Advances in Plant-Derived Extracellular Vesicle Extraction Methods and Pharmacological Effects
Simple Summary
Abstract
1. Introduction
2. Characterization, Identification, and Isolation Techniques for Plant-Derived Extracellular Vesicles (PDEVs)
2.1. Characterization and Identification of PDEVs
2.2. Separation Techniques for PDEVs
2.2.1. Ultracentrifugation (UC)
2.2.2. Density Gradient Ultracentrifugation (DGU)
2.2.3. Ultrafiltration (UF)
2.2.4. Size-Exclusion Chromatography (SEC)
| Extraction Method | Extraction Principle | Merit | Disadvantage | Reference |
|---|---|---|---|---|
| Ultracentrifugation (UC) | Separates vesicles by gradient centrifugation (such as differential centrifugation) combined with ultra-high speed centrifugation (>100,000× g) | Has a relatively high purity and can be used with multiple sample types. | Time-consuming, the equipment is expensive, the yield is low, and it may damage the vesicle structure. | [56] |
| Density gradient ultracentrifugation (DGU) | Separates vesicle and non-vesicle components using different density gradients. | Has higher purity and can distinguish vesicles of different densities | The operation is complex, the yield is low (with some vesicles being lost), and ultracentrifugation equipment is required. | [65] |
| Ultrafiltration (UF) | Utilizes filter membranes with different pore sizes to retain vesicles of specific sizes. | The operation is simple and rapid, and no expensive equipment is required. | Loss may occur due to the adsorption of the filter membrane, and the shear force may damage the vesicle structure. | [79] |
| Size-exclusion chromatography (SEC) | Separation according to the size of vesicles through porous media. | Retains the integrity of the vesicles and there is no damage caused by shear force. | Time-consuming, has a small processing capacity, and the equipment cost is high. | [80] |
2.2.5. The Stability of PDEVs
3. Biological Activities
3.1. Anti-Inflammatory Activity
3.2. Antitumor Activity
3.3. Antioxidant Activity
3.4. Anti-Infectious Activity
| Plants | Research Object | Disease Model | Route of Administration | Results | Potential Mechanism | Reference |
|---|---|---|---|---|---|---|
| Ginger | C57BL/6j mice | Alcohol-induced liver damage | Oral administration | Reduced the levels of ALT, AST, and triglycerides | Inhibit the generation of ROS, activate theTLR4/TRIF pathway, and regulate the activity of Nrf2. | [127] |
| Lemon | BALB/c nude mice | Gastric cancer | Administration by injection | Reduced the tumor weight and inhibits the generation of ROS | Inhibit the generation of ROS and induce apoptosis of cancer cells | [46] |
| Ginseng | Balb/C mice and Wistar rats | Glioma | Wistar rats: IV Balb/Cmice: IC | Reduced the size of the tumor and decreased the luminescence intensity of C6 glioma | Reduce the expression of miRNA and chemokine genes related to cancer-associated fibroblasts (CAFs) | [128] |
| Tea | SD rats | IBS (irritable bowel syndrome) | Oral administration | Increased body weight, relieved defecation, and reduced hypersensitivity reaction | Regulate the CHR pathway to improve irritable bowel syndrome (IBS) | [129] |
| Solanum lycopersicum | C57BL/6J mice | Carotid artery restenosis injury | Oral administration | Reduced the neointimal area and the ratio of neointimal area to medial area, and attenuated the phenotypic transformation | miRNA164a/b-5p weakens phenotypic conversion and improves restenosis injury by activating the Keap1/Nrf2 pathway | [130] |
| Mulberry bark | C57BL/6J mice | Colitis | Oral administration | Increased body weight, inhibited colon shortening, and suppressed the release of inflammatory factors | Regulate the intestinal microbiota and activate the AhR—COPS8 pathway to improve colitis. | [39] |
| Goji | C57BL/6J mice | Muscle atrophy | Inject into the quadriceps femoris muscle | Increased grip strength, the cross-sectional area of the quadriceps femoris muscle, and the expression of myogenic regulatory factors | Improve muscle function by activating the AMPK/SIRT1/PGC1α pathway. | [131] |
| Orange | C57BL/6J mice | Obese (diet-induced) | Gavage | Restored intestinal function, reduced TG content, and regulate the immune response | [132] | |
| Blueberry | C57BL/6J mice | Nonalcoholic fatty liver disease | Gavage | Reduced the mRNA levels of FAS and ACC1, as well as the contents of TC, TG, ALT, AST, and LDL-C, and increased the content of HDL-C | Antioxidative stress and inhibition of cell apoptosis. | [53] |
| Momordica charantia | C57BL/6J mice and Sprague Dawley rats | DOX cardiotoxicity | IV | Reduced cTnT and CK-MB and alleviated myocardial atrophy. Improved the new function indexes, such as EF, FS and HR | Activate the p62/Keap1/Nrf2 pathway to inhibit cell apoptosis. | [133] |
