Establishment and Characteristics of the Spermatogonial Stem Cell Line from the Yellow River Carp (Cyprinus carpio haematopterus)
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animal Ethics
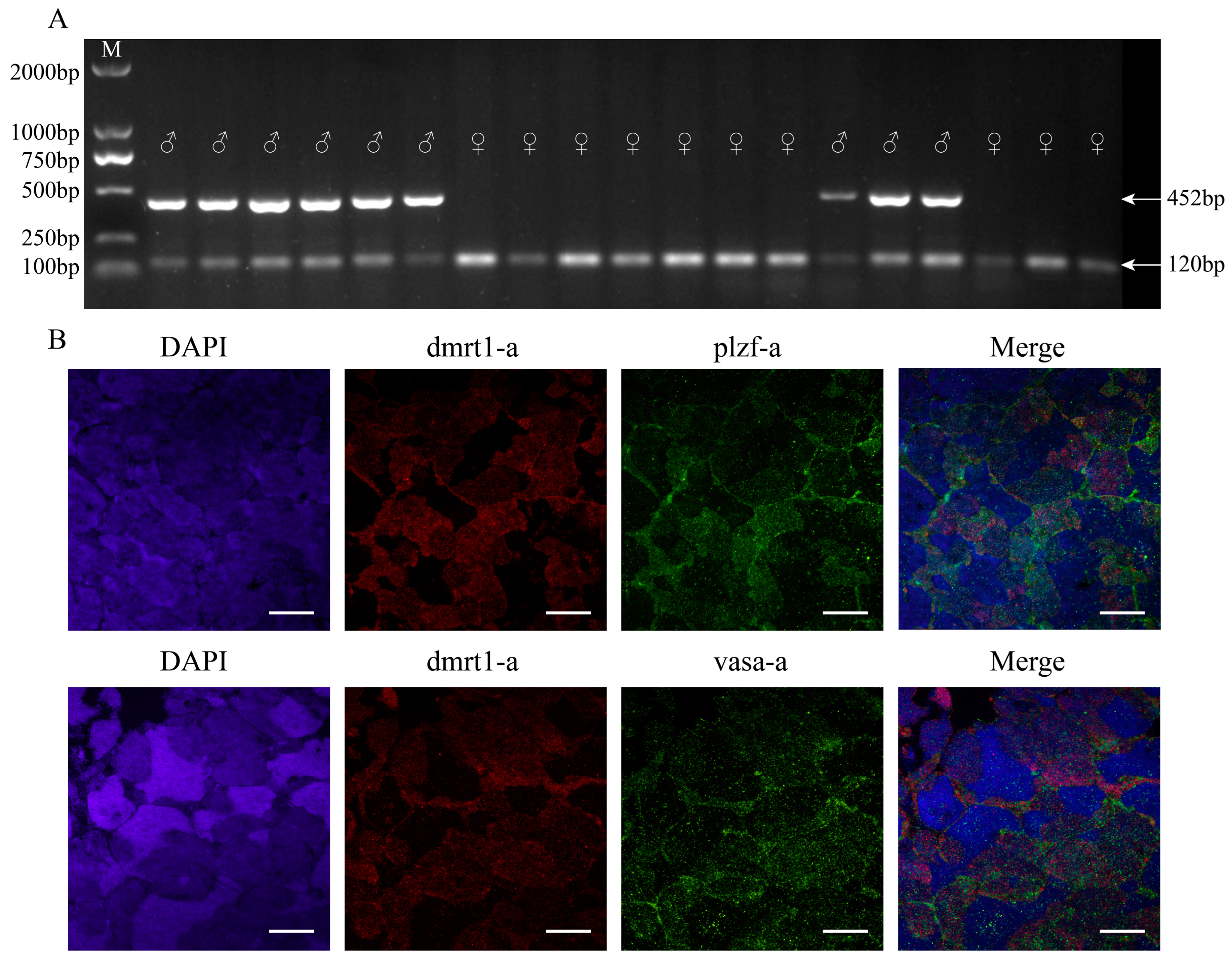
2.2. PCR-Based Sex Identification
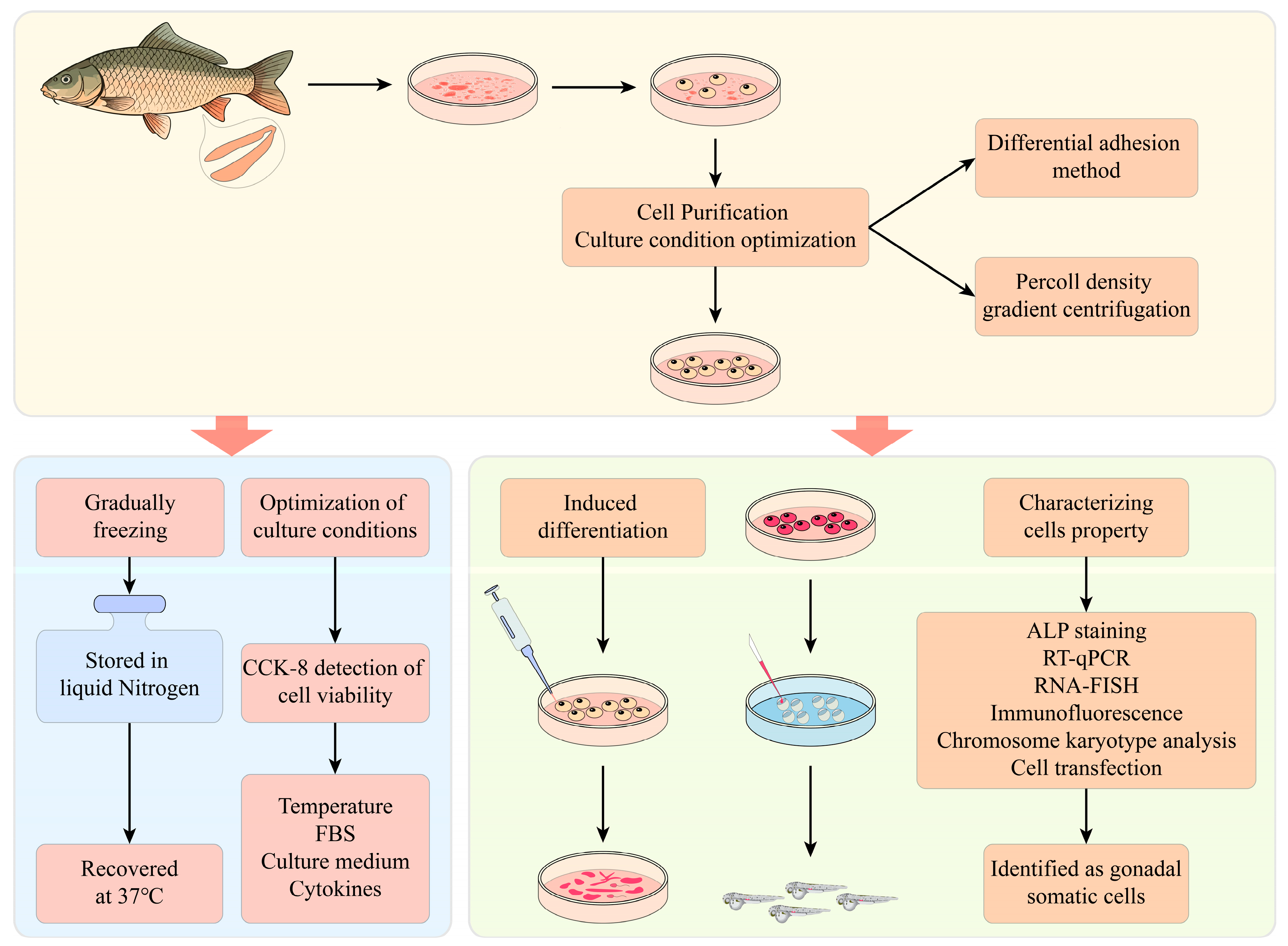
2.3. Isolation of SSCs
2.4. Cell Passaging and Purification
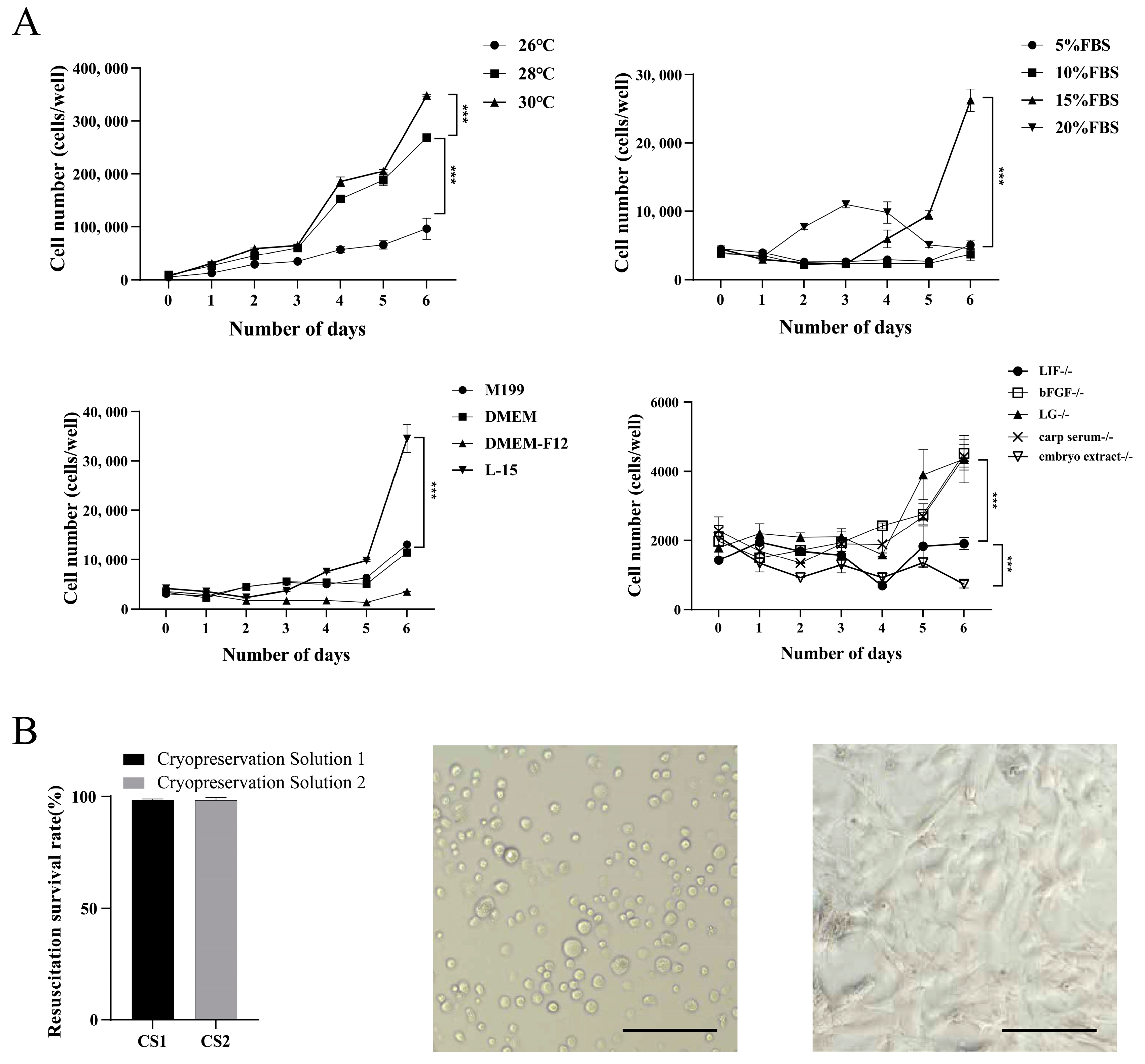
2.5. Cell Cryopreservation and Recovery
2.6. Optimization of Culture Conditions
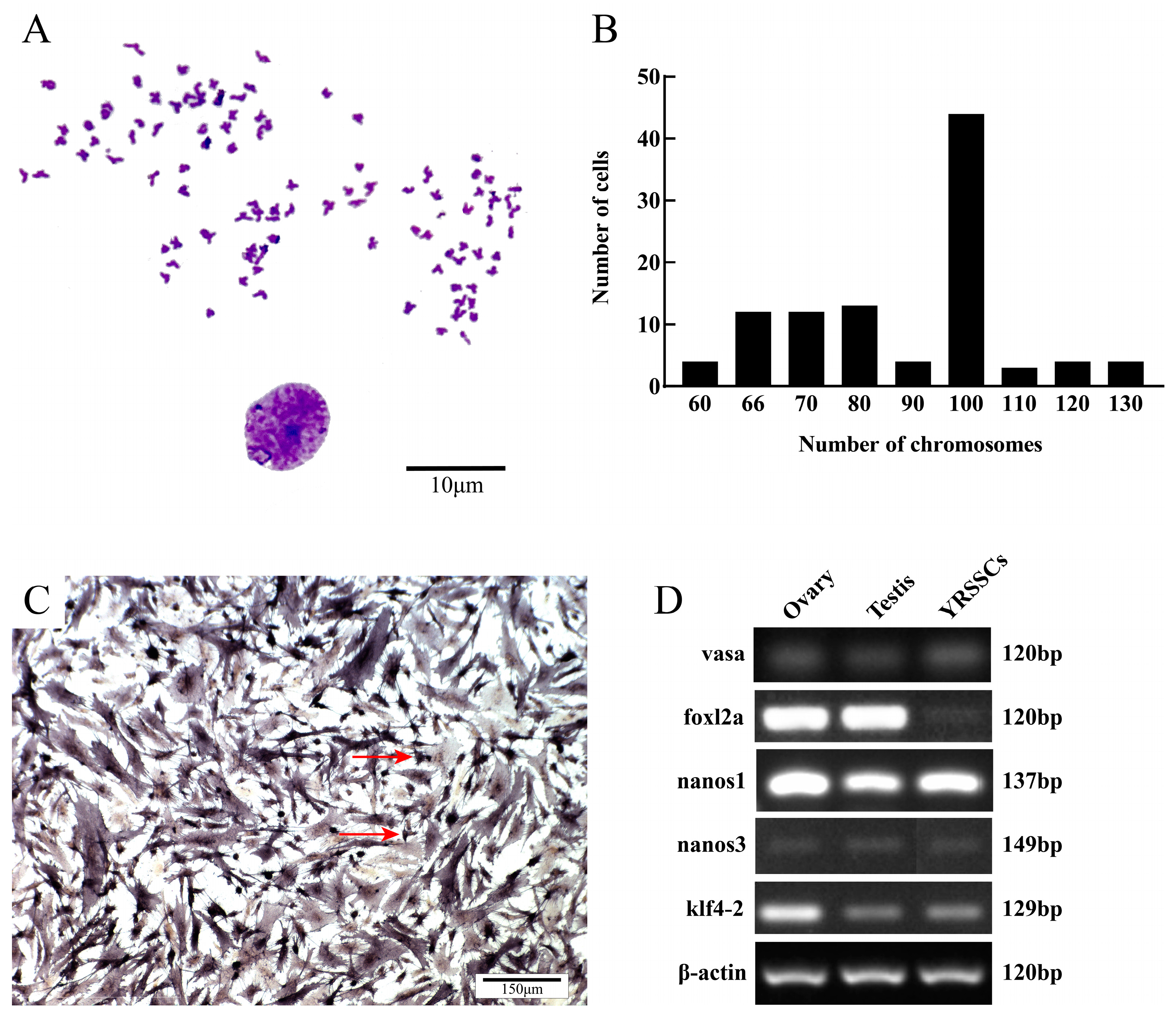
2.7. Karyotype Analysis
2.8. Alkaline Phosphatase Staining
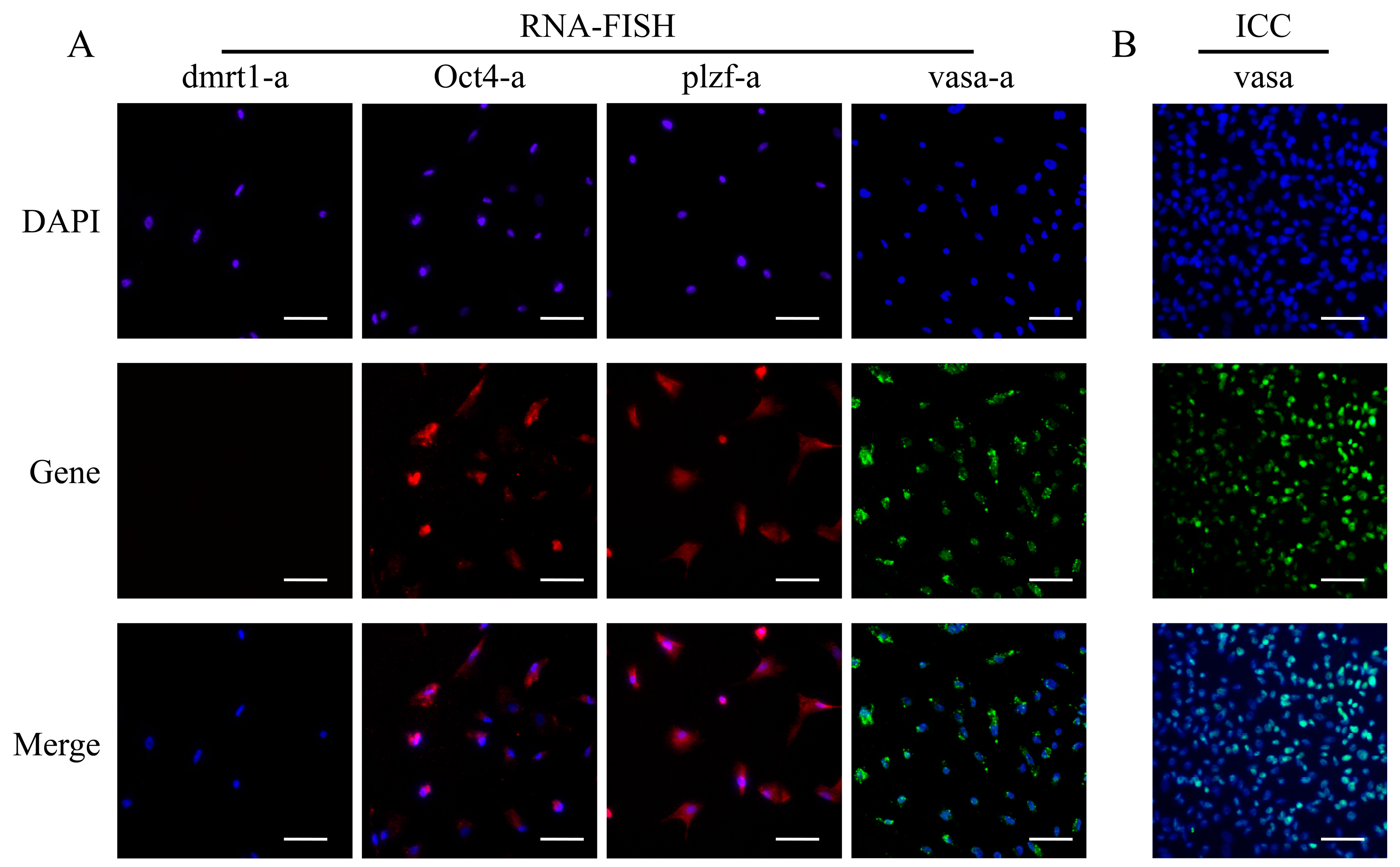
2.9. Expression of Germ Cell Marker Genes
2.10. RNA-FISH
2.11. Flurorescence in Situ Hybridization of Vasa
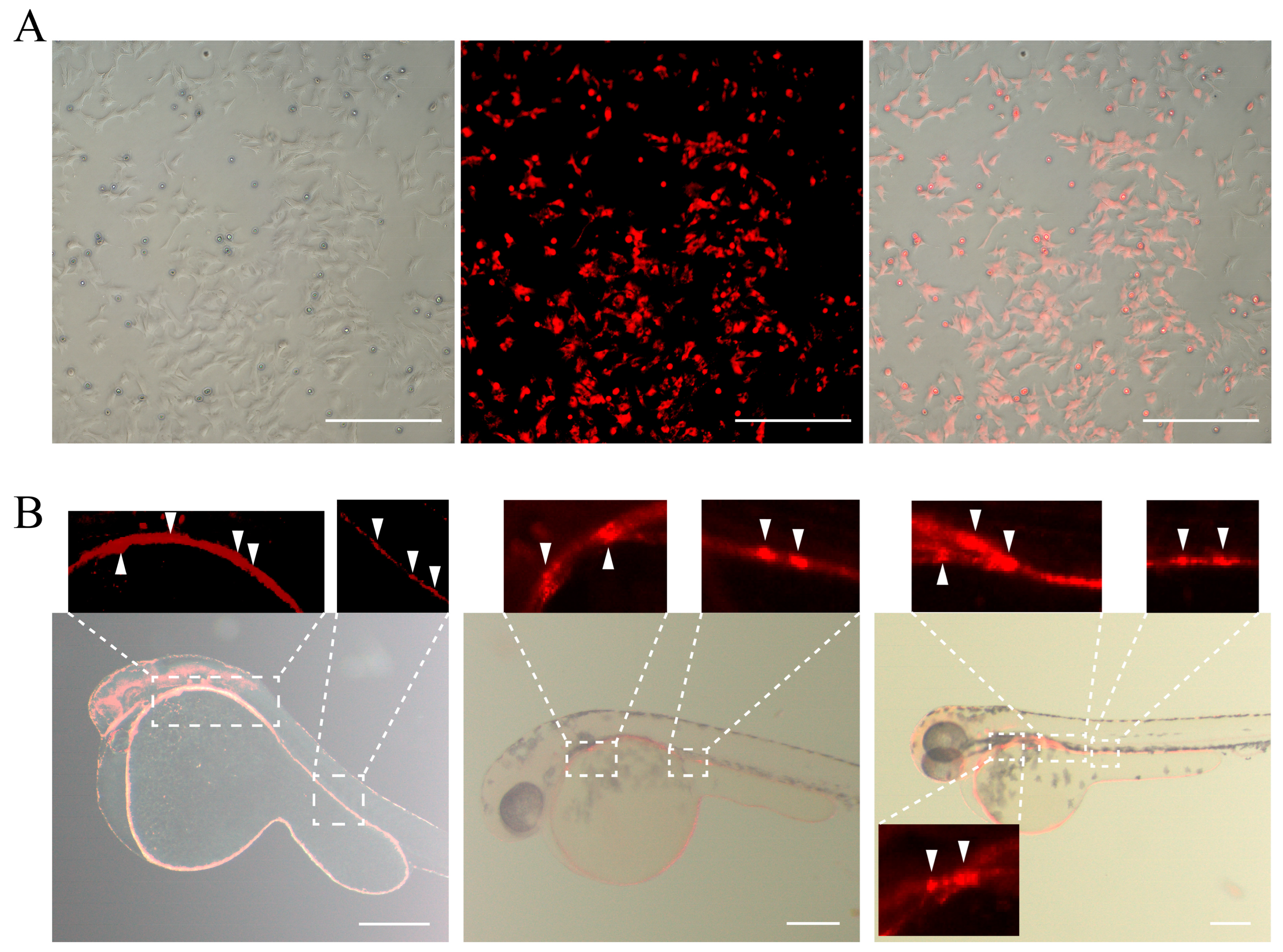
2.12. Analysis of the YRSSCs’ Ability to Express Exogenous Genes
2.13. In Vitro-Induced Differentiation
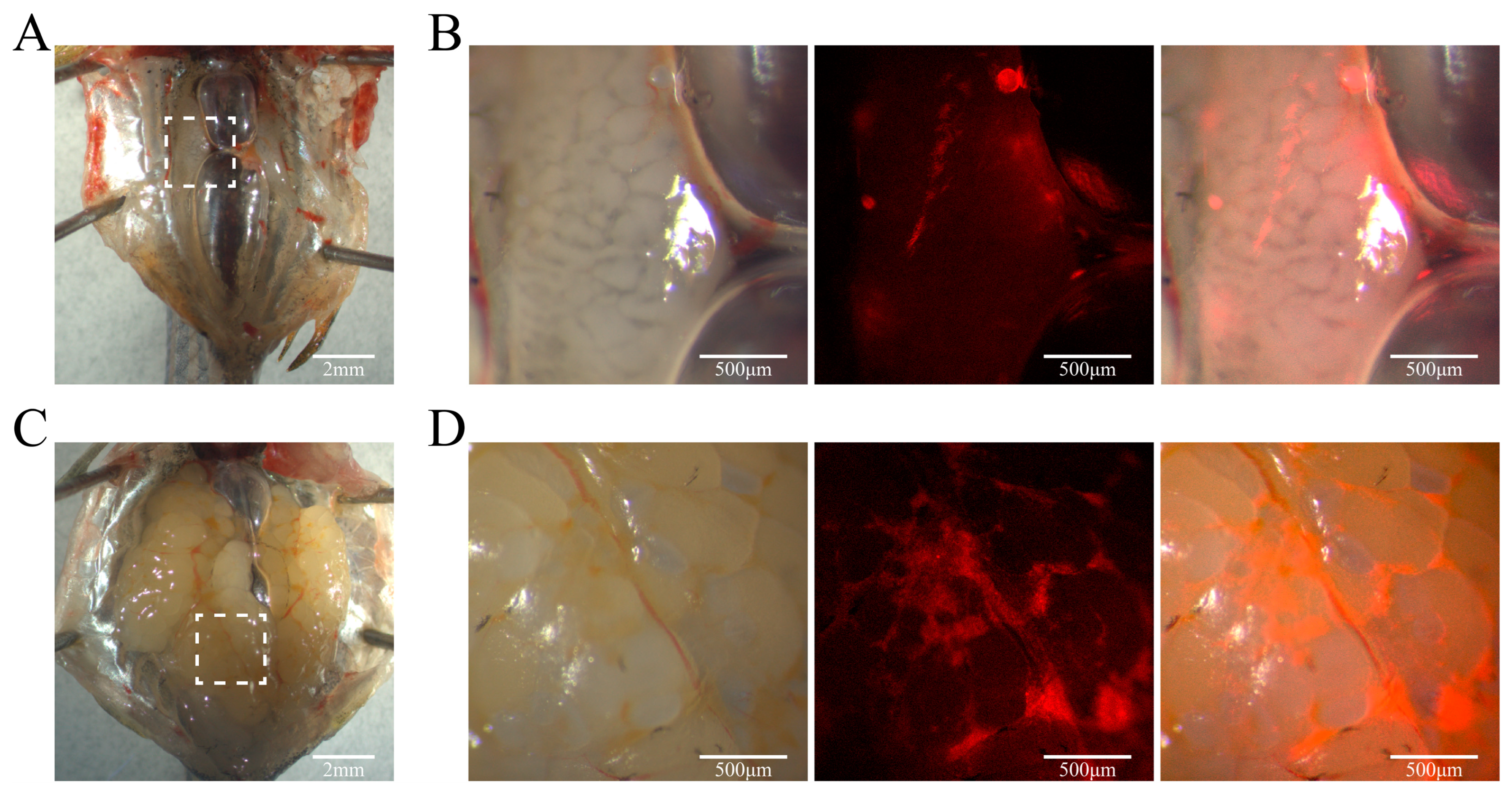
2.14. Cell Transplantation
2.15. Statistical Analysis
3. Results
3.1. Sex Identification of Yellow River Carp
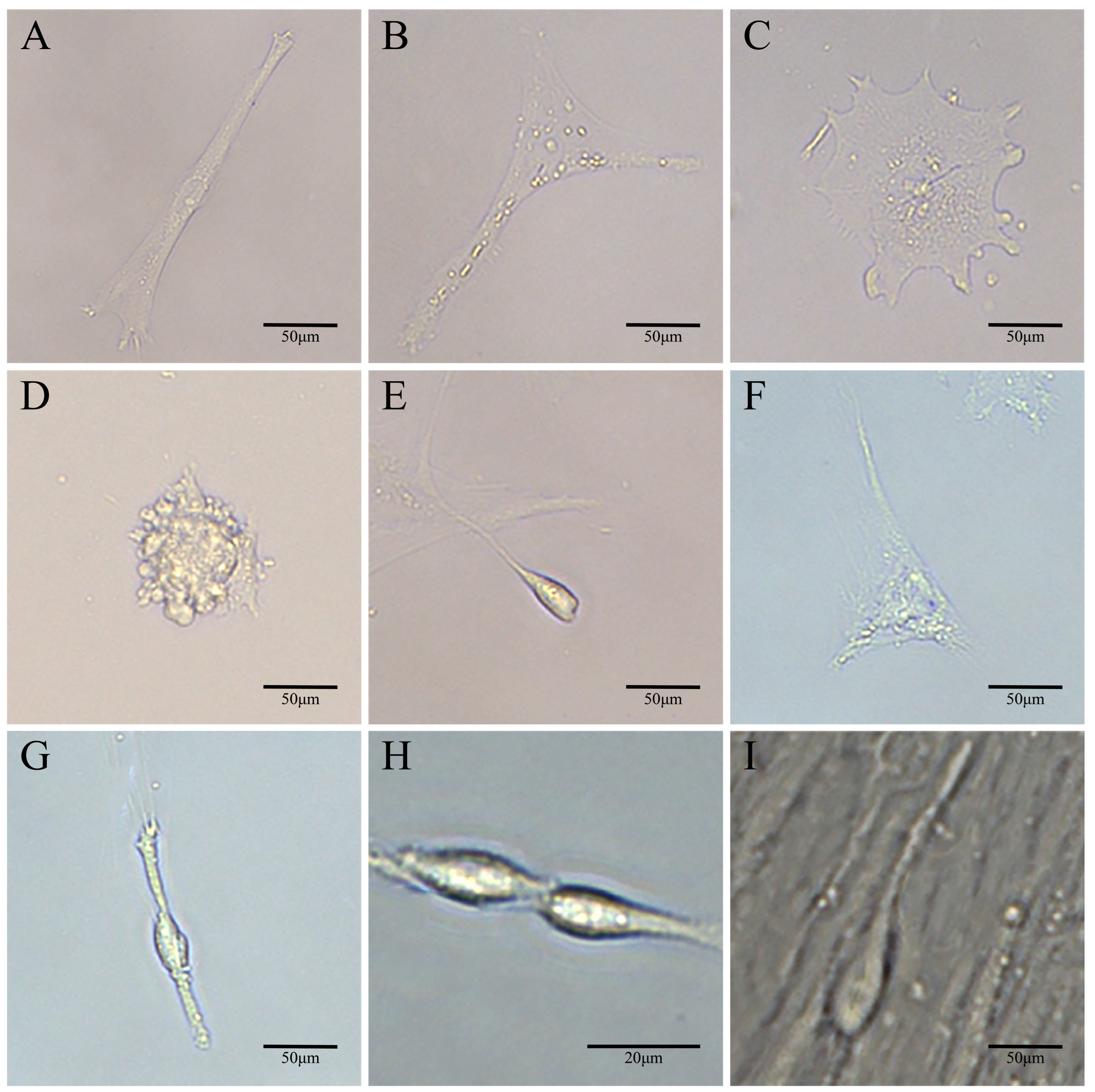
3.2. Establishment of a Spermatogonial Stem Cell Line
3.3. Optimization of Cell Culture and Cryopreservation Conditions
3.4. Characterization of YRSSCs
3.5. Induction of Differentiation In Vitro
3.6. Pluripotency of YRSSCs In Vivo
4. Discussion
4.1. Establishment of the YRSSCs
4.2. Characteristics of YRSSCs
4.3. In Vitro Differentiation of YRSSCs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SSCs | spermatogonial stem cells |
| RNP | ribonucleoprotein |
| PGCs | primordial germ cells |
References
- Nishimura, T.; Tanaka, M. Gonadal development in fish. Sex. Dev. 2014, 8, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Marques-Mari, A.; Lacham-Kaplan, O.; Medrano, J.; Pellicer, A.; Simón, C. Differentiation of germ cells and gametes from stem cells. Hum. Reprod. Update 2009, 15, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, R. Germline stem cells: Origin and destiny. Cell Stem Cell 2012, 10, 729–739. [Google Scholar] [CrossRef]
- Kawasaki, T.; Sakai, N. Establishment of Long-Term Culture Conditions for Zebrafish Spermatogonial Stem Cells; Oxford University Press: Oxford, UK, 2012; Volume 87, p. 586. [Google Scholar]
