Coixol Protects Against Acute Kidney Injury by Reducing Cell Senescence
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.2. Serum Creatinine (SCr) and Blood Urea Nitrogen (BUN)
2.3. RT-qPCR
2.4. SA-β-Gal Staining
2.5. Immunofluorescence Staining
2.6. Identifying Key Pathways of AKI
2.7. Data Sources
2.8. Identifying Key Cellular Senescence-Related Genes
2.9. Using Machine Learning for Screening Hub Genes and Validation
2.10. Molecular Docking
2.11. Molecular Dynamics (MD) Simulation
2.12. Molecular Mechanics Generalized Born Surface Area (MM-GBSA) Studies
2.13. Quantification and Statistical Analysis
3. Results
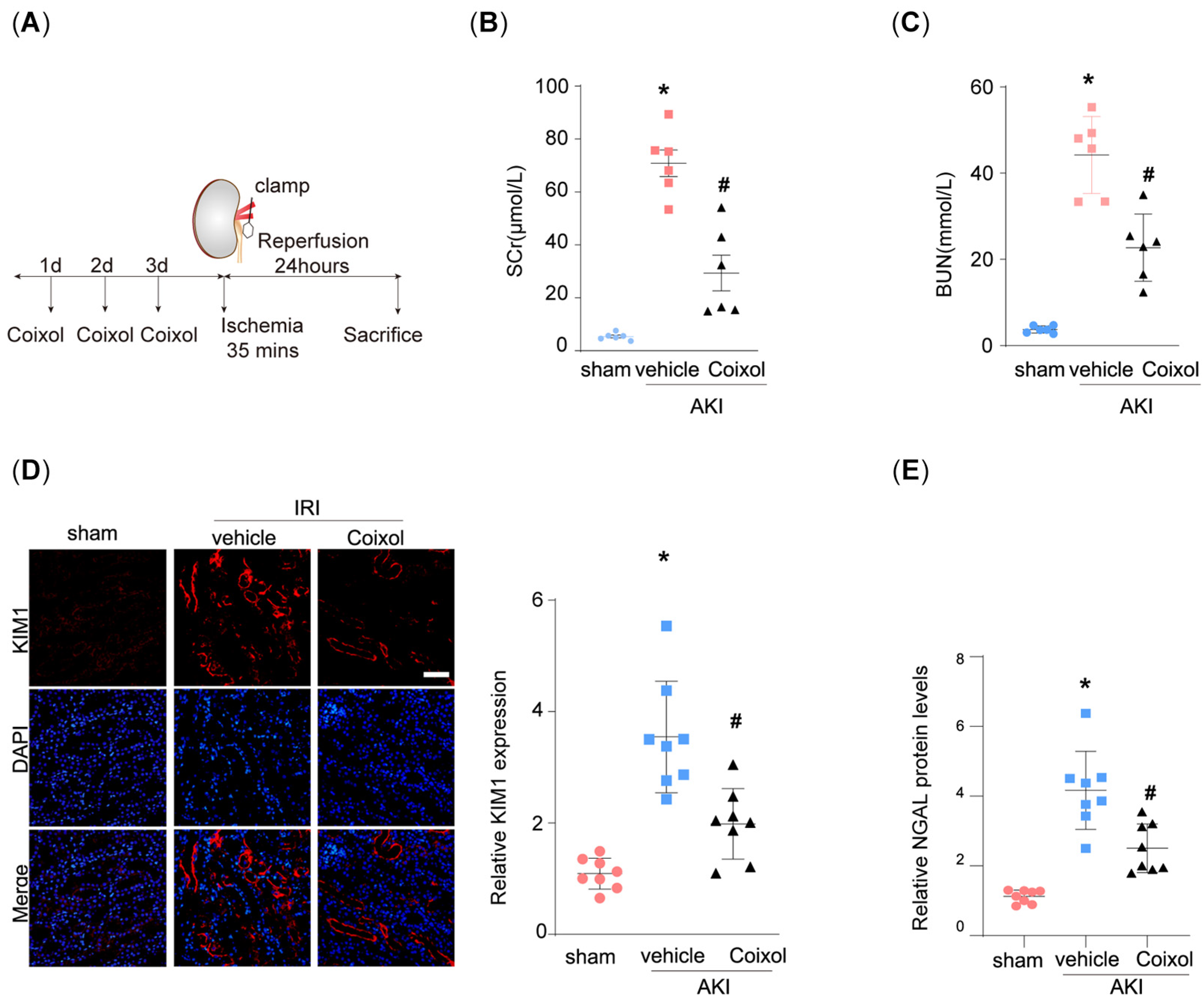
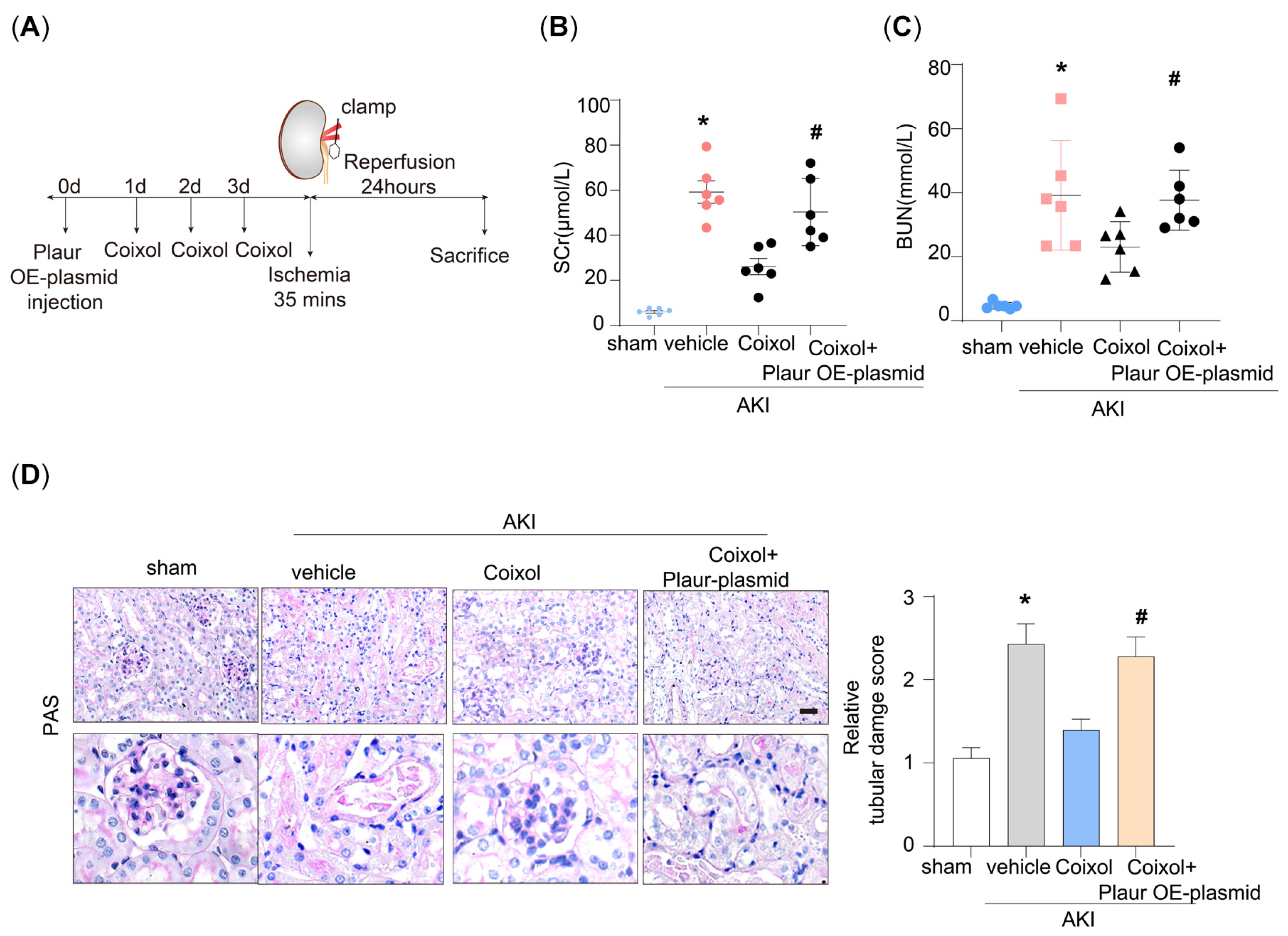
3.1. Coixol Treatment Significantly Ameliorated IRI-Induced Kidney Injury
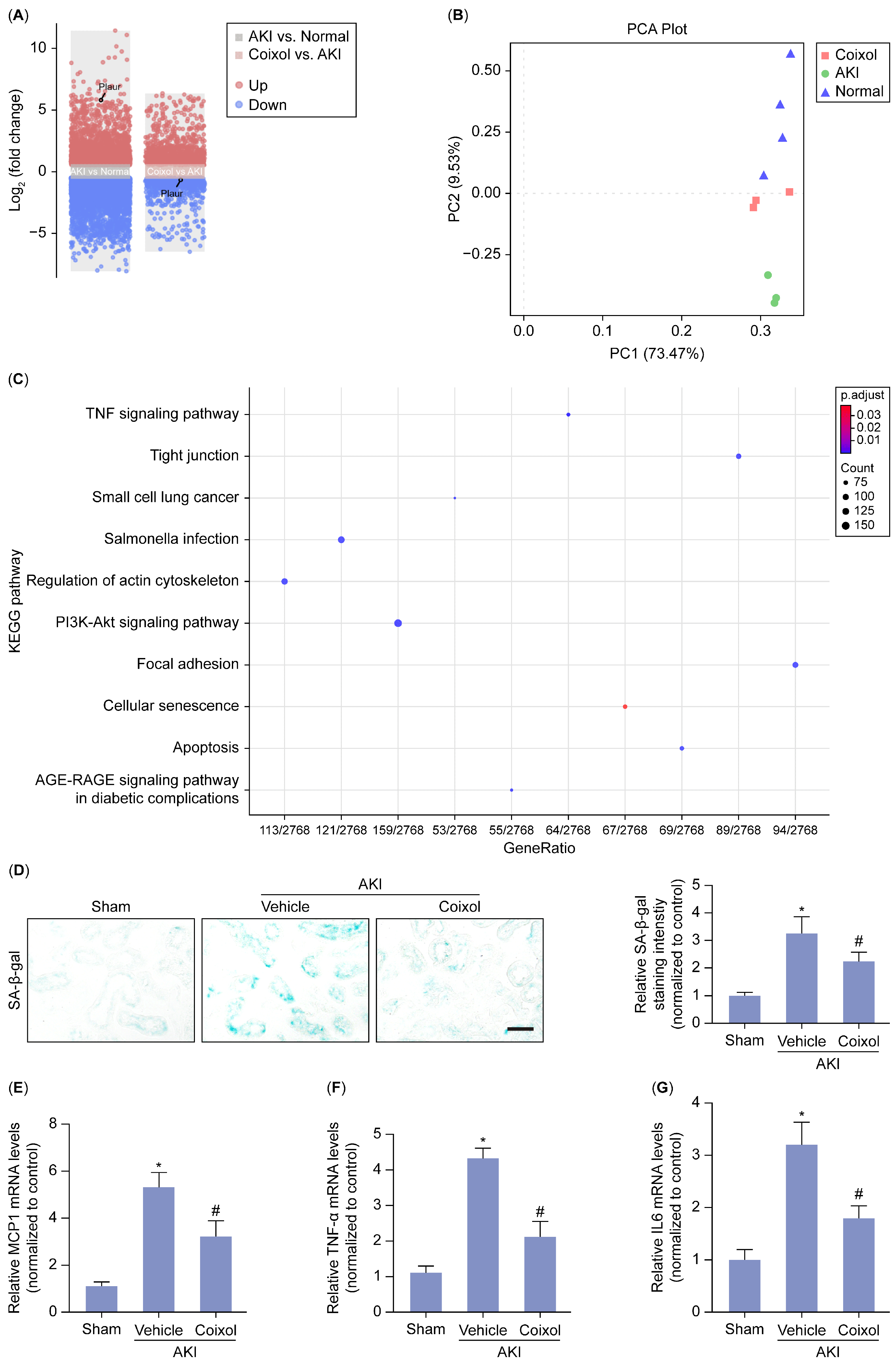
3.2. Cellular Senescence Served as the Major Signaling Pathway Targeted by Coixol in AKI
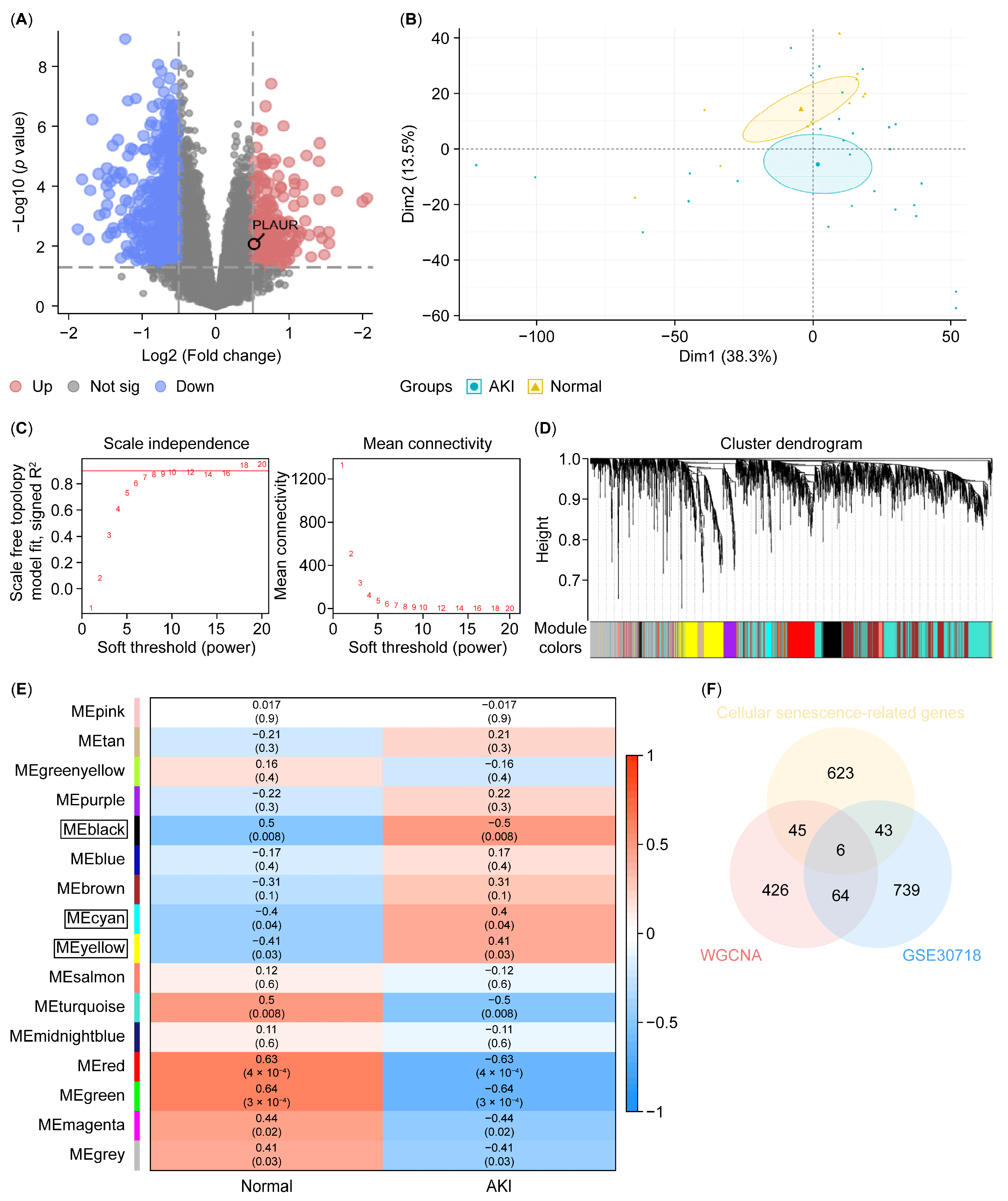
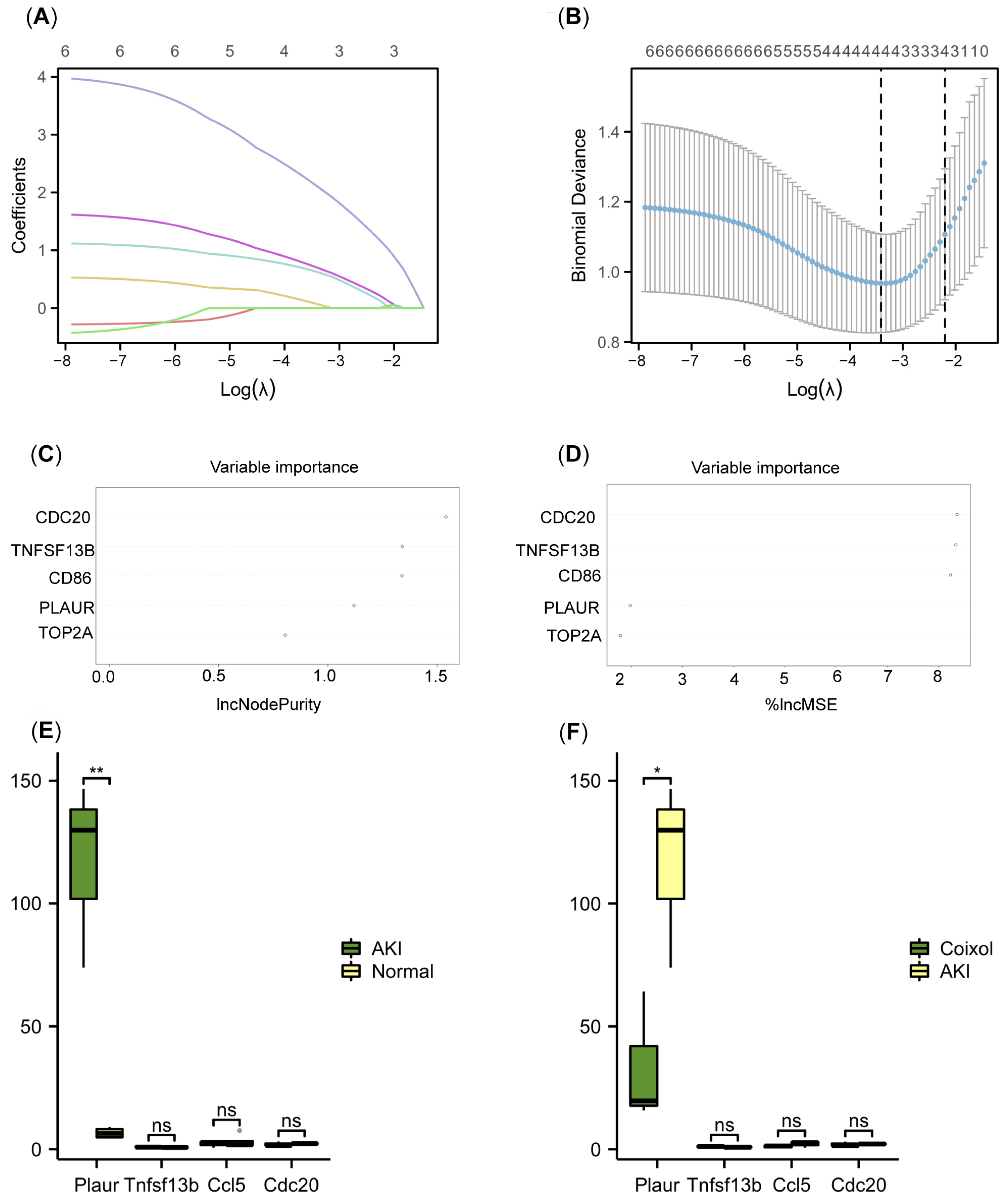
3.3. Plaur Was Identified as the Key Cellular Senescence-Related Gene Associated with the Protective Effect of Coixol
3.4. Plaur Overexpression Diminished the Protective Effect of Coixol on AKI
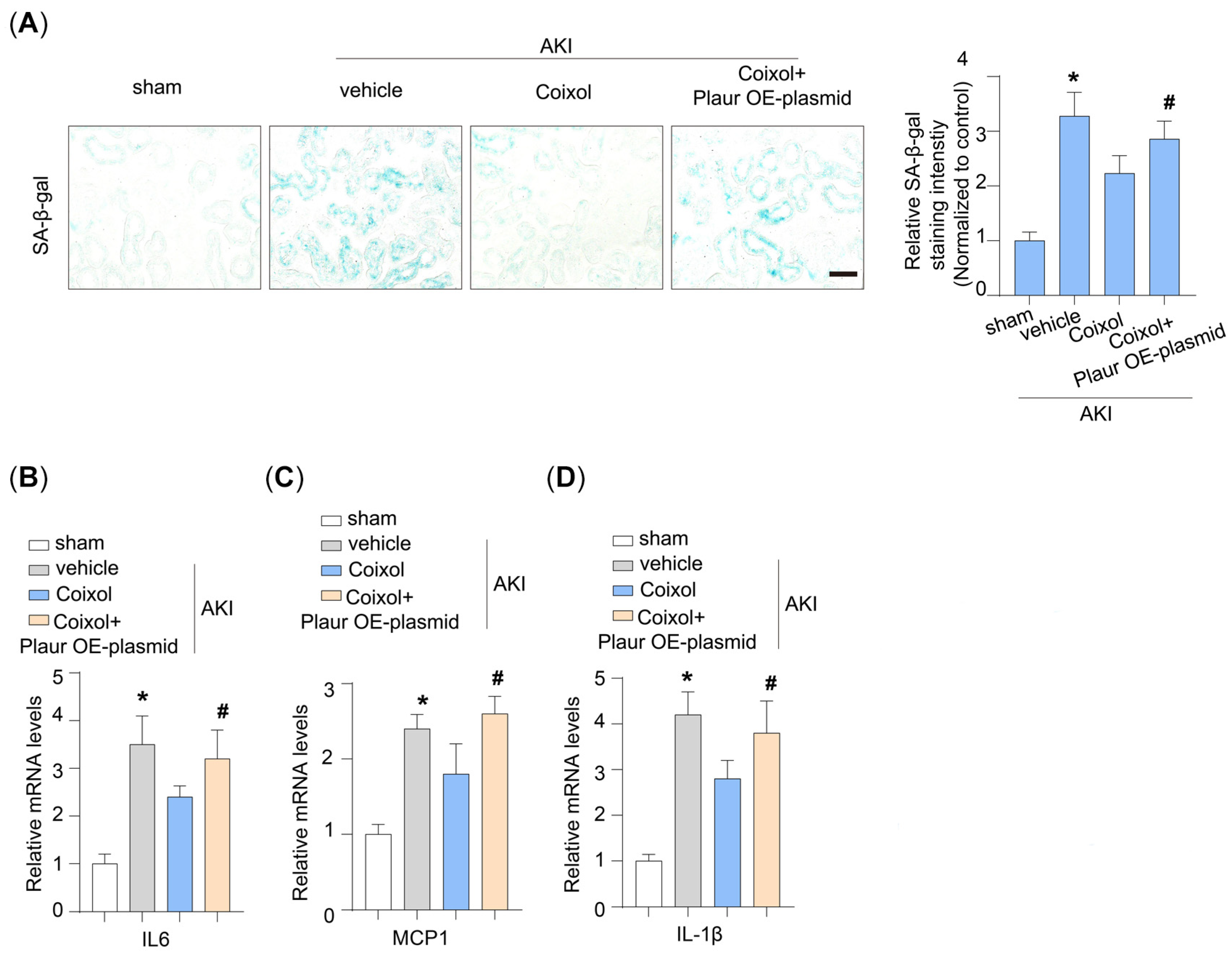
3.5. Cellular Senescence Is a Target of Plaur in Coixol-Treated AKI Mice
3.6. The result of Molecular Docking
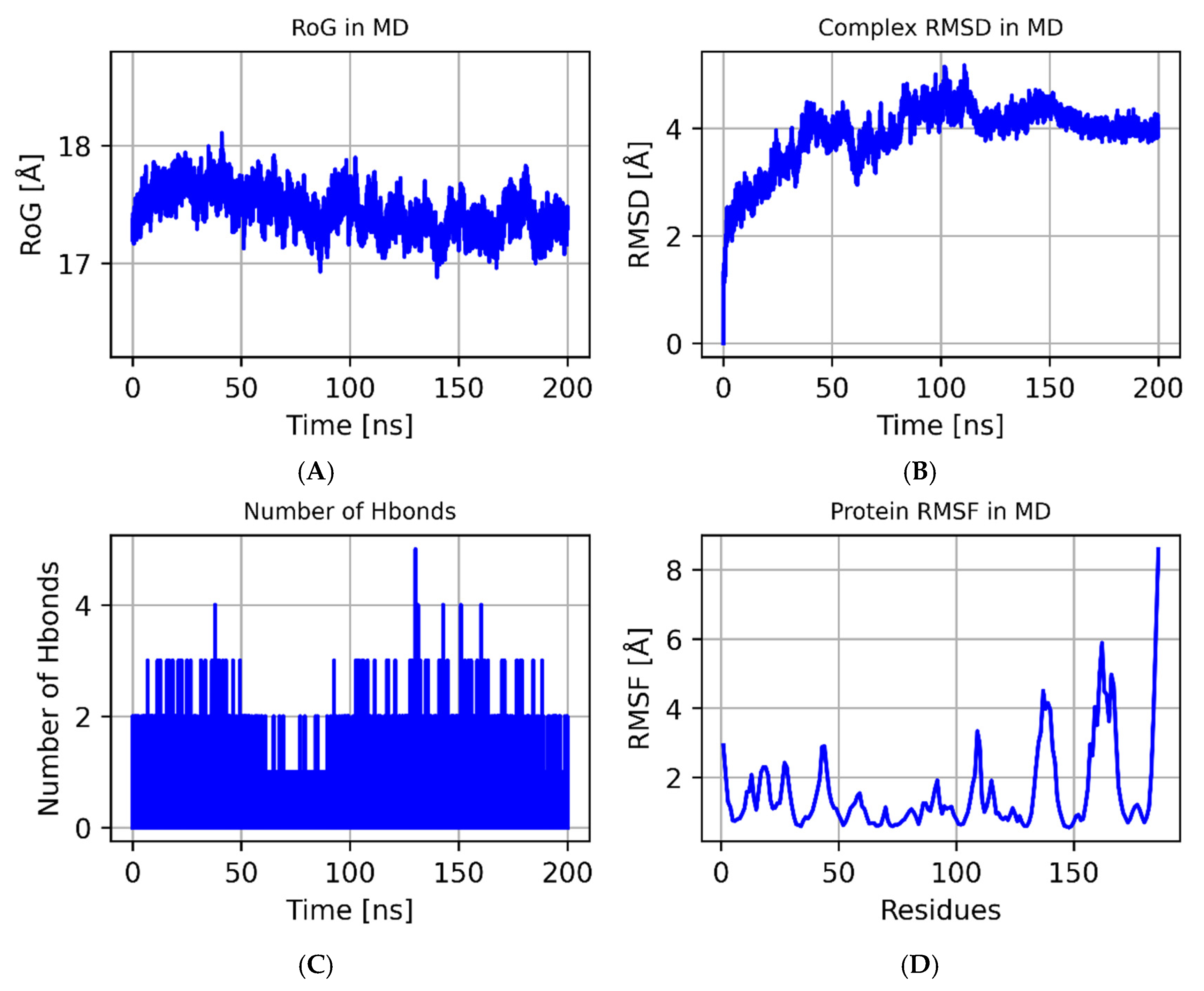
3.7. The Results of the MD Simulations
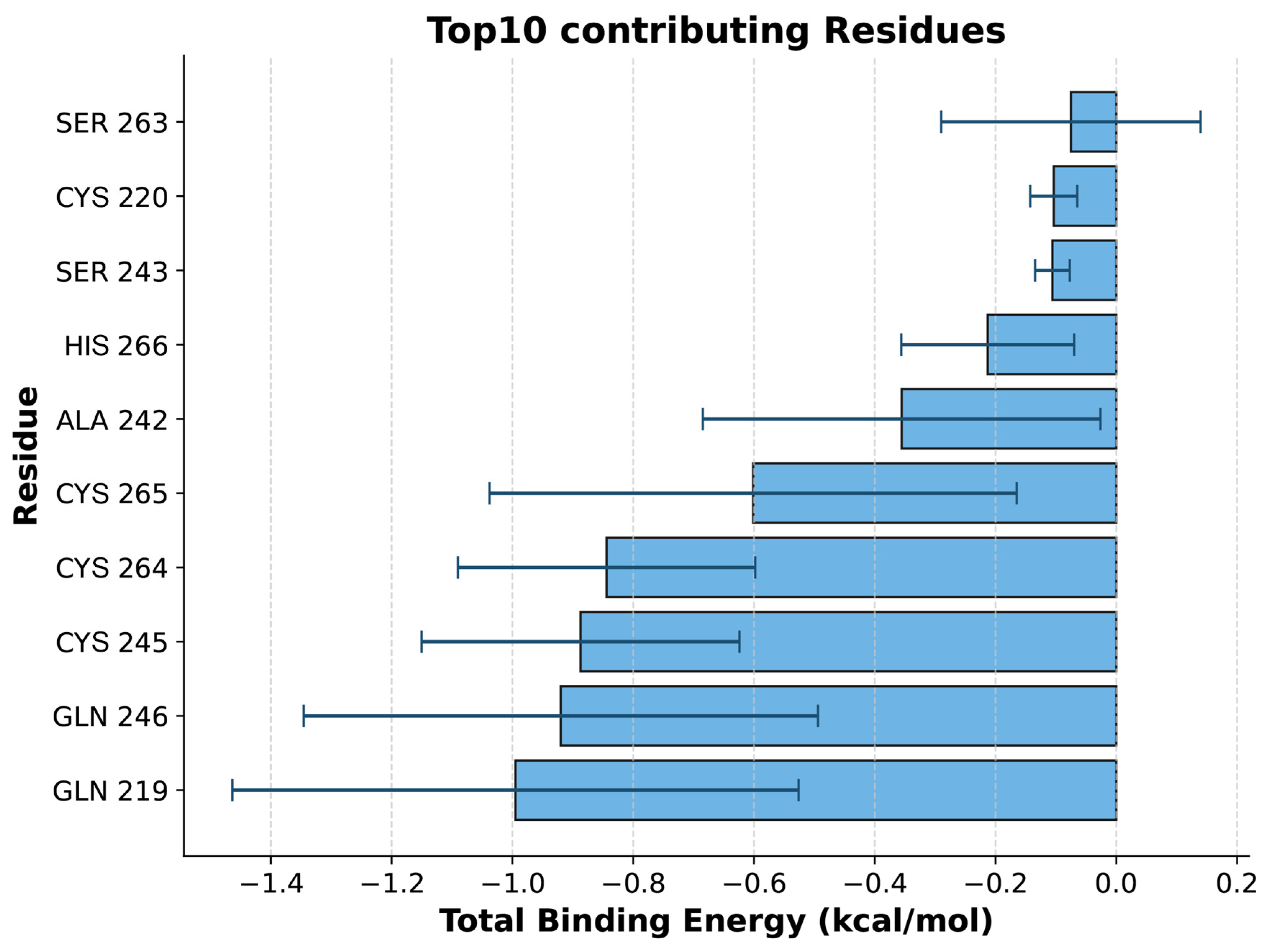
3.8. MM-GBSA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute kidney injury. Nat. Rev. Dis. Prim. 2021, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, M.; Shaw, A.D. Amino Acid Infusion to Protect Kidney Function after Cardiac Surgery. N. Engl. J. Med. 2024, 391, 759–760. [Google Scholar] [CrossRef] [PubMed]