| Panax notoginseng | Sprague–Dawley rats | CI/R (cerebral ischemia–reperfusion injury) | IV | Reduced the area of cerebral infarction and inhibited the apoptosis of brain cells | Activate the PI3K/AKT signaling pathway to reduce the infarct area and improve cerebral ischemia–reperfusion (CI/R). | [37] |
4. The Role of PDEVs as Drug Delivery Vehicles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, K.; Zeng, C. Extracellular Vesicles and Obesity. Adv. Exp. Med. Biol. 2023, 1418, 143–153. [Google Scholar] [CrossRef]
- Krylova, S.V.; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef]
- Laberge, A.; Arif, S.; Moulin, V.J. Microvesicles: Intercellular messengers in cutaneous wound healing. J. Cell Physiol. 2018, 233, 5550–5563. [Google Scholar] [CrossRef]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Santavanond, J.P.; Rutter, S.F.; Atkin-Smith, G.K.; Poon, I.K.H. Apoptotic Bodies: Mechanism of Formation, Isolation and Functional Relevance. Subcell. Biochem. 2021, 97, 61–88. [Google Scholar] [CrossRef]
- de Boer, C.; Davies, N.H. Blood derived extracellular vesicles as regenerative medicine therapeutics. Biochimie 2022, 196, 203–215. [Google Scholar] [CrossRef]
- Liu, Z.; Cauvi, D.M.; Bernardino, E.M.A.; Lara, B.; Lizardo, R.E.; Hawisher, D.; Bickler, S.; De Maio, A. Isolation and characterization of human urine extracellular vesicles. Cell Stress. Chaperones 2018, 23, 943–953. [Google Scholar] [CrossRef]
- Jiang, T.Y.; Shi, Y.Y.; Cui, X.W.; Pan, Y.F.; Lin, Y.K.; Feng, X.F.; Ding, Z.W.; Yang, C.; Tan, Y.X.; Dong, L.W.; et al. PTEN Deficiency Facilitates Exosome Secretion and Metastasis in Cholangiocarcinoma by Impairing TFEB-mediated Lysosome Biogenesis. Gastroenterology 2023, 164, 424–438. [Google Scholar] [CrossRef]
- Yang, L.; Peng, X.; Li, Y.; Zhang, X.; Ma, Y.; Wu, C.; Fan, Q.; Wei, S.; Li, H.; Liu, J. Long non-coding RNA HOTAIR promotes exosome secretion by regulating RAB35 and SNAP23 in hepatocellular carcinoma. Mol. Cancer 2019, 18, 78. [Google Scholar] [CrossRef]
- Valcz, G.; Buzás, E.I.; Kittel, Á.; Krenács, T.; Visnovitz, T.; Spisák, S.; Török, G.; Homolya, L.; Zsigrai, S.; Kiszler, G.; et al. En bloc release of MVB-like small extracellular vesicle clusters by colorectal carcinoma cells. J. Extracell. Vesicles 2019, 8, 1596668. [Google Scholar] [CrossRef]
- Ortiz, A. Extracellular vesicles in cancer progression. Semin. Cancer Biol. 2021, 76, 139–142. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef]
- Huang, C.; Neupane, Y.R.; Lim, X.C.; Shekhani, R.; Czarny, B.; Wacker, M.G.; Pastorin, G.; Wang, J.W. Extracellular vesicles in cardiovascular disease. Adv. Clin. Chem. 2021, 103, 47–95. [Google Scholar] [CrossRef]
- Kubo, H. Extracellular Vesicles in Lung Disease. Chest 2018, 153, 210–216. [Google Scholar] [CrossRef]
- Hill, A.F. Extracellular Vesicles and Neurodegenerative Diseases. J. Neurosci. 2019, 39, 9269–9273. [Google Scholar] [CrossRef]
- Chen, A.; He, B.; Jin, H. Isolation of Extracellular Vesicles from Arabidopsis. Curr. Protoc. 2022, 2, e352. [Google Scholar] [CrossRef]
- He, J.Y.; Wang, F.B.; Hou, J.Q. Research Progress on the Role of Extracellular Vesicles in Tumor Recurrence and Metastasis. Shanghai Med. Pharm. J. 2021, 42, 62–66. [Google Scholar]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-derived extracellular vesicles: A novel nanomedicine approach with advantages and challenges. Cell Commun. Signal 2022, 20, 69. [Google Scholar] [CrossRef]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; Del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J. Plant Physiol. 2018, 229, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Xu, F.; Zhang, X.; Mu, J.; Sayed, M.; Hu, X.; Lei, C.; Sriwastva, M.; Kumar, A.; Sundaram, K.; et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol. Ther. 2021, 29, 2424–2440. [Google Scholar] [CrossRef] [PubMed]
- Urzì, O.; Raimondo, S.; Alessandro, R. Extracellular Vesicles from Plants: Current Knowledge and Open Questions. Int. J. Mol. Sci. 2021, 22, 5366. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zu, M.; Gong, H.; Ma, Y.; Sun, J.; Ran, S.; Shi, X.; Zhang, J.; Xiao, B. Tea leaf-derived exosome-like nanotherapeutics retard breast tumor growth by pro-apoptosis and microbiota modulation. J. Nanobiotechnol. 2023, 21, 6. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Y.; Zhu, G.; Zeng, L.; Xu, S.; Cheng, H.; Ouyang, Z.; Chen, J.; Pathak, J.L.; Wu, L.; et al. The Emerging Role of Plant-Derived Exosomes-Like Nanoparticles in Immune Regulation and Periodontitis Treatment. Front. Immunol. 2022, 13, 896745. [Google Scholar] [CrossRef] [PubMed]
- Tajik, T.; Baghaei, K.; Moghadam, V.E.; Farrokhi, N.; Salami, S.A. Extracellular vesicles of cannabis with high CBD content induce anticancer signaling in human hepatocellular carcinoma. Biomed. Pharmacother. 2022, 152, 113209. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Yin, H.; Bennett, C.; Zhang, H.G.; Guo, P. Arrowtail RNA for Ligand Display on Ginger Exosome-like Nanovesicles to Systemic Deliver siRNA for Cancer Suppression. Sci. Rep. 2018, 8, 14644. [Google Scholar] [CrossRef]
