The Critical Role of Adipocytes in Leukemia
Simple Summary
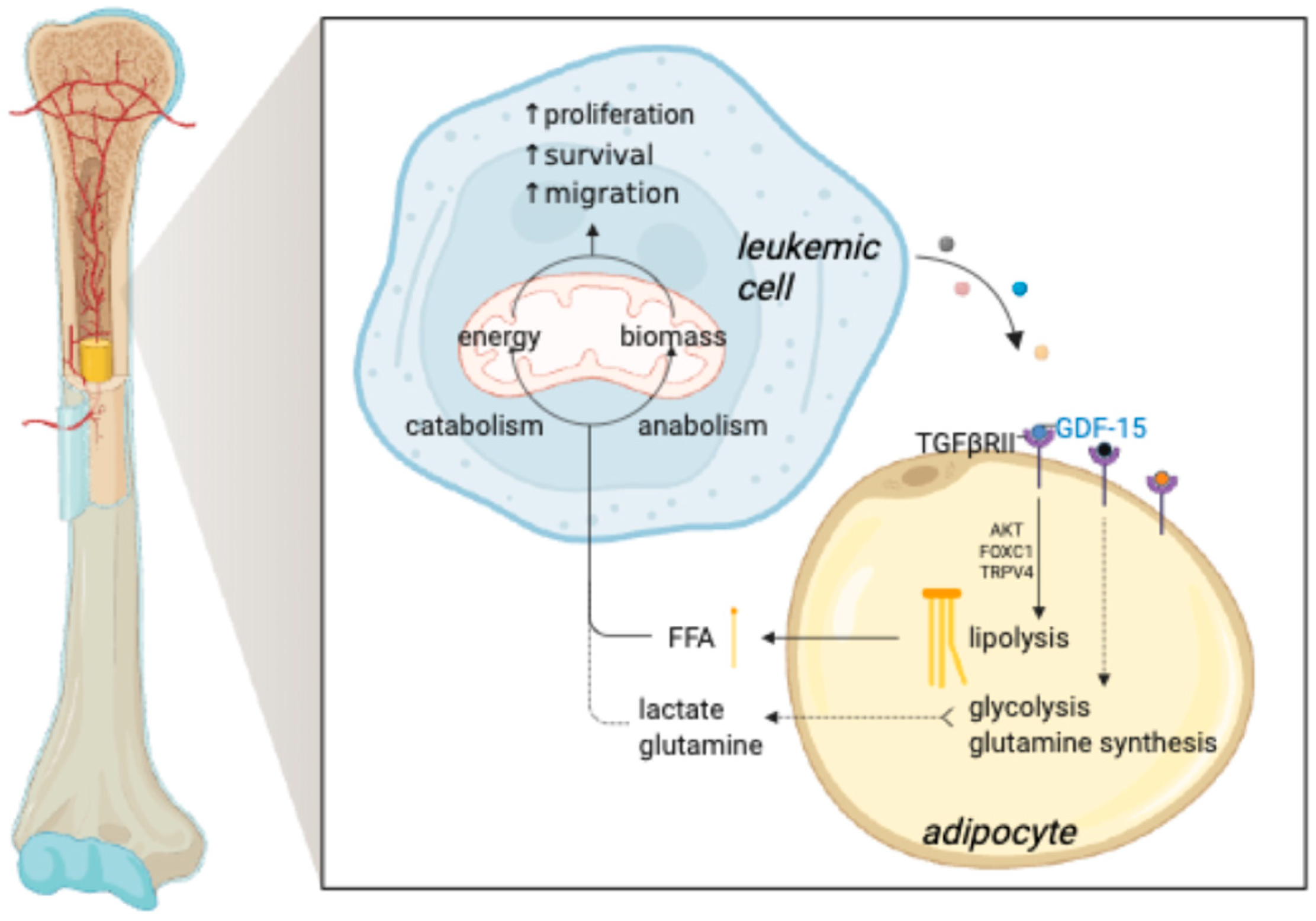
Abstract
1. Introduction
2. What Is Bone Marrow Adipocyte?
3. Adipocytes: Silent Partners in Leukemia’s Journey
4. Adipocytes Support Leukemia Survival and Resistance by Supplying Metabolites
5. Burning Metabolites, Battling Cancer: The Antileukemic Role of Thermogenic Adipocytes
6. Adipocyte-Derived Adipokines in Leukemia Progression
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quail, D.F.; Joyce, J.A. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. CB 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Piotrowska, K.; Tarnowski, M. Bone Marrow Adipocytes-Role in Physiology and Various Nutritional Conditions in Human and Animal Models. Nutrients 2021, 13, 1412. [Google Scholar] [CrossRef]
- Lucas, D. Structural Organization of the Bone Marrow and Its Role in Hematopoiesis. Curr. Opin. Hematol. 2021, 28, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Ghosn, E.; Yoshimoto, M.; Nakauchi, H.; Weissman, I.L.; Herzenberg, L.A. Hematopoietic Stem Cell-Independent Hematopoiesis and the Origins of Innate-like B Lymphocytes. Dev. Camb. Engl. 2019, 146, dev170571. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.d.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Attané, C.; Estève, D.; Chaoui, K.; Iacovoni, J.S.; Corre, J.; Moutahir, M.; Valet, P.; Schiltz, O.; Reina, N.; Muller, C. Human Bone Marrow Is Comprised of Adipocytes with Specific Lipid Metabolism. Cell Rep. 2020, 30, 949–958.e6. [Google Scholar] [CrossRef]
- Fazeli, P.K.; Horowitz, M.C.; MacDougald, O.A.; Scheller, E.L.; Rodeheffer, M.S.; Rosen, C.J.; Klibanski, A. Marrow Fat and Bone--New Perspectives. J. Clin. Endocrinol. Metab. 2013, 98, 935–945. [Google Scholar] [CrossRef]
- Cawthorn, W.P.; Scheller, E.L.; Learman, B.S.; Parlee, S.D.; Simon, B.R.; Mori, H.; Ning, X.; Bree, A.J.; Schell, B.; Broome, D.T.; et al. Bone Marrow Adipose Tissue Is an Endocrine Organ That Contributes to Increased Circulating Adiponectin during Caloric Restriction. Cell Metab. 2014, 20, 368–375. [Google Scholar] [CrossRef]
- Kricun, M.E. Red-Yellow Marrow Conversion: Its Effect on the Location of Some Solitary Bone Lesions. Skeletal Radiol. 1985, 14, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; MacDougald, O.A. Preclinical Models for Investigating How Bone Marrow Adipocytes Influence Bone and Hematopoietic Cellularity. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101547. [Google Scholar] [CrossRef] [PubMed]
- Steiner, R.M.; Mitchell, D.G.; Rao, V.M.; Schweitzer, M.E. Magnetic resonance imaging of diffuse bone marrow disease. Available online: https://www.periodicos.capes.gov.br/index.php/acervo/buscador.html?task=detalhes&id=W1797542295 (accessed on 2 May 2025).