- Landoni, G.; Monaco, F.; Ti, L.K.; Baiardo Redaelli, M.; Bradic, N.; Comis, M.; Kotani, Y.; Brambillasca, C.; Garofalo, E.; Scandroglio, A.M.; et al. A Randomized Trial of Intravenous Amino Acids for Kidney Protection. N. Engl. J. Med. 2024, 391, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Pierson-Marchandise, M.; Gras, V.; Moragny, J.; Micallef, J.; Gaboriau, L.; Picard, S.; Choukroun, G.; Masmoudi, K.; Liabeuf, S.; French National Network of Pharmacovigilance Centres. The drugs that mostly frequently induce acute kidney injury: A case—Noncase study of a pharmacovigilance database. Br. J. Clin. Pharmacol. 2017, 83, 1341–1349. [Google Scholar] [CrossRef]
- Hu, J.; Gu, W.; Ma, N.; Fan, X.; Ci, X. Leonurine alleviates ferroptosis in cisplatin-induced acute kidney injury by activating the Nrf2 signalling pathway. Br. J. Pharmacol. 2022, 179, 3991–4009. [Google Scholar] [CrossRef]
- Li, D.; Liu, B.; Fan, Y.; Liu, M.; Han, B.; Meng, Y.; Xu, X.; Song, Z.; Liu, X.; Hao, Q.; et al. Nuciferine protects against folic acid-induced acute kidney injury by inhibiting ferroptosis. Br. J. Pharmacol. 2021, 178, 1182–1199, Erratum in Br. J. Pharmacol. 2022, 179, 2313–2317. https://doi.org/10.1111/bph.15829. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Lei, Y.; Zhang, X.; Xu, L.; Liu, W.; Fan, Z.; Ma, Z.; Yin, Z.; Li, L.; et al. Multifunctional agents based on benzoxazolone as promising therapeutic drugs for diabetic nephropathy. Eur. J. Med. Chem. 2021, 215, 113269. [Google Scholar] [CrossRef]
- Hameed, A.; Hafizur, R.M.; Khan, M.I.; Jawed, A.; Wang, H.; Zhao, M.; Matsunaga, K.; Izumi, T.; Siddiqui, S.; Khan, F.; et al. Coixol amplifies glucose-stimulated insulin secretion via cAMP mediated signaling pathway. Eur. J. Pharmacol. 2019, 858, 172514. [Google Scholar] [CrossRef]
