Ferroptotic Pathway Activation in Spermatogonia: A Novel Mechanism of Busulfan-Induced Testicular Injury
Simple Summary
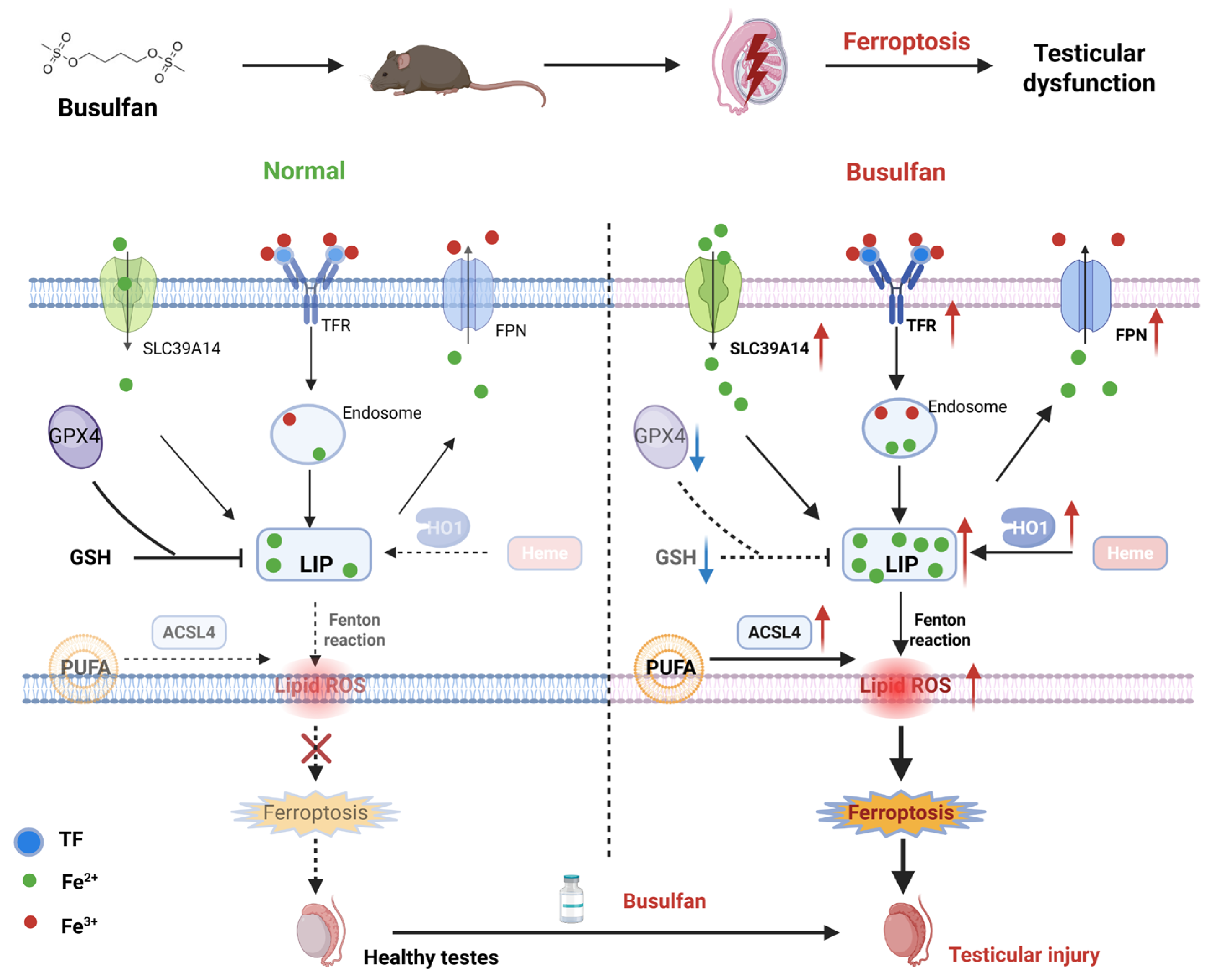
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Experimental Design
2.2. Testicular Function and Histology
2.3. RNA Sequencing (RNA-Seq) Analysis
2.4. Terminal Deoxynucleotidyl Transferase dUTP Nick End-Labeling (TUNEL) Staining
2.5. Cell Lines and Viability Assay
2.6. Transmission Electron Microscopy
2.7. Treatment with Ferroptosis and Heme Oxygenase 1 (HO1) Inhibitors
2.8. Measurement of Iron, GSH, and Malondialdehyde (MDA) Content
2.9. Reverse Transcription Quantitative PCR (RT-qPCR) and Western Blotting
2.10. Measurement of Mitochondrial Membrane Potential
2.11. Assessment of ROS
2.12. Statistical Analysis
3. Results
3.1. Exposure to BU Induces Testicular Injury
3.2. BU Exposure Leads to Ferroptosis in Mouse Testes
3.3. BU Reduces Cell Viability and Induces Ferroptosis in GC-1 Spg Cells
3.4. Ferroptosis Regulates BU-Induced GC-1 Spg Cell Death
3.5. The HO1 Inhibitor ZnPP Suppresses BU-Induced Ferroptosis in GC-1 Spg Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACSL4 | acyl-CoA synthetase long-chain family member 4 |
| BU | Busulfan |
| DFO | Desferrioxamine |
| DHE | Dihydroergotamine |
| DMT1/SLC11A2 | Solute carrier family 11 member 2 |
| Fer-1 | Ferrostatin-1 |
| FPN | Ferroportin |
| FTH | Ferritin, heavy polypeptide |
| FTL | Ferritin, light polypeptide |
| GPX4 | Glutathione peroxidase 4 |
| GSH | Glutathione |
| HO1 | Heme oxygenase 1 |
| LIP | Labile iron pool |
| MDA | Malondialdehyde |
| NAC | N-Acetyl-L-cysteine |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| ROS | Reactive oxygen species |
| TF | Transferrin |
| TFR | Transferrin receptor1 |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
| xCT/SLC7A11 | Solute carrier family 7 member 11 |
| YO-PRO-1 (YP1)/PI | Oxazole yellow/Propidium Iodide |
| ZIP14/SLC39A14 | Solute carrier family 39 member 14 |
| 4HNE | 4-Hydroxynonenal |
References
- Weaver, R.J.; Blomme, E.A.; Chadwick, A.E.; Copple, I.M.; Gerets, H.H.J.; Goldring, C.E.; Guillouzo, A.; Hewitt, P.G.; Ingelman-Sundberg, M.; Jensen, K.G.; et al. Managing the challenge of drug-induced liver injury: A roadmap for the development and deployment of preclinical predictive models. Nat. Rev. Drug Discov. 2020, 19, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.C.; Neelapu, S.S.; Giavridis, T.; Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 2022, 22, 85–96. [Google Scholar] [CrossRef]
- Delessard, M.; Saulnier, J.; Rives, A.; Dumont, L.; Rondanino, C.; Rives, N. Exposure to Chemotherapy During Childhood or Adulthood and Consequences on Spermatogenesis and Male Fertility. Int. J. Mol. Sci. 2020, 21, 1454. [Google Scholar] [CrossRef]
