Diversity of nifH Gene in Culturable Rhizobia from Black Locust (Robinia pseudoacacia L.) Grown in Cadmium-Contaminated Soils
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Preparation and Plant Seeds
2.2. Pot Experiments and Soil and Nodule Sampling
2.3. Determination of Soil Physiochemical Characteristics
2.4. Rhizobial Isolation
2.5. Analysis of nifH Gene Diversity in Culturable Rhizobia
2.6. Statistical Analysis
3. Results
3.1. Soil Physiochemical Characteristics
3.2. Sample Sequencing Quality
3.3. Alpha Diversity of nifH Genes in Culturable Rhizobia Community
3.4. Community Structure of nifH Genes in Culturable Rhizobia
3.5. Characteristics of Culturable Rhizobia Communities in Terms of nifH Gene
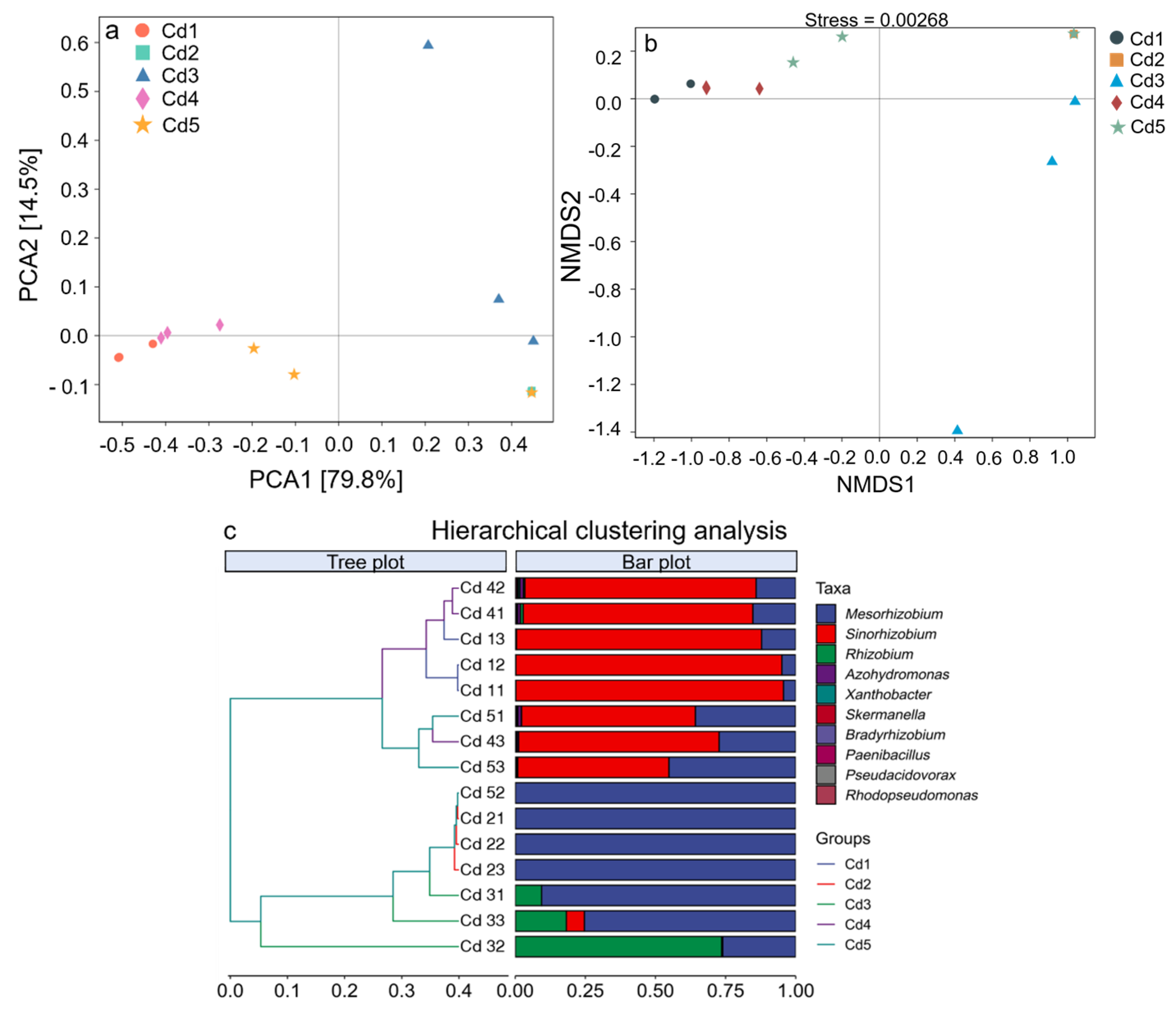
3.5.1. Principal Coordinate Analysis
3.5.2. Nonmetric Multidimensional Scaling
3.5.3. Community Clustering Analysis
3.6. Genera Variation and Marker Genera Analysis
3.7. Relationship Between Diversity of nifH Gene in Culturable Rhizobia and Soil Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, K.M.; Kobayashi, H.; Davies, B.W.; Taga, M.E.; Walker, G.C. How rhizobial symbionts invade plants: The Sinorhizobium–Medicago model. Nat. Rev. Microbiol. 2007, 5, 619–633. [Google Scholar] [PubMed]
- Shao, S.; Chen, M.N.; Liu, W.; Hu, X.K.; Wang, E.T.; Yu, S.; Li, Y. Long-term monoculture reduces the symbiotic rhizobial biodiversity of peanut. Syst. Appl. Microbiol. 2020, 43, 126101. [Google Scholar]
- Alexandre, A.; Oliveira, S. Response to temperature stress in rhizobia. Crit. Rev. Microbiol. 2013, 39, 219–228. [Google Scholar]
- Janczarek, M.; Kozieł, M.; Adamczyk, P.; Buczek, K.; Kalita, M.; Gromada, A.; Mordzińska-Rak, A.; Polakowski, C.; Bieganowski, A. Symbiotic efficiency of Rhizobium leguminosarum sv. trifolii strains originating from the subpolar and temperate climate regions. Sci. Rep. 2024, 14, 6264. [Google Scholar]
- Mng’ong’o, M.; Ojija, F.; Aloo, B. The role of Rhizobia toward food production, food and soil security through microbial agro-input utilization in developing countries. Case Stud. Chem. Environ. Eng. 2023, 8, 100404. [Google Scholar] [CrossRef]
