Neuroprotective Potential of Stevia rebaudiana and Stachys sieboldii: Effects on Oxidative Stress and Locomotor Activity in Male Rats Fed a High-Fat, High-Sucrose Diet
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Animal Experiments and Diets
2.3. Diet Composition
- Control group (Intact): Corn—20.0%, rice—20.0%, bone meal—12.0%, sucrose—0.0%, soy oil—7.5%, lard—0.0%, gluten—20.0%, salt—0.35%, mineral mix—3.5%, vitamin mix—1.65%, inert material—15.0%.
- High-fat, high-sucrose diet group (HFHS): The diet composition was modified by reducing the corn content and eliminating soybean oil. Instead, pork lard was incorporated, increasing the lipid content. Additionally, sucrose was added to elevate carbohydrate levels. Corn—8.0%, rice—20.0%, bone meal—12.0%, sucrose—10.0%, soy oil—0.0%, lard—20.0%, gluten—20.0%, salt—0.35%, mineral mix—3.5%, vitamin mix—1.65%, inert material—4.5%
- HFHS + Stachys group: This diet included an additional 5 g/kg of Stachys sieboldii root powder, while maintaining the overall macronutrient balance similar to the HFHS group.
- HFHS + Stevia group: In this group, 5 g/kg of Stevia rebaudiana leaf powder was added to the diet instead of Stachys sieboldii, keeping the macronutrient composition consistent with the HFHS group [25].
2.4. Determination of Physiological Parameters
2.5. Euthanasia and Brain Tissue Collection
2.6. Biochemical Analysis in Brain Homogenates
2.7. Determination of Conjugated Dienes, Ketodienes, and Schiff Bases
2.8. Measurement of Malondialdehyde (MDA)
2.9. Statistical Analysis
3. Results
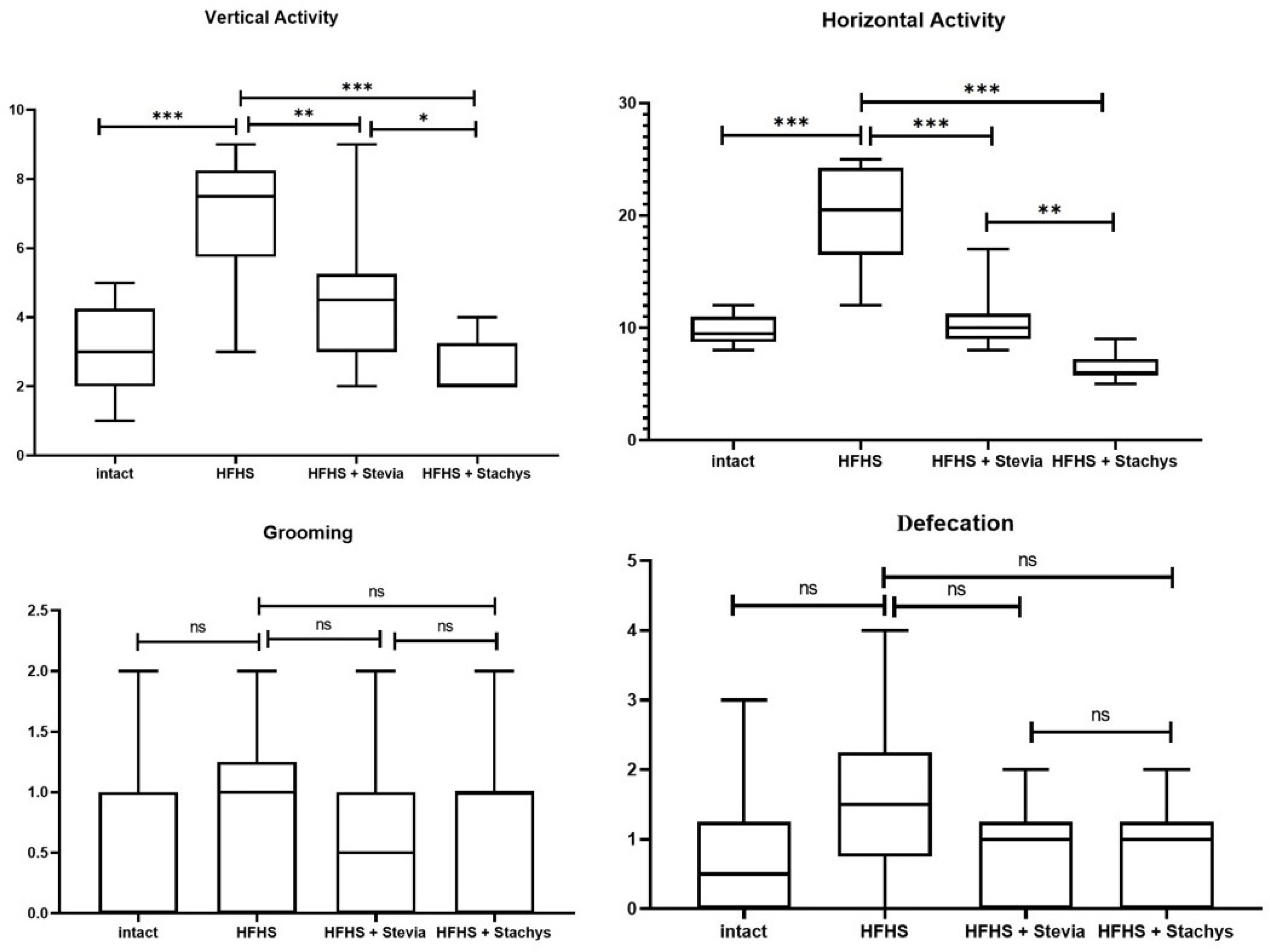
3.1. Changes in Behavioral Responses in Rats Under Dietary Exposure and Correction with Stachys and Stevia
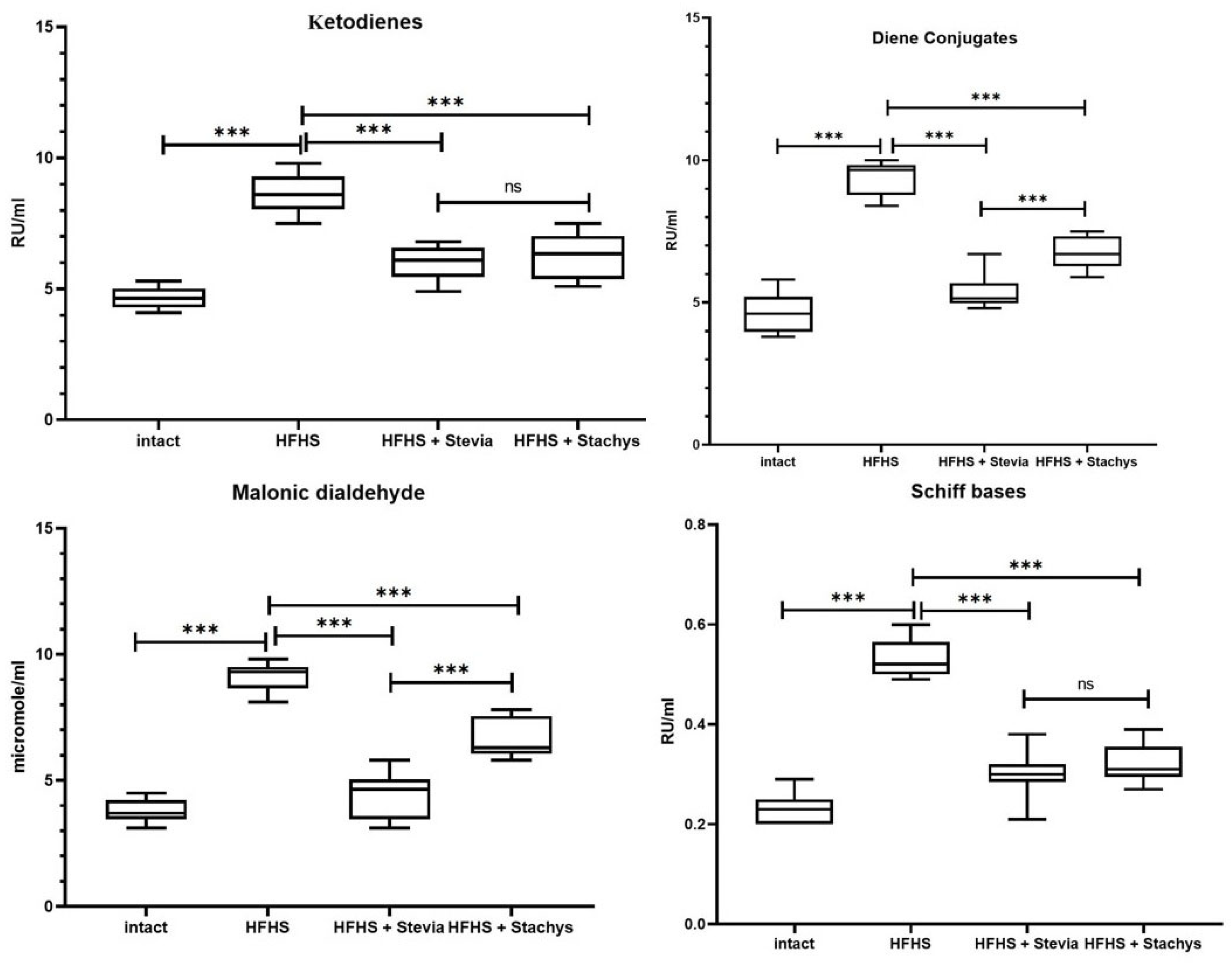
3.2. Levels of Lipid Peroxidation Products in Rat Brain Homogenates
3.3. Correlation Analysis of Behavioral and Biochemical Parameters in Experimental Group
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HFHS | High-fat, high-sugar |
| MDA | Malondialdehyde |
| DK | Conjugated dienes |
| KD | Ketodienes |
| SHO | Schiff bases |
| FDA | U.S. Food and Drug Administration |
| ANOVA | Analysis of variance |
| TBA | Thiobarbituric acid |
| UV–Vis | Ultraviolet–visible (spectroscopy) |
| rpm | Revolutions per minute |
| w/v | Weight/volume ratio |
| v/v | Volume/volume ratio |
| pH | Hydrogen ion concentration |
| r | Pearson’s rank correlation coefficient |