- Qiao, Z.; Zhang, K.; Liu, J.; Cheng, D.; Yu, B.; Zhao, N.; Xu, F.J. Biomimetic electrodynamic nanoparticles comprising ginger-derived extracellular vesicles for synergistic anti-infective therapy. Nat. Commun. 2022, 13, 7164. [Google Scholar] [CrossRef]
- Man, F.; Meng, C.; Liu, Y.; Wang, Y.; Zhou, Y.; Ma, J.; Lu, R. Correction to: The Study of Ginger-Derived Extracellular Vesicles as a Natural Nanoscale Drug Carrier and Their Intestinal Absorption in Rats. AAPS PharmSciTech 2022, 23, 225. [Google Scholar] [CrossRef]
- Bokka, R.; Ramos, A.P.; Fiume, I.; Manno, M.; Raccosta, S.; Turiák, L.; Sugár, S.; Adamo, G.; Csizmadia, T.; Pocsfalvi, G. Biomanufacturing of Tomato-Derived Nanovesicles. Foods 2020, 9, 1852. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Wu, S.C.; Chien, H.Y.; Shen, T.L.; Hsu, W.H. Tomato-fruit-derived extracellular vesicles inhibit Fusobacterium nucleatum via lipid-mediated mechanism. Food Funct. 2023, 14, 8942–8950. [Google Scholar] [CrossRef]
- Mammadova, R.; Maggio, S.; Fiume, I.; Bokka, R.; Moubarak, M.; Gellén, G.; Schlosser, G.; Adamo, G.; Bongiovanni, A.; Trepiccione, F.; et al. Protein Biocargo and Anti-Inflammatory Effect of Tomato Fruit-Derived Nanovesicles Separated by Density Gradient Ultracentrifugation and Loaded with Curcumin. Pharmaceutics 2023, 15, 333. [Google Scholar] [CrossRef]
- You, J.Y.; Kang, S.J.; Rhee, W.J. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact. Mater. 2021, 6, 4321–4332. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yuan, M.; Shao, C.; Ji, N.; Zhang, H.; Li, C. Momordica charantia-Derived Extracellular Vesicles Provide Antioxidant Protection in Ulcerative Colitis. Molecules 2023, 28, 6182. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liao, L.; Gao, M.; Liu, Q. Garlic-derived exosome-like nanovesicles alleviate dextran sulphate sodium-induced mouse colitis via the TLR4/MyD88/NF-κB pathway and gut microbiota modulation. Food Funct. 2023, 14, 7520–7534. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, F.; Fu, L.; Ma, Y.; Ye, L.; Huang, Y.; Fan, W.; Gao, W.; Cai, Y.; Mou, X. Garlic-derived exosome-like nanovesicles as a hepatoprotective agent alleviating acute liver failure by inhibiting CCR2/CCR5 signaling and inflammation. Biomater. Adv. 2023, 154, 213592. [Google Scholar] [CrossRef]
- Kim, D.K.; Rhee, W.J. Antioxidative Effects of Carrot-Derived Nanovesicles in Cardiomyoblast and Neuroblastoma Cells. Pharmaceutics 2021, 13, 1203. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef]
- Li, S.; Zhang, R.; Wang, A.; Li, Y.; Zhang, M.; Kim, J.; Zhu, Y.; Wang, Q.; Zhang, Y.; Wei, Y.; et al. Panax notoginseng: Derived exosome-like nanoparticles attenuate ischemia reperfusion injury via altering microglia polarization. J. Nanobiotechnol. 2023, 21, 416. [Google Scholar] [CrossRef]
- Emmanuela, N.; Muhammad, D.R.; Iriawati; Wijaya, C.H.; Ratnadewi, Y.M.D.; Takemori, H.; Ana, I.D.; Yuniati, R.; Handayani, W.; Wungu, T.D.K.; et al. Isolation of plant-derived exosome-like nanoparticles (PDENs) from Solanum nigrum L. berries and Their Effect on interleukin-6 expression as a potential anti-inflammatory agent. PLoS ONE 2024, 19, e0296259. [Google Scholar] [CrossRef]
- Sriwastva, M.K.; Deng, Z.B.; Wang, B.; Teng, Y.; Kumar, A.; Sundaram, K.; Mu, J.; Lei, C.; Dryden, G.W.; Xu, F.; et al. Exosome-like nanoparticles from Mulberry bark prevent DSS-induced colitis via the AhR/COPS8 pathway. EMBO Rep. 2022, 23, e53365. [Google Scholar] [CrossRef]
- Itakura, S.; Shohji, A.; Amagai, S.; Kitamura, M.; Takayama, K.; Sugibayashi, K.; Todo, H. Gene knockdown in HaCaT cells by small interfering RNAs entrapped in grapefruit-derived extracellular vesicles using a microfluidic device. Sci. Rep. 2023, 13, 3102. [Google Scholar] [CrossRef]
- Stanly, C.; Alfieri, M.; Ambrosone, A.; Leone, A.; Fiume, I.; Pocsfalvi, G. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells 2020, 9, 2722. [Google Scholar] [CrossRef]
- Cao, M.; Yan, H.; Han, X.; Weng, L.; Wei, Q.; Sun, X.; Lu, W.; Wei, Q.; Ye, J.; Cai, X.; et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J. Immunother. Cancer 2019, 7, 326. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.G.; Choi, S.Y.; Kim, H.; Choi, E.J.; Lee, E.J.; Park, P.J.; Ko, J.; Kim, K.P.; Baek, H.S. Panax ginseng-Derived Extracellular Vesicles Facilitate Anti-Senescence Effects in Human Skin Cells: An Eco-Friendly and Sustainable Way to Use Ginseng Substances. Cells 2021, 10, 486. [Google Scholar] [CrossRef]