- Bennis, M.; Perez-Tapia, V.; Alami, S.; Bouhnik, O.; Lamin, H.; Abdelmoumen, H.; Bedmar, E.J.; El Idrissi, M.M. Characterization of plant growth-promoting bacteria isolated from the rhizosphere of Robinia pseudoacacia growing in metal-contaminated mine tailings in eastern Morocco. J. Environ. Manag. 2022, 304, 114321. [Google Scholar]
- Shen, T.; Jin, R.; Yan, J.; Cheng, X.; Zeng, L.; Chen, Q.; Gu, Y.; Zou, L.; Zhao, K.; Xiang, Q.; et al. Study on diversity, nitrogen-fixing capacity, and heavy metal tolerance of culturable Pongamia pinnata rhizobia in the vanadium-titanium magnetite tailings. Front. Microbiol. 2023, 14, 1078333. [Google Scholar]
- Hawkins, J.P.; Oresnik, I.J. The Rhizobium-Legume Symbiosis: Co-opting Sucessful Stress Management. Front. Plant Sci. 2021, 12, 796045. [Google Scholar]
- Yamato, M.; Yoshida, S.; Iwase, K. Cadmium accumulation in Crassocephalum crepidioides (Benth.) S. Moore (Compositae) in heavy-metal polluted soils and Cd-added conditions in hydroponic and pot cultures. J. Soil Sci. Plant Nutr. 2008, 54, 738–743. [Google Scholar]
- Wang, T.Q.; Yao, J.; Yuan, Z.M.; Zhao, Y.; Wang, F.; Chen, H.L. Isolation of lead-resistant Arthrobactor strain GQ-9 and its biosorption mechanism. Environ. Sci. Pollut. Res. 2018, 25, 3527–3538. [Google Scholar]
- Zheng, Y.; Liang, J.; Zhao, D.L.; Meng, C.; Xu, Z.C.; Xie, Z.H.; Zhang, C.S. The Root Nodule Microbiome of Cultivated and Wild Halophytic Legumes Showed Similar Diversity but Distinct Community Structure in Yellow River Delta Saline Soils. Microorganisms 2020, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Fan, M.; Zhang, D.; Yang, R.; Zhang, F.; Xu, L.; Wei, X.; Shen, Y.; Wei, G. Distribution and diversity of rhizobia associated with wild soybean (Glycine soja Sieb. & Zucc.) in Northwest China. Syst. Appl. Microbiol. 2014, 37, 449–456. [Google Scholar] [PubMed]
- Gao, Y.F.; Jia, X.; Zhao, Y.H.; Zhao, J.M.; Ding, X.Y.; Zhang, C.Y.; Feng, X.J. Effect of arbuscular mycorrhizal fungi (Glomus mosseae) and elevated air temperature on Cd migration in the rhizosphere soil of alfalfa. Ecotox. Environ. Saf. 2022, 248, 114342. [Google Scholar] [CrossRef]
