Tricky with Heat and Salt: Soil Factors, Thermotaxis, and Potential for Heat–Saline Agar Trapping of Strongyloides Larvae
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inactivation of Strongyloides stercoralis Infective Larvae by Temperature and pH
2.2. Inactivation of Strongyloides ratti Infective Larvae by pH, Temperature, and Saline Solution
2.3. Measurement of the Inactivation of S. ratti Infective Larvae
2.4. Soil Heat Conduction and the Inactivation of S. ratti Infective Larvae
2.5. Response of S. ratti Infective Larvae to Hazardous Heat Exposure
2.6. Long-Range Heat Attraction of S. ratti Infective Larvae and Heat-High Concentration Saline Trapping
2.7. Thermal Trapping and Removal of S. ratti Infective Larvae from Soil with Saline Agar Cuboid
2.8. Statistical Analysis
3. Results
3.1. Strongyloides stercoralis Infective Larvae Exhibited Susceptibility to 50 °C but Resistance to Varying pH
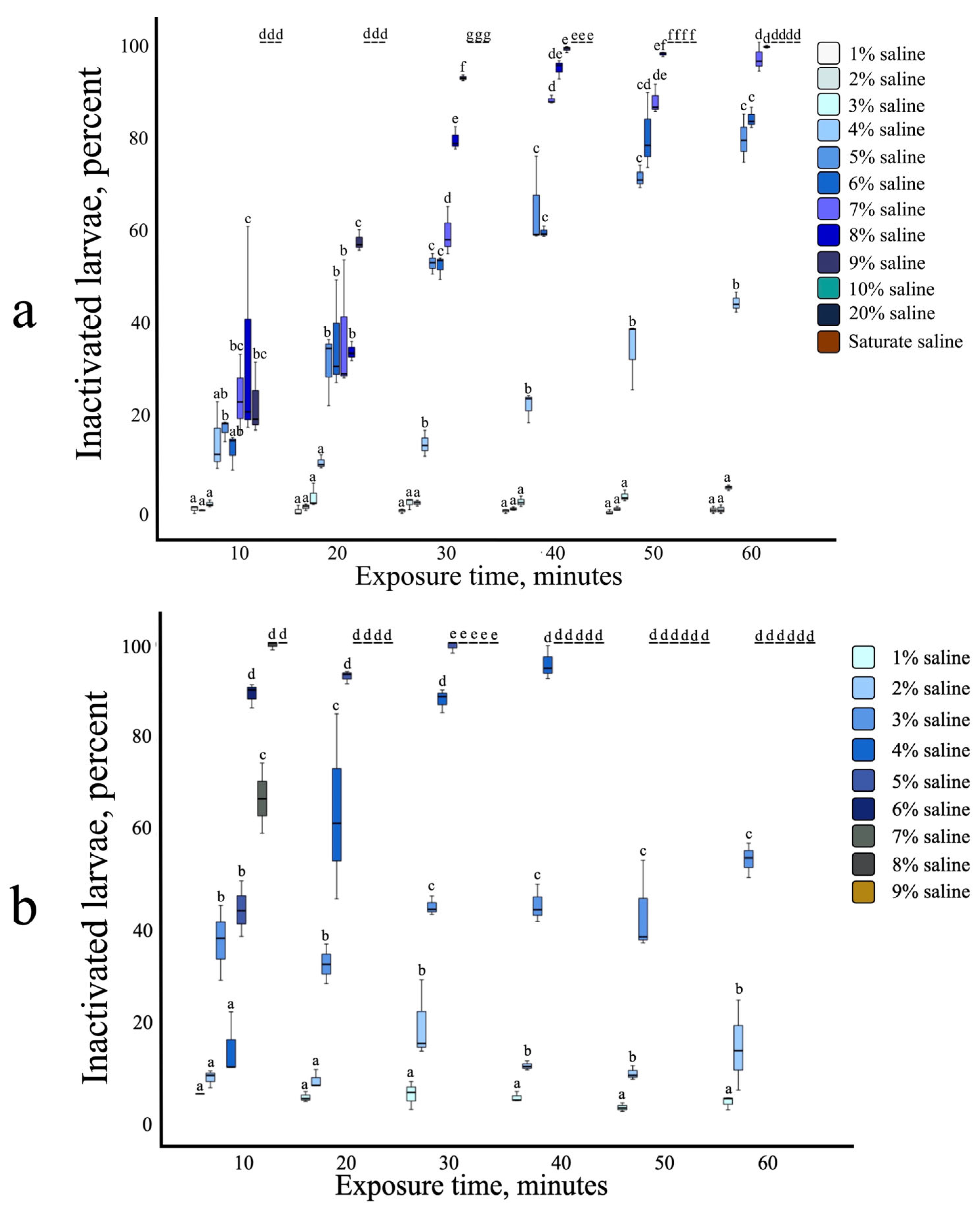
3.2. Strongyloides ratti Infective Larvae: Susceptibility to Heat, Salinity, and Combined Stress
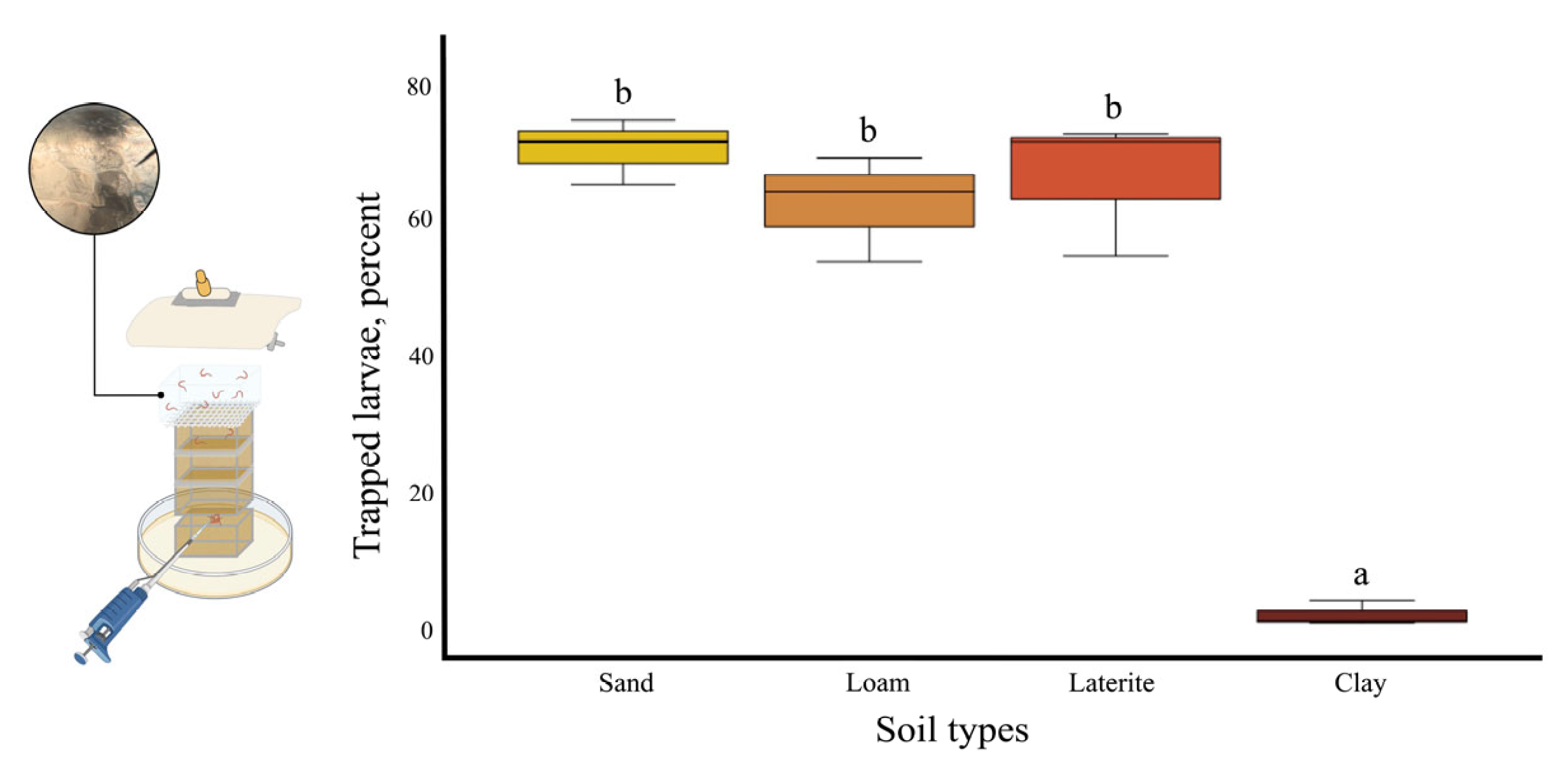
3.3. The Effect of Soil–Heat Conduction and Combined Heat–Salinity Treatment on S. ratti Larval Inactivation
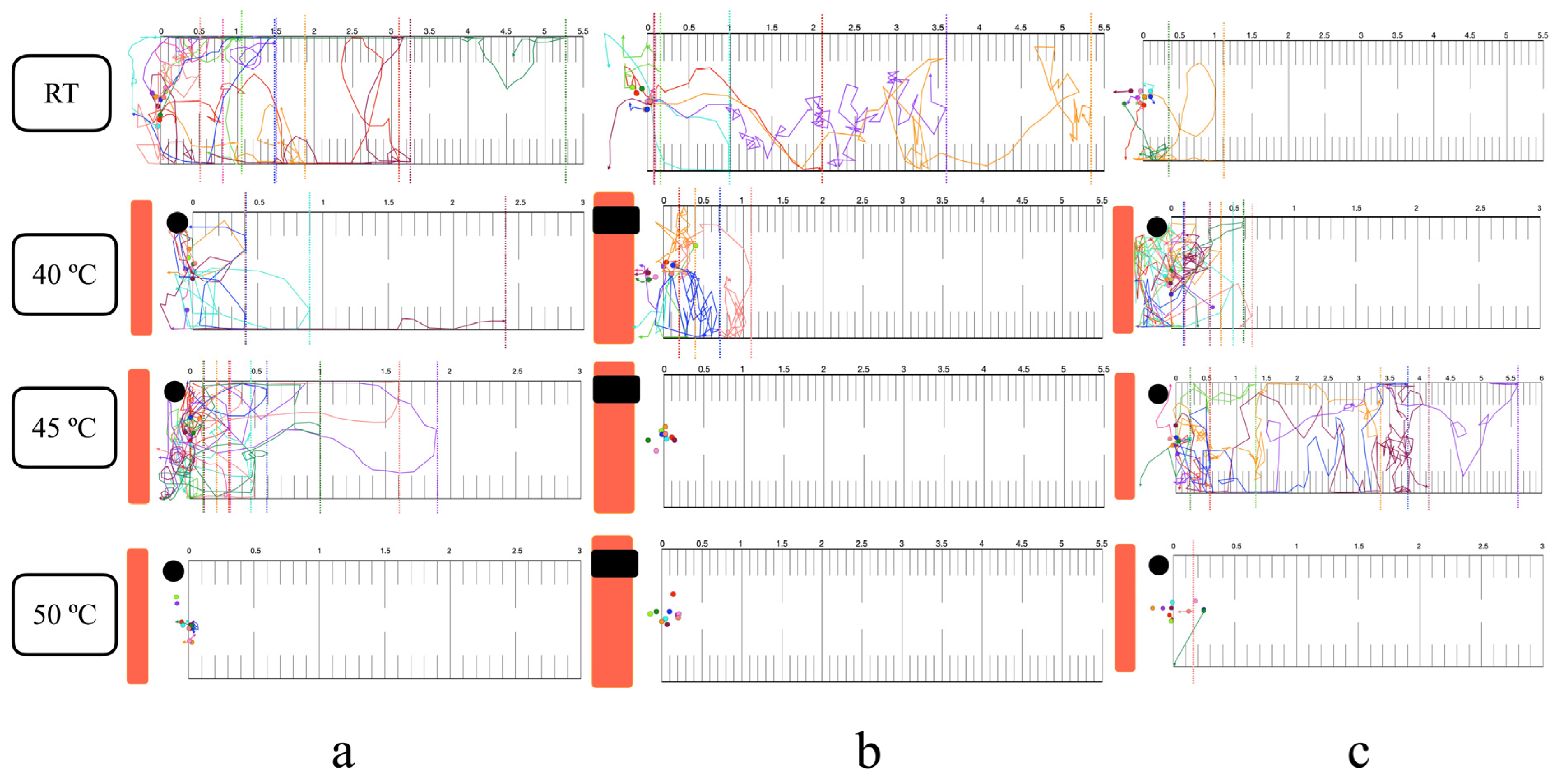
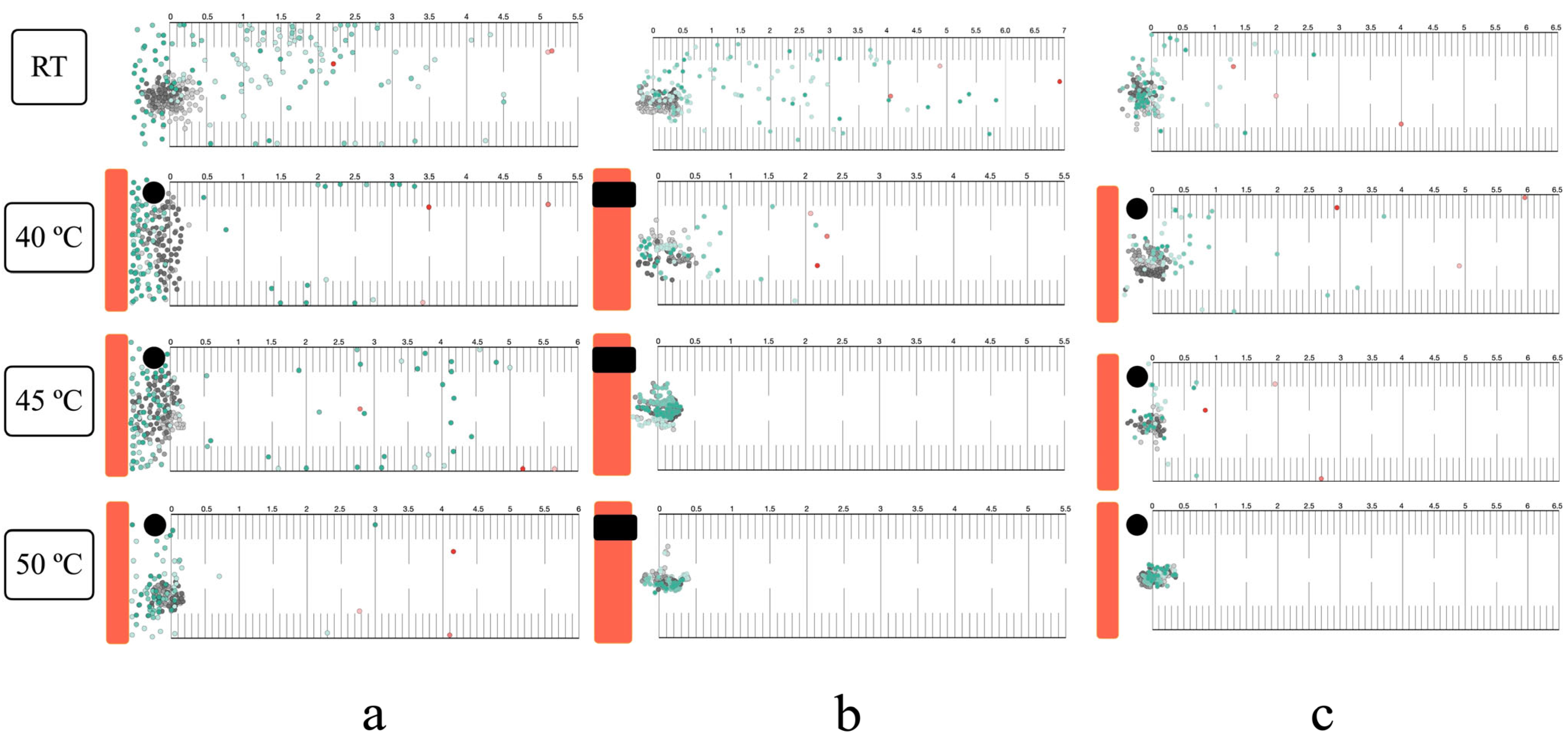
3.4. Thermal Response in the Horizontal Plane
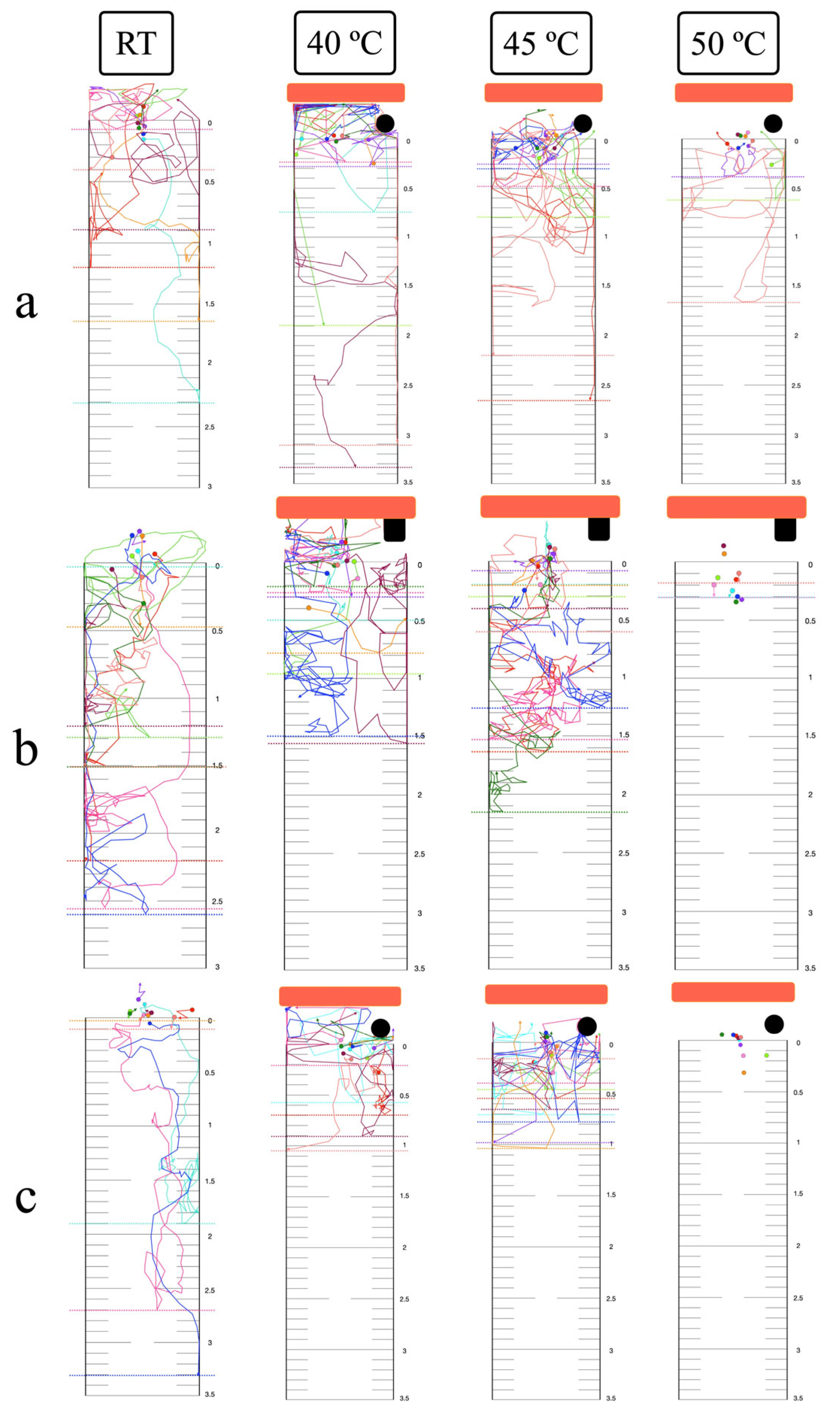
3.5. Thermal Response in the Vertical Plane
3.6. Long-Range Attraction and Response to Noxious Heat
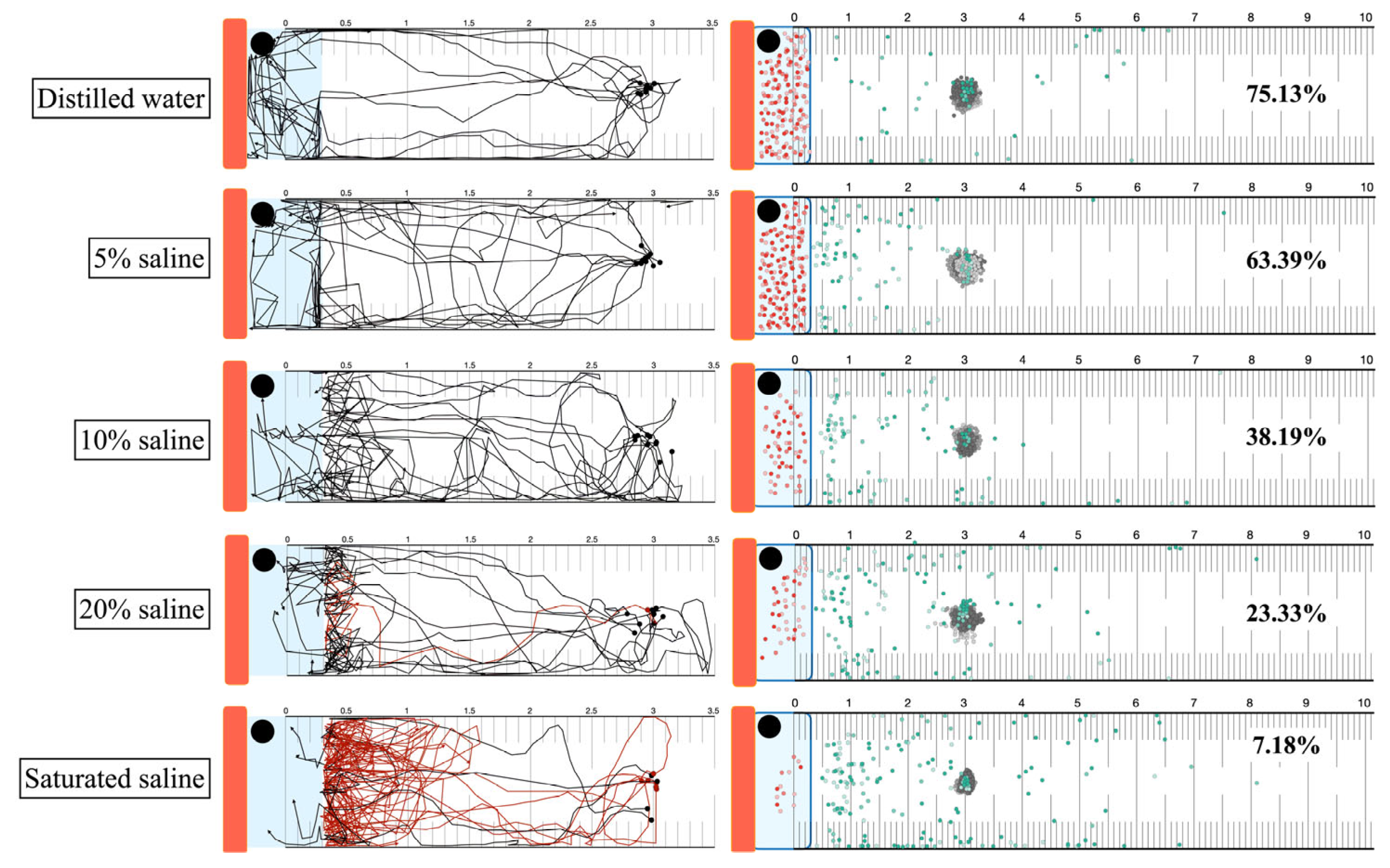
3.7. Heat Attraction and High Concentration Saline Trapping
3.8. Thermal and Saline Agar Trapping for S. ratti Larvae from Soil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
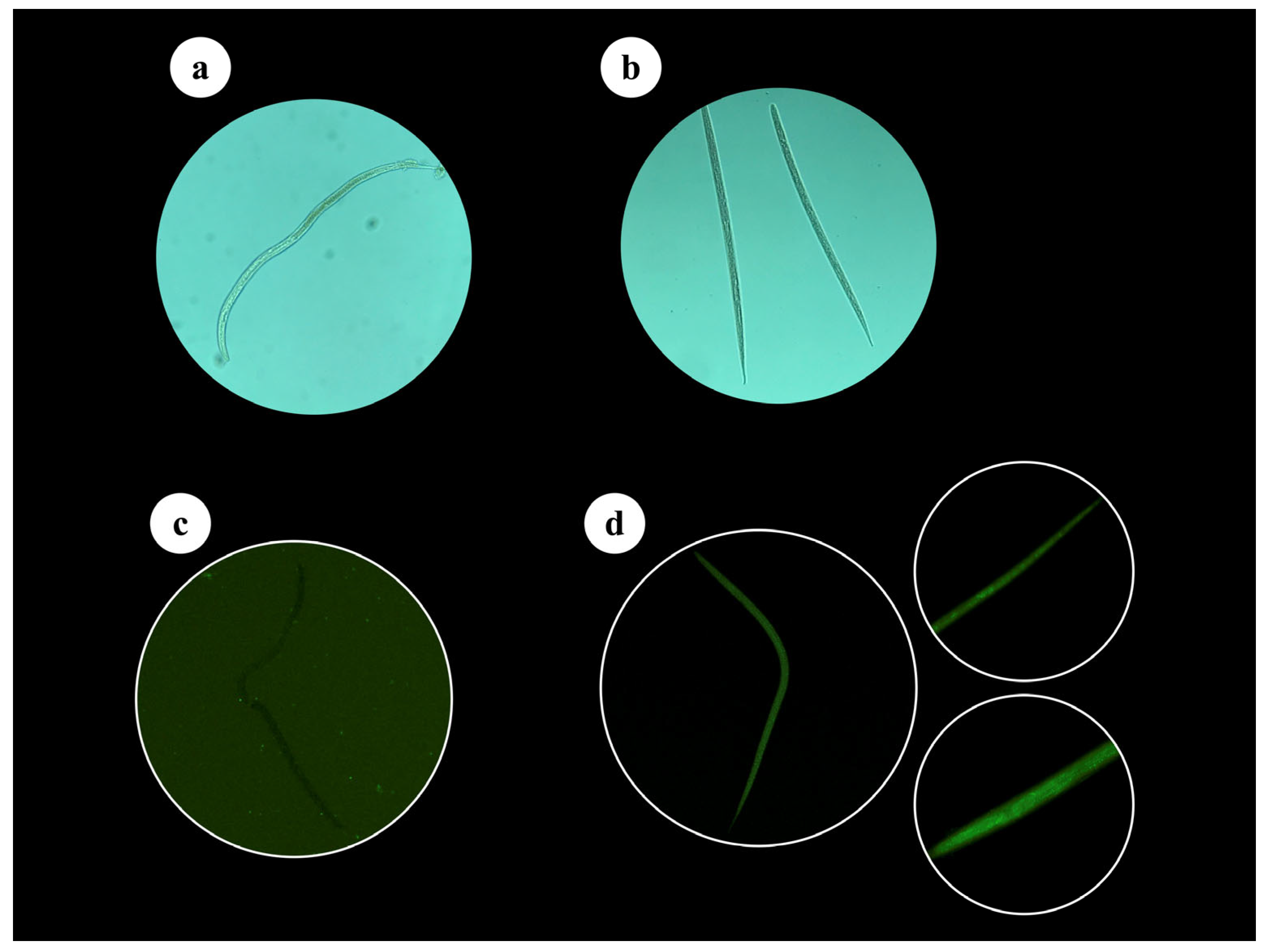
Appendix A.1. Propidium Iodide Staining and Larvae Appearance
Appendix A.2. Maintenance of Strongyloides ratti Life Cycle
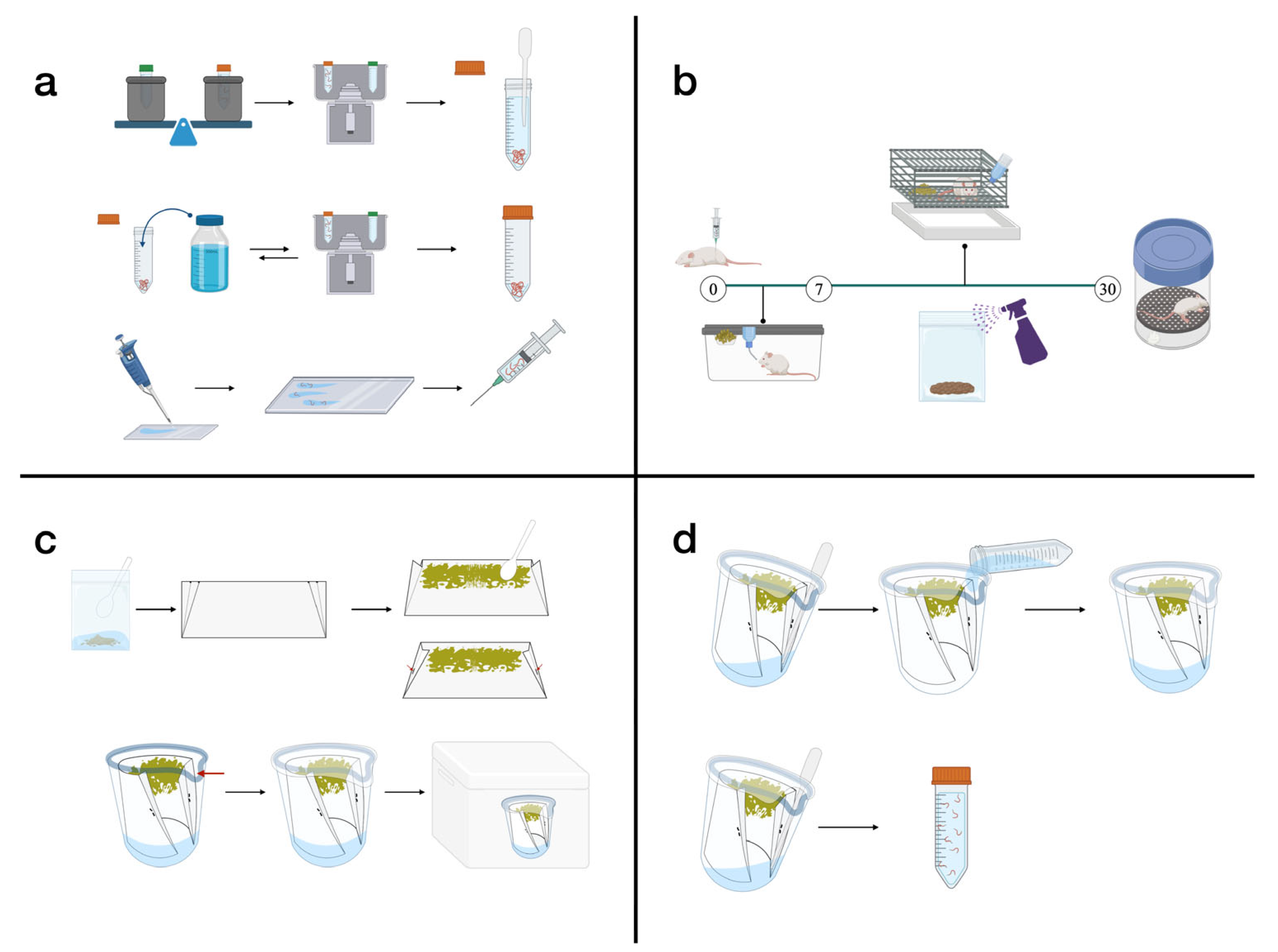
Appendix A.3. Prepare Thermotaxis Agar
References
- Wall, D.H.; Nielsen, U.N.; Six, J. Soil biodiversity and human health. Nature 2015, 528, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Blum, W.E.H.; Zechmeister–Boltenstern, S.; Keiblinger, K.M. Does Soil Contribute to the Human Gut Microbiome? Microorganisms 2019, 7, 287. [Google Scholar] [CrossRef] [PubMed]