- Hong, Y.; Liu, T.; Zhao, H.; Xu, H.; Wang, W.; Liu, R.; Chen, T.; Deng, J.; Gui, J. Establishment of a normal medakafish spermatogonial cell line capable of sperm production in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 8011–8016. [Google Scholar] [CrossRef]
- Shikina, S.; Ihara, S.; Yoshizaki, G. Culture conditions for maintaining the survival and mitotic activity of rainbow trout transplantable type A spermatogonia. Mol. Reprod. Dev. 2008, 75, 529–537. [Google Scholar] [CrossRef]
- Chen, X.; Kan, Y.; Zhong, Y.; Jawad, M.; Wei, W.; Gu, K.; Gui, L.; Li, M. Generation of a normal long-term-cultured Chinese hook snout carp spermatogonial stem cell line capable of sperm production in vitro. Biology 2022, 11, 1069. [Google Scholar] [CrossRef]
- Gu, K.; Zhang, Y.; Zhong, Y.; Kan, Y.; Jawad, M.; Gui, L.; Ren, M.; Xu, G.; Liu, D.; Li, M. Establishment of a Coilia nasus spermatogonial stem cell line capable of spermatogenesis in vitro. Biology 2023, 12, 1175. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Nóbrega, R.; Pšenička, M. Spermatogonial stem cells in fish: Characterization, isolation, enrichment, and recent advances of in vitro culture systems. Biomolecules 2020, 10, 644. [Google Scholar] [CrossRef]
- Wang, S.; Tian, J.; Jiang, X.; Li, C.; Ge, Y.; Hu, X.; Cheng, L.; Shi, X.; Shi, L.; Jia, Z. Effects of different dietary protein levels on the growth performance, physicochemical indexes, quality, and molecular expression of yellow river carp (Cyprinus carpio haematopterus). Animals 2023, 13, 1237. [Google Scholar] [CrossRef]
- Song, D.; Yun, Y.; He, Z.; Mi, J.; Wang, L.; Jin, M.; Zhou, Q.; Nie, G. Fillet texture, physicochemical indexes, muscle cellularity and molecular expression in muscle of Yellow River carp (Cyprinus carpio haematopterus) in response to dietary hydroxyproline supplementation. Aquaculture 2022, 549, 737783. [Google Scholar] [CrossRef]
- Wang, X.; Fu, B.; Yu, X.; Qu, C.; Zhang, Q.; Tong, J. Fine mapping of growth-related quantitative trait loci in Yellow River carp (Cyprinus carpio haematoperus). Aquaculture 2018, 484, 277–285. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Tang, C.; Tao, M.; Zhang, C.; Zhou, Y.; Qin, Q.; Luo, K.; Wu, C.; Hu, F. The Research Advances in Distant Hybridization and Gynogenesis in Fish. Rev. Aquac. 2025, 17, e12972. [Google Scholar] [CrossRef]
