 2021, 59, 101970. [Google Scholar] [CrossRef]
- Li, J.; Seng, S.; Li, D.; Zhang, F.; Liu, Y.; Yao, T.; Liang, J.; Yi, M.; Wu, J. Antagonism between abscisic acid and gibberellin regulates starch synthesis and corm development in gladiolus hybridus. Hortic. Res. 2021, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Jing, S.; Cheng, Z.; Song, B.; Xie, C.; Liu, J.; Zhou, J. DNA methylation affects photoperiodic tuberization in potato (solanum tuberosum l.) by mediating the expression of genes related to the photoperiod and ga pathways. Hortic. Res. 2021, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Jiya, A.L.; Ji, X.; Shao, Y.; Zhao, L.; Zhang, Y.; Huo, X. Effect of endogenous hormones and expressions of relevant genes on tuber growth of bikeqi yam. Fujian J. Agric. Sci. 2020, 35, 964–973. [Google Scholar]
- Wang, J.X.; Ji, X.; Gao, Y.L.; Zhang, Y.F.; Shao, Y.; Xing, L.N.; Huo, X.W. Regulation of CDPK20 on tuber enlargement of yam. Acta Bot. Boreali-Occident. Sin. 2021, 41, 189–196. [Google Scholar]
- Zhou, Y.; Li, Y.; Gong, M.; Qin, F.; Xiao, D.; Zhan, J.; Wang, A.; He, L. Regulatory mechanism of ga3 on tuber growth by della-dependent pathway in yam (Dioscorea opposita). Plant Mol. Biol. 2021, 106, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Wang, Q.; Zhang, F.; Feng, H.; Wang, X.; Cai, C. Advances in the modulation of potato tuber dormancy and sprouting. Int. J. Mol. Sci. 2024, 25, 5078. [Google Scholar] [CrossRef]
- Utsumi, Y.; Tanaka, M.; Utsumi, C.; Takahashi, S.; Matsui, A.; Fukushima, A.; Kobayashi, M.; Sasaki, R.; Oikawa, A.; Kusano, M.; et al. Integrative omics approaches revealed a crosstalk among phytohormones during tuberous root development in cassava. Plant Mol. Biol. 2022, 109, 249–269. [Google Scholar] [CrossRef]
- Huang, L.; Fang, B.; Chen, J.; He, X.; Zhang, X.; Wang, Z. Determination of amylose content in sweet potato by single wavelength colorimetry. J. Chin. Cereals. Oils. Assoc. 2010, 25, 100–104. [Google Scholar]
- Jain, A.; Jain, R.; Jain, S. Quantitative analysis of reducing sugars by 3, 5-dinitrosalicylic acid (dnsa method). In Basic Techniques in Biochemistry, Microbiology and Molecular Biology: Principles and Techniques; Springer: New York, NY, USA, 2020; pp. 181–183. [Google Scholar]
- Cao, T.; Wang, S.; Ali, A.; Shan, N.; Sun, J.; Chen, X.; Wang, P.; Zhu, Q.; Xiao, Y.; Luo, S.; et al. Transcriptome and metabolome analysis reveals the potential mechanism of tuber dynamic development in yam (Dioscorea polystachya turcz.). LWT 2023, 181, 114764. [Google Scholar] [CrossRef]
- Delvallé, D.; Dumez, S.; Wattebled, F.; Roldán, I.; Planchot, V.; Berbezy, P.; Colonna, P.; Vyas, D.; Chatterjee, M.; Ball, S.; et al. Soluble starch synthase i: A major determinant for the synthesis of amylopectin in arabidopsis thaliana leaves. Plant J. 2005, 43, 398–412. [Google Scholar] [CrossRef]
- Tang, Z. Experimental Guide of Modern Plant Physiology; Science Press: Beijing, China, 1999; Volume 95, pp. 1–425. [Google Scholar]
- Shao, Y.; Zhang, Y.; Guo, S.; Zhao, L.; Sun, X.; Huo, X. Enhanced carbohydrate metabolism and salinity tolerance of tobacco plants overexpressing a vacuolar h+-atpase subunit b2 (dovhab2) gene from yam (Dioscorea opposita thunb.). J. Biobased Mater. Bioenergy 2021, 15, 504–513. [Google Scholar] [CrossRef]