- Haahtela, T. A biodiversity hypothesis. Allergy 2019, 74, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, P.M.; Lamberton, P.H.L.; Fenwick, A.; Addiss, D.G. Soil–transmitted helminth infections. Lancet 2018, 391, 252–265. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Buonfrate, D.; Zammarchi, L.; Bisoffi, Z.; Montresor, A.; Boccalini, S. Control programs for strongyloidiasis in areas of high endemicity: An economic analysis of different approaches. Infect. Dis. Poverty 2021, 10, 76. [Google Scholar] [CrossRef]
- Tamarozzi, F.; Martello, E.; Giorli, G.; Fittipaldo, A.; Staffolani, S.; Montresor, A.; Bisoffi, Z.; Buonfrate, D. Morbidity Associated with Chronic Strongyloides stercoralis Infection: A Systematic Review and Meta–Analysis. Am. J. Trop. Med. Hyg. 2019, 100, 1305–1311. [Google Scholar] [CrossRef]
- Vasquez–Rios, G.; Pineda–Reyes, R.; Pineda–Reyes, J.; Marin, R.; Ruiz, E.F.; Terashima, A. Strongyloides stercoralis hyperinfection syndrome: A deeper understanding of a neglected disease. J. Parasit. Dis. 2019, 43, 167–175. [Google Scholar] [CrossRef]
- Mukaigawara, M.; Narita, M.; Shiiki, S.; Takayama, Y.; Takakura, S.; Kishaba, T. Clinical Characteristics of Disseminated Strongyloidiasis, Japan, 1975–2017. Emerg. Infect. Dis. 2020, 26, 401–408. [Google Scholar] [CrossRef]
- Rosadas, C.; Taylor, G.P. HTLV–1 and Co–infections. Front. Med. 2022, 9, 812016. [Google Scholar] [CrossRef]
- GeethaBanu, S.; Arthi, K. Strongyloides hyperinfection in a patient with solid malignant tumor a case report. Indian J. Public Health 2020, 64, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Kosałka–Węgiel, J.; Siwiec, A.; Mastalska, K.; Kłyś, A.; Koźlik, P.; Korkosz, M. Disseminated disease with Strongyloides stercoralis infestation in follicular lymphoma. Pol. Arch. Intern. Med. 2020, 130, 891–892. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.N.; Oliveira, C.L.; Araújo, W.A.C.; Souza, A.B.S.; Silva, M.L.S.; da Cruz, I.D.R.; Sampaio, L.M.; Dos Santos, J.S.B.; Teixeira, M.C.A.; Soares, N.M. Strongyloides stercoralis in Alcoholic Patients: Implications of Alcohol Intake in the Frequency of Infection and Parasite Load. Pathogens 2020, 9, 422. [Google Scholar] [CrossRef]
- Richards, C.; Penner, J.; Colmegna, I.; Loewen, H.; Melaku, Z.; Melkie, A.; Meltzer, M.; Scuccimarri, R.; Mengistu, Y.; Hitchon, C.A. Methotrexate exposure and risk of strongyloidiasis. Trop. Med. Int. Health 2019, 24, 1032–1041. [Google Scholar] [CrossRef]