- Song, H.; Dong, T.; Yan, X.; Wang, W.; Tian, Z.; Sun, A.; Dong, Y.; Zhu, H.; Hu, H. Genomic selection and its research progress in aquaculture breeding. Rev. Aquac. 2023, 15, 274–291. [Google Scholar] [CrossRef]
- Lacerda, S.; Costa, G.; Campos-Junior, P.; Segatelli, T.; Yazawa, R.; Takeuchi, Y.; Morita, T.; Yoshizaki, G.; França, L. Germ cell transplantation as a potential biotechnological approach to fish reproduction. Fish Physiol. Biochem. 2013, 39, 3–11. [Google Scholar] [CrossRef]
- Moran, M.N.; Jones, D.; Jensen, S.; Marcoli, R.; Jerry, D. Optimising commercial traits through gene editing in aquaculture: Strategies for accelerating genetic improvement. Rev. Aquac. 2024, 16, 1127–1159. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.-W.; Mo, C.-Y.; Dong, M.-D.; Jia, K.-T.; Liu, W.; Yi, M.-S. Production of functional sperm from in vitro-cultured premeiotic spermatogonia in a marine fish. Zool. Res. 2022, 43, 537. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.Y.; Goh, C.W.P.; Gan, J.Y.; Lee, Y.S.; Lam, M.K.K.; Hong, N.; Hong, Y.; Chan, W.K.; Shu-Chien, A.C. Derivation and long-term culture of an embryonic stem cell-like line from zebrafish blastomeres under feeder-free condition. Zebrafish 2014, 11, 407–420. [Google Scholar] [CrossRef]
- Saito, T.; Goto-Kazeto, R.; Fujimoto, T.; Kawakami, Y.; Arai, K.; Yamaha, E. Inter-species transplantation and migration of primordial germ cells in cyprinid fish. Int. J. Dev. Biol. 2010, 54, 1481–1486. [Google Scholar] [CrossRef]
- Wong, T.-T.; Collodi, P. Dorsomorphin promotes survival and germline competence of zebrafish spermatogonial stem cells in culture. PLoS ONE 2013, 8, e71332. [Google Scholar] [CrossRef][Green Version]
- Zhang, F.; Hao, Y.; Li, X.; Li, Y.; Ye, D.; Zhang, R.; Wang, X.; He, M.; Wang, H.; Zhu, Z. Surrogate production of genome-edited sperm from a different subfamily by spermatogonial stem cell transplantation. Sci. China Life Sci. 2022, 65, 969–987. [Google Scholar] [CrossRef]
- Franěk, R.; Kašpar, V.; Shah, M.A.; Gela, D.; Pšenička, M. Production of common carp donor-derived offspring from goldfish surrogate broodstock. Aquaculture 2021, 534, 736252. [Google Scholar] [CrossRef]
- Sun, Z.; Ren, Y.; Zhang, Y.; Yang, Y.; Wang, G.; He, Z.; Liu, Y.; Cao, W.; Wang, Y.; Fu, Y. Establishment and characterization of a cell line from the brain of the Japanese flounder (Paralichthys olivaceus) and its application in the study of viral infection. Aquaculture 2023, 562, 738825. [Google Scholar] [CrossRef]