- Miggitsch, C.; Meryk, A.; Naismith, E.; Pangrazzi, L.; Ejaz, A.; Jenewein, B.; Wagner, S.; Nägele, F.; Fenkart, G.; Trieb, K.; et al. Human Bone Marrow Adipocytes Display Distinct Immune Regulatory Properties. EBioMedicine 2019, 46, 387–398. [Google Scholar] [CrossRef]
- Dumas, J.-F.; Brisson, L. Interaction between Adipose Tissue and Cancer Cells: Role for Cancer Progression. Cancer Metastasis Rev. 2021, 40, 31–46. [Google Scholar] [CrossRef]
- Song, Y.; Na, H.; Lee, S.E.; Kim, Y.M.; Moon, J.; Nam, T.W.; Ji, Y.; Jin, Y.; Park, J.H.; Cho, S.C.; et al. Dysfunctional Adipocytes Promote Tumor Progression through YAP/TAZ-Dependent Cancer-Associated Adipocyte Transformation. Nat. Commun. 2024, 15, 4052. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zeng, H.; Li, J.; Zeng, N.; Zhang, Q.; Hou, K.; Li, J.; Yu, J.; Wu, Y. Dissecting the Emerging Role of Cancer-Associated Adipocyte-Derived Cytokines in Remodeling Breast Cancer Progression. Heliyon 2024, 10, e35200. [Google Scholar] [CrossRef]
- Salamanna, F.; Contartese, D.; Errani, C.; Sartori, M.; Borsari, V.; Giavaresi, G. Role of Bone Marrow Adipocytes in Bone Metastasis Development and Progression: A Systematic Review. Front. Endocrinol. 2023, 14, 1207416. [Google Scholar] [CrossRef]
- Zinngrebe, J.; Debatin, K.-M.; Fischer-Posovszky, P. Adipocytes in Hematopoiesis and Acute Leukemia: Friends, Enemies, or Innocent Bystanders? Leukemia 2020, 34, 2305–2316. [Google Scholar] [CrossRef]
- Cohen, P.; Kajimura, S. The Cellular and Functional Complexity of Thermogenic Fat. Nat. Rev. Mol. Cell Biol. 2021, 22, 393–409. [Google Scholar] [CrossRef]
- Ricquier, D. UCP1, the Mitochondrial Uncoupling Protein of Brown Adipocyte: A Personal Contribution and a Historical Perspective. Biochimie 2017, 134, 3–8. [Google Scholar] [CrossRef]
- Scheller, E.L.; Cawthorn, W.P.; Burr, A.A.; Horowitz, M.C.; MacDougald, O.A. Marrow Adipose Tissue: Trimming the Fat. Trends Endocrinol. Metab. TEM 2016, 27, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Scheller, E.L.; Rosen, C.J. What’s the Matter with MAT? Marrow Adipose Tissue, Metabolism, and Skeletal Health. Ann. N. Y. Acad. Sci. 2014, 1311, 14–30. [Google Scholar] [CrossRef]
- Sebo, Z.L.; Rendina-Ruedy, E.; Ables, G.P.; Lindskog, D.M.; Rodeheffer, M.S.; Fazeli, P.K.; Horowitz, M.C. Bone Marrow Adiposity: Basic and Clinical Implications. Endocr. Rev. 2019, 40, 1187–1206. [Google Scholar] [CrossRef]
- Ackert-Bicknell, C.L.; Shockley, K.R.; Horton, L.G.; Lecka-Czernik, B.; Churchill, G.A.; Rosen, C.J. Strain-Specific Effects of Rosiglitazone on Bone Mass, Body Composition, and Serum Insulin-like Growth Factor-I. Endocrinology 2009, 150, 1330–1340. [Google Scholar] [CrossRef]
- Tavassoli, M. Ultrastructural Development of Bone Marrow Adipose Cell. Acta Anat. 1976, 94, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shi, Y.; Regan, J.; Karuppaiah, K.; Ornitz, D.M.; Long, F. Osx-Cre Targets Multiple Cell Types besides Osteoblast Lineage in Postnatal Mice. PLoS ONE 2014, 9, e85161. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Pinho, S.; Ahmed, J.; Kunisaki, Y.; Hanoun, M.; Mendelson, A.; Ono, N.; Kronenberg, H.M.; Frenette, P.S. Osterix Marks Distinct Waves of Primitive and Definitive Stromal Progenitors during Bone Marrow Development. Dev. Cell 2014, 29, 340–349. [Google Scholar] [CrossRef]
- Zhou, B.O.; Yue, R.; Murphy, M.M.; Peyer, J.G.; Morrison, S.J. Leptin-Receptor-Expressing Mesenchymal Stromal Cells Represent the Main Source of Bone Formed by Adult Bone Marrow. Cell Stem Cell 2014, 15, 154–168. [Google Scholar] [CrossRef]
- Worthley, D.L.; Churchill, M.; Compton, J.T.; Tailor, Y.; Rao, M.; Si, Y.; Levin, D.; Schwartz, M.G.; Uygur, A.; Hayakawa, Y.; et al. Gremlin 1 Identifies a Skeletal Stem Cell with Bone, Cartilage, and Reticular Stromal Potential. Cell 2015, 160, 269–284. [Google Scholar] [CrossRef]
- Zhang, X.; Robles, H.; Magee, K.L.; Lorenz, M.R.; Wang, Z.; Harris, C.A.; Craft, C.S.; Scheller, E.L. A Bone-Specific Adipogenesis Pathway in Fat-Free Mice Defines Key Origins and Adaptations of Bone Marrow Adipocytes with Age and Disease. eLife 2021, 10, e66275. [Google Scholar] [CrossRef]
- Gunaratnam, K.; Vidal, C.; Gimble, J.M.; Duque, G. Mechanisms of Palmitate-Induced Lipotoxicity in Human Osteoblasts. Endocrinology 2014, 155, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Scheller, E.L.; Doucette, C.R.; Learman, B.S.; Cawthorn, W.P.; Khandaker, S.; Schell, B.; Wu, B.; Ding, S.-Y.; Bredella, M.A.; Fazeli, P.K.; et al. Region-Specific Variation in the Properties of Skeletal Adipocytes Reveals Regulated and Constitutive Marrow Adipose Tissues. Nat. Commun. 2015, 6, 7808. [Google Scholar] [CrossRef]