- Cui, E.; Qian, S.; Li, J.; Jiang, X.; Wang, H.; Du, S.; Du, L. Discovery of Coixol Derivatives as Potent Anti-inflammatory Agents. J. Nat. Prod. 2023, 86, 1950–1959. [Google Scholar] [CrossRef]
- Shen, X.Y.; Lu, J.M.; Lu, Y.N.; Jin, G.N.; Ma, J.W.; Wang, J.H.; Wang, Y.; Xu, X.; Piao, L.X. Coixol ameliorates Toxoplasma gondii infection-induced lung injury by interfering with T. gondii HSP70/TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2023, 118, 110031. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Lu, Y.N.; Shen, X.Y.; Quan, Y.Z.; Lu, J.M.; Jin, G.N.; Liu, Y.M.; Zhang, S.H.; Xu, G.H.; Xu, X.; et al. Coixol mitigates Toxoplasma gondii infection-induced liver injury by inhibiting the Toxoplasma gondii HSP70/TLR4/NF-κB signaling pathway in hepatic macrophages. J. Ethnopharmacol. 2024, 335, 118694. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lerman, L.O. Cellular Senescence: A New Player in Kidney Injury. Hypertension 2020, 76, 1069–1075. [Google Scholar] [CrossRef]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Lin, X.; Jin, H.; Chai, Y.; Shou, S. Cellular senescence and acute kidney injury. Pediatr. Nephrol. 2022, 37, 3009–3018. [Google Scholar] [CrossRef]
- Pulido, T.; Velarde, M.C.; Alimirah, F. The senescence-associated secretory phenotype: Fueling a wound that never heals. Mech. Ageing Dev. 2021, 199, 111561. [Google Scholar] [CrossRef]
- Mohamad Kamal, N.S.; Safuan, S.; Shamsuddin, S.; Foroozandeh, P. Aging of the cells: Insight into cellular senescence and detection Methods. Eur. J. Cell Biol. 2020, 99, 151108. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Yang, M.; Ma, X. Implication of cellular senescence in the progression of chronic kidney disease and the treatment potencies. Biomed. Pharmacother. 2021, 135, 111191. [Google Scholar] [CrossRef]
