Long-Term Management and Monitoring of the Bladder After Spinal Cord Injury in a Rodent Model
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Permanent Bladder Catheter Implantation
2.3. Spinal Cord Contusion
2.4. Catheter Flushing Protocol
2.5. Animal Welfare
2.6. Locomotion Scoring
2.7. Awake Cystometry
2.8. µCT of Spinal Cord
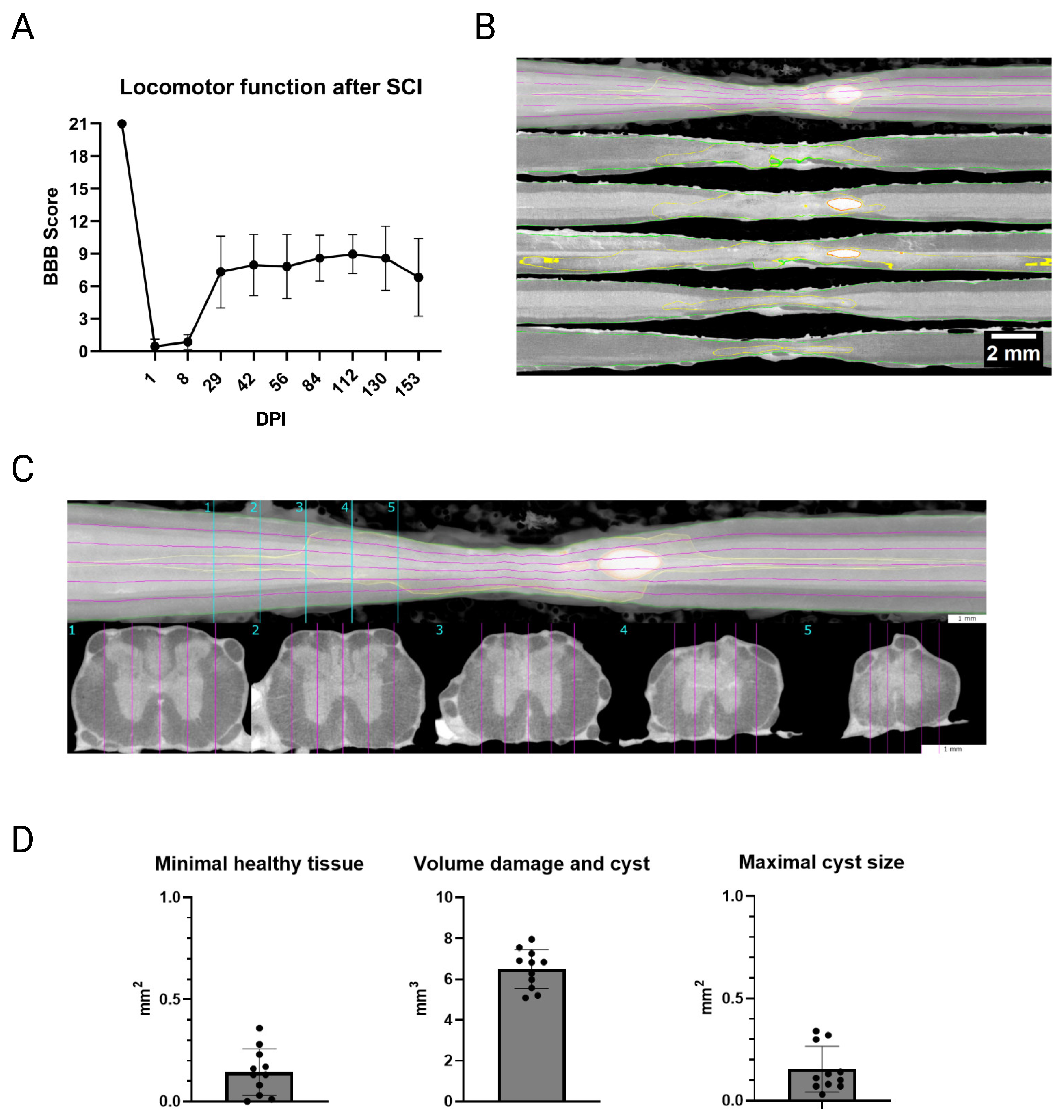
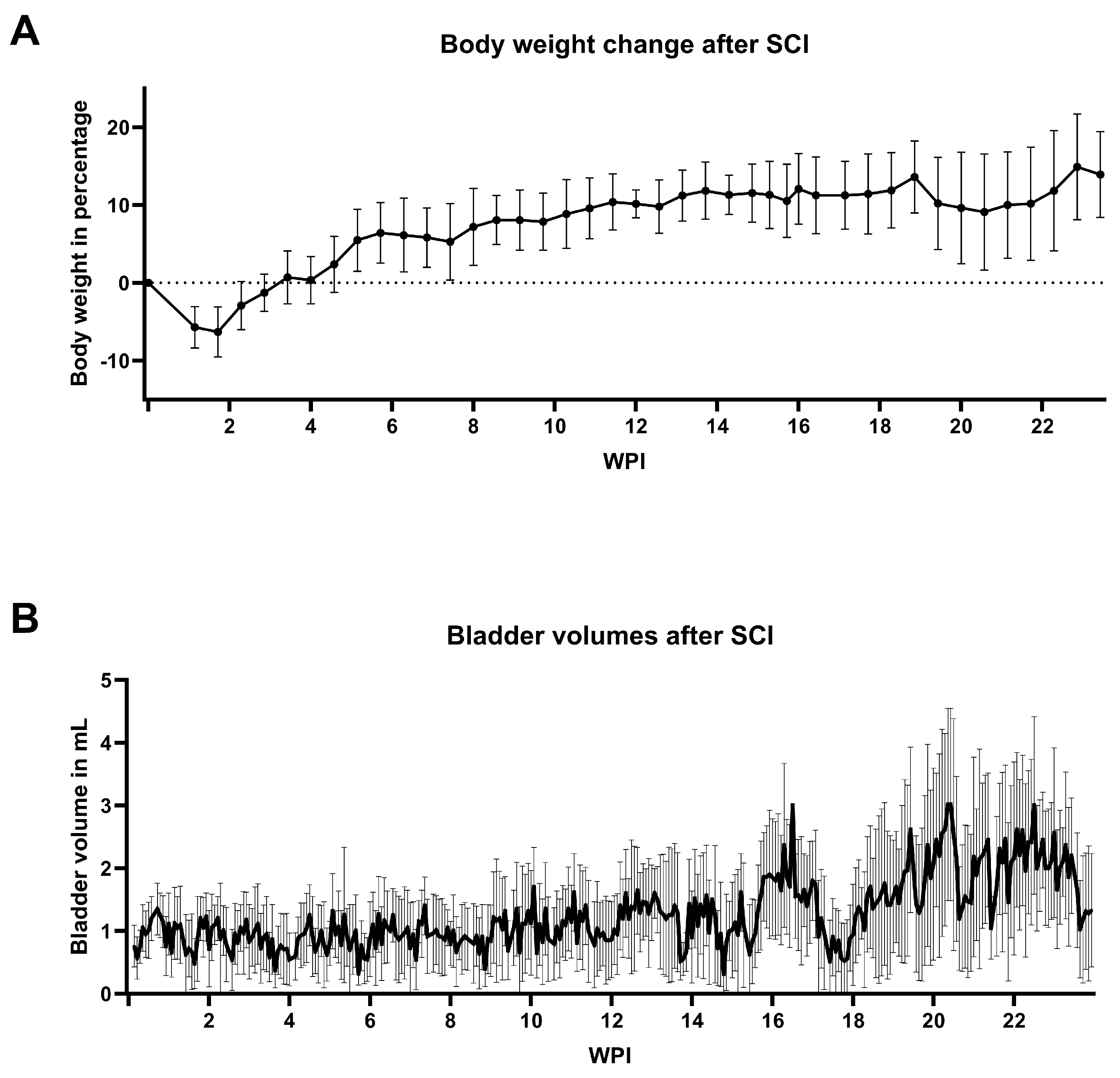
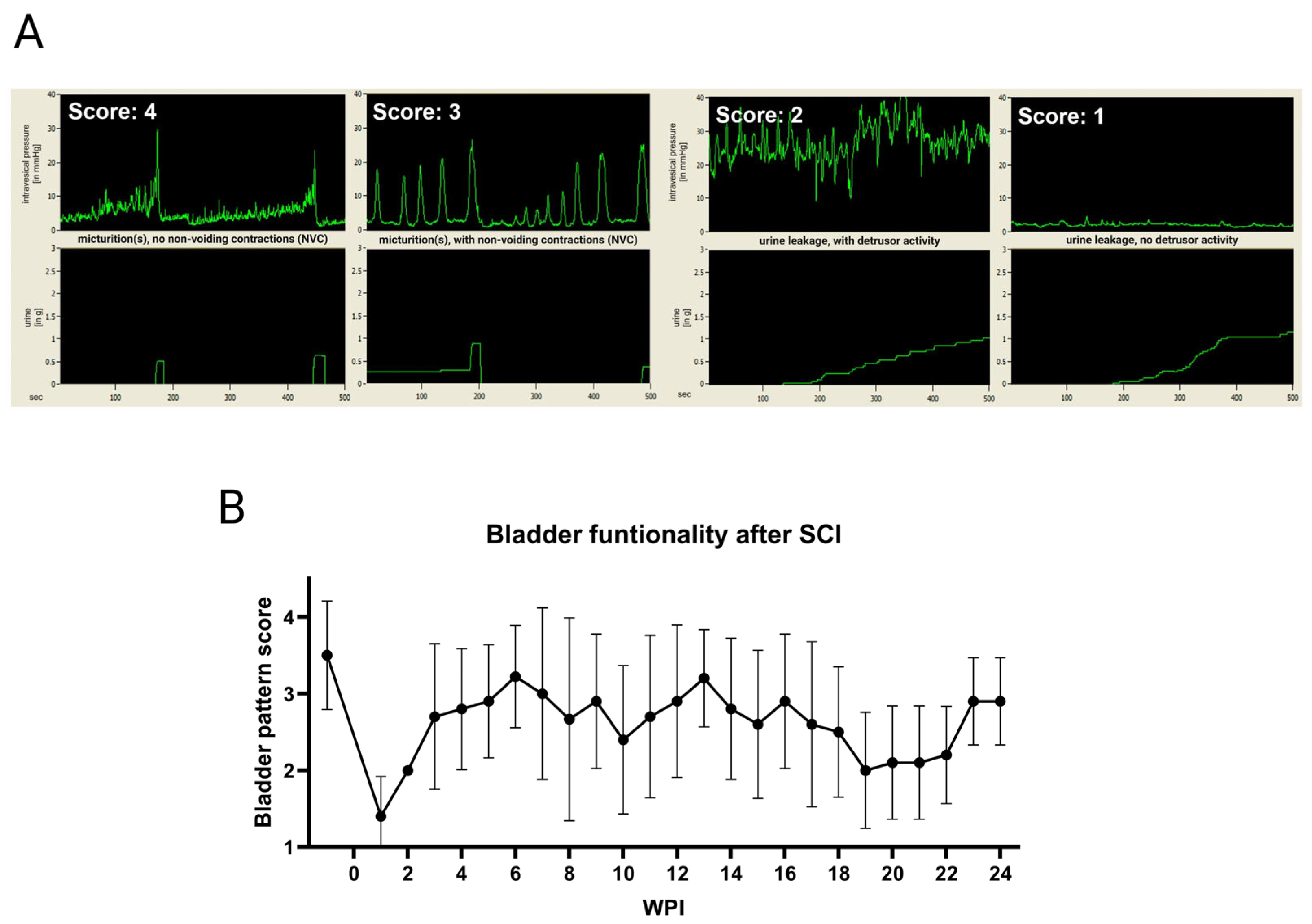
3. Results
3.1. Confirmation of Spinal Cord Contusion
3.2. General Animal Welfare
3.3. Bladder Welfare
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBB | Basso, Beattie, and Bresnahan |
| CCS | Catamount cystometry station |
| CNS | Central nervous system |
| DPI | Days post-injury |
| FUP | Follow-up period |
| IH | Infinite horizon |
| nLUTD | Neurogenic lower urinary tract dysfunction |
| NVC | Non-voiding contractions |
| PBS | Phosphate-buffered saline |
| PFA | Paraformaldehyde |
| QoL | Quality of life |
| SCI | Spinal cord injury |
| UTI | Urinary tract infection |
| UUT | Upper urinary tract |
| WPI | Weeks post-injury |
References
- Ding, W.; Hu, S.; Wang, P.; Kang, H.; Peng, R.; Dong, Y.; Li, F. Spinal cord injury: The global incidence, prevalence, and disability from the Global Burden of Disease Study 2019. Spine (Phila Pa 1976) 2022, 47, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Huebner, E.A.; Strittmatter, S.M. Axon Regeneration in the Peripheral and Central Nervous Systems. In Cell Biology of the Axon; Results and Problems in Cell Differentiation; Springer: Berlin/Heidelberg, Germany, 2009; Volume 48, pp. 305–360. [Google Scholar] [CrossRef]
- Taylor, E.C.; Fitzpatrick, C.E.; Thompson, S.E.; Justice, S.B. Acute traumatic spinal cord injury. Adv. Emerg. Nurs. J. 2022, 44, 272–280. [Google Scholar] [CrossRef]