- Suchacki, K.J.; Tavares, A.A.S.; Mattiucci, D.; Scheller, E.L.; Papanastasiou, G.; Gray, C.; Sinton, M.C.; Ramage, L.E.; McDougald, W.A.; Lovdel, A.; et al. Bone Marrow Adipose Tissue Is a Unique Adipose Subtype with Distinct Roles in Glucose Homeostasis. Nat. Commun. 2020, 11, 3097. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.; Ivaska, K.K.; Hannukainen, J.C.; Virtanen, K.A.; Lidell, M.E.; Enerbäck, S.; Mäkelä, K.; Parkkola, R.; Piirola, S.; Oikonen, V.; et al. Human Bone Marrow Adipose Tissue Is a Metabolically Active and Insulin-Sensitive Distinct Fat Depot. J. Clin. Endocrinol. Metab. 2020, 105, 2300–2310. [Google Scholar] [CrossRef] [PubMed]
- Krings, A.; Rahman, S.; Huang, S.; Lu, Y.; Czernik, P.J.; Lecka-Czernik, B. Bone Marrow Fat Has Brown Adipose Tissue Characteristics, Which Are Attenuated with Aging and Diabetes. Bone 2012, 50, 546–552. [Google Scholar] [CrossRef]
- Lindsey, R.C.; Mohan, S. Thyroid Hormone Acting via TRβ Induces Expression of Browning Genes in Mouse Bone Marrow Adipose Tissue. Endocrine 2017, 56, 109–120. [Google Scholar] [CrossRef]
- Liu, L.-F.; Shen, W.-J.; Ueno, M.; Patel, S.; Kraemer, F.B. Characterization of Age-Related Gene Expression Profiling in Bone Marrow and Epididymal Adipocytes. BMC Genomics 2011, 12, 212. [Google Scholar] [CrossRef]
- Lecoutre, S.; Lambert, M.; Drygalski, K.; Dugail, I.; Maqdasy, S.; Hautefeuille, M.; Clément, K. Importance of the Microenvironment and Mechanosensing in Adipose Tissue Biology. Cells 2022, 11, 2310. [Google Scholar] [CrossRef]
- Laharrague, P.; Larrouy, D.; Fontanilles, A.M.; Truel, N.; Campfield, A.; Tenenbaum, R.; Galitzky, J.; Corberand, J.X.; Pénicaud, L.; Casteilla, L. High Expression of Leptin by Human Bone Marrow Adipocytes in Primary Culture. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1998, 12, 747–752. [Google Scholar] [CrossRef]
- Laharrague, P.; Truel, N.; Fontanilles, A.M.; Corberand, J.X.; Pénicaud, L.; Casteilla, L. Regulation by Cytokines of Leptin Expression in Human Bone Marrow Adipocytes. Horm. Metab. Res. Horm. Stoffwechselforschung Horm. Metab. 2000, 32, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Zhou, B.O.; Shimada, I.S.; Zhao, Z.; Morrison, S.J. Leptin Receptor Promotes Adipogenesis and Reduces Osteogenesis by Regulating Mesenchymal Stromal Cells in Adult Bone Marrow. Cell Stem Cell 2016, 18, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.; Han, S.-J.; Enamorado, M.; Link, V.M.; Huang, B.; Moseman, E.A.; Kishton, R.J.; Shannon, J.P.; Dixit, D.; Schwab, S.R.; et al. The Bone Marrow Protects and Optimizes Immunological Memory during Dietary Restriction. Cell 2019, 178, 1088-1101.e15. [Google Scholar] [CrossRef] [PubMed]
- Naveiras, O.; Nardi, V.; Wenzel, P.L.; Hauschka, P.V.; Fahey, F.; Daley, G.Q. Bone-Marrow Adipocytes as Negative Regulators of the Haematopoietic Microenvironment. Nature 2009, 460, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, T.H.; Scialdone, A.; Graja, A.; Gohlke, S.; Jank, A.-M.; Bocian, C.; Woelk, L.; Fan, H.; Logan, D.W.; Schürmann, A.; et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell 2017, 20, 771-784.e6. [Google Scholar] [CrossRef]
- Zhu, R.-J.; Wu, M.-Q.; Li, Z.-J.; Zhang, Y.; Liu, K.-Y. Hematopoietic Recovery Following Chemotherapy Is Improved by BADGE-Induced Inhibition of Adipogenesis. Int. J. Hematol. 2013, 97, 58–72. [Google Scholar] [CrossRef]
- Doucette, C.R.; Horowitz, M.C.; Berry, R.; MacDougald, O.A.; Anunciado-Koza, R.; Koza, R.A.; Rosen, C.J. A High Fat Diet Increases Bone Marrow Adipose Tissue (MAT) But Does Not Alter Trabecular or Cortical Bone Mass in C57BL/6J Mice. J. Cell. Physiol. 2015, 230, 2032–2037. [Google Scholar] [CrossRef]
- Poloni, A.; Maurizi, G.; Serrani, F.; Mancini, S.; Zingaretti, M.C.; Frontini, A.; Cinti, S.; Olivieri, A.; Leoni, P. Molecular and Functional Characterization of Human Bone Marrow Adipocytes. Exp. Hematol. 2013, 41, 558-566.e2. [Google Scholar] [CrossRef]
- Spindler, T.J.; Tseng, A.W.; Zhou, X.; Adams, G.B. Adipocytic Cells Augment the Support of Primitive Hematopoietic Cells in Vitro but Have No Effect in the Bone Marrow Niche under Homeostatic Conditions. Stem Cells Dev. 2014, 23, 434–441. [Google Scholar] [CrossRef]