- Li, L.; Xiang, T.; Guo, J.; Guo, F.; Wu, Y.; Feng, H.; Liu, J.; Tao, S.; Fu, P.; Ma, L. Inhibition of ACSS2-mediated histone crotonylation alleviates kidney fibrosis via IL-1β-dependent macrophage activation and tubular cell senescence. Nat. Commun. 2024, 15, 3200. [Google Scholar] [CrossRef]
- Zhou, B.; Wan, Y.; Chen, R.; Zhang, C.; Li, X.; Meng, F.; Glaser, S.; Wu, N.; Zhou, T.; Li, S.; et al. The emerging role of cellular senescence in renal diseases. J. Cell. Mol. Med. 2020, 24, 2087–2097. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, Y.; Ding, Q.; Wang, S.S.; Rastogi, P.; Dai, D.-F.; Lu, D.; Purvis, M.; Cao, C.; Wang, A.; et al. Epithelial innate immunity mediates tubular cell senescence after kidney injury. JCI Insight 2019, 4, e125490. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, X.; Zhu, R.; Chen, S. miR-21-5p in extracellular vesicles obtained from adipose tissue-derived stromal cells facilitates tubular epithelial cell repair in acute kidney injury. Cytotherapy 2023, 25, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Rayego-Mateos, S.; Marquez-Expósito, L.; Rodrigues-Diez, R.; Sanz, A.B.; Guiteras, R.; Doladé, N.; Rubio-Soto, I.; Manonelles, A.; Codina, S.; Ortiz, A.; et al. Molecular Mechanisms of Kidney Injury and Repair. Int. J. Mol. Sci. 2022, 23, 1542. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Shi, E.; Li, J.; Li, Y.; Qiao, Z.; Wang, Z.; Liu, M.; Tang, W.; Sun, Y.; Zhang, Y.; et al. GPR87 promotes renal tubulointerstitial fibrosis by accelerating glycolysis and mitochondrial injury. Free Radic. Biol. Med. 2022, 189, 58–70. [Google Scholar] [CrossRef]
- Huang, W.; Wang, B.O.; Hou, Y.F.; Fu, Y.; Cui, S.J.; Zhu, J.H.; Zhan, X.Y.; Li, R.K.; Tang, W.; Wu, J.C.; et al. JAML promotes acute kidney injury mainly through a macrophage-dependent mechanism. JCI Insight 2022, 7, e158571. [Google Scholar] [CrossRef]