- Spears, N.; Lopes, F.; Stefansdottir, A.; Rossi, V.; De Felici, M.; Anderson, R.A.; Klinger, F.G. Ovarian damage from chemotherapy and current approaches to its protection. Hum. Reprod. Update 2019, 25, 673–693. [Google Scholar] [CrossRef]
- Tharmalingam, M.D.; Matilionyte, G.; Wallace, W.H.B.; Stukenborg, J.B.; Jahnukainen, K.; Oliver, E.; Goriely, A.; Lane, S.; Guo, J.; Cairns, B.; et al. Cisplatin and carboplatin result in similar gonadotoxicity in immature human testis with implications for fertility preservation in childhood cancer. BMC Med. 2020, 18, 374. [Google Scholar] [CrossRef]
- Tefferi, A.; Barbui, T. Polycythemia vera: 2024 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2023, 98, 1465–1487. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Moon, S.; Rhee, S.J. Optimal Once-Daily Busulfan Administration in Pediatric Patients: A Simulation-Based Investigation of Intravenous Infusion Times. Drug Des. Dev. Ther. 2024, 18, 871–879. [Google Scholar] [CrossRef]
- Grochow, L.B.; Krivit, W.; Whitley, C.B.; Blazar, B. Busulfan disposition in children. Blood 1990, 75, 1723–1727. [Google Scholar] [CrossRef]
- Galaup, A.; Paci, A. Pharmacology of dimethanesulfonate alkylating agents: Busulfan and treosulfan. Expert Opin. Drug Metab. Toxicol. 2013, 9, 333–347. [Google Scholar] [CrossRef]
- Oliner, H.; Schwartz, R.; Rubio, F.; Dameshek, W. Interstitial pulmonary fibrosis following busulfan therapy. Am. J. Med. 1961, 31, 134–139. [Google Scholar] [CrossRef]
- Meng, A.; Wang, Y.; Brown, S.A.; Van Zant, G.; Zhou, D. Ionizing radiation and busulfan inhibit murine bone marrow cell hematopoietic function via apoptosis-dependent and -independent mechanisms. Exp. Hematol. 2003, 31, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Dong, Y.; Chen, H.; Ye, T.; Chen, G.; Fan, B.; Wang, X.; Shi, J.; Wang, C. Ursonic acid attenuates spermatogenesis in oligozoospermia mice through inhibiting ferroptosis. Bioorg. Chem. 2024, 144, 107174. [Google Scholar] [CrossRef]
- Pu, R.; Liu, J.; Zhang, A.; Yang, J.; Zhang, W.; Long, X.; Ren, X.; Hua, H.; Shi, D.; Zhang, W.; et al. Modeling methods for busulfan-induced oligospermia and asthenozoospermia in mice: A systematic review and meta-analysis. J. Assist. Reprod. Genet. 2023, 40, 19–32. [Google Scholar] [CrossRef]
- Xie, Y.; Deng, C.C.; Ouyang, B.; Lv, L.Y.; Yao, J.H.; Zhang, C.; Chen, H.C.; Li, X.Y.; Sun, X.Z.; Deng, C.H.; et al. Establishing a nonlethal and efficient mouse model of male gonadotoxicity by intraperitoneal busulfan injection. Asian J. Androl. 2020, 22, 184–191. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Hirschhorn, T.; Stockwell, B.R. The development of the concept of ferroptosis. Free Radic. Biol. Med. 2019, 133, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Lei, G.; Zhuang, L.; Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021, 28, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Sun, Z.; Ji, G.; Hu, H. Emerging roles of ferroptosis in male reproductive diseases. Cell Death Discov. 