- Sahdo, B.; Evans, A.L.; Arnemo, J.M.; Fröbert, O.; Särndahl, E.; Blanc, S. Body Temperature during Hibernation Is Highly Correlated with a Decrease in Circulating Innate Immune Cells in the Brown Bear (Ursus Arctos): A Common Feature among Hibernators? Int. J. Med. Sci. 2013, 10, 508–514. [Google Scholar] [CrossRef]
- Reagan, M.R.; Rosen, C.J. Navigating the Bone Marrow Niche: Translational Insights and Cancer-Driven Dysfunction. Nat. Rev. Rheumatol. 2016, 12, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Schepers, K.; Campbell, T.B.; Passegué, E. Normal and Leukemic Stem Cell Niches: Insights and Therapeutic Opportunities. Cell Stem Cell 2015, 16, 254–267. [Google Scholar] [CrossRef]
- Nagasawa, T. CXC Chemokine Ligand 12 (CXCL12) and Its Receptor CXCR4. J. Mol. Med. Berl. Ger. 2014, 92, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Ponte, F.; Kim, H.-N.; Iyer, S.; Han, L.; Almeida, M.; Manolagas, S.C. Cxcl12 Deletion in Mesenchymal Cells Increases Bone Turnover and Attenuates the Loss of Cortical Bone Caused by Estrogen Deficiency in Mice. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2020, 35, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Fukuhara, A.; Onodera, T.; Kita, S.; Yokoyama, C.; Otsuki, M.; Shimomura, I. SDF-1 Is an Autocrine Insulin-Desensitizing Factor in Adipocytes. Diabetes 2018, 67, 1068–1078. [Google Scholar] [CrossRef]
- Periyasamy-Thandavan, S.; Herberg, S.; Arounleut, P.; Upadhyay, S.; Dukes, A.; Davis, C.; Johnson, M.; McGee-Lawrence, M.; Hamrick, M.W.; Isales, C.M.; et al. Caloric Restriction and the Adipokine Leptin Alter the SDF-1 Signaling Axis in Bone Marrow and in Bone Marrow Derived Mesenchymal Stem Cells. Mol. Cell. Endocrinol. 2015, 410, 64–72. [Google Scholar] [CrossRef]
- Battula, V.L.; Chen, Y.; Cabreira, M.dG.; Ruvolo, V.; Wang, Z.; Ma, W.; Konoplev, S.; Shpall, E.; Lyons, K.; Strunk, D.; et al. Connective Tissue Growth Factor Regulates Adipocyte Differentiation of Mesenchymal Stromal Cells and Facilitates Leukemia Bone Marrow Engraftment. Blood 2013, 122, 357–366. [Google Scholar] [CrossRef]
- Segerberg, F.; Lambert, M.; Sanz-Ortega, L.; Andersson, A.; Childs, R.W.; Carlsten, M. Improved Leukemia Clearance After Adoptive Transfer of NK Cells Expressing the Bone Marrow Homing Receptor CXCR4R334X. HemaSphere 2023, 7, e974. [Google Scholar] [CrossRef]
- Levy, E.; Reger, R.; Segerberg, F.; Lambert, M.; Leijonhufvud, C.; Baumer, Y.; Carlsten, M.; Childs, R. Enhanced Bone Marrow Homing of Natural Killer Cells Following mRNA Transfection with Gain-of-Function Variant CXCR4R334X. Front. Immunol. 2019, 10, 1262. [Google Scholar] [CrossRef]
- Jia, X.; Liao, N.; Yao, Y.; Guo, X.; Chen, K.; Shi, P. Dynamic Evolution of Bone Marrow Adipocyte in B Cell Acute Lymphoblastic Leukemia: Insights from Diagnosis to Post-Chemotherapy. Cancer Biol. Ther. 2024, 25, 2323765. [Google Scholar] [CrossRef]
- Liu, H.; Zhai, Y.; Zhao, W.; Wan, Y.; Lu, W.; Yang, S.; Yu, Y.; Wei, Y.; Li, Z.; Shi, J. Consolidation Chemotherapy Prevents Relapse by Indirectly Regulating Bone Marrow Adipogenesis in Patients with Acute Myeloid Leukemia. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 45, 2389–2400. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Weng, W.; Zhu, Q.; Zhai, Y.; Wan, Y.; Liu, H.; Yang, S.; Yu, Y.; Wei, Y.; Shi, J. Small Bone Marrow Adipocytes Predict Poor Prognosis in Acute Myeloid Leukemia. Haematologica 2018, 103, e21–e24. [Google Scholar] [CrossRef]
- Masamoto, Y.; Arai, S.; Sato, T.; Kubota, N.; Takamoto, I.; Kadowaki, T.; Kurokawa, M. Adiponectin Enhances Quiescence Exit of Murine Hematopoietic Stem Cells and Hematopoietic Recovery Through mTORC1 Potentiation. Stem Cells Dayt. Ohio 2017, 35, 1835–1848. [Google Scholar] [CrossRef]
- Boyd, A.L.; Reid, J.C.; Salci, K.R.; Aslostovar, L.; Benoit, Y.D.; Shapovalova, Z.; Nakanishi, M.; Porras, D.P.; Almakadi, M.; Campbell, C.J.V.; et al. Acute Myeloid Leukaemia Disrupts Endogenous Myelo-Erythropoiesis by Compromising the Adipocyte Bone Marrow Niche. Nat. Cell Biol. 2017, 19, 1336–1347. [Google Scholar] [CrossRef]
- van Zoelen, E.J.; Duarte, I.; Hendriks, J.M.; van der Woning, S.P. TGFβ-Induced Switch from Adipogenic to Osteogenic Differentiation of Human Mesenchymal Stem Cells: Identification of Drug Targets for Prevention of Fat Cell Differentiation. Stem Cell Res. Ther. 2016, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lu, W.; Zhao, C.; Zhai, Y.; Wei, Y.; Liu, J.; Yu, Y.; Li, Z.; Shi, J. Leukemia Cells Remodel Marrow Adipocytes via TRPV4-Dependent Lipolysis. Haematologica 2020, 105, 2572–2583. [Google Scholar] [CrossRef]