- Luo, C.; Zhou, S.; Zhou, Z.; Liu, Y.; Yang, L.; Liu, J.; Zhang, Y.; Li, H.; Liu, Y.; Hou, F.F.; et al. Wnt9a Promotes Renal Fibrosis by Accelerating Cellular Senescence in Tubular Epithelial Cells. J. Am. Soc. Nephrol. 2018, 29, 1238–1256. [Google Scholar] [CrossRef]
- Jia, M.; Li, L.; Chen, R.; Du, J.; Qiao, Z.; Zhou, D.; Liu, M.; Wang, X.; Wu, J.; Xie, Y.; et al. Targeting RNA oxidation by ISG20-mediated degradation is a potential therapeutic strategy for acute kidney injury. Mol. Ther. 2023, 31, 3034–3051. [Google Scholar] [CrossRef]
- Chu, Q.; Li, Y.; Wu, J.; Gao, Y.; Guo, X.; Li, J.; Lv, H.; Liu, M.; Tang, W.; Zhan, P.; et al. Oxysterol Sensing Through GPR183 Triggers Endothelial Senescence in Hypertension. Circ. Res. 2024, 135, 708–721. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, W.; Li, T.; Yang, G.; Zhu, W.; Chen, N.; Jin, H. Interrelationship between 2019-nCov receptor DPP4 and diabetes mellitus targets based on protein interaction network. Sci. Rep. 2022, 12, 188. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Liu, S.; Wang, R.; Lou, Y.; Liu, J. Uncovering the Mechanism of the Effects of Pien-Tze-Huang on Liver Cancer Using Network Pharmacology and Molecular Docking. Evid.-Based Complement. Altern. Med. 2020, 2020, 4863015. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Yin, X.; Tao, D.; Wang, X.; Zhang, J. Identification and Validation of Diagnostic Model Based on Angiogenesis- and Epithelial Mesenchymal Transition-Related Genes in Myocardial Infarction. Int. J. Gen. Med. 2024, 17, 3239–3255. [Google Scholar] [CrossRef] [PubMed]
- Salomon-Ferrer, R.; Case, D.A.; Walker, R.C. An overview of the Amber biomolecular simulation package. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3, 198–210. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Sagui, C.; Darden, T.A. Molecular dynamics simulations of biomolecules: Long-range electrostatic effects. Annu. Rev. Biophys. Biomol. Struct. 1999, 28, 155–179. [Google Scholar] [CrossRef]