- Miglioli–Galvão, L.; Pestana, J.O.M.; Santoro–Lopes, G.; Torres Gonçalves, R.; Requião Moura, L.R.; Pacheco Silva, Á.; Camera Pierrotti, L.; David Neto, E.; Santana Girão, E.; Costa de Oliveira, C.M.; et al. Severe Strongyloides stercoralis infection in kidney transplant recipients: A multicenter case–control study. PLoS Negl. Trop. Dis. 2020, 14, e0007998. [Google Scholar] [CrossRef]
- Paltridge, M.; Traves, A. The Health Effects of Strongyloidiasis on Pregnant Women and Children: A Systematic Literature Review. Trop. Med. Infect. Dis. 2018, 3, 50. [Google Scholar] [CrossRef]
- Lee, J.D.; Yen, C.M.; Wang, J.J.; Lin, R.J.; Chung, L.Y. A school–based soil–transmitted helminths survey in the Guadalcanal Province, the Solomon Islands. Trop. Doct. 2021, 51, 167–170. [Google Scholar] [CrossRef]
- Greenaway, C.; Castelli, F. Migration Medicine. Infect. Dis. Clin. N. Am. 2019, 33, 265–287. [Google Scholar] [CrossRef]
- Laoraksawong, P.; Sanpool, O.; Rodpai, R.; Thanchomnang, T.; Kanarkard, W.; Maleewong, W.; Kraiklang, R.; Intapan, P.M. Impact of the health education and preventive equipment package (HEPEP) on prevention of Strongyloides stercoralis infection among rural communities in Northeast Thailand: A cluster randomized controlled trial. BMC Public Health 2018, 18, 1184. [Google Scholar] [CrossRef]
- Amato, V.S.; Tuon, F.F. Mass Drug Administration for the Control of Strongyloides stercoralis Infection: Progress and Challenges. Clin. Infect. Dis. 2020, 71, 3229–3231. [Google Scholar] [CrossRef]
- Werkman, M.; Wright, J.E.; Truscott, J.E.; Oswald, W.E.; Halliday, K.E.; Papaiakovou, M.; Farrell, S.H.; Pullan, R.L.; Anderson, R.M. The impact of community–wide, mass drug administration on aggregation of soil–transmitted helminth infection in human host populations. Parasit. Vectors 2020, 13, 290. [Google Scholar] [CrossRef] [PubMed]