- Seo, K.; Yoo, J.H.; Kim, J.; Min, S.J.; Heo, D.N.; Kwon, I.K.; Moon, H.J. Ginseng-derived exosome-like nanovesicles extracted by sucrose gradient ultracentrifugation to inhibit osteoclast differentiation. Nanoscale 2023, 15, 5798–5808. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Urzì, O.; Meraviglia, S.; Di Simone, M.; Corsale, A.M.; Rabienezhad Ganji, N.; Palumbo Piccionello, A.; Polito, G.; Lo Presti, E.; Dieli, F.; et al. Anti-inflammatory properties of lemon-derived extracellular vesicles are achieved through the inhibition of ERK/NF-κB signalling pathways. J. Cell Mol. Med. 2022, 26, 4195–4209. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, X.; Luo, Q.; Xu, L.; Chen, F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J. Nanobiotechnol. 2020, 18, 100. [Google Scholar] [CrossRef]
- Urzì, O.; Cafora, M.; Ganji, N.R.; Tinnirello, V.; Gasparro, R.; Raccosta, S.; Manno, M.; Corsale, A.M.; Conigliaro, A.; Pistocchi, A.; et al. Lemon-derived nanovesicles achieve antioxidant and anti-inflammatory effects activating the AhR/Nrf2 signaling pathway. iScience 2023, 26, 107041. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, Y.; Li, X.; Luo, J.; Zhou, Z.; Yu, L.; Wang, G. Orange-derived and dexamethasone-encapsulated extracellular vesicles reduced proteinuria and alleviated pathological lesions in IgA nephropathy by targeting intestinal lymphocytes. Front. Immunol. 2022, 13, 900963. [Google Scholar] [CrossRef]
- Long, F.; Pan, Y.; Li, J.; Sha, S.; Shi, X.; Guo, H.; Huang, C.; Xiao, Q.; Fan, C.; Zhang, X.; et al. Orange-derived extracellular vesicles nanodrugs for efficient treatment of ovarian cancer assisted by transcytosis effect. Acta Pharm. Sin. B 2023, 13, 5121–5134. [Google Scholar] [CrossRef]
- Wang, X.; Wu, B.; Sun, G.; He, W.; Gao, J.; Huang, T.; Liu, J.; Zhou, Q.; He, X.; Zhang, S.; et al. Selenium Biofortification Enhanced miR167a Expression in Broccoli Extracellular Vesicles Inducing Apoptosis in Human Pancreatic Cancer Cells by Targeting IRS1. Int. J. Nanomed. 2023, 18, 2431–2446. [Google Scholar] [CrossRef]
- Del Pozo-Acebo, L.; López de Las Hazas, M.C.; Tomé-Carneiro, J.; Del Saz-Lara, A.; Gil-Zamorano, J.; Balaguer, L.; Chapado, L.A.; Busto, R.; Visioli, F.; Dávalos, A. Therapeutic potential of broccoli-derived extracellular vesicles as nanocarriers of exogenous miRNAs. Pharmacol. Res. 2022, 185, 106472. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.J.; Bian, Y.P.; Wang, Q.H.; Yin, F.; Yin, L.; Zhang, Y.L.; Liu, J.H. Blueberry-derived exosomes-like nanoparticles ameliorate nonalcoholic fatty liver disease by attenuating mitochondrial oxidative stress. Acta Pharmacol. Sin. 2022, 43, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, C.M.; Manzaneque-López, M.C.; Pérez-Bermúdez, P.; Soler, C.; Marcilla, A. Characterization and bioactivity of extracellular vesicles isolated from pomegranate. Food Funct. 2022, 13, 12870–12882. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Ma, J.; Zhou, Y.; Lu, R. Focusing on Future Applications and Current Challenges of Plant Derived Extracellular Vesicles. Pharmaceuticals 2022, 15, 708. [Google Scholar] [CrossRef]
- Alzahrani, F.A.; Khan, M.I.; Kameli, N.; Alsahafi, E.; Riza, Y.M. Plant-Derived Extracellular Vesicles and Their Exciting Potential as the Future of Next-Generation Drug Delivery. Biomolecules 2023, 13, 839. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed. Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Vestuto, V.; Conte, M.; Vietri, M.; Mensitieri, F.; Santoro, V.; Di Muro, A.; Alfieri, M.; Moros, M.; Miranda, M.R.; Amante, C.; et al. Multiomic Profiling and Neuroprotective Bioactivity of Salvia Hairy Root-Derived Extracellular Vesicles in a Cellular Model of Parkinson’s Disease. Int. J. Nanomed. 2024, 19, 9373–9393. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Trachtenberg, A.J.; Hochberg, F.H.; Skog, J.; Kuo, W.P. Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front. Physiol. 2012, 3, 162. [Google Scholar] [CrossRef]
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorun, V.M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015, 5, 17319. [Google Scholar] [CrossRef]
- Coughlan, C.; Bruce, K.D.; Burgy, O.; Boyd, T.D.; Michel, C.R.; Garcia-Perez, J.E.; Adame, V.; Anton, P.; Bettcher, B.M.; Chial, H.J.; et al. Exosome Isolation by Ultracentrifugation and Precipitation and Techniques for Downstream Analyses. Curr. Protoc. Cell Biol. 2020, 88, e110. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wong, D.K.; Hong, K.Y.; Raffai, R.L. Cushioned-Density Gradient Ultracentrifugation (C-DGUC): A Refined and High Performance Method for the Isolation, Characterization, and Use of Exosomes. Methods Mol. Biol. 2018, 1740, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Alzhrani, G.N.; Alanazi, S.T.; Alsharif, S.Y.; Albalawi, A.M.; Alsharif, A.A.; Abdel-Maksoud, M.S.; Elsherbiny, N. Exosomes: Isolation, characterization, and biomedical applications. Cell Biol. Int. 2021, 45, 1807–1831. [Google Scholar] [CrossRef]