- Kräutler, V.; Van Gunsteren, W.F.; Hünenberger, P.H. A fast SHAKE algorithm to solve distance constraint equations for small molecules in molecular dynamics simulations. J. Comput. Chem. 2001, 22, 501–508. [Google Scholar] [CrossRef]
- Larini, L.; Mannella, R.; Leporini, D. Langevin stabilization of molecular-dynamics simulations of polymers by means of quasisymplectic algorithms. J. Chem. Phys. 2007, 126, 104101. [Google Scholar] [CrossRef]
- Hou, T.; Wang, J.; Li, Y.; Wang, W. Assessing the performance of the MM/PBSA and MM/GBSA methods. 1. The accuracy of binding free energy calculations based on molecular dynamics simulations. J. Chem. Inf. Model. 2011, 51, 69–82. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Rastelli, G.; Del Rio, A.; Degliesposti, G.; Sgobba, M. Fast and accurate predictions of binding free energies using MM-PBSA and MM-GBSA. J. Comput. Chem. 2010, 31, 797–810. [Google Scholar] [CrossRef]
- Nguyen, H.; Roe, D.R.; Simmerling, C. Improved Generalized Born Solvent Model Parameters for Protein Simulations. J. Chem. Theory Comput. 2013, 9, 2020–2034. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.; Shenkin, P.S.; Still, W.C. Approximate atomic surfaces from linear combinations of pairwise overlaps (LCPO). J. Comput. Chem. 1999, 20, 217–230. [Google Scholar] [CrossRef]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: The good, the bad and the unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Yi, X.; Dou, Q.; Yang, X.; He, Y.; Chen, J.; Chen, K. Cellular senescence of renal tubular epithelial cells in acute kidney injury. Cell Death Discov. 2024, 10, 62. [Google Scholar] [CrossRef]