- Welk, B. Quality of life in neurourology patients. Eur. Urol. Focus 2020, 6, 531–533. [Google Scholar] [CrossRef]
- Costa, P.; Costa, P.; Perrouin-Verbe, B.; Perrouin-Verbe, B.; Colvez, A.; Colvez, A.; Didier, J.P.; Didier, J.P.; Marquis, P.; Marquis, P.; et al. Quality of life in spinal cord injury patients with urinary difficulties. Development and validation of QUALIVEEN. Eur. Urol. 2001, 39, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Weld, K.J.; Dmochowski, R.R. Association of level of injury and bladder behavior in patients with post-traumatic spinal cord injury. Urology 2000, 55, 490–494. [Google Scholar] [CrossRef]
- Panicker, J.N. Neurogenic Bladder: Epidemiology, Diagnosis, and Management. Semin. Neurol. 2020, 40, 569–579. [Google Scholar] [CrossRef]
- Taweel, W.A.; Seyam, R. Neurogenic bladder in spinal cord injury patients. Res. Rep. Urol. 2015, 7, 85–99. [Google Scholar] [CrossRef]
- Doelman, A.W.; Streijger, F.; Majerus, S.J.; Damaser, M.S.; Kwon, B.K. Assessing neurogenic lower urinary tract dysfunction after spinal cord injury: Animal models in preclinical neuro-urology research. Biomedicines 2023, 11, 1539. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, K.; Aquarius, R.; Klymov, A.; Wever, K.E.; Damveld, L.; Leeuwenburgh, S.C.; Bartels, R.H.; Hooijmans, C.R.; Walboomers, X.F. Systematic evaluation of spinal cord injury animal models in the field of biomaterials. Tissue Eng. Part B Rev. 2022, 28, 1169–1179. [Google Scholar] [CrossRef]
- Liu, F.; Huang, Y.; Wang, H. Rodent models of spinal cord injury: From pathology to application. Neurochem. Res. 2023, 48, 340–361. [Google Scholar] [CrossRef]
- Verma, R.; Virdi, J.K.; Singh, N.; Jaggi, A.S. Animal models of spinal cord contusion injury. Korean J. Pain. 2019, 32, 12–21. [Google Scholar] [CrossRef]