- Jia, X.; Gao, Y.F.; Li, X.D.; Zhao, Y.H.; Wang, L.; Zhang, C.Y. Effects of cadmium on soil nitrification in the rhizosphere of Robinia pseudoacacia L. seedlings under elevated atmospheric CO2 scenarios. Sci. Total Environ. 2021, 772, 145023. [Google Scholar] [CrossRef]
- Xu, H.; Huang, Y.; Xiong, X.; Zhu, H.; Lin, J.; Shi, J.; Tang, C.; Xu, J. Changes in soil Cd contents and microbial communities following Cd-containing straw return. Environ. Pollut. 2023, 330, 121753. [Google Scholar] [CrossRef]
- Choppala, G.; Saifullah; Bolan, N.; Bibi, S.; Iqbal, M.; Rengel, Z.; Kunhikrishnan, A.; Ashwath, N.; Ok, Y.S. Cellular Mechanisms in Higher Plants Governing Tolerance to Cadmium Toxicity. Crit. Rev. Plant Sci. 2014, 33, 374–391. [Google Scholar] [CrossRef]
- Sarwar, N.; Saifullah; Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar] [CrossRef]
- Wu, Q.; Su, N.; Cai, J.; Shen, Z.; Cui, J. Hydrogen-rich water enhances cadmium tolerance in Chinese cabbage by reducing cadmium uptake and increasing antioxidant capacities. J. Plant Physiol. 2015, 175, 174–182. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 2017, 601–602, 1591–1605. [Google Scholar]
- Renella, G.; Egamberdiyeva, D.; Landi, L.; Mench, M.; Nannipieri, P. Microbial activity and hydrolase activities during decomposition of root exudates released by an artificial root surface in Cd-contaminated soils. Soil Biol. Biochem. 2006, 38, 702–708. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar]
- Hao, X.L.; Xie, P.; Zhu, Y.G.; Taghavi, S.; Wei, G.H.; Rensing, C. Copper Tolerance Mechanisms of Mesorhizobium amorphae and Its Role in Aiding Phytostabilization by Robinia pseudoacacia in Copper Contaminated Soil. Environ. Sci. Technol. 2015, 49, 2328–2340. [Google Scholar] [PubMed]
- Carrasco, J.A.; Armario, P.; Pajuelo, E.; Burgos, A.; Caviedes, M.A.; López, R.; Chamber, M.A.; Palomares, A.J. Isolation and characterisation of symbiotically effective Rhizobium resistant to arsenic and heavy metals after the toxic spill at the Aznalcóllar pyrite mine. Soil Biol. Biochem. 2005, 37, 1131–1140. [Google Scholar]