- Sengthong, C.; Yingklang, M.; Intuyod, K.; Haonon, O.; Pinlaor, P.; Jantawong, C.; Hongsrichan, N.; Laha, T.; Anutrakulchai, S.; Cha’on, U.; et al. Repeated Ivermectin Treatment Induces Ivermectin Resistance in Strongyloides ratti by Upregulating the Expression of ATP–Binding Cassette Transporter Genes. Am. J. Trop. Med. Hyg. 2021, 105, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Steinbaum, L.; Mboya, J.; Mahoney, R.; Njenga, S.M.; Null, C.; Pickering, A.J. Effect of a sanitation intervention on soil–transmitted helminth prevalence and concentration in household soil: A cluster–randomized controlled trial and risk factor analysis. PLoS Negl. Trop. Dis. 2019, 13, e0007180. [Google Scholar] [CrossRef] [PubMed]
- Incani, R.N.; Grillet, M.E.; Mughini–Gras, L. Hotspots and correlates of soil–transmitted helminth infections in a Venezuelan rural community: Which are the “wormy” houses? J. Infect. 2021, 82, 143–149. [Google Scholar] [CrossRef]
- Beknazarova, M.; Whiley, H.; Ross, K. Advocating for both Environmental and Clinical Approaches to Control Human Strongyloidiasis. Pathogens 2016, 5, 59. [Google Scholar] [CrossRef]
- Ntalli, N.; Adamski, Z.; Doula, M.; Monokrousos, N. Nematicidal Amendments and Soil Remediation. Plants 2020, 9, 429. [Google Scholar] [CrossRef]
- Silva, L.P.C.; Ferraz, C.M.; Aguiar, A.R.; Araújo, J.V.; Ribeiro, S.R.; Rossi, D.G.; Mendes, L.Q.; Pereira, F.E.L.; Moreira, N.I.B.; Braga, F.R. Viability of Strongyloides venezuelensis eggs and larvae in vermiculite containing the fungus Duddingtonia flagrans. Parasitol. Res. 2017, 116, 2047–2051. [Google Scholar] [CrossRef]
- Braga, F.R.; Ferraz, C.M.; da Silva, E.N.; de Araújo, J.V. Efficiency of the Bioverm® (Duddingtonia flagrans) fungal formulation to control in vivo and in vitro of Haemonchus contortus and Strongyloides papillosus in sheep. 3 Biotech 2020, 10, 62. [Google Scholar] [CrossRef]
- Forrer, A.; Khieu, V.; Vounatsou, P.; Sithithaworn, P.; Ruantip, S.; Huy, R.; Muth, S.; Odermatt, P. Strongyloides stercoralis: Spatial distribution of a highly prevalent and ubiquitous soil–transmitted helminth in Cambodia. PLoS Negl. Trop. Dis. 2019, 13, e0006943. [Google Scholar] [CrossRef]
- Etewa, S.E.; Abdel–Rahman, S.A.; Abd El–Aal, N.F.; Fathy, G.M.; El–Shafey, M.A.; Ewis, A.M. Geohelminths distribution as affected by soil properties, physicochemical factors and climate in Sharkyia governorate Egypt. J. Parasit. Dis. 2016, 40, 496–504. [Google Scholar] [CrossRef]
- Sedionoto, B.; Wasessombat, S.; Punsawad, C.; Anamnart, W. Environmental Factors and Prevalence of Hookworm infection and Strongyloidiasis in Rural East Kalimantan, Indonesia. E3S Web Conf. 2019, 125, 04001. [Google Scholar] [CrossRef]