- Kishi, J.Y.; Lapan, S.W.; Beliveau, B.J.; West, E.R.; Zhu, A.; Sasaki, H.M.; Saka, S.K.; Wang, Y.; Cepko, C.L.; Yin, P. SABER amplifies FISH: Enhanced multiplexed imaging of RNA and DNA in cells and tissues. Nat. Methods 2019, 16, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Morishima, K.; Fujimoto, T.; Saito, T.; Kobayashi, T.; Yamaha, E.; Arai, K. Chromosome doubling in early spermatogonia produces diploid spermatozoa in a natural clonal fish. Biol. Reprod. 2009, 80, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.D.; Yoshimizu, M.; Ezura, Y.; Kimura, T. Comparative growth response of fish cell lines in different media, temperatures, and sodium chloride concentrations. Fish Pathol. 1993, 28, 27–34. [Google Scholar] [CrossRef]
- Lakra, W.; Swaminathan, T.R.; Joy, K. Development, characterization, conservation and storage of fish cell lines: A review. Fish Physiol. Biochem. 2011, 37, 1–20. [Google Scholar] [CrossRef]
- Kumar, G.S.; Singh, I.B.; Philip, R. Development of a cell culture system from the ovarian tissue of African catfish (Clarias gariepinus). Aquaculture 2001, 194, 51–62. [Google Scholar] [CrossRef]
- Zhong, C.; Tao, Y.; Liu, M.; Wu, X.; Yang, Y.; Wang, T.; Meng, Z.; Xu, H.; Liu, X. Establishment of a spermatogonial stem cell line with potential of meiosis in a Hermaphroditic fish, Epinephelus coioides. Cells 2022, 11, 2868. [Google Scholar] [CrossRef]
- Nicola, N.A.; Babon, J.J. Leukemia inhibitory factor (LIF). Cytokine Growth Factor Rev. 2015, 26, 533–544. [Google Scholar] [CrossRef]
- Hashimoto, H.; Toyohara, H.; Yokoyama, Y.; Sakaguchi, M.; Ozato, K.; Wakamatsu, Y. Effects of carp serum on the growth of goldfish fin cells in early passage. J. Fish Biol. 1997, 50, 201–207. [Google Scholar] [CrossRef]
- Kondo, H.; Watabe, S. Growth promoting effects of carp serum components on goldfish culture cells. Fish. Sci. 2006, 72, 884–888. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Y.; Zhu, T.; Song, P.; Guo, J.; Zhong, Y.; Gui, L.; Li, M. Vasa identifies germ cells in embryos and gonads of Oryzias celebensis. Gene 2022, 823, 146369. [Google Scholar] [CrossRef]