- Sharif-Alhoseini, M.; Khormali, M.; Rezaei, M.; Safdarian, M.; Hajighadery, A.; Khalatbari, M.M.; Meknatkhah, S.; Rezvan, M.; Chalangari, M.; Derakhshan, P.; et al. Animal models of spinal cord injury: A systematic review. Spinal Cord 2017, 55, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.; Bennett, B.; de Groat, W. Effect of Urinary Diversion on the Recovery of Micturition Reflexes after Spinal Cord Injury in the Rat. J. Urol. 1994, 151, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.S.; Wheeler, J.S.; Cai, W.; Wurster, R.D. Direct bladder stimulation with suture electrodes promotes voiding in a spinal animal model: A technical report. J. Rehabil. Res. Dev. 1997, 34, 72–81. [Google Scholar] [PubMed]
- Lam Van Ba, O.; Barbe, M.F.; Caremel, R.; Aharony, S.; Loutochin, O.; Jacques, L.; Wood, M.W.; Tiwari, E.; Tuite, G.F.; Campeau, L.; et al. Lumbar to sacral root rerouting to restore bladder function in a feline spinal cord injury model: Urodynamic and retrograde nerve tracing results from a pilot study. Neurourol. Urodyn. 2018, 37, 153–162. [Google Scholar] [CrossRef]
- Tang, P.C.; Walter, J.S. Voiding in anesthetized spinal dogs induced by stimulating sacral and coccygeal roots with the “volume conduction” method. Neurourol. Urodyn. 1984, 3, 51–61. [Google Scholar] [CrossRef]
- Keller, E.E.; Patras, I.; Hutu, I.; Roider, K.; Sievert, K.D.; Aigner, L.; Janetschek, G.; Lusuardi, L.; Zimmermann, R.; Bauer, S. Early sacral neuromodulation ameliorates urinary bladder function and structure in complete spinal cord injury minipigs. Neurourol. Urodyn. 2020, 39, 586–593. [Google Scholar] [CrossRef]
- Schneider, M.P.; Hughes, F.M., Jr.; Engmann, A.K.; Purves, J.T.; Kasper, H.; Tedaldi, M.; Spruill, L.S.; Gullo, M.; Schwab, M.E.; Kessler, T.M. A novel urodynamic model for lower urinary tract assessment in awake rats. BJU Int. 2015, 115, 8–15. [Google Scholar] [CrossRef]