- Rhim, W.K.; Kim, J.Y.; Lee, S.Y.; Cha, S.G.; Park, J.M.; Park, H.J.; Park, C.G.; Han, D.K. Recent advances in extracellular vesicle engineering and its applications to regenerative medicine. Biomater. Res. 2023, 27, 130. [Google Scholar] [CrossRef]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Kim, K.; Park, J.; Jung, J.H.; Lee, R.; Park, J.H.; Yuk, J.M.; Hwang, H.; Yeon, J.H. Cyclic tangential flow filtration system for isolation of extracellular vesicles. APL Bioeng. 2021, 5, 016103. [Google Scholar] [CrossRef]
- Ko, K.W.; Yoo, Y.I.; Kim, J.Y.; Choi, B.; Park, S.B.; Park, W.; Rhim, W.K.; Han, D.K. Attenuation of Tumor Necrosis Factor-α Induced Inflammation by Umbilical Cord-Mesenchymal Stem Cell Derived Exosome-Mimetic Nanovesicles in Endothelial Cells. Tissue Eng. Regen. Med. 2020, 17, 155–163. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol. Ther. 2018, 26, 2838–2847. [Google Scholar] [CrossRef]
- Sarwareddy, K.K.; Singh, A.D.; Patnam, S.; Sesuraj, B.A.; Ponamgi, S.; Thakur, B.K.; Manda, V.S. Harnessing tomato-derived small extracellular vesicles as drug delivery system for cancer therapy. Future Sci. OA 2025, 11, 2461956. [Google Scholar] [CrossRef]
- Blans, K.; Hansen, M.S.; Sørensen, L.V.; Hvam, M.L.; Howard, K.A.; Möller, A.; Wiking, L.; Larsen, L.B.; Rasmussen, J.T. Pellet-free isolation of human and bovine milk extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2017, 6, 1294340. [Google Scholar] [CrossRef]
- Kreimer, S.; Ivanov, A.R. Rapid Isolation of Extracellular Vesicles from Blood Plasma with Size-Exclusion Chromatography Followed by Mass Spectrometry-Based Proteomic Profiling. Methods Mol. Biol. 2017, 1660, 295–302. [Google Scholar] [CrossRef]
- Takov, K.; Yellon, D.M.; Davidson, S.M. Comparison of small extracellular vesicles isolated from plasma by ultracentrifugation or size-exclusion chromatography: Yield, purity and functional potential. J. Extracell. Vesicles 2019, 8, 1560809. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.E.; Korbie, D.; Trau, M.; Hill, M.M. Optimizing Size Exclusion Chromatography for Extracellular Vesicle Enrichment and Proteomic Analysis from Clinically Relevant Samples. Proteomics 2019, 19, e1800156. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.M.; Li, A.; Chen, J.J.; Sun, E.J. Research Development on Exosome Separation Technology. J. Membr. Biol. 2023, 256, 25–34. [Google Scholar] [CrossRef]
- Karimi, N.; Cvjetkovic, A.; Jang, S.C.; Crescitelli, R.; Hosseinpour Feizi, M.A.; Nieuwland, R.; Lötvall, J.; Lässer, C. Detailed analysis of the plasma extracellular vesicle proteome after separation from lipoproteins. Cell Mol. Life Sci. 2018, 75, 2873–2886. [Google Scholar] [CrossRef] [PubMed]
- López de Las Hazas, M.C.; Tomé-Carneiro, J.; Del Pozo-Acebo, L.; Del Saz-Lara, A.; Chapado, L.A.; Balaguer, L.; Rojo, E.; Espín, J.C.; Crespo, C.; Moreno, D.A.; et al. Therapeutic potential of plant-derived extracellular vesicles as nanocarriers for exogenous miRNAs. Pharmacol. Res. 2023, 198, 106999. [Google Scholar] [CrossRef]
- Yu, L.L.; Zhu, J.; Liu, J.X.; Jiang, F.; Ni, W.K.; Qu, L.S.; Ni, R.Z.; Lu, C.H.; Xiao, M.B. A Comparison of Traditional and Novel Methods for the Separation of Exosomes from Human Samples. Biomed. Res. Int. 2018, 2018, 3634563. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, opportunity, and perspective on exosome isolation—Efforts for efficient exosome-based theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Kim, K.; Park, J.; Sohn, Y.; Oh, C.E.; Park, J.H.; Yuk, J.M.; Yeon, J.H. Stability of Plant Leaf-Derived Extracellular Vesicles According to Preservative and Storage Temperature. Pharmaceutics 2022, 14, 457. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Xu, J.; Huang, C.; Hu, J.X.; Xu, H.M.; Guo, X.; Zhang, Y.; Xu, J.K.; Peng, Y.; Zhang, Y.; et al. Houttuynia cordata-Derived Exosome-Like Nanoparticles Mitigate Colitis in Mice via Inhibition of the NLRP3 Signaling Pathway and Modulation of the Gut Microbiota. Int. J. Nanomed. 2024, 19, 13991–14018. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Wang, H.; Tie, H.; Liao, J.; Luo, Y.; Huang, W.; Yu, R.; Song, L.; Zhu, J. Novel plant-derived exosome-like nanovesicles from Catharanthus roseus: Preparation, characterization, and immunostimulatory effect via TNF-α/NF-κB/PU.1 axis. J. Nanobiotechnol. 2023, 21, 160. [Google Scholar] [CrossRef]
- Krainer, J.; Siebenhandl, S.; Weinhäusel, A. Systemic autoinflammatory diseases. J. Autoimmun. 2020, 109, 102421. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Man, J.J.; Beckman, J.A.; Jaffe, I.Z. Sex as a Biological Variable in Atherosclerosis. Circ. Res. 2020, 126, 1297–1319. [Google Scholar] [CrossRef]