- Xu, W.; Fu, W.; Long, M.; Yuan, X.; Zhao, K.; Hu, X.; Liu, J.; Liu, W.; Peng, L.; Xiao, Y. Rapid establishment of Oct4: EGFP transgenic zebrafish homozygote through gynogenesis for monitoring the pluripotency during induction of pluripotent stem cells. Reprod. Breed. 2022, 2, 106–111. [Google Scholar] [CrossRef]
- Walsh, C.J.; Rhody, N.; Main, K.L.; Restivo, J.; Tarnecki, A.M. Advances in development of long-term embryonic stem cell-like cultures from a marine fish, Sciaenops ocellatus. Curr. Res. Food Sci. 2024, 9, 100841. [Google Scholar] [CrossRef] [PubMed]
- Chênais, N.; Le Cam, A.; Guillet, B.; Lareyre, J.-J.; Labbé, C. Reprogramming of fish somatic cells for nuclear transfer is primed by Xenopus egg extract. bioRxiv 2022. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, X.; Liu, Q.; Yang, J.; Xu, S.; Wu, Z.; Wang, Y.; You, F.; Song, Z.; Li, J. Successful spermatogonial stem cells transplantation within pleuronectiformes: First breakthrough at inter-family level in marine fish. Int. J. Biol. Sci. 2021, 17, 4426. [Google Scholar] [CrossRef]
- Hu, Y.; Tan, R.; Zhu, X.; Wang, B.; Wang, J.; Guo, B.; Li, Y.; Du, H.; Yang, Y. Genome-wide identification, phylogeny and expressional profile of the Dmrt gene family in Chinese sturgeon (Acipenser sinensis). Sci. Rep. 2024, 14, 4231. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, X.; Li, W.; Tang, Z.; Zhao, Y.; Wu, X. Isolation and in vitro culture of ovarian stem cells in Chinese soft-shell turtle (Pelodiscus sinensis). J. Cell. Biochem. 2018, 119, 7667–7677. [Google Scholar] [CrossRef]
- Beer, R.L.; Draper, B.W. nanos3 maintains germline stem cells and expression of the conserved germline stem cell gene nanos2 in the zebrafish ovary. Dev. Biol. 2013, 374, 308–318. [Google Scholar] [CrossRef]
- Haraguchi, S.; Tsuda, M.; Kitajima, S.; Sasaoka, Y.; Nomura-Kitabayashid, A.; Kurokawa, K.; Saga, Y. nanos1: A mouse nanos gene expressed in the central nervous system is dispensable for normal development. Mech. Dev. 2003, 120, 721–731. [Google Scholar] [CrossRef]
- Higuchi, K.; Goto, R.; Konishi, J.; Ina, Y.; Kazeto, Y.; Gen, K. Early development of primordial germ cells in Pacific bluefin tuna Thunnus orientalis. Theriogenology 2019, 131, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Psenicka, M. Novel technique for visualizing primordial germ cells in sturgeons (Acipenser ruthenus, A. gueldenstaedtii, A. baerii, and Huso huso). Biol. Reprod. 2015, 93, 96. [Google Scholar] [CrossRef]