- Schneider, M.P.; Sartori, A.M.; Tampé, J.; Moors, S.; Engmann, A.K.; Ineichen, B.V.; Hofer, A.S.; Schwab, M.E.; Kessler, T.M. Urodynamic measurements reflect physiological bladder function in rats. Neurourol. Urodyn. 2018, 37, 1266–1271. [Google Scholar] [CrossRef]
- Foditsch, E.E.; Roider, K.; Sartori, A.M.; Kessler, T.M.; Kayastha, S.R.; Aigner, L.; Schneider, M.P. Cystometric and external urethral sphincter measurements in awake rats with implanted catheter and electrodes allowing for repeated measurements. J. Vis. Exp. 2018, 131, 56506. [Google Scholar] [CrossRef]
- Rinwa, P.; Eriksson, M.; Cotgreave, I.; Bäckberg, M. 3R-refinement principles: Elevating rodent well-being and research quality. Lab. Anim. Res. 2024, 40, 11. [Google Scholar] [CrossRef] [PubMed]
- Sotocina, S.G.; Sorge, R.E.; Zaloum, A.; Tuttle, A.H.; Martin, L.J.; Wieskopf, J.S.; Mapplebeck, J.C.; Wei, P.; Zhan, S.; Zhang, S.; et al. The rat grimace scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain 2011, 7, 1744–8069. [Google Scholar] [CrossRef]
- Onifer, S.M.; Rabchevsky, A.G.; Scheff, S.W. Rat models of traumatic spinal cord injury to assess motor recovery. ILAR J. 2007, 48, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Noyes, D.H. Electromechanical impactor for producing experimental spinal cord injury in animals. Med. Biol. Eng. Comput. 1987, 25, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Acton, C.; Ivins, N.; Bainbridge, P.; Browning, P. Management of incontinence-associated dermatitis patients using a skin protectant in acute care: A case series. J. Wound Care 2020, 29, 18–26. [Google Scholar] [CrossRef]
