Seminal F2-IsoP and RvD1 Levels in Idiopathic Infertile Men
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Semen Analysis
2.3. F2-Isoprostanes (F2-IsoPs) Determination
2.4. Resolvin D1 (RvD1) Determination
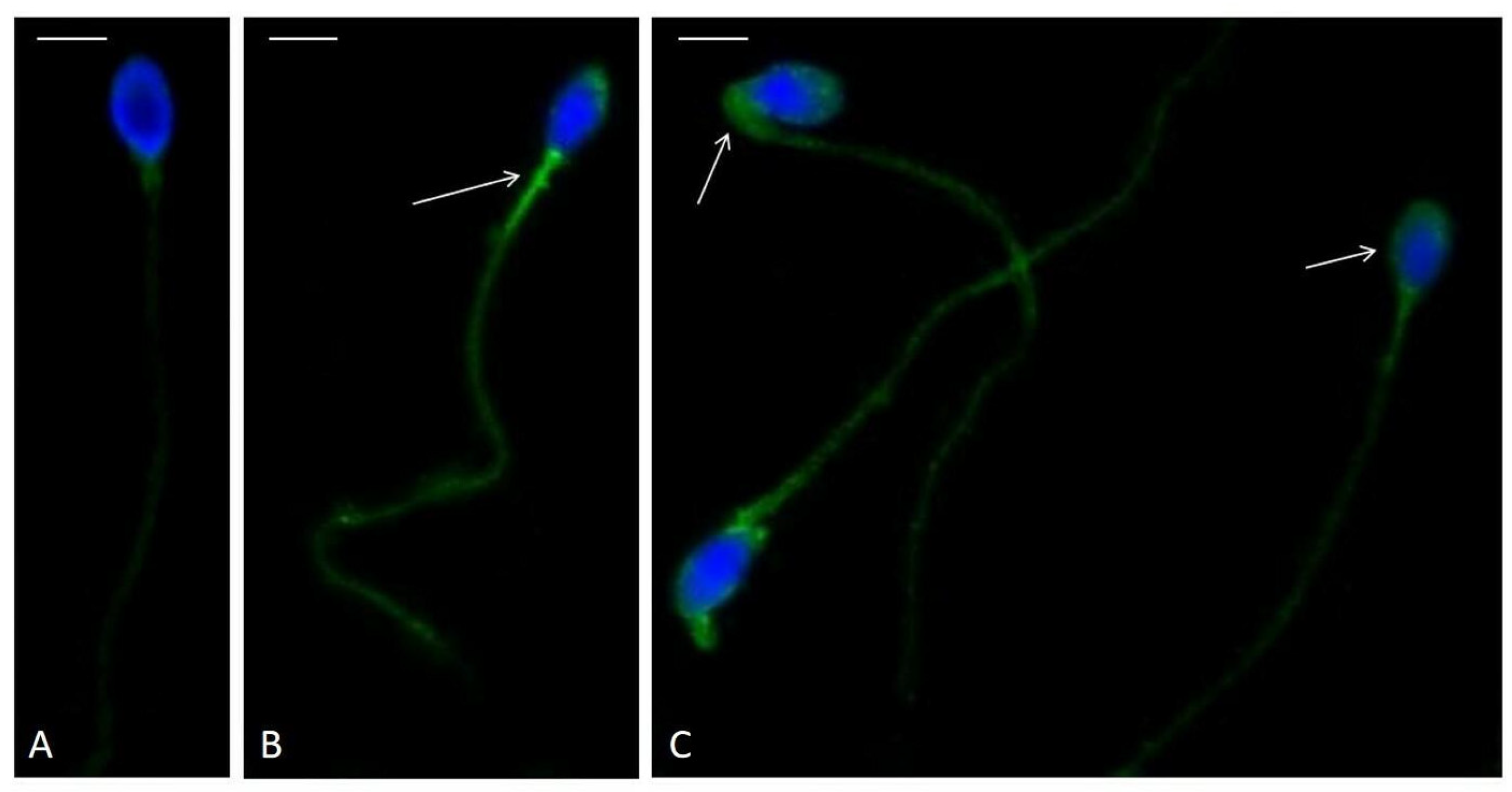
2.5. Immunolocalization of F2-IsoPs
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OS | Oxidative stress |
| F2-IsoP | F2-Isoprostane |
| RvD1 | Resolvin D1 |
| WHO | World Health Organization |
| MOSI | Male oxidative stress infertility |
| ROS | Reactive oxygen species |
| PGF2α | Prostaglandin F2α |
| PUFAs | Polyunsaturated fatty acids |
| LPO | Lipid peroxidation |
| SPMs | Specialized pro-resolvin mediators |
| PBS | Phosphate-buffered saline |
| GC/NICI-MS/MS | Gas chromatography/negative ion chemical ionization tandem mass spectrometry |
| BSA | Bovine serum albumin |
| NGS | Normal goat serum |
| DAPI | 4,6-diamidino-2-phenylindole |
References
- Moretti, E.; Signorini, C.; Ferretti, F.; Noto, D.; Collodel, G. A Study to Validate the Relevance of Semen F2-Isoprostanes on Human Male Infertility. Int. J. Environ. Res. Public Health 2022, 19, 1642. [Google Scholar] [CrossRef] [PubMed]
- Fainberg, J.; Kashanian, J.A. Recent advances in understanding and managing male infertility. F1000Research 2019, 8, 670. [Google Scholar] [CrossRef] [PubMed]
- Corsini, C.; Boeri, L.; Candela, L.; Pozzi, E.; Belladelli, F.; Capogrosso, P.; Fallara, G.; Schifano, N.; Cignoli, D.; Ventimiglia, E.; et al. Is There a Relevant Clinical Impact in Differentiating Idiopathic versus Unexplained Male Infertility? World J. Mens Health 2023, 41, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Roychoudhury, S.; Nath, M.; Dutta, S. Oxidative Stress and Idiopathic Male Infertility. Adv. Exp. Med. Biol. 2022, 1358, 181–204. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO Press: Geneva, Switzerland, 2021. [Google Scholar]