| RU/ml | Relative units per milliliter |
| μmol/ml | Micromoles per milliliter |
References
- Sinclair, D.A.; LaPlante, M.D. Lifespan: Why We Age and Why We Don’t Have To; Atria Books: New York, NY, USA, 2019. [Google Scholar]
- Volkert, D.; Delzenne, N.; Demirkan, K.; Schneider, S.; Abbasoglu, O.; Bahat, G.; Barazzoni, R.; Bauer, J.; Cuerda, C.; de van der Schueren, M.; et al. Nutrition for the older adult—Current concepts. Report from an ESPEN symposium. Clin. Nutr. 2024, 43, 1815–1824. [Google Scholar] [CrossRef]
- Whitelock, E.; Ensaff, H. On your own: Older adults’ food choice and dietary habits. Nutrients 2018, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Gropper, S.S. The role of nutrition in chronic disease. Nutrients 2023, 15, 664. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.L.; Gong, Y.; Qi, Y.J.; Shao, Z.M.; Jiang, Y.Z. Effects of dietary intervention on human diseases: Molecular mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2024, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, J. The hidden dangers of fast and processed food. Am. J. Lifestyle Med. 2018, 12, 375–381. [Google Scholar] [CrossRef]
- Fanzo, J.; Rudie, C.; Sigman, I.; Grinspoon, S.; Benton, T.G.; Brown, M.E.; Covic, N.; Fitch, K.; Golden, C.D.; Grace, D.; et al. Sustainable food systems and nutrition in the 21st century: A report from the 22nd annual Harvard Nutrition Obesity Symposium. Am. J. Clin. Nutr. 2022, 115, 18–33. [Google Scholar] [CrossRef]
- Tadesse, A.D.; Anto, T.G.; Birhanu, M.Y.; Agedew, E.; Yimer, B.; Abejie, A.N. Prevalence of undernutrition and its associated factors among older adults using Mini Nutritional Assessment tool in Womberma district, West Gojjam Zone, Amhara Region, North West Ethiopia, 2020. PLoS ONE 2023, 18, e0274557. [Google Scholar] [CrossRef]
- Temba, M.; Njobeh, P.; Adebo, O.; Omoyajowo, A.; Kayitesi, E. The role of compositing cereals with legumes to alleviate protein energy malnutrition in Africa. Int. J. Food Sci. Technol. 2016, 51, 543–554. [Google Scholar] [CrossRef]
- Kazantseva, O.A.; Leonova, A.A. Comparative assessment of the diets of elderly people in the Russian Federation and the Republic of Kazakhstan. In Proceedings of the 53rd Annual All-Russian Conference of Students and Young Scientists on Current Problems of Theoretical, Experimental, Clinical Medicine and Pharmacy, Tyumen, Russia, 27–28 March 2019; pp. 163–164. [Google Scholar]