- Liu, X.; Fan, Y.; Long, J.; Wei, R.; Kjelgren, R.; Gong, C.; Zhao, J. Effects of soil water and nitrogen availability on photosynthesis and water use efficiency of Robinia pseudoacacia seedlings. J. Environ. Sci. 2013, 25, 585–595. [Google Scholar]
- Yang, J.; Lan, L.Y.; Jin, Y.; Yu, N.; Wang, D.; Wang, E. Mechanisms underlying legume-rhizobium symbioses. J. Integr. Plant Biol. 2022, 64, 244–267. [Google Scholar]
- Umapathi, M.; Chandrasekhar, C.N.; Senthil, A.; Kalaiselvi, T.; Santhi, R.; Ravikesavan, R. Isolation, characterization and plant growth-promoting effects of sorghum [Sorghum bicolor (L.) moench] root-associated rhizobacteria and their potential role in drought mitigation. Arch. Microbiol. 2022, 204, 354. [Google Scholar]
- Rahal, S.; Menaa, B.; Chekireb, D. Screening of heavy metal-resistant rhizobial and non-rhizobial microflora isolated from Trifolium sp. growing in mining areas. Environ. Monit. Assess. 2024, 196, 283. [Google Scholar]
- Li, T.Q.; Tao, Q.; Liang, C.F.; Yang, X.E. Elevated CO2 concentration increase the mobility of Cd and Zn in the rhizosphere of hyperaccumulator Sedum alfredii. Sci. Pollut. Res. 2014, 21, 5899–5908. [Google Scholar]
- Júnior, P.I.; de Lima, A.A.; Passos, S.R.; Tuão Gava, C.A.; de Oliveira, P.J.; Rumjanek, N.G.; Xavier, G.R. Phenotypic diversity and amylolytic activity of fast growing rhizobia from pigeonpea [Cajanus cajan (L.) Millsp]. Braz. J. Microbiol. 2012, 43, 1604–1612. [Google Scholar]
- Turdahon, M.; Osman, G.; Hamdun, M.; Yusuf, K.; Abdurehim, Z.; Abaydulla, G.; Abdukerim, M.; Fang, C.; Rahman, E. Rhizobium tarimense sp. nov., isolated from soil in the ancient Khiyik River. Int. J. Syst. Evol. Microbiol. 2013, 63, 2424–2429. [Google Scholar]
- Jia, X.; Zhao, J.; Zhang, N.; Zhao, Y.; Zhang, C.; Wang, L.; Cao, K.; Gao, Y. Elevated atmospheric CO2 generally improved soluble sugars content in the rhizosphere soil of black locust seedlings under cadmium exposure. Plant Soil 2021, 468, 197–209. [Google Scholar]
- Jia, X.; Zhao, Y.; He, Y.; Chang, Y. Glomalin-related soil protein in the rhizosphere of Robinia pseudoacacia L. seedlings under higher air temperature combined with Cd-contaminated soil. Eur. J. Soil Sci. 2018, 69, 634–645. [Google Scholar]