- Tüttelmann, F.; Ruckert, C.; Röpke, A. Disorders of spermatogenesis: Perspectives for novel genetic diagnostics after 20 years of unchanged routine. Med. Genet. 2018, 30, 12–20. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; Duca, Y.; La Vignera, S.; Calogero, A.E. New insights into the genetics of spermatogenic failure: A review of the literature. Hum. Genet. 2019, 138, 125–140. [Google Scholar] [CrossRef]
- Cassidy, F.C.; Charalambous, M. Genomic imprinting, growth and maternal-fetal interactions. J. Exp. Biol. 2018, 221 (Suppl. S1), jeb164517. [Google Scholar] [CrossRef]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Mens Health 2019, 37, 296–312. [Google Scholar] [CrossRef]
- Calogero, A.E.; Cannarella, R.; Agarwal, A.; Hamoda, T.A.A.; Rambhatla, A.; Saleh, R.; Boitrelle, F.; Ziouziou, I.; Toprak, T.; Gul, M.; et al. The Renaissance of Male Infertility Management in the Golden Age of Andrology. World J. Mens Health 2023, 41, 237–254. [Google Scholar] [CrossRef]
- Atig, F.; Raffa, M.; Habib, B.A.; Kerkeni, A.; Saad, A.; Ajina, M. Impact of seminal trace element and glutathione levels on semen quality of Tunisian infertile men. BMC Urol. 2012, 12, 6. [Google Scholar] [CrossRef]
- Benedetti, S.; Tagliamonte, M.C.; Catalani, S.; Primiterra, M.; Canestrari, F.; De Stefani, S.; Palini, S.; Bulletti, C. Differences in blood and semen oxidative status in fertile and infertile men, and their relationship with sperm quality. Reprod. Biomed. Online 2012, 25, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Aktan, G.; Doğru-Abbasoğlu, S.; Küçükgergin, C.; Kadıoğlu, A.; Ozdemirler-Erata, G.; Koçak-Toker, N. Mystery of idiopathic male infertility: Is oxidative stress an actual risk? Fertil. Steril. 2013, 99, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Collodel, G.; Castellini, C.; Iacoponi, F.; Noto, D.; Signorini, C. Cytosolic phospholipase A2 and F2 isoprostanes are involved in semen quality and human infertility-A study on leucocytospermia, varicocele and idiopathic infertility. Andrologia 2020, 52, e13465. [Google Scholar] [CrossRef] [PubMed]