- Sataeva, Z.I.; Musabekova, A.N. Comparative analysis of nutrition in the elderly population of Kazakhstan. Mech. Technol. 2021, 3, 5–13. [Google Scholar] [CrossRef]
- Dosmagambetova, R.S.; Terekhin, S.P.; Akhmetova, S.V. On the issue of healthy nutrition in old age. Med. Ecol. 2017, 3, 32–36. [Google Scholar]
- Espinosa-Salas, S.; Gonzalez-Arias, M. Nutrition: Macronutrient intake, imbalances, and interventions. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef] [PubMed]
- Al-Shami, I.; Al-Dalaeen, A.; Alkhatib, B.; Agraib, L.M. Dietary fat types consumption association with obesity and coronary indices. J. Nutr. Sci. 2023, 12, e110. [Google Scholar] [CrossRef] [PubMed]
- Muth, A.K.; Park, S.Q. The impact of dietary macronutrient intake on cognitive function and the brain. Clin. Nutr. 2021, 40, 3999–4010. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Sha, Q.; Zhang, X.; Liu, P.; Rong, S.; Han, T.; Liu, P.; Pan, H. The evaluation of the oxidative stress parameters in patients with primary angle-closure glaucoma. PLoS ONE 2011, 6, e27218. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Darenskaya, M.; Kolesnikova, L.; Kolesnikov, S. Oxidative stress: Pathogenetic role in diabetes mellitus and its complications development, therapeutic approaches to correction. Bull. Exp. Biol. Med. 2021, 171, 136–149. [Google Scholar] [CrossRef]
- Cobb, C.A.; Cole, M.P. Oxidative and nitrative stress in neurodegeneration. Neurobiol. Dis. 2015, 84, 4–21. [Google Scholar] [CrossRef]
- Wang, R.; Lan, Z.; Luo, Y.; Deng, Z. The complete chloroplast genome of Stachys geobombycis and comparative analysis with related Stachys species. Sci. Rep. 2024, 14, 8523. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic potential of phenolic compounds in medicinal plants—Natural health products for human health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Sarsenbaev, B.A.; Mursalieva, V.K.; Sultanova, N.A.; Mamontov, L.C.; Usenbekov, B.N. Phytochemical analysis of stevia and stachys for the content of biologically active substances. Bull. Kazakh Natl. Univ. Chem. Ser. 2012, 1, 370–374. [Google Scholar] [CrossRef]