- Chen, K.; Chen, J.; Wang, L.; Yang, J.; Xiao, F.; Wang, X.; Yuan, J.; Wang, L.; He, Y. Parkin ubiquitinates GATA4 and attenuates the GATA4/GAS1 signaling and detrimental effects on diabetic nephropathy. FASEB J. 2020, 34, 8858–8875. [Google Scholar] [CrossRef]
- Tang, L.; Han, X. The urokinase plasminogen activator system in breast cancer invasion and metastasis. Biomed. Pharmacother. 2013, 67, 179–182. [Google Scholar] [CrossRef]
- Hayek, S.S.; Leaf, D.E.; Samman Tahhan, A.; Raad, M.; Sharma, S.; Waikar, S.S.; Sever, S.; Camacho, A.; Wang, X.; Dande, R.R.; et al. Soluble Urokinase Receptor and Acute Kidney Injury. N. Engl. J. Med. 2020, 382, 416–426. [Google Scholar] [CrossRef]
- Hahm, E.; Wei, C.; Fernandez, I.; Li, J.; Tardi, N.J.; Tracy, M.; Wadhwani, S.; Cao, Y.; Peev, V.; Zloza, A.; et al. Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease. Nat. Med. 2017, 23, 100–106. [Google Scholar] [CrossRef]
- Su, Z.; Li, Y.; Lv, H.; Cui, X.; Liu, M.; Wang, Z.; Zhang, Y.; Zhen, J.; Tang, W.; Wang, X.; et al. CLEC14A protects against podocyte injury in mice with adriamycin nephropathy. FASEB J. 2021, 35, e21711. [Google Scholar] [CrossRef]
- Elwakiel, A.; Gupta, D.; Rana, R.; Manoharan, J.; Al-Dabet, M.M.; Ambreen, S.; Fatima, S.; Zimmermann, S.; Mathew, A.; Li, Z.; et al. Factor XII signaling via uPAR-integrin β1 axis promotes tubular senescence in diabetic kidney disease. Nat. Commun. 2024, 15, 7963. [Google Scholar] [CrossRef]
- Amor, C.; Feucht, J.; Leibold, J.; Ho, Y.J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T.; et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020, 583, 127–132, Erratum in Nature 2024, 627, E9. https://doi.org/10.1038/s41586-024-07197-3. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, T. Step further towards targeted senolytic therapy: Therapeutic potential of uPAR-CAR T cells for senescence-related diseases. Signal Transduct. Target. Ther. 2020, 5, 155. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.; Zhen, J.; Zhang, C.; Wan, Q.; Liu, G.; Wei, X.; Zhang, Y.; Wang, Z.; Han, H.; et al. Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney Int. 2014, 86, 712–725. [Google Scholar] [CrossRef]