- Mahmud, Z.H.; Das, P.K.; Khanum, H.; Hossainey, M.R.; Islam, E.; Mahmud, H.A.; Islam, S.; Imran, K.M.; Dey, D.; Islam, S. Time–Temperature Model for Bacterial and Parasitic Annihilation from Cow Dung and Human Faecal Sludge: A Forthcoming Bio–Fertilizer. J. Bacteriol. Parasitol. 2016, 7, 10–4172. [Google Scholar] [CrossRef]
- Saqer, A.S.; Seham, H.; Ragaa, G.; Yehia Ael, W.; Wafaa, S. Optimum methods of inactivation of Strongyloides stercoralis larvae from reclaimed wastewater. Environ. Monit. Assess. 2007, 130, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, E.B.; King, W.C. Observations on the reactions of the larvae of Ancylostoma canium and Ancylostoma braziliense to various drugs and chemical compounds based on in vitro tests. J. Investig. Dermatol. 1953, 20, 337–341. [Google Scholar] [CrossRef]
- Nolan, T.J.; Brenes, M.; Ashton, F.T.; Zhu, X.; Forbes, W.M.; Boston, R.; Schad, G.A. The amphidial neuron pair ALD controls the temperature-sensitive choice of alternative developmental pathways in the parasitic nematode, Strongyloides stercoralis. Parasitology 2004, 129, 753–759. [Google Scholar] [CrossRef]
- Bryant, A.S.; Ruiz, F.; Gang, S.S.; Castelletto, M.L.; Lopez, J.B.; Hallem, E.A. A Critical Role for Thermosensation in Host Seeking by Skin–Penetrating Nematodes. Curr. Biol. 2018, 28, 2338–2347. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Rekanovic, E.; Hrustic, J.; Grahovac, M.; Pešić, B. Methods for management of soilborne plant pathogens. Pestic. Phytomed. 2017, 32, 9–24. [Google Scholar] [CrossRef]
- Ekobol, N.; Boonjaraspinyo, S.; Artchayasawat, A.; Boonmars, T. Monks: A Population at Risk for Liver Fluke and Skin–Penetrating Helminths. Trop. Med. Infect. Dis. 2023, 8, 135. [Google Scholar] [CrossRef]
- Coughlan, K.; Cresswell, H.; McKenzie, N. Soil Physical Measurement and Interpretation for Land Evaluation; CSIRO Publishing: Clayton, Australia, 2002; ISBN 978-064-306-987-9. [Google Scholar]
- Food and Agriculture Organization of the United Nations/Global Soil Partnership. Soil Testing Methods–Global Soil Doctors Programme—A Farmer–to–Farmer Training Programme, 1st ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; pp. 10–14. ISBN 978-925-131-195-0. [Google Scholar]