- Carrizales-Sepúlveda, E.F.; Ordaz-Farías, A.; Vera-Pineda, R.; Flores-Ramírez, R. Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular Disease. Heart Lung Circ. 2018, 27, 1327–1334. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Detection of inflammatory biomarkers in saliva and urine: Potential in diagnosis, prevention, and treatment for chronic diseases. Exp. Biol. Med. 2016, 241, 783–799. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Lv, C.; Cheng, T.; Zhang, B.; Sun, K.; Lu, K. Triptolide protects against podocyte injury in diabetic nephropathy by activating the Nrf2/HO-1 pathway and inhibiting the NLRP3 inflammasome pathway. Ren. Fail. 2023, 45, 2165103. [Google Scholar] [CrossRef] [PubMed]
- Marcum, Z.A.; Hanlon, J.T. Recognizing the Risks of Chronic Nonsteroidal Anti-Inflammatory Drug Use in Older Adults. Ann. Longterm Care 2010, 18, 24–27. [Google Scholar] [PubMed]
- Wei, Z.N.; Zou, T.; Qiu, Z.; Luo, Y.; Wang, N.; Wang, L.O. Analysis of 43 Cases of Adverse Reaction Reports of Non-steroidal Anti-inflammatory Drugs. Chin. J. Clin. Ration. Drug Use 2023, 16, 158–160. [Google Scholar] [CrossRef]
- Li, A.; Li, D.; Gu, Y.; Liu, R.; Tang, X.; Zhao, Y.; Qi, F.; Wei, J.; Liu, J. Plant-derived nanovesicles: Further exploration of biomedical function and application potential. Acta Pharm. Sin. B 2023, 13, 3300–3320. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Basak, D.; Arrighi, S.; Darwiche, Y.; Deb, S. Comparison of Anticancer Drug Toxicities: Paradigm Shift in Adverse Effect Profile. Life 2021, 12, 48. [Google Scholar] [CrossRef]
- Yang, M.; Luo, Q.; Chen, X.; Chen, F. Bitter melon derived extracellular vesicles enhance the therapeutic effects and reduce the drug resistance of 5-fluorouracil on oral squamous cell carcinoma. J. Nanobiotechnol. 2021, 19, 259. [Google Scholar] [CrossRef]
- Garaeva, L.; Kamyshinsky, R.; Kil, Y.; Varfolomeeva, E.; Verlov, N.; Komarova, E.; Garmay, Y.; Landa, S.; Burdakov, V.; Myasnikov, A.; et al. Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Sci. Rep. 2021, 11, 6489. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef]
- Liu, P.F.; Cai, C.Z.; Feng, X.Q.; Ye, X.Q.; Xu, Z.f. Study on the Mechanism of the Nrf2-ARE Signaling Pathway Affecting Oxidative Stress in Diabetic Myocardial Infarction. Jilin Med. J. 2023, 44, 3021–3024. [Google Scholar]
- Kimball, J.S.; Johnson, J.P.; Carlson, D.A. Oxidative Stress and Osteoporosis. J. Bone Joint Surg. Am. 2021, 103, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef]
- Kowalska, M.; Wize, K.; Prendecki, M.; Lianeri, M.; Kozubski, W.; Dorszewska, J. Genetic Variants and Oxidative Stress in Alzheimer’s Disease. Curr. Alzheimer Res. 2020, 17, 208–223. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, X.; Cao, T.; Chen, E.; Li, Y.; Lei, W.; Hu, Y.; He, B.; Liu, S. Endoplasmic Reticulum Stress and Oxidative Stress in Inflammatory Diseases. DNA Cell Biol. 2022, 41, 924–934. [Google Scholar] [CrossRef]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Huang, G.; Mei, X.; Hu, J. The Antioxidant Activities of Natural Polysaccharides. Curr. Drug Targets 2017, 18, 1296–1300. [Google Scholar] [CrossRef]
- Ozawa, H.; Miyazawa, T.; Burdeos, G.C.; Miyazawa, T. Biological Functions of Antioxidant Dipeptides. J. Nutr. Sci. Vitaminol. 2022, 68, 162–171. [Google Scholar] [CrossRef]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Fais, S. The Potentiality of Plant-Derived Nanovesicles in Human Health-A Comparison with Human Exosomes and Artificial Nanoparticles. Int. J. Mol. Sci. 2022, 23, 4919. [Google Scholar] [CrossRef]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Fais, S. Nanovesicles from Organic Agriculture-Derived Fruits and Vegetables: Characterization and Functional Antioxidant Content. Int. J. Mol. Sci. 2021, 22, 8170. [Google Scholar] [CrossRef]
- Castelli, G.; Logozzi, M.; Mizzoni, D.; Di Raimo, R.; Cerio, A.; Dolo, V.; Pasquini, L.; Screnci, M.; Ottone, T.; Testa, U.; et al. Ex Vivo Anti-Leukemic Effect of Exosome-like Grapefruit-Derived Nanovesicles from Organic Farming-The Potential Role of Ascorbic Acid. Int. J. Mol. Sci. 2023, 24, 15663. [Google Scholar] [CrossRef] [PubMed]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-Derived Exosome-Like Nanoparticles Prevent Oxidative Stress in Human Mesenchymal Stromal Cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Di Raimo, R.; Mizzoni, D.; Spada, M.; Dolo, V.; Fais, S.; Logozzi, M. Oral Treatment with Plant-Derived Exosomes Restores Redox Balance in H(2)O(2)-Treated Mice. Antioxidants 2023, 12, 1169. [Google Scholar] [CrossRef]
- Di Raimo, R.; Mizzoni, D.; Aloi, A.; Pietrangelo, G.; Dolo, V.; Poppa, G.; Fais, S.; Logozzi, M. Antioxidant Effect of a Plant-Derived Extracellular Vesicles’ Mix on Human Skin Fibroblasts: Induction of a Reparative Process. Antioxidants 2024, 13, 1373. [Google Scholar] [CrossRef]
- Jaffer, H.; Andrabi, S.S.; Petro, M.; Kuang, Y.; Steinmetz, M.P.; Labhasetwar, V. Catalytic antioxidant nanoparticles mitigate secondary injury progression and promote functional recovery in spinal cord injury model. J. Control Release 2023, 364, 109–123. [Google Scholar] [CrossRef]