- Šimura, J.; Antoniadi, I.; Široká, J.; Tarkowská, D.e.; Strnad, M.; Ljung, K.; Novák, O. Plant hormonomics: Multiple phytohormone profiling by targeted metabolomics. Plant Physiol. 2018, 177, 476–489. [Google Scholar] [CrossRef]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. Metax: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yin, F.; Zhu, W.; Zhang, M.; Xiao, X.; Yao, Y.; Ge, F.; Wang, W. Transcriptomics integrated with targeted metabolomics reveals endogenous hormone changes in tuberous root expansion of pueraria. BMC Genom. 2024, 25, 1112. [Google Scholar]
- Gao, Y.; Zhang, Y.; Wang, J.; Sun, X.; Suo, N.; Yang, F.; Huo, X. Transcriptome analysis reveals the accumulation mechanism of starch in chinese yam (Dioscorea opposite thunb.) tubers using rna-seq and iso-seq. Sci. Adv. Mater. 2022, 14, 305–318. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Shao, Y.; Zhao, L.; Xing, L.; Ge, M.; Huo, X. Transciptome analysis molecular mechanism of starch synthesis during tuber development in chinese yam (Dioscorea opposita). J. Biobased Mater. Bioenergy 2021, 15, 589–597. [Google Scholar] [CrossRef]
- Li, T.; Xu, Y.; Zhang, L.; Ji, Y.; Tan, D.; Yuan, H.; Wang, A. The jasmonate-activated transcription factor mdmyc2 regulates ethylene response factor and ethylene biosynthetic genes to promote ethylene biosynthesis during apple fruit ripening. Plant Cell 2017, 29, 1316–1334. [Google Scholar] [CrossRef]
- Wise, A.A.; Liu, Z.; Binns, A.N. Three methods for the introduction of foreign DNA into agrobacterium. In Agrobacterium Protocols; Springer: Berlin/Heidelberg, Germany, 2006; pp. 43–54. [Google Scholar]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Shao, Y.; Xing, L.; Ge, M.; Huo, X. Overexpression of dobam1 from yam (Dioscorea opposita thunb.) enhances cold tolerance in transgenic tobacco. Genes 2022, 13, 2296. [Google Scholar] [CrossRef]
- Fu, D.; Liu, R.; Gao, F. Yeast one-hybrid screening for regulators of ibwd40 in purple-fleshed sweet potato (Ipomoea batatas [L] Lam.). Front. Biosci. 2023, 28, 200. [Google Scholar] [CrossRef] [PubMed]
- Gietz, R.D.; Schiestl, R.H. High-efficiency yeast transformation using the liac/ss carrier DNA/peg method. Nat. Protoc. 2007, 2, 31–34. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, C.; Zhang, J.; Ren, Y.; He, L.; Tang, R.; Wang, W.; Jia, X. The sweet potato b-box transcription factor gene ibbbx28 negatively regulates drought tolerance in transgenic arabidopsis. Front. Genet. 2022, 13, 1077958. [Google Scholar] [CrossRef] [PubMed]
- Mabhaudhi, T.; Chimonyo, V.G.P.; Hlahla, S.; Massawe, F.; Mayes, S.; Nhamo, L.; Modi, A.T. Prospects of orphan crops in climate change. Planta 2019, 250, 695–708. [Google Scholar] [CrossRef]
- Wang, M.; Chen, J.; Zhang, X.; Li, S.; Zhang, T.; Li, W.; Lin, L. Gibberellin a3 induces polyaerial shoot formation and increases the propagation rate in paris polyphylla rhizomes. Ind. Crops Prod. 2021, 167, 113511. [Google Scholar] [CrossRef]
- Chen, P.; Yang, R.; Bartels, D.; Dong, T.; Duan, H. Roles of abscisic acid and gibberellins in stem/root tuber development. Int. J. Mol. Sci. 2022, 23, 4955. [Google Scholar] [CrossRef]
- Ma, P.a.; Chen, X.; Liu, C.; Meng, Y.; Xia, Z.; Zeng, C.; Lu, C.; Wang, W. Mesaur1, encoded by a small auxin-up rna gene, acts as a transcription regulator to positively regulate adp-glucose pyrophosphorylase small subunit1a gene in cassava. Front. Plant Sci. 2017, 8, 1315. [Google Scholar] [CrossRef] [PubMed]