- Arora, K.R. Soil Mechanics and Foundation Engineering (Geotechnical Engineering): In Si Units, 7th ed.; Standard Publishers Distributors: Delhi, India, 2008; ISBN 818-014-112-8. [Google Scholar]
- Selker, J.; Or, D. Soil Hydrology and Biophysics, 1st ed.; Oregon State University: Corvallis, OR, USA, 2019. [Google Scholar]
- Shinn–Thomas, J.H.; Scanga, S.E.; Spica, P.S.; Nariya, H.K.; Klempic, E.; Brockett, M.R. Wrapping culture plates with Parafilm M® increases Caenorhabditis elegans growth. BMC Res. Notes 2019, 12, 818. [Google Scholar] [CrossRef]
- Wessolek, G.; Bohne, K.; Trinks, S. Validation of Soil Thermal Conductivity Models. Int. J. Thermophys. 2023, 44, 20. [Google Scholar] [CrossRef]
- Boyko, O.O.; Gavrilina, O.G.; Gavrilin, P.N.; Gugosyan, Y.A.; Brygadyrenko, V.V. Influence of formic acid on the viability of Strongyloides papillosus. Regul. Mech. Biosyst. 2018, 9, 435–439. [Google Scholar] [CrossRef]
- Boyko, O.O.; Brygadyrenko, V.V. Nematicidal Activity of Inorganic Food Additives. Diversity 2022, 14, 663. [Google Scholar] [CrossRef]
- Aleuy, O.A.; Kutz, S. Adaptations, life–history traits and ecological mechanisms of parasites to survive extremes and environmental unpredictability in the face of climate change. Int. J. Parasitol. Parasites Wildl. 2020, 12, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Khathwayo, Z.; Ramakuwela, T.; Hatting, J.; Shapiro–Ilan, D.I.; Cochrane, N. Quantification of pH tolerance levels among entomopathogenic nematodes. J. Nematol. 2021, 53, e2021-62. [Google Scholar] [CrossRef]
- Forbes, W.M.; Ashton, F.T.; Boston, R.; Schad, G.A. Chemotactic behaviour of Strongyloides stercoralis infective larvae on a sodium chloride gradient. Parasitology 2003, 127, 189–197. [Google Scholar] [CrossRef]
- Choe, K.P. Physiological and molecular mechanisms of salt and water homeostasis in the nematode Caenorhabditis elegans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, 175–186. [Google Scholar] [CrossRef]
- Gao, X.; Guo, W.; Wu, N.; Yao, Y.; Du, H.; Xu, M.; Zhao, Y.; Tu, Y. Effects of salt and heat treatment on the physicochemical properties, microstructure, secondary structure, and simulated in vitro gastrointestinal digestion of duck egg white. J. Sci. Food Agric. 2021, 101, 6093–6103. [Google Scholar] [CrossRef]