- Liu, M.; Liang, K.; Zhen, J.; Zhou, M.; Wang, X.; Wang, Z.; Wei, X.; Zhang, Y.; Sun, Y.; Zhou, Z.; et al. Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat. Commun. 2017, 8, 413. [Google Scholar] [CrossRef]
- Sharma, K.R.; Adhikari, A.; Hafizur, R.M.; Hameed, A.; Raza, S.A.; Kalauni, S.K.; Miyazaki, J.; Choudhary, M.I. Potent Insulin Secretagogue from Scoparia dulcis Linn of Nepalese Origin. Phytother. Res. 2015, 29, 1672–1675. [Google Scholar] [CrossRef]

| System Name | Plaur_Coixol |
|---|---|
| ΔEvdw | −14.10 ± 1.00 |
| ΔEelec | −5.78 ± 5.50 |
| ΔGGB | 10.54 ± 4.09 |
| ΔGSA | −1.83 ± 0.16 |
| ΔGbind | −11.17 ± 1.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Wang, X.; Tang, H.; Wang, F.; Qu, Z.; Wang, X.; Li, X.; Cao, X. Coixol Protects Against Acute Kidney Injury by Reducing Cell Senescence. Biology 2025, 14, 560. https://doi.org/10.3390/biology14050560
Li K, Wang X, Tang H, Wang F, Qu Z, Wang X, Li X, Cao X. Coixol Protects Against Acute Kidney Injury by Reducing Cell Senescence. Biology. 2025; 14(5):560. https://doi.org/10.3390/biology14050560
Chicago/Turabian StyleLi, Kang, Xiaoxue Wang, Huidi Tang, Feifan Wang, Zetong Qu, Xiaojie Wang, Xiang Li, and Xiaoqing Cao. 2025. "Coixol Protects Against Acute Kidney Injury by Reducing Cell Senescence" Biology 14, no. 5: 560. https://doi.org/10.3390/biology14050560
APA StyleLi, K., Wang, X., Tang, H., Wang, F., Qu, Z., Wang, X., Li, X., & Cao, X. (2025). Coixol Protects Against Acute Kidney Injury by Reducing Cell Senescence. Biology, 14(5), 560. https://doi.org/10.3390/biology14050560