2023, 9, 358. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Z.; Gao, J.; Li, H.; Wang, X.; Li, Y.; Sun, F. Inhibition of ferroptosis attenuates busulfan-induced oligospermia in mice. Toxicology 2020, 440, 152489. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, L.; Si, Y.; Huang, W.; Liu, R.; Liu, Z.; Jiang, Z.; Xu, F. Ferritinophagy-mediated ferroptosis of spermatogonia is involved in busulfan-induced oligospermia in the mice. Chem. Biol. Interact. 2024, 390, 110870. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, M.J.; Park, N.C.; Park, H.J. Effect of N-acetyl-L-cysteine on Testicular Tissue in Busulfan-Induced Dysfunction in the Male Reproductive System. World J. Men’s Health 2023, 41, 882–891. [Google Scholar] [CrossRef]
- Nam, H.J.; Park, M.J.; Joo, B.S.; Koo, Y.K.; Kim, S.; Lee, S.D.; Park, H.J. Effects of Perilla frutescens Var. Acuta in Busulfan-Induced Spermatogenesis Dysfunction Mouse Model. World J. Men’s Health 2024, 42, 810–820. [Google Scholar] [CrossRef]
- Almasry, S.M.; Hassan, Z.A.; Elsaed, W.M.; Elbastawisy, Y.M. Structural evaluation of the peritubular sheath of rat’s testes after administration of ribavirin: A possible impact on the testicular function. Int. J. Immunopathol. Pharmacol. 2017, 30, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Karimi-Dehkordi, M.; Pouriayevali, F. Evaluation of the sperm parameters, oxidative stress, and histopathological effects of vitamin B12 in preventing Helicobacter pylori-induced testicular toxicity: An experimental study. Int. J. Reprod. BioMed. (IJRM) 2024, 22, 383–394. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Zhao, T.; Chen, J.; Kang, L.; Wei, Y.; Han, L.; Shen, L.; Long, C.; Wu, S.; et al. Di-(2-ethylhexyl) phthalate exposure leads to ferroptosis via the HIF-1α/HO-1 signaling pathway in mouse testes. J. Hazard. Mater. 2022, 426, 127807. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, M.; Wang, Y.; Xu, J.; Ma, C.; Tang, Y.; Luo, Q.; Zhang, H.; Xu, F. Endoplasmic reticulum stress promotes blood-testis barrier impairment in mice with busulfan-induced oligospermia through PERK-eIF2α signaling pathway. Toxicology 2022, 473, 153193. [Google Scholar] [CrossRef]
- Hu, K.; Zhu, Q.; Zou, J.; Li, X.; Ye, M.; Yang, J.; Chen, S.; Li, F.; Ding, B.; Yang, S.; et al. Proteomic analysis for busulfan-induced spermatogenesis disorder. Ann. Med. 2025, 57, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017, 106, 1559s–1566s. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, L.; Wang, H.; Shen, Z.; Cheng, Q.; Zhang, P.; Wang, J.; Wu, Q.; Fang, X.; Duan, L.; et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood 2020, 136, 726–739. [Google Scholar] [CrossRef]