- Jia, X.; Zhao, Y.; Liu, T.; Huang, S.; Chang, Y. Elevated CO2 increases glomalin-related soil protein (GRSP) in the rhizosphere of Robinia pseudoacacia L. seedlings in Pb- and Cd-contaminated soils. Environ. Pollut. 2016, 218, 349–357. [Google Scholar]
- Wang, Y.; Zhang, C.; Wang, L.; Zhao, Y.; Gao, Y.; Jia, X. Influence of arbuscular mycorrhizal fungi on low molecular weight soluble compounds in the rhizosphere soil of black locust seedlings grown in cadmium-contaminated soils under elevated CO2 scenarios. Plant Soil 2023, 486, 469–486. [Google Scholar]
- Cui, S.; Xiao, H.; Miao, D.; Yang, W. Metal uptake and translocation by Chinese brake fern (Pteris vittata) and diversity of rhizosphere microbial communities under single and combined arsenic and cadmium stress. Environ. Sci. Pollut. Res. 2023, 30, 85198–85209. [Google Scholar]
- Zhou, N.; Li, X.; Zheng, Z.; Liu, J.; Downie, J.A.; Xie, F. RinRK1 enhances NF receptors accumulation in nanodomain-like structures at root-hair tip. Nat. Commun. 2024, 15, 3568. [Google Scholar]
- Jia, X.; Li, X.D.; Zhao, Y.H.; Wang, L.; Zhang, C.Y. Soil microbial community structure in the rhizosphere of Robinia pseudoacacia L. seedlings exposed to elevated air temperature and cadmium-contaminated soils for 4 years. Sci. Total Environ. 2019, 650, 2355–2363. [Google Scholar]
- Motaharpoor, Z.; Taheri, H.; Nadian, H. Rhizophagus irregularis modulates cadmium uptake, metal transporter, and chelator gene expression in Medicago sativa. Mycorrhiza 2019, 29, 389–395. [Google Scholar]
- Wang, L.; Jia, X.; Zhao, Y.; Zhang, C.; Zhao, J. Effect of arbuscular mycorrhizal fungi in roots on antioxidant enzyme activity in leaves of Robinia pseudoacacia L. seedlings under elevated CO2 and Cd exposure. Environ. Pollut. 2022, 294, 118652. [Google Scholar]
- Zhang, C.; Jia, X.; Zhao, Y.; Wang, L.; Wang, Y. Adaptive response of flavonoids in Robinia pseudoacacia L. affected by the contamination of cadmium and elevated CO2 to arbuscular mycorrhizal symbiosis. Ecotox. Environ. Saf. 2023, 263, 115379. [Google Scholar]