- Peters, E.; Hanssens, V.; De Henau, M.; Dupont, Y.; Spinnael, J.; Giunta, G.; Zeltzer, A.; De Baerdemaeker, R.; Hamdi, M. Using an elastomeric skin protectant to manage donor site wounds of split-thickness skin grafts: A case series. Adv. Ski. Wound Care 2023, 36, 1–5. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Romanelli, P.; Bieler, L.; Heimel, P.; Škokić, S.; Jakubecova, D.; Kreutzer, C.; Zaunmair, P.; Smolčić, T.; Benedetti, B.; Rohde, E.; et al. Enhancing functional recovery through intralesional application of extracellular vesicles in a rat model of traumatic spinal cord injury. Front. Cell Neurosci. 2022, 15, 795008. [Google Scholar] [CrossRef]
- Romero-Ramírez, L.; Wu, S.; de Munter, J.; Wolters, E.C.; Kramer, B.W.; Mey, J. Treatment of rats with spinal cord injury using human bone marrow-derived stromal cells prepared by negative selection. J. Biomed. Sci. 2020, 27, 1–18. [Google Scholar] [CrossRef]
- Chiu, C.W.; Cheng, H.; Hsieh, S.L. Contusion spinal cord injury rat model. Bio Protoc. 2017, 7, e2337. [Google Scholar] [CrossRef] [PubMed]
- Krishna, V.; Andrews, H.; Jin, X.; Yu, J.; Varma, A.; Wen, X.; Kindy, M. A contusion model of severe spinal cord injury in rats. J. Vis. Exp. 2013, 78, 50111. [Google Scholar] [CrossRef]
- Corbee, R.J.; Van Kerkhoven, W.J. Nutritional support of dogs and cats after surgery or illness. Open J. Vet. Med. 2014, 4, 44–57. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, J.H.; Oh, S.K.; Baek, S.R.; Min, J.; Kim, Y.W.; Kim, S.T.; Woo, C.W.; Jeon, S.R. Analysis of equivalent parameters of two spinal cord injury devices: The New York University impactor versus the Infinite Horizon impactor. Spine J. 2016, 16, 1392–1403. [Google Scholar] [CrossRef]
- Van Gorp, S.; Leerink, M.; Nguyen, S.; Platoshyn, O.; Marsala, M.; Joosten, E.A. Translation of the rat thoracic contusion model; Part 2—Forward versus backward locomotion testing. Spinal Cord 2014, 52, 529–535. [Google Scholar] [CrossRef]