- Saito, T.; Fujimoto, T.; Maegawa, S.; Inoue, K.; Tanaka, M.; Arai, K.; Yamaha, E. Visualization of primordial germ cells in vivo using GFP-nos1 3′UTR mRNA. Int. J. Dev. Biol. 2006, 50, 691. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Liao, B.-K.; Lu, Y.-F.; Liu, Y.-H.; Hsieh, F.-C.; Hwang, P.-P.; Hwang, S.-P.L. Zebrafish Klf4 maintains the ionocyte progenitor population by regulating epidermal stem cell proliferation and lateral inhibition. PLoS Genet. 2019, 15, e1008058. [Google Scholar] [CrossRef]
- Rowland, B.D.; Bernards, R.; Peeper, D.S. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat. Cell Biol. 2005, 7, 1074–1082. [Google Scholar] [CrossRef]
- Issigonis, M.; Redkar, A.B.; Rozario, T.; Khan, U.W.; Mejia-Sanchez, R.; Lapan, S.W.; Reddien, P.W.; Newmark, P.A. A Krüppel-like factor is required for development and regeneration of germline and yolk cells from somatic stem cells in planarians. PLoS Biol. 2022, 20, e3001472. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.W.; Bhat, I.A.; Rather, M.A.; Khan, I.A.; Bhat, R.A.H.; Iqbal, G. Beyond the petri dish: Fish cell lines pioneering advances in biotechnology, genetic engineering, toxicity and disease solutions. Blue Biotechnol. 2025, 2, 1. [Google Scholar] [CrossRef]
- Xu, H.; Li, M.; Gui, J.; Hong, Y. Fish germ cells. Sci. China Life Sci. 2010, 53, 435–446. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Salas-Vidal, E.; Rueb, S.; Krens, S.G.; Meijer, A.H.; Snaar-Jagalska, B.E.; Spaink, H.P. Genetic and transcriptome characterization of model zebrafish cell lines. Zebrafish 2006, 3, 441–453. [Google Scholar] [CrossRef]
- Jung, K.M.; Kim, Y.M.; Kim, J.L.; Han, J.Y. Efficient gene transfer into zebra finch germline-competent stem cells using an adenoviral vector system. Sci. Rep. 2021, 11, 14746. [Google Scholar] [CrossRef]
- Dehghan, Z.; Darya, G.; Mehdinejadiani, S.; Derakhshanfar, A. Comparison of two methods of sperm-and testis-mediated gene transfer in production of transgenic animals: A systematic review. Anim. Genet. 2024, 55, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Liu, Q.; He, Y.; Zhang, J.; Hou, J.; Ren, Y.; Ma, W.; Wang, Q.; Shao, C. Establishment and Characterization of a Spermatogonial Stem Cell Line from Tiger Puffer Fish (Takifugu rubripes). Animals 2023, 13, 2959. [Google Scholar] [CrossRef]