- Pontes-Quero, G.M.; Benito-Garzón, L.; Pérez Cano, J.; Aguilar, M.R.; Vázquez-Lasa, B. Amphiphilic polymeric nanoparticles encapsulating curcumin: Antioxidant, anti-inflammatory and biocompatibility studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111793. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzyńska, K. The Influence of Synthesis Conditions on the Antioxidant Activity of Selenium Nanoparticles. Molecules 2022, 27, 2486. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, L.; Fan, Y.; Zhang, J.; Dai, J.; Zhong, H.; Fu, G.; Hu, Y. Taurine inhibits hydrogen peroxide-induced oxidative stress, inflammatory response and apoptosis in liver of Monopterus albus. Fish. Shellfish. Immunol. 2022, 128, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Pham, C.V.; Chowdhury, R.; Patel, S.; Jaysawal, S.K.; Hou, Y.; Xu, H.; Jia, L.; Duan, A.; Tran, P.H.; et al. Development of Blueberry-Derived Extracellular Nanovesicles for Immunomodulatory Therapy. Pharmaceutics 2023, 15, 2115. [Google Scholar] [CrossRef]
- Mielko, K.A.; Jabłoński, S.J.; Milczewska, J.; Sands, D.; Łukaszewicz, M.; Młynarz, P. Metabolomic studies of Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2019, 35, 178. [Google Scholar] [CrossRef]
- Chai, Y.H.; Xu, J.F. How does Pseudomonas aeruginosa affect the progression of bronchiectasis? Clin. Microbiol. Infect. 2020, 26, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, C.; Norman, J.; Singh, A.; Sanderson, F. Community-acquired Pseudomonas aeruginosa meningitis. BMJ Case Rep. 2017, 2017, bcr-2017-221839. [Google Scholar] [CrossRef]
- Berube, B.J.; Rangel, S.M.; Hauser, A.R. Pseudomonas aeruginosa: Breaking down barriers. Curr. Genet. 2016, 62, 109–113. [Google Scholar] [CrossRef]
- Sharma, A.; Ahmad Farouk, I.; Lal, S.K. COVID-19: A Review on the Novel Coronavirus Disease Evolution, Transmission, Detection, Control and Prevention. Viruses 2021, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Seyed Hosseini, E.; Riahi Kashani, N.; Nikzad, H.; Azadbakht, J.; Hassani Bafrani, H.; Haddad Kashani, H. The novel coronavirus Disease-2019 (COVID-19): Mechanism of action, detection and recent therapeutic strategies. Virology 2020, 551, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hatfull, G.F.; Dedrick, R.M.; Schooley, R.T. Phage Therapy for Antibiotic-Resistant Bacterial Infections. Annu. Rev. Med. 2022, 73, 197–211. [Google Scholar] [CrossRef]
- Zhuang, X.; Deng, Z.B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Zhu, Y.; Chen, S.; Wang, D.; Zhang, S.; Xia, J.; Li, S.; Qiu, Q.; Lee, H.; Wang, J. Anti-glioma effect of ginseng-derived exosomes-like nanoparticles by active blood-brain-barrier penetration and tumor microenvironment modulation. J. Nanobiotechnol. 2023, 21, 253. [Google Scholar] [CrossRef]
- Gong, Q.; Xiong, F.; Zheng, Y.; Guo, Y. Tea-derived exosome-like nanoparticles prevent irritable bowel syndrome induced by water avoidance stress in rat model. J. Gastroenterol. Hepatol. 2024, 39, 2690–2699. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, M.; Liu, D.; Liang, X.; Chang, Y.; Hu, X.; Gao, W. Solanum lycopersicum derived exosome-like nanovesicles alleviate restenosis after vascular injury through the Keap1/Nrf2 pathway. Food Funct. 2025, 16, 539–553. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, S.; Zhang, Z.; Tang, M.; Meng, Z.; Peng, Z.; Liao, Y.; Yang, X.; Nüssler, A.K.; Liu, L.; et al. Gouqi-derived nanovesicles (GqDNVs) inhibited dexamethasone-induced muscle atrophy associating with AMPK/SIRT1/PGC1α signaling pathway. J. Nanobiotechnol. 2024, 22, 276. [Google Scholar] [CrossRef]
- Berger, E.; Colosetti, P.; Jalabert, A.; Meugnier, E.; Wiklander, O.P.B.; Jouhet, J.; Errazurig-Cerda, E.; Chanon, S.; Gupta, D.; Rautureau, G.J.P.; et al. Use of Nanovesicles from Orange Juice to Reverse Diet-Induced Gut Modifications in Diet-Induced Obese Mice. Mol. Ther. Methods Clin. Dev. 2020, 18, 880–892. [Google Scholar] [CrossRef]
- Ye, C.; Yan, C.; Bian, S.J.; Li, X.R.; Li, Y.; Wang, K.X.; Zhu, Y.H.; Wang, L.; Wang, Y.C.; Wang, Y.Y.; et al. Momordica charantia L.-derived exosome-like nanovesicles stabilize p62 expression to ameliorate doxorubicin cardiotoxicity. J. Nanobiotechnol. 2024, 22, 464. [Google Scholar] [CrossRef] [PubMed]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, M.; Lyu, Z.; Shan, K.; Chen, Z.; Chen, B.; Chen, Y.; Hu, X.; Dou, B.; Zhang, J.; et al. Medicinal plant-based drug delivery system for inflammatory bowel disease. Front. Pharmacol. 2023, 14, 1158945. [Google Scholar] [CrossRef] [PubMed]
- Shahjin, F.; Chand, S.; Yelamanchili, S.V. Extracellular Vesicles as Drug Delivery Vehicles to the Central Nervous System. J. Neuroimmune Pharmacol. 2020, 15, 443–458. [Google Scholar] [CrossRef]
- Kim, I.K.; Kim, S.H.; Choi, S.M.; Youn, B.S.; Kim, H.S. Extracellular Vesicles as Drug Delivery Vehicles for Rheumatoid Arthritis. Curr. Stem Cell Res. Ther. 2016, 11, 329–342. [Google Scholar] [CrossRef]