- Fouad, K.; Hurd, C.; Magnuson, D.S. Functional testing in animal models of spinal cord injury: Not as straightforward as one would think. Front. Integr. Neurosci. 2013, 7, 85. [Google Scholar] [CrossRef]
- Vrinten, D.H.; Hamers, F.F. ‘CatWalk’ automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat; a comparison with von Frey testing. Pain 2003, 102, 203–209. [Google Scholar] [CrossRef]
- Smith, R.R.; Burke, D.A.; Baldini, A.D.; Shum-Siu, A.; Baltzley, R.; Bunger, M.; Magnuson, D.S. The Louisville Swim Scale: A novel assessment of hindlimb function following spinal cord injury in adult rats. J. Neurotrauma 2006, 23, 1654–1670. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleindorfer, M.; Keller, E.E.; Roider, K.; Beyerer, E.; Heimel, P.; Hercher, D.; Brandtner, M.G.; Lusuardi, L.; Aigner, L.; Bauer, S. Long-Term Management and Monitoring of the Bladder After Spinal Cord Injury in a Rodent Model. Biology 2025, 14, 373. https://doi.org/10.3390/biology14040373
Kleindorfer M, Keller EE, Roider K, Beyerer E, Heimel P, Hercher D, Brandtner MG, Lusuardi L, Aigner L, Bauer S. Long-Term Management and Monitoring of the Bladder After Spinal Cord Injury in a Rodent Model. Biology. 2025; 14(4):373. https://doi.org/10.3390/biology14040373
Chicago/Turabian StyleKleindorfer, Michael, Elena Esra Keller, Karin Roider, Evelyn Beyerer, Patrick Heimel, David Hercher, Martha Georgina Brandtner, Lukas Lusuardi, Ludwig Aigner, and Sophina Bauer. 2025. "Long-Term Management and Monitoring of the Bladder After Spinal Cord Injury in a Rodent Model" Biology 14, no. 4: 373. https://doi.org/10.3390/biology14040373
APA StyleKleindorfer, M., Keller, E. E., Roider, K., Beyerer, E., Heimel, P., Hercher, D., Brandtner, M. G., Lusuardi, L., Aigner, L., & Bauer, S. (2025). Long-Term Management and Monitoring of the Bladder After Spinal Cord Injury in a Rodent Model. Biology, 14(4), 373. https://doi.org/10.3390/biology14040373