- Li, L.; Lu, Y.; Ren, T.; Horton, R. Quartz contents derived from particle density measurements improve the accuracy of soil thermal conductivity estimates. Geoderma 2023, 436, 116526. [Google Scholar] [CrossRef]
- Gregory, B.T.; Desouky, M.; Slaughter, J.; Hallem, E.A.; Bryant, A.S. Thermosensory behaviors of the free–living life stages of Strongyloides species support parasitism in tropical environments. PLoS Negl. Trop. Dis. 2024, 18, e0012529. [Google Scholar] [CrossRef]
- Pan, T.; Ronan, E.A.; Xu, X.Z.S. Thermosensation: How a human–infective nematode finds its host. Curr. Biol. 2022, 32, 464–466. [Google Scholar] [CrossRef]
- Takeishi, A. Environmental–temperature and internal–state dependent thermotaxis plasticity of nematodes. Curr. Opin. Neurobiol. 2022, 74, 102541. [Google Scholar] [CrossRef] [PubMed]
- Redlich, O. Intensive and extensive properties. J. Chem. Educ. 1970, 47, 154. [Google Scholar] [CrossRef]
- Smith, J.M.; Van Ness, H.C.; Abbott, M.M.; Swihart, M.T. Introduction to Chemical Engineering Thermodynamics, 8th ed.; McGraw–Hill Education: New York, NY, USA, 2018; pp. 24–59. ISBN 978-125-969-652-7. [Google Scholar]
- Erdogan, H.; Cruzado-Gutierrez, K.; Stevens, G.; Shapiro-Ilan, D.; Kaplan, F.; Alborn, H.; Lewis, E. Nematodes Follow a Leader. Front. Ecol. Evol. 2021, 9, 740351. [Google Scholar] [CrossRef]
- Stevens, G.; Usman, M.; Gulzar, S.; Stevens, C.; Pimentel, E.; Erdogan, H.; Schliekelman, P.; Kaplan, F.; Alborn, H.; Wakil, W.; et al. Group Movement in Entomopathogenic Nematodes: Aggregation Levels Vary Based on Context. J. Nematol. 2024, 56, 20240002. [Google Scholar] [CrossRef]
- Fincher, G.T.; Stewart, T.B. Vertical migration by nematode larvae of cattle parasites through soil. Proc. Helminthol. Soc. Wash. 1979, 46, 43–46. [Google Scholar]
- Gruner, L.; Mauleon, H.; Sauve, C. Migrations of trichostrongyle infective larvae experiments with ovine parasites in soil. Ann. Rech. Vet. 1982, 13, 51–59. [Google Scholar]
- Sciacca, J.; Ketschek, A.; Forbes, W.M.; Boston, R.; Guerrero, J.; Ashton, F.T.; Gamble, H.R.; Schad, G.A. Vertical migration by the infective larvae of three species of parasitic nematodes: Is the behaviour really a response to gravity? Parasitology 2002, 125, 553–560. [Google Scholar] [CrossRef]