- Jahani, R.; Khaledyan, D.; Jahani, A.; Jamshidi, E.; Kamalinejad, M.; Khoramjouy, M.; Faizi, M. Evaluation and comparison of the antidepressant-like activity of Artemisia dracunculus and Stachys lavandulifolia ethanolic extracts: An in vivo study. Res. Pharm. Sci. 2019, 14, 544. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakova, Y.; Solyanov, D.; Tatina, Y.; Britko, V.; Omarbekova, N.; Korshukova, M. Influence of Stachys sieboldii Miq. root powder on changes in neural system parameters in growing male rats on a high-fat and sucrose diet. Asian J. Agric. Biol. 2024, 4, 1–11. [Google Scholar] [CrossRef]
- Azizi, V.; Allahyari, F.; Hosseini, A. The anxiolytic and antidepressant effect of Stachys lavandulifolia in the experimental pentylenetetrazole-induced rat model of acute seizure. World J. Tradit. Chin. Med. 2023, 10, 114–120. [Google Scholar] [CrossRef]
- Harada, S.; Tsujita, T.; Ono, A.; Miyagi, K.; Mori, T.; Tokuyama, S. Stachys sieboldii (Labiatae, Chorogi) protects against learning and memory dysfunction associated with ischemic brain injury. J. Nutr. Sci. Vitaminol. 2015, 61, 167–174. [Google Scholar] [CrossRef]
- Jahangir Chughtai, M.F.; Pasha, I.; Zahoor, T.; Khaliq, A.; Ahsan, S.; Wu, Z.; Tanweer, S. Nutritional and therapeutic perspectives of Stevia rebaudiana as an emerging sweetener: A way forward for the sweetener industry. CyTA J. Food 2020, 18, 164–177. [Google Scholar] [CrossRef]
- Simlat, M.; Ptak, A.; Wójtowicz, T.; Szewczyk, A. The content of phenolic compounds in Stevia rebaudiana (Bertoni) plants derived from melatonin- and NaCl-treated seeds. Plants 2023, 12, 780. [Google Scholar] [CrossRef]
- Singh, D.; Kumari, M.; Prakash, H.G.; Rao, G.; Solomon, S. Phytochemical and pharmacological importance of Stevia: A calorie-free natural sweetener. Sugar Tech. 2019, 21, 227–234. [Google Scholar] [CrossRef]
- El Nashar, E.M.; Obydah, W.; Alghamdi, M.A.; Saad, S.; Yehia, A.; Maryoud, A.; Kiwan, N.A.; Alasmari, W.A.; Hussein, A.M. Effects of Stevia rebaudiana Bertoni extracts in the rat model of epilepsy induced by pentylenetetrazol: Sirt-1, at the crossroads between inflammation and apoptosis. J. Integr. Neurosci. 2022, 21, 21. [Google Scholar] [CrossRef]
- Dandin, E.; Ünal, İ.; Beler, M.; Ustundag, U.; Cansiz, D.; Ates-Kalkan, P.; Emekli-Alturfan, E. Stevioside improves brain oxidant-antioxidant status in overfed zebrafish. Eur. J. Biol. 2023, 82, 258–262. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Chan, P.; Sue, Y.M.; Liu, J.C.; Liang, T.H.; Huang, T.Y.; Tomlinson, B.; Chow, M.S.; Kao, P.F.; Chen, Y.J. Efficacy and tolerability of oral stevioside in patients with mild essential hypertension: A two-year, randomized, placebo-controlled study. Clin. Ther. 2003, 25, 2797–2808. [Google Scholar] [CrossRef]