- Ali, B.; Gill, R.A.; Yang, S.; Gill, M.B.; Ali, S.; Rafiq, M.T.; Zhou, W. Hydrogen sulfide alleviates cadmium-induced morpho-physiological and ultrastructural changes in Brassica napus. Ecotoxicol. Environ. Saf. 2014, 110, 197–207. [Google Scholar] [PubMed]
- Mierzwa, B.; Łotocka, B.; Wdowiak-Wróbel, S.; Kalita, M.; Gnat, S.; Małek, W. Insight into the evolutionary history of symbiotic genes of Robinia pseudoacacia rhizobia deriving from Poland and Japan. Arch. Microbiol. 2010, 192, 341–350. [Google Scholar]
- Wei, G.; Chen, W.; Zhu, W.; Chen, C.; Young, J.P.; Bontemps, C. Invasive Robinia pseudoacacia in China is nodulated by Mesorhizobium and Sinorhizobium species that share similar nodulation genes with native American symbionts. FEMS Microbiol. Ecol. 2009, 68, 320–328. [Google Scholar]
- Ghnaya, T.; Mnassri, M.; Ghabriche, R.; Wali, M.; Poschenrieder, C.; Lutts, S.; Abdelly, C. Nodulation by Sinorhizobium meliloti originated from a mining soil alleviates Cd toxicity and increases Cd-phytoextraction in Medicago sativa L. Front. Plant Sci. 2015, 6, 863. [Google Scholar]
- Pueppke, S.G. The Genetic and Biochemical Basis for Nodulation of Legumes by Rhizobia. Crit. Rev. Biotechnol. 1996, 16, 1–51. [Google Scholar]
- Szewczuk-Karpisz, K.; Bajda, T.; Tomczyk, A.; Kuśmierz, M.; Komaniecka, I. Immobilization mechanism of Cd2+/HCrO4−/CrO42− ions and carboxin on montmorillonite modified with Rhizobium leguminosarum bv. trifolii exopolysaccharide. J. Hazard. Mater. 2022, 428, 128228. [Google Scholar]
- Vidal, C.; Chantreuil, C.; Berge, O.; Mauré, L.; Escarré, J.; Béna, G.; Brunel, B.; Cleyet-Marel, J.C. Mesorhizobium metallidurans sp. nov., a metal-resistant symbiont of Anthyllis vulneraria growing on metallicolous soil in Languedoc, France. Int. J. Syst. Evol. Microbiol. 2009, 59, 850–855. [Google Scholar]
- Cao, Y.; Tie, D.; Zhao, J.L.; Wang, X.B.; Yi, J.J.; Chai, Y.F.; Wang, K.F.; Wang, E.T.; Yue, M. Diversity and distribution of Sophora davidii rhizobia in habitats with different irradiances and soil traits in Loess Plateau area of China. Syst. Appl. Microbiol. 2021, 44, 126224. [Google Scholar]