- Shafat, M.S.; Oellerich, T.; Mohr, S.; Robinson, S.D.; Edwards, D.R.; Marlein, C.R.; Piddock, R.E.; Fenech, M.; Zaitseva, L.; Abdul-Aziz, A.; et al. Leukemic Blasts Program Bone Marrow Adipocytes to Generate a Protumoral Microenvironment. Blood 2017, 129, 1320–1332. [Google Scholar] [CrossRef]
- Heydt, Q.; Xintaropoulou, C.; Clear, A.; Austin, M.; Pislariu, I.; Miraki-Moud, F.; Cutillas, P.; Korfi, K.; Calaminici, M.; Cawthorn, W.; et al. Adipocytes Disrupt the Translational Programme of Acute Lymphoblastic Leukaemia to Favour Tumour Survival and Persistence. Nat. Commun. 2021, 12, 5507. [Google Scholar] [CrossRef] [PubMed]
- Cahu, X.; Calvo, J.; Poglio, S.; Prade, N.; Colsch, B.; Arcangeli, M.-L.; Leblanc, T.; Petit, A.; Baleydier, F.; Baruchel, A.; et al. Bone Marrow Sites Differently Imprint Dormancy and Chemoresistance to T-Cell Acute Lymphoblastic Leukemia. Blood Adv. 2017, 1, 1760–1772. [Google Scholar] [CrossRef]
- Behan, J.W.; Avramis, V.I.; Yun, J.P.; Louie, S.G.; Mittelman, S.D. Diet-Induced Obesity Alters Vincristine Pharmacokinetics in Blood and Tissues of Mice. Pharmacol. Res. 2010, 61, 385–390. [Google Scholar] [CrossRef]
- Behan, J.W.; Yun, J.P.; Proektor, M.P.; Ehsanipour, E.A.; Arutyunyan, A.; Moses, A.S.; Avramis, V.I.; Louie, S.G.; Butturini, A.; Heisterkamp, N.; et al. Adipocytes Impair Leukemia Treatment in Mice. Cancer Res. 2009, 69, 7867–7874. [Google Scholar] [CrossRef] [PubMed]
- Ehsanipour, E.A.; Sheng, X.; Behan, J.W.; Wang, X.; Butturini, A.; Avramis, V.I.; Mittelman, S.D. Adipocytes Cause Leukemia Cell Resistance to L-Asparaginase via Release of Glutamine. Cancer Res. 2013, 73, 2998–3006. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The Hallmarks of Cancer Metabolism: Still Emerging. Cell Metab. 2022, 34, 355–377. [Google Scholar] [CrossRef]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting Cancer Metabolism in the Era of Precision Oncology. Nat. Rev. Drug Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef]
- Saito, Y.; Chapple, R.H.; Lin, A.; Kitano, A.; Nakada, D. AMPK Protects Leukemia-Initiating Cells in Myeloid Leukemias from Metabolic Stress in the Bone Marrow. Cell Stem Cell 2015, 17, 585–596. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Jones, C.L.; Stevens, B.M.; D’Alessandro, A.; Reisz, J.A.; Culp-Hill, R.; Nemkov, T.; Pei, S.; Khan, N.; Adane, B.; Ye, H.; et al. Inhibition of Amino Acid Metabolism Selectively Targets Human Leukemia Stem Cells. Cancer Cell 2019, 35, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Brody, J.I.; Oski, F.A.; Singer, D.E. Impaired Pentose Phosphate Shunt and Decreased Glycolytic Activity in Lymphocytes of Chronic Lymphocytic Leukemia. Metabolic Pathway. Blood 1969, 34, 421–429. [Google Scholar] [CrossRef]
- Brody, J.I.; Merlie, K. Metabolic and Biosynthetic Features of Lymphocytes from Patients with Diabetes Mellitus: Similarities to Lymphocytes in Chronic Lymphocytic Leukaemia. Br. J. Haematol. 1970, 19, 193–201. [Google Scholar] [CrossRef]
- Ryland, L.K.; Doshi, U.A.; Shanmugavelandy, S.S.; Fox, T.E.; Aliaga, C.; Broeg, K.; Baab, K.T.; Young, M.; Khan, O.; Haakenson, J.K.; et al. C6-Ceramide Nanoliposomes Target the Warburg Effect in Chronic Lymphocytic Leukemia. PLoS ONE 2013, 8, e84648. [Google Scholar] [CrossRef] [PubMed]
- Rozovski, U.; Hazan-Halevy, I.; Barzilai, M.; Keating, M.J.; Estrov, Z. Metabolism Pathways in Chronic Lymphocytic Leukemia. Leuk. Lymphoma 2016, 57, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Doughty, C.A.; Bleiman, B.F.; Wagner, D.J.; Dufort, F.J.; Mataraza, J.M.; Roberts, M.F.; Chiles, T.C. Antigen Receptor-Mediated Changes in Glucose Metabolism in B Lymphocytes: Role of Phosphatidylinositol 3-Kinase Signaling in the Glycolytic Control of Growth. Blood 2006, 107, 4458–4465. [Google Scholar] [CrossRef] [PubMed]
- Adekola, K.U.A.; Dalva Aydemir, S.; Ma, S.; Zhou, Z.; Rosen, S.T.; Shanmugam, M. Investigating and Targeting Chronic Lymphocytic Leukemia Metabolism with the Human Immunodeficiency Virus Protease Inhibitor Ritonavir and Metformin. Leuk. Lymphoma 2015, 56, 450–459. [Google Scholar] [CrossRef]
- Galicia-Vázquez, G.; Smith, S.; Aloyz, R. Del11q-Positive CLL Lymphocytes Exhibit Altered Glutamine Metabolism and Differential Response to GLS1 and Glucose Metabolism Inhibition. Blood Cancer J. 2018, 8, 13. [Google Scholar] [CrossRef]