- Bracke, A.; Peeters, K.; Punjabi, U.; Hoogewijs, D.; Dewilde, S. A search for molecular mechanisms underlying male idiopathic infertility. Reprod. Biomed. Online 2018, 36, 327–339. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Roychoudhury, S.; Chakravarthi, S.; Wang, C.W.; Slama, P. Antioxidant Paradox in Male Infertility: ‘A Blind Eye’ on Inflammation. Antioxidants 2022, 11, 167. [Google Scholar] [CrossRef]
- Milne, G.L.; Dai, Q.; Roberts, L.J., 2nd. The isoprostanes—25 years later. Biochim. Biophys. Acta 2015, 1851, 433–445. [Google Scholar] [CrossRef]
- Signorini, C.; Moretti, E.; Collodel, G. Role of isoprostanes in human male infertility. Syst. Biol. Reprod. Med. 2020, 66, 291–299. [Google Scholar] [CrossRef]
- Signorini, C.; Moretti, E.; Noto, D.; Micheli, L.; Ponchia, R.; Collodel, G. Fatty Acid Oxidation and Pro-Resolving Lipid Mediators Are Related to Male Infertility. Antioxidants 2022, 11, 107. [Google Scholar] [CrossRef]
- Collodel, G.; Moretti, E.; Longini, M.; Pascarelli, N.A.; Signorini, C. Increased F2-isoprostane levels in semen and immunolocalization of the 8-iso prostaglandin F2α in spermatozoa from infertile patients with varicocele. Oxidative Med. Cell. Longev. 2018, 2018, 7508014. [Google Scholar] [CrossRef]
- Agarwal, A.; Rana, M.; Qiu, E.; AlBunni, H.; Bui, A.D.; Henkel, R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia 2018, 50, e13126. [Google Scholar] [CrossRef]
- Farsimadan, M.; Motamedifar, M. Bacterial infection of the male reproductive system causing infertility. J. Reprod. Immunol. 2020, 142, 103183. [Google Scholar] [CrossRef] [PubMed]
- Minas, A.; Costa, L.V.S.; Miyazaki, M.A.; Antoniassi, M.P. Insight toward inflammasome complex contribution to male infertility. Am. J. Reprod. Immunol. 2023, 90, e13734. [Google Scholar] [CrossRef] [PubMed]