- Mares, D.J.; Marscfaner, H.; Krauss, A. Effect of gibberellic acid on growth and carbohydrate metabolism of developing tubers of potato (solanum tuberosum). Physiol. Plant. 1981, 52, 267–274. [Google Scholar] [CrossRef]
- Murcia, G.; Pontin, M.; Reinoso, H.; Baraldi, R.; Bertazza, G.; Gómez-Talquenca, S.; Bottini, R.; Piccoli, P.N. Aba and ga increase carbon allocation in different organs of grapevine plants by inducing accumulation of non-structural carbohydrates in leaves, enhancement of phloem area and expression of sugar transporters. Physiol. Plant. 2016, 156, 323–337. [Google Scholar] [CrossRef]
- Hong, C.P.; Kim, J.; Lee, J.; Yoo, S.-i.; Bae, W.; Geem, K.R.; Yu, J.; Jang, I.; Jo, I.H.; Cho, H.; et al. Gibberellin signaling promotes the secondary growth of storage roots in panax ginseng. Int. J. Mol. Sci. 2021, 22, 8694. [Google Scholar] [CrossRef]
- Hu, J.; Su, H.; Cao, H.; Wei, H.; Fu, X.; Jiang, X.; Song, Q.; He, X.; Xu, C.; Luo, K. Auxin response factor7 integrates gibberellin and auxin signaling via interactions between della and aux/iaa proteins to regulate cambial activity in poplar. Plant Cell 2022, 34, 2688–2707. [Google Scholar]
- Greenboim-Wainberg, Y.; Maymon, I.; Borochov, R.; Alvarez, J.; Olszewski, N.; Ori, N.; Eshed, Y.; Weiss, D. Cross talk between gibberellin and cytokinin: The arabidopsis ga response inhibitor spindly plays a positive role in cytokinin signaling. Plant Cell 2005, 17, 92–102. [Google Scholar]
- Que, F.; Khadr, A.; Wang, G.-L.; Li, T.; Wang, Y.-H.; Xu, Z.-S.; Xiong, A.-S. Exogenous brassinosteroids altered cell length, gibberellin content, and cellulose deposition in promoting carrot petiole elongation. Plant Sci. 2018, 277, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Phokas, A.; Coates, J.C. Evolution of della function and signaling in land plants. Evol. Dev. 2021, 23, 137–154. [Google Scholar]
- He, H.; Liang, G.; Lu, S.; Wang, P.; Liu, T.; Ma, Z.; Zuo, C.; Sun, X.; Chen, B.; Mao, J. Genome-wide identification and expression analysis of ga2ox, ga3ox, and ga20ox are related to gibberellin oxidase genes in grape (Vitis vinifera L.). Genes 2019, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, C.; Du, T.; Li, A.; Qin, Z.; Zhang, L.; Dong, S.; Wang, Q.; Hou, F. Overexpression of 9-cis-epoxycarotenoid dioxygenase gene, ibnced1, negatively regulates plant height in transgenic sweet potato. Int. J. Mol. Sci. 2023, 24, 10421. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Okada, K.; Fukazawa, J.; Takahashi, Y. Della-dependent and -independent gibberellin signaling. Plant Signal. Behav. 2018, 13, e1445933. [Google Scholar] [CrossRef]
- Ci, J.; Wang, X.; Wang, Q.; Zhao, F.; Yang, W.; Cui, X.; Jiang, L.; Ren, X.; Yang, W. Genome-wide analysis of gibberellin-dioxygenases gene family and their responses to ga applications in maize. PLoS ONE 2021, 16, e0250349. [Google Scholar]
- Nelson, S.K.; Steber, C.M. Gibberellin hormone signal perception: Down-regulating della repressors of plant growth and development. In Annual Plant Reviews; Wiley: Hoboken, NJ, USA, 2016; Volume 49, pp. 153–188. [Google Scholar]
- Ma, J.; Wang, X.-q.; Ni, H.-f.; Huang, T.-y.; Yang, B.-x. Gras transcription factors mediate flowering through signaling pathways of gibberellin and circadian rhythm in lonicera japonica thunb. Plant Gene 2021, 28, 100340. [Google Scholar]