- Mayers, J.R.; Vander Heiden, M.G. Famine versus Feast: Understanding the Metabolism of Tumors In Vivo. Trends Biochem. Sci. 2015, 40, 130–140. [Google Scholar] [CrossRef]
- Jones, C.L.; Inguva, A.; Jordan, C.T. Targeting Energy Metabolism in Cancer Stem Cells: Progress and Challenges in Leukemia and Solid Tumors. Cell Stem Cell 2021, 28, 378–393. [Google Scholar] [CrossRef]
- Figueroa, M.E.; Abdel-Wahab, O.; Lu, C.; Ward, P.S.; Patel, J.; Shih, A.; Li, Y.; Bhagwat, N.; Vasanthakumar, A.; Fernandez, H.F.; et al. Leukemic IDH1 and IDH2 Mutations Result in a Hypermethylation Phenotype, Disrupt TET2 Function, and Impair Hematopoietic Differentiation. Cancer Cell 2010, 18, 553–567. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic Reprogramming and Cancer Progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef]
- Prentice, K.J.; Saksi, J.; Hotamisligil, G.S. Adipokine FABP4 Integrates Energy Stores and Counterregulatory Metabolic Responses. J. Lipid Res. 2019, 60, 734–740. [Google Scholar] [CrossRef]
- Yan, F.; Shen, N.; Pang, J.X.; Zhang, Y.W.; Rao, E.Y.; Bode, A.M.; Al-Kali, A.; Zhang, D.E.; Litzow, M.R.; Li, B.; et al. Fatty Acid-Binding Protein FABP4 Mechanistically Links Obesity with Aggressive AML by Enhancing Aberrant DNA Methylation in AML Cells. Leukemia 2017, 31, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, O.; Gong, G.; Olipitz, W.; Muthupalani, S.; Engelward, B.P. Inflammation-Induced Cell Proliferation Potentiates DNA Damage-Induced Mutations in Vivo. PLoS Genet. 2015, 11, e1004901. [Google Scholar] [CrossRef] [PubMed]
- Zechner, R.; Strauss, J.; Frank, S.; Wagner, E.; Hofmann, W.; Kratky, D.; Hiden, M.; Levak-Frank, S. The Role of Lipoprotein Lipase in Adipose Tissue Development and Metabolism. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2000, 24 (Suppl. 4), S53–S56. [Google Scholar] [CrossRef]
- Rosenwald, A.; Alizadeh, A.A.; Widhopf, G.; Simon, R.; Davis, R.E.; Yu, X.; Yang, L.; Pickeral, O.K.; Rassenti, L.Z.; Powell, J.; et al. Relation of Gene Expression Phenotype to Immunoglobulin Mutation Genotype in B Cell Chronic Lymphocytic Leukemia. J. Exp. Med. 2001, 194, 1639–1647. [Google Scholar] [CrossRef]
- Cahill, N.; Rosenquist, R. Uncovering the DNA Methylome in Chronic Lymphocytic Leukemia. Epigenetics 2013, 8, 138–148. [Google Scholar] [CrossRef]
- Cahill, N.; Bergh, A.-C.; Kanduri, M.; Göransson-Kultima, H.; Mansouri, L.; Isaksson, A.; Ryan, F.; Smedby, K.E.; Juliusson, G.; Sundström, C.; et al. 450K-Array Analysis of Chronic Lymphocytic Leukemia Cells Reveals Global DNA Methylation to Be Relatively Stable over Time and Similar in Resting and Proliferative Compartments. Leukemia 2013, 27, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Rozovski, U.; Grgurevic, S.; Bueso-Ramos, C.; Harris, D.M.; Li, P.; Liu, Z.; Wu, J.Y.; Jain, P.; Wierda, W.; Burger, J.; et al. Aberrant LPL Expression, Driven by STAT3, Mediates Free Fatty Acid Metabolism in CLL Cells. Mol. Cancer Res. MCR 2015, 13, 944–953. [Google Scholar] [CrossRef]
- Kroemer, G.; Pouyssegur, J. Tumor Cell Metabolism: Cancer’s Achilles’ Heel. Cancer Cell 2008, 13, 472–482. [Google Scholar] [CrossRef]
- Emadi, A.; Zokaee, H.; Sausville, E.A. Asparaginase in the Treatment of Non-ALL Hematologic Malignancies. Cancer Chemother. Pharmacol. 2014, 73, 875–883. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine Reliance in Cell Metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Lecoutre, S.; Maqdasy, S.; Rizo-Roca, D.; Renzi, G.; Vlassakev, I.; Alaeddine, L.M.; Higos, R.; Jalkanen, J.; Zhong, J.; Zareifi, D.S.; et al. Reduced Adipocyte Glutaminase Activity Promotes Energy Expenditure and Metabolic Health. Nat. Metab. 2024, 6, 1329–1346. [Google Scholar] [CrossRef] [PubMed]
- Lecoutre, S.; Maqdasy, S.; Petrus, P.; Ludzki, A.; Couchet, M.; Mejhert, N.; Rydén, M. Glutamine Metabolism in Adipocytes: A Bona Fide Epigenetic Modulator of Inflammation. Adipocyte 2020, 9, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Willems, L.; Jacque, N.; Jacquel, A.; Neveux, N.; Maciel, T.T.; Lambert, M.; Schmitt, A.; Poulain, L.; Green, A.S.; Uzunov, M.; et al. Inhibiting Glutamine Uptake Represents an Attractive New Strategy for Treating Acute Myeloid Leukemia. Blood 2013, 122, 3521–3532. [Google Scholar] [CrossRef]
- Gregory, M.A.; Nemkov, T.; Park, H.J.; Zaberezhnyy, V.; Gehrke, S.; Adane, B.; Jordan, C.T.; Hansen, K.C.; D’Alessandro, A.; DeGregori, J. Targeting Glutamine Metabolism and Redox State for Leukemia Therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 4079–4090. [Google Scholar] [CrossRef]