- Kawabata, H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef]
- Gao, M.; Monian, P.; Pan, Q.; Zhang, W.; Xiang, J.; Jiang, X. Ferroptosis is an autophagic cell death process. Cell Res. 2016, 26, 1021–1032. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef] [PubMed]
- Badgley, M.A.; Kremer, D.M.; Maurer, H.C.; DelGiorno, K.E.; Lee, H.J.; Purohit, V.; Sagalovskiy, I.R.; Ma, A.; Kapilian, J.; Firl, C.E.M.; et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 2020, 368, 85–89. [Google Scholar] [CrossRef]
- Yang, G.; Ren, D.; Yu, T.; Fang, J. Biodegradable copper-doped calcium phosphate nanoplatform enables tumor microenvironment modulations for amplified ferroptosis in cervical carcinoma treatment. Int. J. Pharm. X 2025, 9, 100315. [Google Scholar] [CrossRef]
- Chang, L.C.; Chiang, S.K.; Chen, S.E.; Yu, Y.L.; Chou, R.H.; Chang, W.C. Heme oxygenase-1 mediates BAY 11-7085 induced ferroptosis. Cancer Lett. 2018, 416, 124–137. [Google Scholar] [CrossRef]
- Kwon, M.Y.; Park, E.; Lee, S.J.; Chung, S.W. Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget 2015, 6, 24393–24403. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Feng, B.; Yu, J.; Yan, L.; Che, L.; Zhuo, Y.; Luo, Y.; Yu, B.; Wu, D.; Chen, D. Fibroblast growth factor 21 attenuates iron overload-induced liver injury and fibrosis by inhibiting ferroptosis. Redox Biol. 2021, 46, 102131. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Li, J.; Zhu, J.; Wang, R.; Xi, Q.; Wu, H.; Shi, T.; Chen, W. Astragalus polysaccharide prevents ferroptosis in a murine model of experimental colitis and human Caco-2 cells via inhibiting NRF2/HO-1 pathway. Eur. J. Pharmacol. 2021, 911, 174518. [Google Scholar] [CrossRef]
- Wang, H.; An, P.; Xie, E.; Wu, Q.; Fang, X.; Gao, H.; Zhang, Z.; Li, Y.; Wang, X.; Zhang, J.; et al. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology 2017, 66, 449–465. [Google Scholar] [CrossRef]
- Berberat, P.O.; Dambrauskas, Z.; Gulbinas, A.; Giese, T.; Giese, N.; Künzli, B.; Autschbach, F.; Meuer, S.; Büchler, M.W.; Friess, H. Inhibition of heme oxygenase-1 increases responsiveness of pancreatic cancer cells to anticancer treatment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 3790–3798. [Google Scholar] [CrossRef]
- Sharawy, N.; Aboulhoda, B.E.; Khalifa, M.M.; Morcos, G.N.; Morsy, S.; Alghamdi, M.A.; Khalifa, I.M.; Abd Algaleel, W.A. Amelioration of nephrotoxicity by targeting ferroptosis: Role of NCOA4, IREB2, and SLC7a11 signaling. Braz. J. Med. Biol. Res. = Rev. Bras. Pesqui. Medicas Biol. 2024, 57, e13116. [Google Scholar] [CrossRef]
- Shi, H.; Hou, B.; Li, H.; Zhou, H.; Du, B. Cyclophosphamide Induces the Ferroptosis of Tumor Cells Through Heme Oxygenase-1. Front. Pharmacol. 2022, 13, 839464. [Google Scholar] [CrossRef]