- Alfaro Gómez, M.; Fernández-Santos, M.D.R.; Jurado-Campos, A.; Soria-Meneses, P.J.; Montoro Angulo, V.; Soler, A.J.; Garde, J.J.; Rodríguez-Robledo, V. On Males, Antioxidants and Infertility (MOXI): Certitudes, Uncertainties and Trends. Antioxidants 2023, 12, 1626. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, T.; Usui, K.; Mori, K.; Asai, T.; Yasuda, K.; Kuroda, S.; Yumura, Y. Oxidative stress and male infertility. RBM 2021, 20, 41–52. [Google Scholar] [CrossRef]
- Agarwal, A.; Majzoub, A. Laboratory tests for oxidative stress. Indian J. Urol. 2017, 33, 199–206. [Google Scholar] [CrossRef]
- Aitken, R.J. Antioxidant trials-the need to test for stress. Hum. Reprod. Open 2021, 2021, hoab007. [Google Scholar] [CrossRef]
- Milne, G.L. Classifying oxidative stress by F2-Isoprostane levels in human disease: The re-imagining of a biomarker. Redox Biol. 2017, 12, 897–898. [Google Scholar] [CrossRef]
- Khosrowbeygi, A.; Zarghami, N. Levels of oxidative stress biomarkers in seminal plasma and their relationship with seminal parameters. BMC Clin. Pathol. 2007, 7, 6. [Google Scholar] [CrossRef]
- Collodel, G.; Moretti, E.; Noto, D.; Corsaro, R.; Signorini, C. Oxidation of Polyunsaturated Fatty Acids as a Promising Area of Research in Infertility. Antioxidants 2022, 11, 1002. [Google Scholar] [CrossRef]
- Wathes, D.C.; Abayasekara, D.R.; Aitken, R.J. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 2007, 77, 190–201. [Google Scholar] [CrossRef]
- Stafforini, D.M.; Sheller, J.R.; Blackwell, T.S.; Sapirstein, A.; Yull, F.E.; McIntyre, T.M.; Bonventre, J.V.; Prescott, S.M.; Roberts, L.J., 2nd. Release of free F2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. J. Biol. Chem. 2006, 281, 4616–4623. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Signorini, C.; Menchiari, S.; Liguori, L.; Corsaro, R.; Gambera, L.; Collodel, G. Are F2-isoprostanes a better marker of semen lipid peroxidation than MDA in reproductive pathologies with inflammatory basis? Cytokine 2025, 188, 156889. [Google Scholar] [CrossRef] [PubMed]