- Knox, O.; Polain, K.; Fortescue, E.; Griffiths, B. Distribution and Restricted Vertical Movement of Nematodes in a Heavy Clay Soil. Agronomy 2020, 10, 221. [Google Scholar] [CrossRef]
- Ferreira, S.R.; Mendes, T.A.; Bueno, L.L.; de Araújo, J.V.; Bartholomeu, D.C.; Fujiwara, R.T. A new methodology for evaluation of nematode viability. Biomed. Res. Int. 2015, 2015, 879263. [Google Scholar] [CrossRef]
- Crowley, L.C.; Scott, A.P.; Marfell, B.J.; Boughaba, J.A.; Chojnowski, G.; Waterhouse, N.J. Measuring Cell Death by Propidium Iodide Uptake and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016, pdb-prot087163. [Google Scholar] [CrossRef]
- Goodman, M.B.; Klein, M.; Lasse, S.; Luo, L.; Mori, I.; Samuel, A.; Sengupta, P.; Wang, D. Thermotaxis navigation behavior. WormBook 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Croze, O.A.; Ferguson, G.P.; Cates, M.E.; Poon, W.C. Migration of chemotactic bacteria in soft agar: Role of gel concentration. Biophys. J. 2011, 101, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, D.; Schüler, D. Quantifying the Benefit of a Dedicated “Magnetoskeleton” in Bacterial Magnetotaxis by Live–Cell Motility Tracking and Soft Agar Swimming Assay. Appl. Environ. Microbiol. 2020, 86, e01976-19. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekobol, N.; Boonjaraspinyo, S.; Eamudomkarn, C.; Boonmars, T. Tricky with Heat and Salt: Soil Factors, Thermotaxis, and Potential for Heat–Saline Agar Trapping of Strongyloides Larvae. Biology 2025, 14, 559. https://doi.org/10.3390/biology14050559
Ekobol N, Boonjaraspinyo S, Eamudomkarn C, Boonmars T. Tricky with Heat and Salt: Soil Factors, Thermotaxis, and Potential for Heat–Saline Agar Trapping of Strongyloides Larvae. Biology. 2025; 14(5):559. https://doi.org/10.3390/biology14050559
Chicago/Turabian StyleEkobol, Nuttapon, Sirintip Boonjaraspinyo, Chatanun Eamudomkarn, and Thidarut Boonmars. 2025. "Tricky with Heat and Salt: Soil Factors, Thermotaxis, and Potential for Heat–Saline Agar Trapping of Strongyloides Larvae" Biology 14, no. 5: 559. https://doi.org/10.3390/biology14050559
APA StyleEkobol, N., Boonjaraspinyo, S., Eamudomkarn, C., & Boonmars, T. (2025). Tricky with Heat and Salt: Soil Factors, Thermotaxis, and Potential for Heat–Saline Agar Trapping of Strongyloides Larvae. Biology, 14(5), 559. https://doi.org/10.3390/biology14050559