- Sasso-Cerri, E.; Oliveira, B.; de Santi, F.; Beltrame, F.L.; Caneguim, B.H.; Cerri, P.S. The antineoplastic busulphan impairs peritubular and Leydig cells, and vitamin B(12) stimulates spermatogonia proliferation and prevents busulphan-induced germ cell death. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 95, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.; Wu, M.; Liu, Y.; Hao, J.; Wu, Y.; Liao, X.; Li, G. Increased Sat2 expression is associated with busulfan-induced testicular Sertoli cell injury. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2017, 43, 47–57. [Google Scholar] [CrossRef]
- Chen, P.H.; Tseng, W.H.; Chi, J.T. The Intersection of DNA Damage Response and Ferroptosis-A Rationale for Combination Therapeutics. Biology 2020, 9, 187. [Google Scholar] [CrossRef]
- Myers, A.L.; Kawedia, J.D.; Champlin, R.E.; Kramer, M.A.; Nieto, Y.; Ghose, R.; Andersson, B.S. Clarifying busulfan metabolism and drug interactions to support new therapeutic drug monitoring strategies: A comprehensive review. Expert Opin. Drug Metab. Toxicol. 2017, 13, 901–923. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-S.; Li, M.-X.; Liu, J.; Jiao, H.; Xia, J.-M.; Shi, X.-J.; Zhao, H.; Chu, L.; Liu, J.; Qi, W.; et al. Competitive oxidation and ubiquitylation on the evolutionarily conserved cysteine confer tissue-specific stabilization of Insig-2. Nat. Commun. 2020, 11, 379. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vargas, M.P.A.; Chipuk, J.E. Physiological and Pharmacological Control of BAK, BAX, and Beyond. Trends Cell Biol. 2016, 26, 906–917. [Google Scholar] [CrossRef]
- Heo, A.J.; Kim, S.B.; Ji, C.H.; Han, D.; Lee, S.J.; Lee, S.H.; Lee, M.J.; Lee, J.S.; Ciechanover, A.; Kim, B.Y.; et al. The N-terminal cysteine is a dual sensor of oxygen and oxidative stress. Proc. Natl. Acad. Sci. USA 2021, 118, e2107993118. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Yuan, W.; Wang, Y.; Dong, Z.; Chen, G. Ferroptotic Pathway Activation in Spermatogonia: A Novel Mechanism of Busulfan-Induced Testicular Injury. Biology 2025, 14, 594. https://doi.org/10.3390/biology14060594
Hu H, Yuan W, Wang Y, Dong Z, Chen G. Ferroptotic Pathway Activation in Spermatogonia: A Novel Mechanism of Busulfan-Induced Testicular Injury. Biology. 2025; 14(6):594. https://doi.org/10.3390/biology14060594
Chicago/Turabian StyleHu, Huanhuan, Wenzheng Yuan, Yulin Wang, Zimei Dong, and Guangwen Chen. 2025. "Ferroptotic Pathway Activation in Spermatogonia: A Novel Mechanism of Busulfan-Induced Testicular Injury" Biology 14, no. 6: 594. https://doi.org/10.3390/biology14060594
APA StyleHu, H., Yuan, W., Wang, Y., Dong, Z., & Chen, G. (2025). Ferroptotic Pathway Activation in Spermatogonia: A Novel Mechanism of Busulfan-Induced Testicular Injury. Biology, 14(6), 594. https://doi.org/10.3390/biology14060594