- Lacerda, S.M.S.N.; Costa, G.M.J.; da Silva, M.d.A.; Campos-Junior, P.H.A.; Segatelli, T.M.; Peixoto, M.T.D.; Resende, R.R.; de França, L.R. Phenotypic characterization and in vitro propagation and transplantation of the Nile tilapia (Oreochromis niloticus) spermatogonial stem cells. Gen. Comp. Endocrinol. 2013, 192, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, G.; Lee, S. Production of live fish derived from frozen germ cells via germ cell transplantation. Stem Cell Res. 2018, 29, 103–110. [Google Scholar] [CrossRef]
- Hryhorowicz, M.; Lipiński, D.; Zeyland, J. Evolution of CRISPR/cas systems for precise genome editing. Int. J. Mol. Sci. 2023, 24, 14233. [Google Scholar] [CrossRef] [PubMed]
- Veillet, F.; Durand, M.; Kroj, T.; Cesari, S.; Gallois, J.-L. Precision breeding made real with CRISPR: Illustration through genetic resistance to pathogens. Plant Commun. 2020, 1, 100102. [Google Scholar] [CrossRef]
- Puthumana, J.; Chandrababu, A.; Sarasan, M.; Joseph, V.; Singh, I.B. Genetic improvement in edible fish: Status, constraints, and prospects on CRISPR-based genome engineering. 3 Biotech 2024, 14, 44. [Google Scholar] [CrossRef]
- Edvardsen, R.B.; Leininger, S.; Kleppe, L.; Skaftnesmo, K.O.; Wargelius, A. Targeted mutagenesis in Atlantic salmon (Salmo salar L.) using the CRISPR/Cas9 system induces complete knockout individuals in the F0 generation. PLoS ONE 2014, 9, e108622. [Google Scholar] [CrossRef]
- Li, M.; Sun, Y.; Zhao, J.; Shi, H.; Zeng, S.; Ye, K.; Jiang, D.; Zhou, L.; Sun, L.; Tao, W. A tandem duplicate of anti-Müllerian hormone with a missense SNP on the Y chromosome is essential for male sex determination in Nile tilapia, Oreochromis niloticus. PLoS Genet. 2015, 11, e1005678. [Google Scholar] [CrossRef]
- Khalil, K.; Elayat, M.; Khalifa, E.; Daghash, S.; Elaswad, A.; Miller, M.; Abdelrahman, H.; Ye, Z.; Odin, R.; Drescher, D. Generation of myostatin gene-edited channel catfish (Ictalurus punctatus) via zygote injection of CRISPR/Cas9 system. Sci. Rep. 2017, 7, 7301. [Google Scholar] [CrossRef]
- Kishimoto, K.; Washio, Y.; Yoshiura, Y.; Toyoda, A.; Ueno, T.; Fukuyama, H.; Kato, K.; Kinoshita, M. Production of a breed of red sea bream Pagrus major with an increase of skeletal muscle mass and reduced body length by genome editing with CRISPR/Cas9. Aquaculture 2018, 495, 415–427. [Google Scholar] [CrossRef]