- Sarnowska, E.A.; Rolicka, A.T.; Bucior, E.; Cwiek, P.; Tohge, T.; Fernie, A.R.; Jikumaru, Y.; Kamiya, Y.; Franzen, R.; Schmelzer, E.; et al. Della-interacting swi3c core subunit of switch/sucrose nonfermenting chromatin remodeling complex modulates gibberellin responses and hormonal cross talk in Arabidopsis. Plant Physiol. 2013, 163, 305–317. [Google Scholar] [CrossRef]
- Lu, Y.; Feng, Z.; Meng, Y.; Bian, L.; Xie, H.; Mysore, K.S.; Liang, J. Slender rice1 and oryza sativa indeterminate domain2 regulating osmir396 are involved in stem elongation1. Plant Physiol. 2020, 182, 2213–2227. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, S.; Zhang, B.; Shang-Guan, K.; Shi, Y.; Zhang, D.; Liu, X.; Wu, K.; Xu, Z.; Fu, X.; et al. A gibberellin-mediated della-nac signaling cascade regulates cellulose synthesis in rice. Plant Cell 2015, 27, 1681–1696. [Google Scholar] [PubMed]
- Liu, X.; Li, Y.; Zhong, S. Interplay between light and plant hormones in the control of arabidopsis seedling chlorophyll biosynthesis. Front. Plant Sci. 2017, 8, 1433. [Google Scholar] [CrossRef] [PubMed]
- Sauter, M.; Moffatt, B.; Saechao, M.C.; Hell, R.; Wirtz, M. Methionine salvage and s-adenosylmethionine: Essential links between sulfur, ethylene and polyamine biosynthesis. Biochem. J. 2013, 451, 145–154. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L.; Yu, J. Maize wrky28 interacts with the della protein d8 to affect skotomorphogenesis and participates in the regulation of shade avoidance and plant architecture. J. Exp. Bot. 2023, 74, 3122–3141. [Google Scholar] [CrossRef]













| ID No. | Functional Comments | Number of Clones | NCBI Blast Serial Number | Comparison Rate/% |
|---|---|---|---|---|
| Y2H-3 | 1,2-dihydroxy-3-keto-5-methylthiopentene dioxygenase (MTCPB) | 1 | XM_039258858 | 97 |
| Y2H-4 | Low-temperature-induced cysteine proteinase | 1 | XM_020237188 | 79 |
| Y2H-5 | Heme oxygenase 1 | 1 | XM_039286673.1 | 97 |
| Y2H-7 | Histone H2A | 1 | XM_039280286 | 91 |
| Y2H-14 | Protein DEFECTIVE IN EXINE FORMATION (DEX1) | 1 | XM_039288871 | 98 |
| Y2H-15 | Triosephosphate isomerase | 1 | XM_039273555.1 | 97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, M.; Zhang, Y.; Xing, Y.; Xing, L.; Miao, H.; Huo, X. DoDELLA-GAI2 Integrates Gibberellin and Ethylene Signaling to Regulate Chinese Yam (Dioscorea opposita) Tuber Development. Biology 2025, 14, 635. https://doi.org/10.3390/biology14060635
Ge M, Zhang Y, Xing Y, Xing L, Miao H, Huo X. DoDELLA-GAI2 Integrates Gibberellin and Ethylene Signaling to Regulate Chinese Yam (Dioscorea opposita) Tuber Development. Biology. 2025; 14(6):635. https://doi.org/10.3390/biology14060635
Chicago/Turabian StyleGe, Mingran, Yanfang Zhang, Yanping Xing, Linan Xing, Huiqin Miao, and Xiuwen Huo. 2025. "DoDELLA-GAI2 Integrates Gibberellin and Ethylene Signaling to Regulate Chinese Yam (Dioscorea opposita) Tuber Development" Biology 14, no. 6: 635. https://doi.org/10.3390/biology14060635
APA StyleGe, M., Zhang, Y., Xing, Y., Xing, L., Miao, H., & Huo, X. (2025). DoDELLA-GAI2 Integrates Gibberellin and Ethylene Signaling to Regulate Chinese Yam (Dioscorea opposita) Tuber Development. Biology, 14(6), 635. https://doi.org/10.3390/biology14060635