- Mao, Y.; Han, M.; Chen, C.; Wang, X.; Han, J.; Gao, Y.; Wang, S. A biomimetic nanocomposite made of a ginger-derived exosome and an inorganic framework for high-performance delivery of oral antibodies. Nanoscale 2021, 13, 20157–20169. [Google Scholar] [CrossRef]
- Feng, W.; Teng, Y.; Zhong, Q.; Zhang, Y.; Zhang, J.; Zhao, P.; Chen, G.; Wang, C.; Liang, X.J.; Ou, C. Biomimetic Grapefruit-Derived Extracellular Vesicles for Safe and Targeted Delivery of Sodium Thiosulfate against Vascular Calcification. ACS Nano 2023, 17, 24773–24789. [Google Scholar] [CrossRef]
- Pomatto, M.A.C.; Gai, C.; Negro, F.; Massari, L.; Deregibus, M.C.; De Rosa, F.G.; Camussi, G. Oral Delivery of mRNA Vaccine by Plant-Derived Extracellular Vesicle Carriers. Cells 2023, 12, 1826. [Google Scholar] [CrossRef]
- Fan, S.J.; Chen, J.Y.; Tang, C.H.; Zhao, Q.Y.; Zhang, J.M.; Qin, Y.C. Edible plant extracellular vesicles: An emerging tool for bioactives delivery. Front. Immunol. 2022, 13, 1028418. [Google Scholar] [CrossRef]
- Yan, G.; Xiao, Q.; Zhao, J.; Chen, H.; Xu, Y.; Tan, M.; Peng, L. Brucea javanica derived exosome-like nanovesicles deliver miRNAs for cancer therapy. J. Control Release 2024, 367, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, O.; Pomareda, F.; Olivares, B.; Huang, Y.L.; Zavala, G.; Carrasco-Rojas, J.; Álvarez, S.; Leiva-Sabadini, C.; Hidalgo, V.; Romo, P.; et al. Aloe vera peel-derived nanovesicles display anti-inflammatory properties and prevent myofibroblast differentiation. Phytomedicine 2024, 122, 155108. [Google Scholar] [CrossRef] [PubMed]

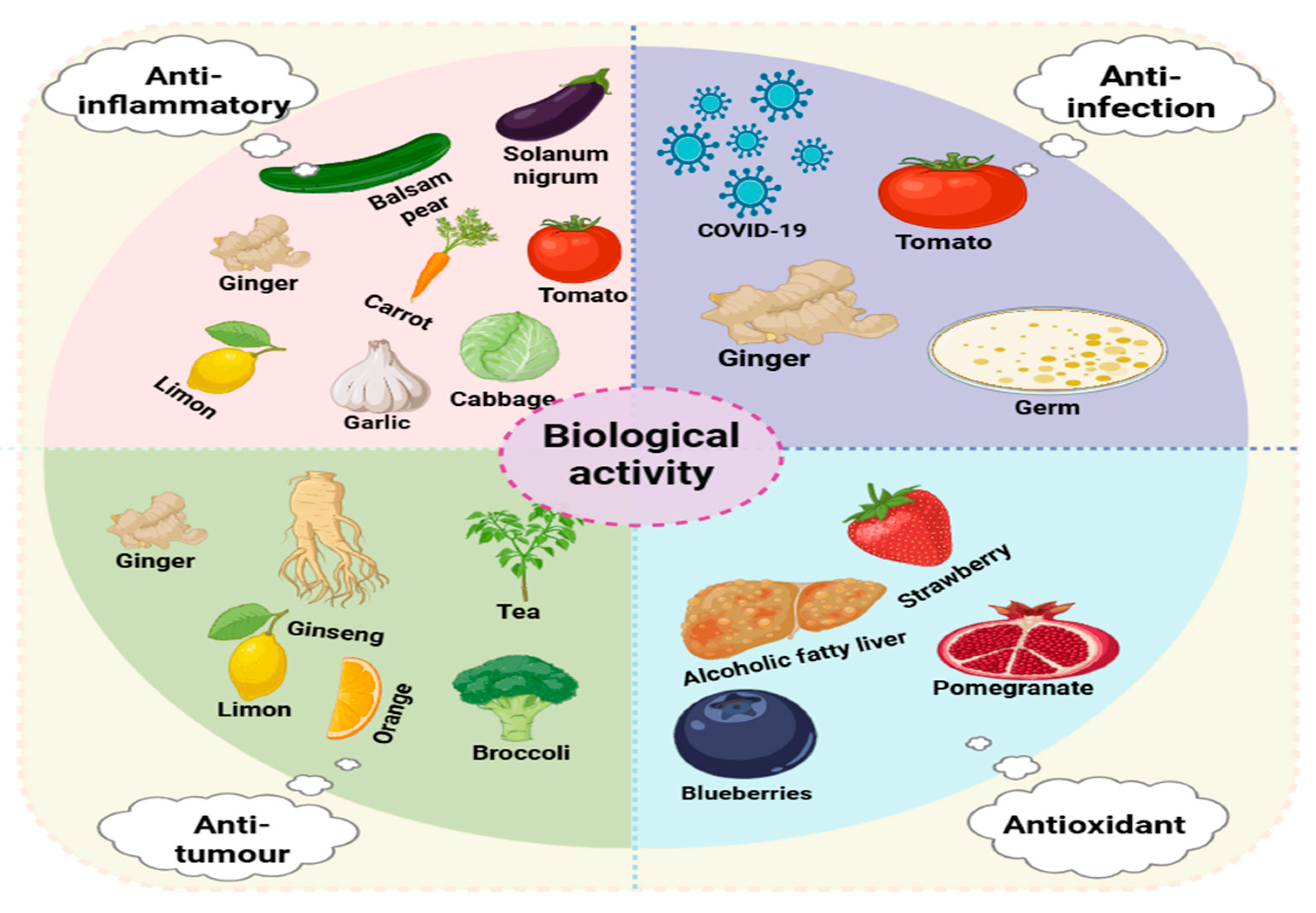
| Plants | Source | Extraction Techniques | Biological Function | Illnesses | Average Particle Size (nm) | References |
|---|---|---|---|---|---|---|
| Ginger | Vegetable | UC | Anti-inflammatory, anti-infection, antitumor | COVID-19 | 70.09 ± 19.24 | [25,26,27] |
| Tomato | Vegetable | UC | Anti-inflammatory, anti-infection | Inflammatory-related diseases | 110 ± 10 | [28,29,30] |
| Cabbage | Vegetable | UF, SEC | Anti-inflammatory | Inflammatory-related diseases | 100 | [31] |
| Momordica charantia | Vegetable | Density gradient centrifugation | Anti-inflammatory | Colitis | 106.0 | [32] |
| Garlic | Vegetable | UC, density gradient centrifugation | Anti-inflammatory, liver protection | Colitis | 43.82–396.1 | [33,34] |
| Carrot | Vegetable | SEC, UF | Anti-inflammatory | 143.9 | [35,36] | |
| Panax notoginseng | Root | UC, density gradient centrifugation | Anti-inflammatory | Cerebral ischemia–reperfusion injury | 151.3 | [37] |
| Solanum nigrum L. | Vegetable | PEG | Anti-inflammatory | Inflammatory-related diseases | 107.0 | [38] |
| Mulberry bark | Bark | UC | Anti-inflammatory | colitis | 151.3 ± 45.4 | [39] |
| Grapefruit | Fruit | Density gradient centrifugation | Antitumor | Melanoma | 210.8 ± 48.62 | [40,41] |
| Ginseng | Vegetable | Density gradient centrifugation | Antitumor, regenerative | Melanoma | 92.04 ± 4.85 | [42,43,44] |
| Lemon | Fruit | UC | Antitumor, anti-inflammatory | Gastric cancer, chronic inflammation | 65 ± 2.7 | [45,46,47] |
| Orange | Fruit | UC | Antitumor | Ovarian cancer | 91 | [48,49] |
| Tea | Leaf | UC, gradient centrifugation | Antitumor | Breast cancer | 166.9 | [22] |
| Broccoli | Vegetable | UC, SEC | Antitumor | Pancreatic cancer | 146.7 ± 7.2 | [50,51] |
| Strawberry | Fruit | UC | Antioxidant | Inflammatory-related diseases | 30–191 | [52] |
| Blueberry | Fruit | UC | Antioxidant | Alcoholic fatty liver disease | 189.62 | [53] |
| Pomegranate | Fruit | SEC | Antioxidant | Inflammatory-related diseases | 148.7 ± 9.2 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nueraihemaiti, N.; Dilimulati, D.; Baishan, A.; Hailati, S.; Maihemuti, N.; Aikebaier, A.; Paerhati, Y.; Zhou, W. Advances in Plant-Derived Extracellular Vesicle Extraction Methods and Pharmacological Effects. Biology 2025, 14, 377. https://doi.org/10.3390/biology14040377
Nueraihemaiti N, Dilimulati D, Baishan A, Hailati S, Maihemuti N, Aikebaier A, Paerhati Y, Zhou W. Advances in Plant-Derived Extracellular Vesicle Extraction Methods and Pharmacological Effects. Biology. 2025; 14(4):377. https://doi.org/10.3390/biology14040377
Chicago/Turabian StyleNueraihemaiti, Nuerbiye, Dilihuma Dilimulati, Alhar Baishan, Sendaer Hailati, Nulibiya Maihemuti, Alifeiye Aikebaier, Yipaerguli Paerhati, and Wenting Zhou. 2025. "Advances in Plant-Derived Extracellular Vesicle Extraction Methods and Pharmacological Effects" Biology 14, no. 4: 377. https://doi.org/10.3390/biology14040377
APA StyleNueraihemaiti, N., Dilimulati, D., Baishan, A., Hailati, S., Maihemuti, N., Aikebaier, A., Paerhati, Y., & Zhou, W. (2025). Advances in Plant-Derived Extracellular Vesicle Extraction Methods and Pharmacological Effects. Biology, 14(4), 377. https://doi.org/10.3390/biology14040377