- Nies, D.H. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 2003, 27, 313–339. [Google Scholar]
- Khanal, A.; Han, S.R.; Lee, J.H.; Oh, T.J. Comparative Genome Analysis of Polar Mesorhizobium sp. PAMC28654 to Gain Insight into Tolerance to Salinity and Trace Element Stress. Microorganisms 2024, 12, 120. [Google Scholar] [CrossRef]
- Lu, M.; Li, Z.; Liang, J.; Wei, Y.; Rensing, C.; Wei, G. Zinc Resistance Mechanisms of P1B-type ATPases in Sinorhizobium meliloti CCNWSX0020. Sci. Rep. 2016, 6, 29355. [Google Scholar]
- Bonaldi, D.S.; Funnicelli, M.I.G.; Fernandes, C.C.; Laurito, H.F.; Pinheiro, D.G.; Alves, L.M.C. Genetic and biochemical determinants in potentially toxic metals resistance and plant growth promotion in Rhizobium sp. LBMP-C04. World J. Microbiol. Biotechnol. 2024, 41, 7. [Google Scholar]
- Sun, H.X.; Chen, M.M.; Wei, L.; Xue, P.Y.; Zhao, Q.L.; Gao, P.P.; Geng, L.P.; Wen, Q.X.; Liu, W.J. Roots recruited distinct rhizo-microbial communities to adapt to long-term Cd and As co-contaminated soil in wheat-maize rotation. Environ. Pollut. 2024, 342, 123052. [Google Scholar]
- Pueppke, S.G.; Broughton, W.J. Rhizobium sp. strain NGR234 and R. fredii USDA257 share exceptionally broad, nested host ranges. Mol. Plant Microbe. Interact. 1999, 12, 293–318. [Google Scholar]

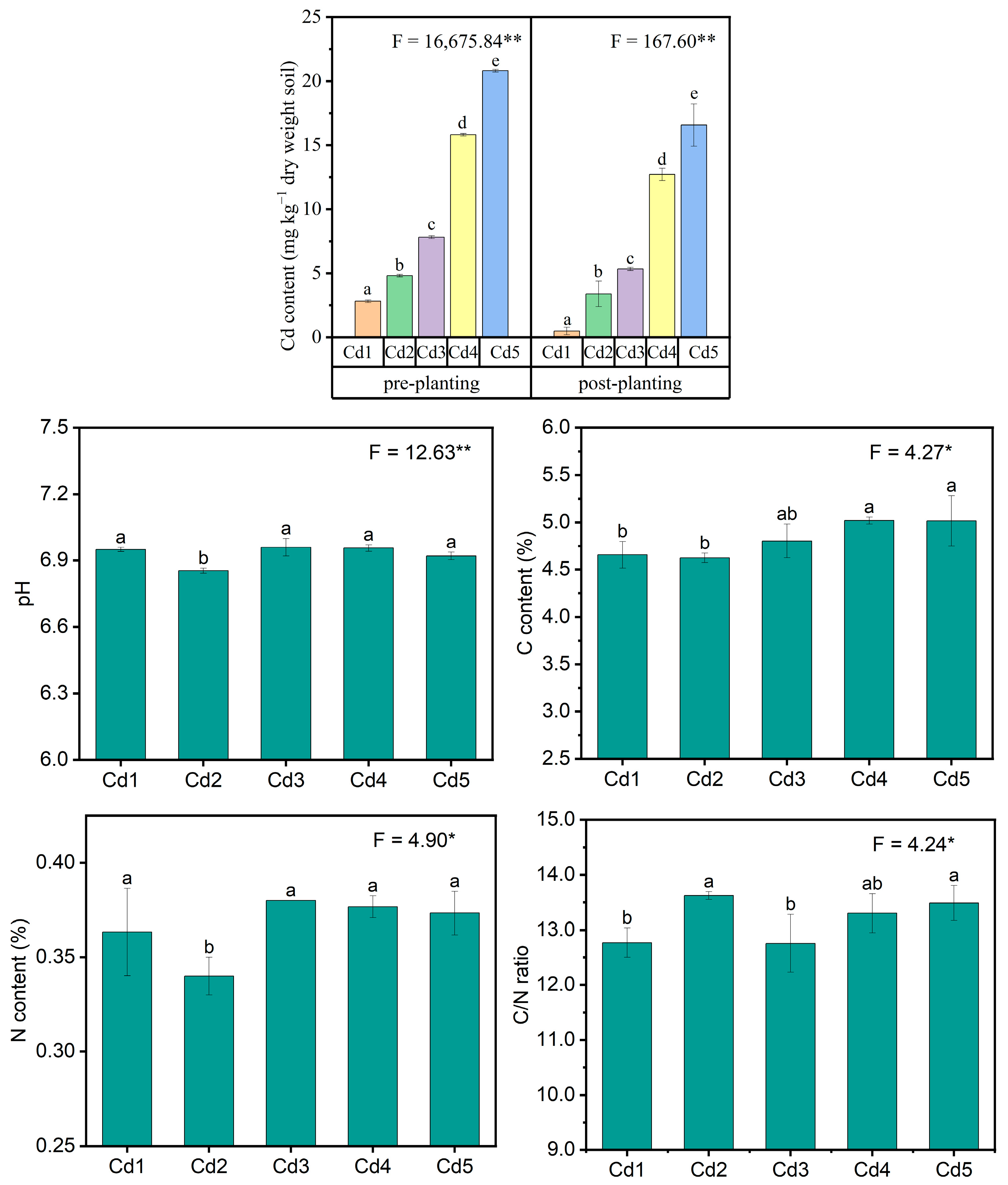
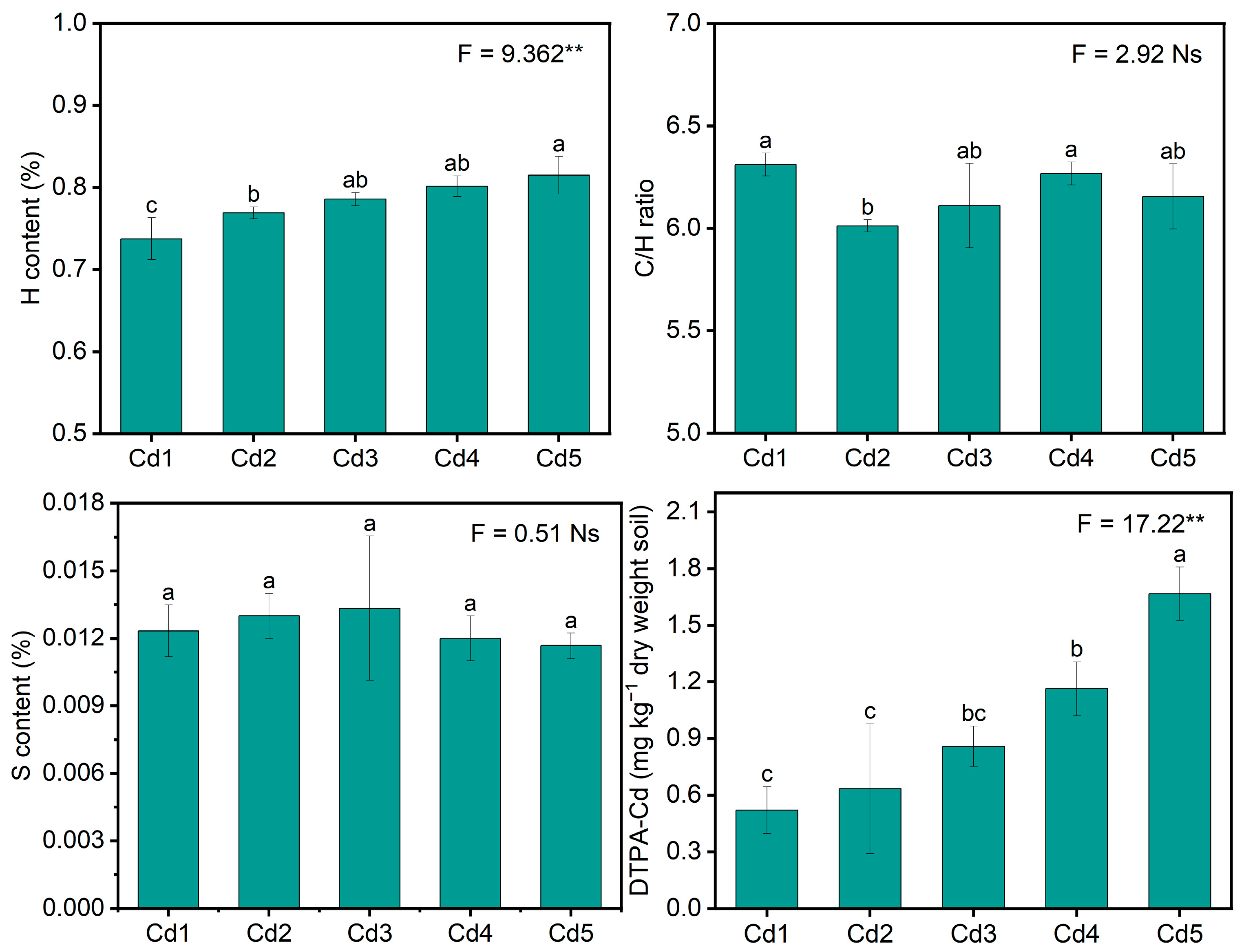


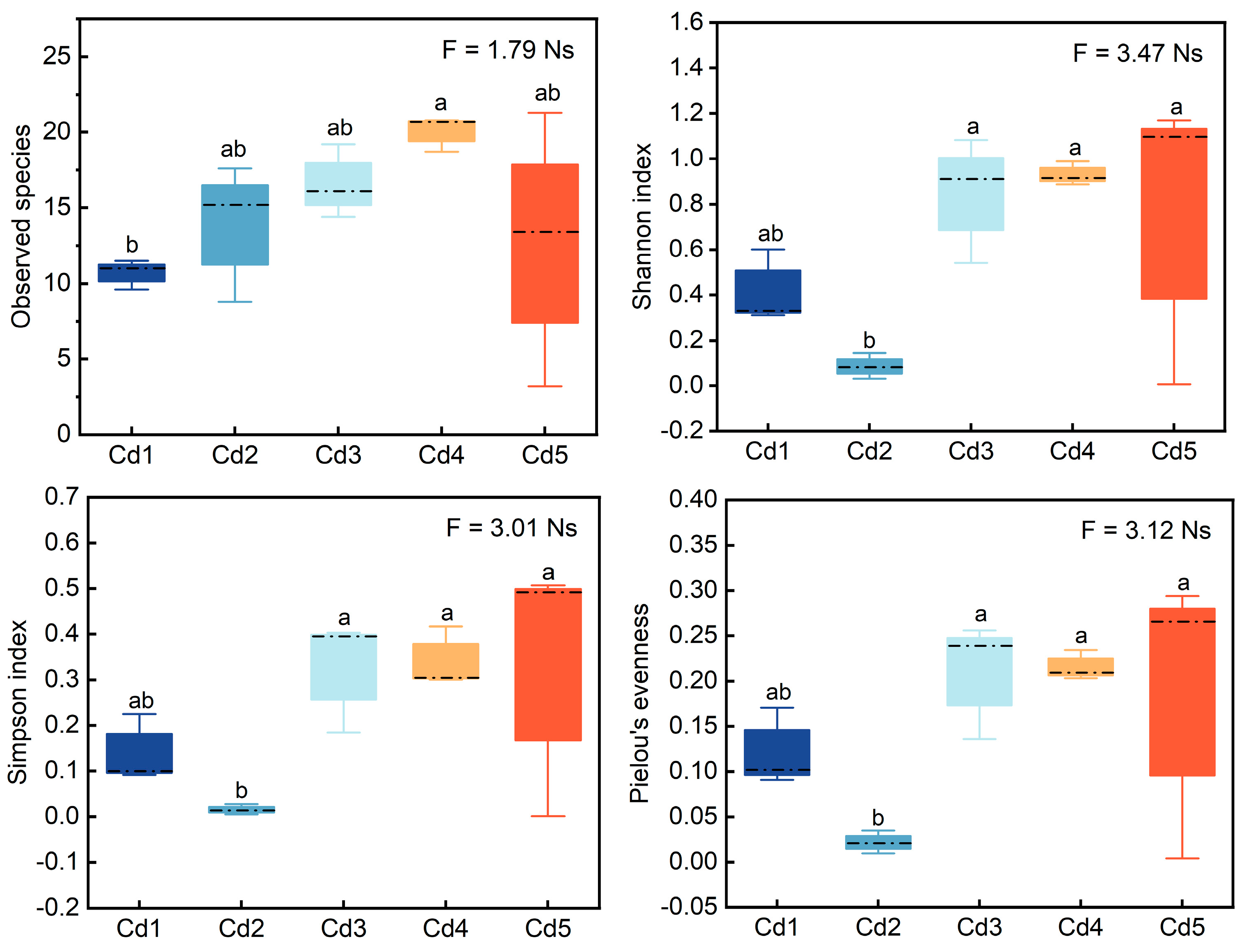

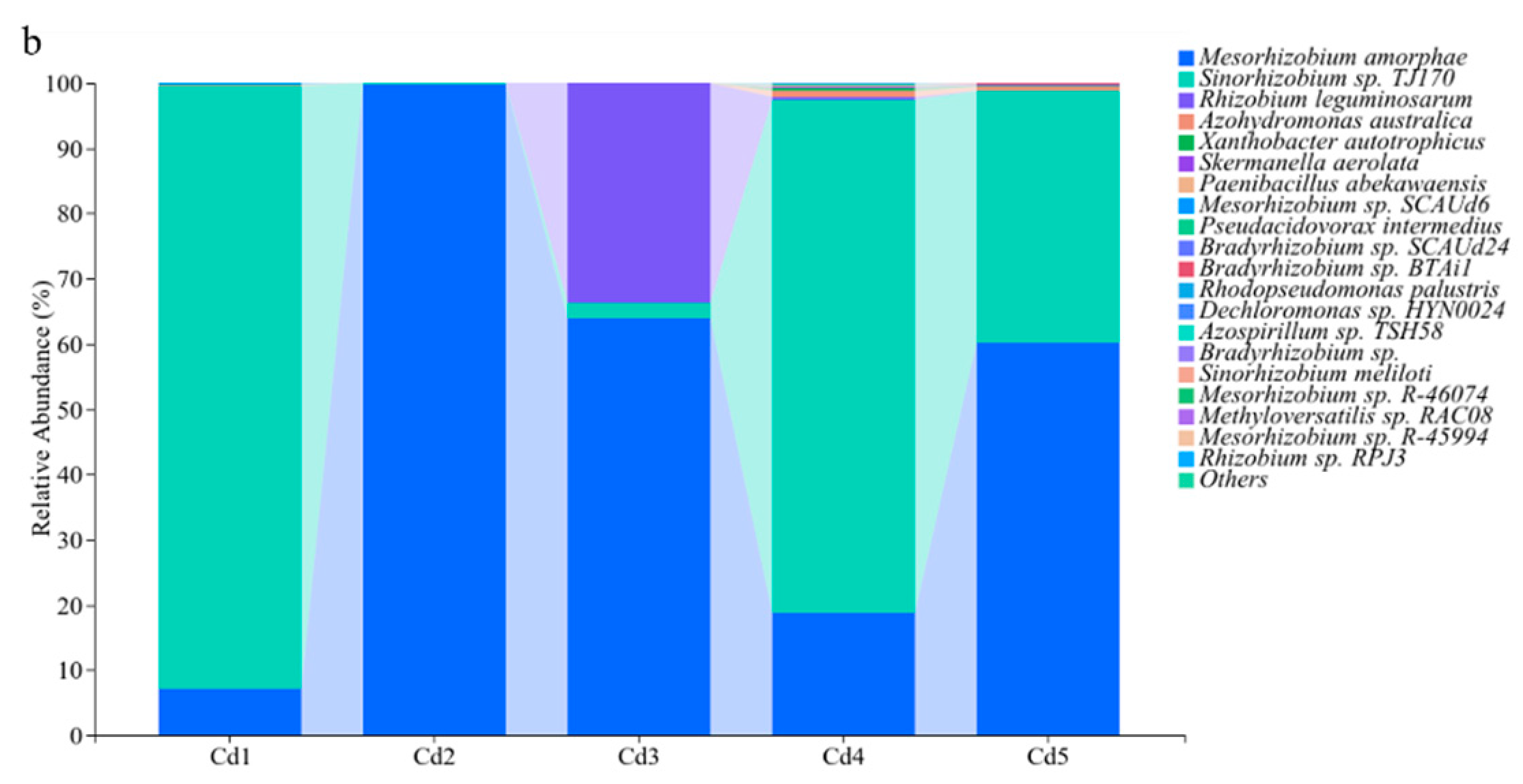


| Index | pH | Total Cd | DTPA-Cd | C/N Ratio | C/H Ratio | TS | H | TN | TC |
|---|---|---|---|---|---|---|---|---|---|
| Chao 1 | 0.123 | 0.174 | 0.208 | −0.034 | −0.434 | 0.278 | 0.179 | 0.003 | −0.076 |
| Observed species | 0.204 | 0.289 | 0.188 | 0.095 | 0.017 | 0.086 | 0.232 | 0.157 | 0.212 |
| Shannon | 0.528 * | 0.539 * | 0.453 | −0.292 | 0.042 | −0.019 | 0.319 | 0.506 | 0.300 |
| Simpson | 0.436 | 0.580 * | 0.497 | −0.272 | −0.025 | −0.090 | 0.334 | 0.475 | 0.277 |
| Pielou’s evenness | 0.54 * | 0.488 | 0.422 | −0.372 | 0.031 | −0.055 | 0.239 | 0.489 | 0.223 |