- Jin, J.; Byun, J.-K.; Choi, Y.-K.; Park, K.-G. Targeting Glutamine Metabolism as a Therapeutic Strategy for Cancer. Exp. Mol. Med. 2023, 55, 706–715. [Google Scholar] [CrossRef]
- Petrus, P.; Lecoutre, S.; Dollet, L.; Wiel, C.; Sulen, A.; Gao, H.; Tavira, B.; Laurencikiene, J.; Rooyackers, O.; Checa, A.; et al. Glutamine Links Obesity to Inflammation in Human White Adipose Tissue. Cell Metab. 2020, 31, 375-390.e11. [Google Scholar] [CrossRef] [PubMed]
- Lien, E.C.; Westermark, A.M.; Zhang, Y.; Yuan, C.; Li, Z.; Lau, A.N.; Sapp, K.M.; Wolpin, B.M.; Vander Heiden, M.G. Low Glycaemic Diets Alter Lipid Metabolism to Influence Tumour Growth. Nature 2021, 599, 302–307. [Google Scholar] [CrossRef]
- Seki, T.; Yang, Y.; Sun, X.; Lim, S.; Xie, S.; Guo, Z.; Xiong, W.; Kuroda, M.; Sakaue, H.; Hosaka, K.; et al. Brown-Fat-Mediated Tumour Suppression by Cold-Altered Global Metabolism. Nature 2022, 608, 421–428. [Google Scholar] [CrossRef]
- Malik, M.; van Gelderen, E.M.; Lee, J.H.; Kowalski, D.L.; Yen, M.; Goldwater, R.; Mujais, S.K.; Schaddelee, M.P.; de Koning, P.; Kaibara, A.; et al. Proarrhythmic Safety of Repeat Doses of Mirabegron in Healthy Subjects: A Randomized, Double-Blind, Placebo-, and Active-Controlled Thorough QT Study. Clin. Pharmacol. Ther. 2012, 92, 696–706. [Google Scholar] [CrossRef]
- O’Mara, A.E.; Johnson, J.W.; Linderman, J.D.; Brychta, R.J.; McGehee, S.; Fletcher, L.A.; Fink, Y.A.; Kapuria, D.; Cassimatis, T.M.; Kelsey, N.; et al. Chronic Mirabegron Treatment Increases Human Brown Fat, HDL Cholesterol, and Insulin Sensitivity. J. Clin. Investig. 2020, 130, 2209–2219. [Google Scholar] [CrossRef]
- Sun, X.; Sui, W.; Mu, Z.; Xie, S.; Deng, J.; Li, S.; Seki, T.; Wu, J.; Jing, X.; He, X.; et al. Mirabegron Displays Anticancer Effects by Globally Browning Adipose Tissues. Nat. Commun. 2023, 14, 7610. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Cheng, T.; Xie, S.; Sun, X.; Chen, M.; Zhao, S.; Ruan, Q.; Ni, X.; Rao, M.; Quan, X.; et al. Effective Prevention and Treatment of Acute Leukemias in Mice by Activation of Thermogenic Adipose Tissues. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2024, 11, e2402332. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Posovszky, P.; Zinngrebe, J. Brown Adipose Tissue Fights the Battle against Leukaemia. Nat. Rev. Endocrinol. 2024, 20, 699–700. [Google Scholar] [CrossRef]
- Nguyen, H.P.; An, K.; Ito, Y.; Kharbikar, B.N.; Sheng, R.; Paredes, B.; Murray, E.; Pham, K.; Bruck, M.; Zhou, X.; et al. Implantation of Engineered Adipocytes Suppresses Tumor Progression in Cancer Models. Nat. Biotechnol. 2025. [Google Scholar] [CrossRef]
- Kohler, J.A.; Moon, R.J.; Wright, S.; Willows, E.; Davies, J.H. Increased Adiposity and Altered Adipocyte Function in Female Survivors of Childhood Acute Lymphoblastic Leukaemia Treated without Cranial Radiation. Horm. Res. Paediatr. 2011, 75, 433–440. [Google Scholar] [CrossRef]
- Tabe, Y.; Konopleva, M.; Munsell, M.F.; Marini, F.C.; Zompetta, C.; McQueen, T.; Tsao, T.; Zhao, S.; Pierce, S.; Igari, J.; et al. PML-RARalpha Is Associated with Leptin-Receptor Induction: The Role of Mesenchymal Stem Cell-Derived Adipocytes in APL Cell Survival. Blood 2004, 103, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Crotty, B.H.; Fargnoli, J.; Papadavid, E.; Lekka, A.; Triantafilli, M.; Karmaniolas, K.; Migdalis, I.; Dionyssiou-Asteriou, A.; Mantzoros, C.S. B-Cell Chronic Lymphocytic Leukemia Risk in Association with Serum Leptin and Adiponectin: A Case-Control Study in Greece. Cancer Causes Control CCC 2010, 21, 1451–1459. [Google Scholar] [CrossRef][Green Version]
- Lu, Z.; Xie, J.; Wu, G.; Shen, J.; Collins, R.; Chen, W.; Kang, X.; Luo, M.; Zou, Y.; Huang, L.J.-S.; et al. Fasting Selectively Blocks Development of Acute Lymphoblastic Leukemia via Leptin-Receptor Upregulation. Nat. Med. 2017, 23, 79–90. [Google Scholar] [CrossRef]
- Yun, J.P.; Behan, J.W.; Heisterkamp, N.; Butturini, A.; Klemm, L.; Ji, L.; Groffen, J.; Müschen, M.; Mittelman, S.D. Diet-Induced Obesity Accelerates Acute Lymphoblastic Leukemia Progression in Two Murine Models. Cancer Prev. Res. Phila. Pa 2010, 3, 1259–1264. [Google Scholar] [CrossRef]
- Myers, M.G.; Leibel, R.L.; Seeley, R.J.; Schwartz, M.W. Obesity and Leptin Resistance: Distinguishing Cause from Effect. Trends Endocrinol. Metab. TEM 2010, 21, 643–651. [Google Scholar] [CrossRef]