- Zhong, Z.; Niu, P.; Wang, M.; Huang, G.; Xu, S.; Sun, Y.; Xu, X.; Hou, Y.; Sun, X.; Yan, Y. Targeted disruption of sp7 and myostatin with CRISPR-Cas9 results in severe bone defects and more muscular cells in common carp. Sci. Rep. 2016, 6, 22953. [Google Scholar] [CrossRef] [PubMed]
- Simora, R.M.C.; Xing, D.; Bangs, M.R.; Wang, W.; Ma, X.; Su, B.; Khan, M.G.; Qin, Z.; Lu, C.; Alston, V. CRISPR/Cas9-mediated knock-in of alligator cathelicidin gene in a non-coding region of channel catfish genome. Sci. Rep. 2020, 10, 22271. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yuan, Y.; Zhu, F.; Hong, Y.; Ge, R. Efficient genome editing using CRISPR/Cas9 ribonucleoprotein approach in cultured Medaka fish cells. Biol. Open 2018, 7, bio035170. [Google Scholar] [CrossRef]
- Pan, Q.; Luo, J.; Jiang, Y.; Wang, Z.; Lu, K.; Chen, T. Efficient gene editing in a medaka (Oryzias latipes) cell line and embryos by SpCas9/tRNA-gRNA. J. Zhejiang Univ.-Sci. B 2022, 23, 74–83. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Liu, T.; Zhang, T.; Sun, Z.; Xu, H.; Zhang, T.; Yin, Y.; Li, N.; Yan, T.; Kuang, Y. Establishment and Characteristics of the Spermatogonial Stem Cell Line from the Yellow River Carp (Cyprinus carpio haematopterus). Biology 2025, 14, 536. https://doi.org/10.3390/biology14050536
Zhou H, Liu T, Zhang T, Sun Z, Xu H, Zhang T, Yin Y, Li N, Yan T, Kuang Y. Establishment and Characteristics of the Spermatogonial Stem Cell Line from the Yellow River Carp (Cyprinus carpio haematopterus). Biology. 2025; 14(5):536. https://doi.org/10.3390/biology14050536
Chicago/Turabian StyleZhou, Huijie, Tianqi Liu, Tan Zhang, Zhipeng Sun, Huan Xu, Tingting Zhang, Yashan Yin, Na Li, Ting Yan, and Youyi Kuang. 2025. "Establishment and Characteristics of the Spermatogonial Stem Cell Line from the Yellow River Carp (Cyprinus carpio haematopterus)" Biology 14, no. 5: 536. https://doi.org/10.3390/biology14050536
APA StyleZhou, H., Liu, T., Zhang, T., Sun, Z., Xu, H., Zhang, T., Yin, Y., Li, N., Yan, T., & Kuang, Y. (2025). Establishment and Characteristics of the Spermatogonial Stem Cell Line from the Yellow River Carp (Cyprinus carpio haematopterus). Biology, 14(5), 536. https://doi.org/10.3390/biology14050536