- Sanocka, D.; Jędrzejczak, P.; Szumała-Kaękol, A.; Frączek, M.; Kurpisz, M. Male genital tract inflammation: The role of selected interleukins in regulation of pro-oxidant and antioxidant enzymatic substances in seminal plasma. J. Androl. 2003, 24, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Zhou, D.; Deng, R. Serum resolvin D1 potentially predicts neurofunctional recovery, the risk of recurrence and death in patients with acute ischemic stroke. Biomed. Rep. 2023, 20, 10. [Google Scholar] [CrossRef]
- Aitken, R.J.; Wilkins, A.; Harrison, N.; Bahrami, M.; Gibb, Z.; McIntosh, K.; Vuong, Q.; Lambourne, S. A Comparative Analysis of the Antioxidant Profiles Generated by the RoXstaTM System for Diverse Biological Fluids Highlights the Powerful Protective Role of Human Seminal Plasma. Antioxidants 2025, 14, 90. [Google Scholar] [CrossRef]
- Oehninger, S.; Franken, D.R.; Ombelet, W. Sperm functional tests. Fertil. Steril. 2014, 102, 1528–1533. [Google Scholar] [CrossRef]
- Barbăroșie, C.; Agarwal, A.; Henkel, R. Diagnostic value of advanced semen analysis in evaluation of male infertility. Andrologia 2021, 53, e13625. [Google Scholar] [CrossRef]
| Semen Parameters | Median (25th–75th Percentile) | 5th Percentile Values [5] |
|---|---|---|
| Volume (mL) | 3.60 (3.00–4.35) | 1.40 |
| Sperm concentration (106× mL) | 16.50 (2.03–36.25) | 16.00 |
| Sperm progressive motility (%) | 28.00 (22.00–35.00) | 30.00 |
| Sperm normal morphology (%) | 6.00 (4.00–9.00) | 4.00 |
| Sperm vitality (%) | 70.00 (64.00–75.00) | 54.00 |
| Seminal levels | Median (25th–75th percentile) | Control values |
| F2-IsoPs (ng/mL) | 29.80 (13.33–44.70) | 29.96 [20] |
| RvD1 (pg/mL) | 42.51 (31.88–54.65) | 31.20 [18] |
| Sperm Concentration (106× mL) | Sperm Progressive Motility (%) | Sperm Normal Morphology (%) | Sperm Vitality (%) | F2-IsoPs (ng/mL) | RvD1 (pg/mL) | |
|---|---|---|---|---|---|---|
| Sperm concentration (106× mL) | 1 | |||||
| Sperm progressive motility (%) | r = 0.34 * | 1 | ||||