- Pramanik, R.; Sheng, X.; Ichihara, B.; Heisterkamp, N.; Mittelman, S.D. Adipose Tissue Attracts and Protects Acute Lymphoblastic Leukemia Cells from Chemotherapy. Leuk. Res. 2013, 37, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Oritani, K.; Takahashi, I.; Ishikawa, J.; Matsuyama, A.; Ouchi, N.; Kihara, S.; Funahashi, T.; Tenner, A.J.; Tomiyama, Y.; et al. Adiponectin, a New Member of the Family of Soluble Defense Collagens, Negatively Regulates the Growth of Myelomonocytic Progenitors and the Functions of Macrophages. Blood 2000, 96, 1723–1732. [Google Scholar] [CrossRef]
- Barb, D.; Williams, C.J.; Neuwirth, A.K.; Mantzoros, C.S. Adiponectin in Relation to Malignancies: A Review of Existing Basic Research and Clinical Evidence. Am. J. Clin. Nutr. 2007, 86, s858–s866. [Google Scholar] [CrossRef]
- Körner, A.; Pazaitou-Panayiotou, K.; Kelesidis, T.; Kelesidis, I.; Williams, C.J.; Kaprara, A.; Bullen, J.; Neuwirth, A.; Tseleni, S.; Mitsiades, N.; et al. Total and High-Molecular-Weight Adiponectin in Breast Cancer: In Vitro and in Vivo Studies. J. Clin. Endocrinol. Metab. 2007, 92, 1041–1048. [Google Scholar] [CrossRef]
- Cong, L.; Gasser, J.; Zhao, J.; Yang, B.; Li, F.; Zhao, A.Z. Human Adiponectin Inhibits Cell Growth and Induces Apoptosis in Human Endometrial Carcinoma Cells, HEC-1-A and RL95 2. Endocr. Relat. Cancer 2007, 14, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Bråkenhielm, E.; Veitonmäki, N.; Cao, R.; Kihara, S.; Matsuzawa, Y.; Zhivotovsky, B.; Funahashi, T.; Cao, Y. Adiponectin-Induced Antiangiogenesis and Antitumor Activity Involve Caspase-Mediated Endothelial Cell Apoptosis. Proc. Natl. Acad. Sci. USA 2004, 101, 2476–2481. [Google Scholar] [CrossRef]
- Juarez, J.; Bradstock, K.F.; Gottlieb, D.J.; Bendall, L.J. Effects of Inhibitors of the Chemokine Receptor CXCR4 on Acute Lymphoblastic Leukemia Cells in Vitro. Leukemia 2003, 17, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Butler, D. Translational Research: Crossing the Valley of Death. Nature 2008, 453, 840–842. [Google Scholar] [CrossRef]
- Torisawa, Y.; Spina, C.S.; Mammoto, T.; Mammoto, A.; Weaver, J.C.; Tat, T.; Collins, J.J.; Ingber, D.E. Bone Marrow-on-a-Chip Replicates Hematopoietic Niche Physiology in Vitro. Nat. Methods 2014, 11, 663–669. [Google Scholar] [CrossRef]
- Nelson, M.R.; Ghoshal, D.; Mejías, J.C.; Rubio, D.F.; Keith, E.; Roy, K. A Multi-Niche Microvascularized Human Bone Marrow (hBM) on-a-Chip Elucidates Key Roles of the Endosteal Niche in hBM Physiology. Biomaterials 2021, 270, 120683. [Google Scholar] [CrossRef]
- Ma, C.; Wang, H.; Liu, L.; Tong, J.; Witkowski, M.T.; Aifantis, I.; Ghassemi, S.; Chen, W. A Bioengineered Immunocompetent Human Leukemia Chip for Preclinical Screening of CAR T Cell Immunotherapy. Res. Sq. 2023, rs.3.rs-2762929. [Google Scholar] [CrossRef]
- Ma, C.; Witkowski, M.T.; Harris, J.; Dolgalev, I.; Sreeram, S.; Qian, W.; Tong, J.; Chen, X.; Aifantis, I.; Chen, W. Leukemia-on-a-Chip: Dissecting the Chemoresistance Mechanisms in B Cell Acute Lymphoblastic Leukemia Bone Marrow Niche. Sci. Adv. 2020, 6, eaba5536. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Dove, R.; Gebeyehu, A.; Merenich, D.; Collier, P.; Crowgey, E.L.; Schaffer, M.; Shuey, D.; Macarrón, R.; Amador Arjona, A. Establishment of Human Bone Marrow-on-Chip as a Preclinical Model to Evaluate Drug-Induced Toxicities and Myelofibrosis Patient-Specific Pathophysiology. Blood 2023, 142, 5611. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higos, R.; Saitoski, K.; Hautefeuille, M.; Marcelin, G.; Clément, K.; Varin-Blank, N.; Breton, C.; Lecoutre, S.; Lambert, M. The Critical Role of Adipocytes in Leukemia. Biology 2025, 14, 624. https://doi.org/10.3390/biology14060624
Higos R, Saitoski K, Hautefeuille M, Marcelin G, Clément K, Varin-Blank N, Breton C, Lecoutre S, Lambert M. The Critical Role of Adipocytes in Leukemia. Biology. 2025; 14(6):624. https://doi.org/10.3390/biology14060624
Chicago/Turabian StyleHigos, Romane, Kevin Saitoski, Mathieu Hautefeuille, Geneviève Marcelin, Karine Clément, Nadine Varin-Blank, Christophe Breton, Simon Lecoutre, and Mélanie Lambert. 2025. "The Critical Role of Adipocytes in Leukemia" Biology 14, no. 6: 624. https://doi.org/10.3390/biology14060624
APA StyleHigos, R., Saitoski, K., Hautefeuille, M., Marcelin, G., Clément, K., Varin-Blank, N., Breton, C., Lecoutre, S., & Lambert, M. (2025). The Critical Role of Adipocytes in Leukemia. Biology, 14(6), 624. https://doi.org/10.3390/biology14060624