| Genus | pH | Cd | DTPA-Cd | C/N Ratio | C/H Ratio | TS | H | TN | TC |
|---|---|---|---|---|---|---|---|---|---|
| Mesorhizobium | −0.529 * | 0.022 | 0.026 | 0.493 | −0.533 * | 0.299 | 0.256 | −0.344 | −0.054 |
| Sinorhizobium | 0.454 | 0.033 | 0.006 | −0.220 | 0.634 * | −0.246 | −0.247 | 0.191 | 0.115 |
| Rhizobium | 0.089 | −0.149 | −0.093 | −0.546 * | −0.299 | −0.072 | −0.011 | 0.275 | −0.167 |
| Azohydromonas | 0.223 | 0.648 ** | 0.553 * | 0.286 | 0.361 | −0.182 | 0.494 | 0.354 | 0.626 * |
| Xanthobacter | 0.176 | 0.703 ** | 0.629 * | 0.170 | 0.252 | −0.288 | 0.434 | 0.331 | 0.515 * |
| Skermanella | 0.266 | 0.645 ** | 0.562 * | 0.178 | 0.262 | −0.233 | 0.330 | 0.216 | 0.427 |
| Bradyrhizobium | 0.324 | 0.372 | 0.257 | 0.221 | 0.286 | 0.026 | 0.343 | 0.235 | 0.451 |
| Paenibacillus | 0.271 | 0.215 | 0.120 | −0.146 | 0.226 | −0.087 | 0.070 | 0.216 | 0.184 |
| Pseudacidovorax | 0.175 | 0.446 | 0.418 | 0.186 | 0.426 | −0.101 | 0.308 | 0.309 | 0.500 |
| Rhodopseudomonas | 0.160 | 0.635 * | 0.523 * | 0.284 | 0.397 | −0.241 | 0.501 | 0.380 | 0.653 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Jia, X.; Zhao, Y.; Xie, Y.; Meng, X.; Wang, F. Diversity of nifH Gene in Culturable Rhizobia from Black Locust (Robinia pseudoacacia L.) Grown in Cadmium-Contaminated Soils. Biology 2025, 14, 362. https://doi.org/10.3390/biology14040362
Wang X, Jia X, Zhao Y, Xie Y, Meng X, Wang F. Diversity of nifH Gene in Culturable Rhizobia from Black Locust (Robinia pseudoacacia L.) Grown in Cadmium-Contaminated Soils. Biology. 2025; 14(4):362. https://doi.org/10.3390/biology14040362
Chicago/Turabian StyleWang, Xiaomeng, Xia Jia, Yonghua Zhao, Yuan Xie, Xiuxin Meng, and Fang Wang. 2025. "Diversity of nifH Gene in Culturable Rhizobia from Black Locust (Robinia pseudoacacia L.) Grown in Cadmium-Contaminated Soils" Biology 14, no. 4: 362. https://doi.org/10.3390/biology14040362
APA StyleWang, X., Jia, X., Zhao, Y., Xie, Y., Meng, X., & Wang, F. (2025). Diversity of nifH Gene in Culturable Rhizobia from Black Locust (Robinia pseudoacacia L.) Grown in Cadmium-Contaminated Soils. Biology, 14(4), 362. https://doi.org/10.3390/biology14040362