| Sperm normal morphology (%) | r = 0.38 * | r = 0.30 * | 1 | |||
| Sperm vitality (%) | r = 0.35 * | r = 0.43 * | r = 0.49 * | 1 | ||
| F2-IsoPs (ng/mL) | r = 0.14 | r = −0.18 | r = −0.06 | r = −0.11 | 1 | |
| RvD1 (pg/mL) | r = −0.01 | r = −0.21 | r = 0.25 | r = −0.05 | r = 0.13 | 1 |
| Semen Parameters | Median (25th–75th Percentile) | Statistics | |
|---|---|---|---|
| Group 1 | Group 2 | ||
| Volume (mL) | 3.50 (3.00–4.50) | 3.80 (3.00–4.00) | ns |
| Sperm concentration (106× mL) | 9.55 (1.55–29.50) | 18.00 (7.25–47.50) | ns |
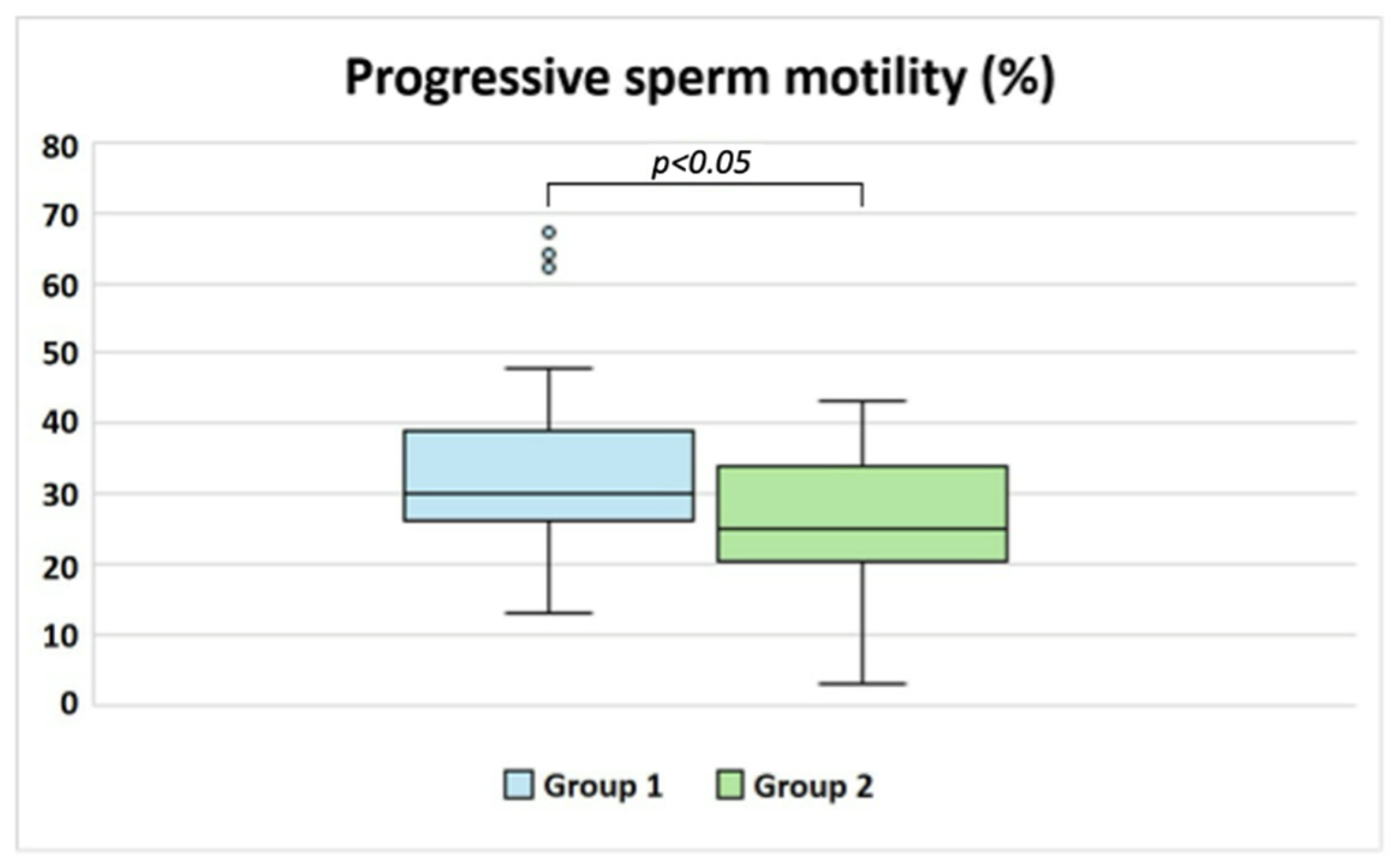
| Sperm progressive motility (%) | 30.00 (26.50–38.50) | 25.00 (21.00–33.75) | p < 0.05 |
| Sperm normal morphology (%) | 6.00 (4.00–9.00) | 5.00 (4.25–8.00) | ns |
| Sperm vitality (%) | 72.00 (65.00–78.00) | 69.50 (59.25–75.00) | ns |
| Seminal levels | |||
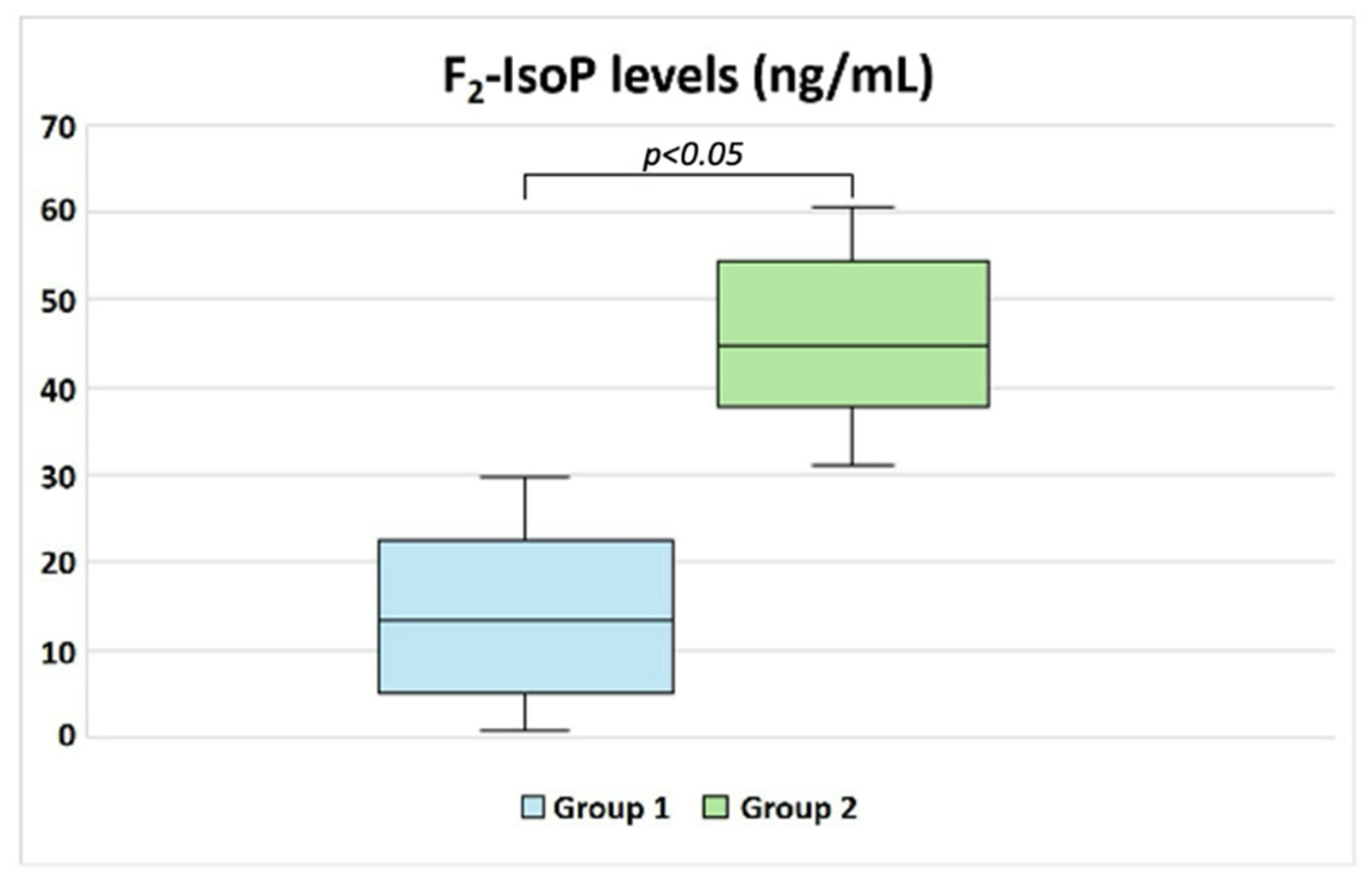
| F2-IsoPs (ng/mL) | 13.33 (5.30–22.11) | 44.80 (37.87–53.94) | p < 0.05 |
| RvD1 (pg/mL) | 36.09 (31.85–46.47) | 44.94 (34.00–56.48) | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretti, E.; Collodel, G.; Marcucci, C.; Liguori, L.; Gambera, L.; Signorini, C. Seminal F2-IsoP and RvD1 Levels in Idiopathic Infertile Men. Biology 2025, 14, 450. https://doi.org/10.3390/biology14040450
Moretti E, Collodel G, Marcucci C, Liguori L, Gambera L, Signorini C. Seminal F2-IsoP and RvD1 Levels in Idiopathic Infertile Men. Biology. 2025; 14(4):450. https://doi.org/10.3390/biology14040450
Chicago/Turabian StyleMoretti, Elena, Giulia Collodel, Caterina Marcucci, Laura Liguori, Laura Gambera, and Cinzia Signorini. 2025. "Seminal F2-IsoP and RvD1 Levels in Idiopathic Infertile Men" Biology 14, no. 4: 450. https://doi.org/10.3390/biology14040450
APA StyleMoretti, E., Collodel, G., Marcucci, C., Liguori, L., Gambera, L., & Signorini, C. (2025). Seminal F2-IsoP and RvD1 Levels in Idiopathic Infertile Men. Biology, 14(4), 450. https://doi.org/10.3390/biology14040450