- Yepifantseva, Y.V.; Abdrakhmanova, M.G.; Pozdnyakova, Y.V.; Semenikhina, P.S.; Belyayev, R.A.; Stupina, T.A.; Beisembayeva, M.; Adekenov, S.M. The level of reactive carbonyl derivatives of proteins, malondialdehyde, and catalase activity in the brain of rats after therapy following chronic unpredictable moderate stress. Open Access Maced. J. Med. Sci. 2020, 8, 691–698. [Google Scholar] [CrossRef]
- Ushkalova, V.N.; Kadochnikova, G.D. Use of parameters characterizing lipid peroxidation activity in studying human adaptation to new climatic and geographic conditions. Bull. Exp. Biol. Med. 1987, 103, 571–573. [Google Scholar]
- Madiyeva, L.S.; Mamaygalieva, S.B.; Rakhmetova, A.M.; Bakirova, R.E.; Nursultanova, S.D.; Ashirbekova, B.D.; Li, V.V. Clinical features and assessment of modified proteins in bronchial asthma of varying severity. Med. Ecol. 2023, 1, 27–31. [Google Scholar] [CrossRef]
- Kobi, J.B.B.S.; Matias, A.M.; Gasparini, P.V.F.; Torezani-Sales, S.; Madureira, A.R.; da Silva, D.S.; Correa, C.R.; Garcia, J.L.; Haese, D.; Nogueira, B.V.; et al. High-fat, high-sucrose, and combined high-fat/high-sucrose diets effects in oxidative stress and inflammation in male rats under presence or absence of obesity. Physiol. Rep. 2023, 11, e15635. [Google Scholar] [CrossRef]
- Melo, B.F.; Sacramento, J.F.; Ribeiro, M.J.; Prego, C.S.; Correia, M.C.; Coelho, J.C.; Cunha-Guimaraes, J.P.; Rodrigues, T.; Martins, I.B.; Guarino, M.P.; et al. Evaluating the impact of different hypercaloric diets on weight gain, insulin resistance, glucose intolerance, and its comorbidities in rats. Nutrients 2019, 11, 1197. [Google Scholar] [CrossRef]
- Gasparini, P.V.F.; Matias, A.M.; Torezani-Sales, S.; Kobi, J.B.B.S.; Siqueira, J.S.; Corrêa, C.R.; Lima-Leopoldo, A.P.; Leopoldo, A.S. High-fat and combined high-fat and sucrose diets promote cardiac oxidative stress independent of Nox2 redox regulation and obesity in rats. Cell Physiol. Biochem. 2021, 55, 618–634. [Google Scholar] [CrossRef]
- Santiago Santana, J.M.; Vega-Torres, J.D.; Ontiveros-Angel, P.; Bin Lee, J.; Arroyo Torres, Y.; Cruz Gonzalez, A.Y.; Aponte Boria, E.; Zabala Ortiz, D.; Alvarez Carmona, C.; Figueroa, J.D. Oxidative stress and neuroinflammation in a rat model of co-morbid obesity and psychogenic stress. Behav. Brain Res. 2021, 400, 112995. [Google Scholar] [CrossRef]
- Mabrok, H.B.; Ramadan, A.A.; Hamed, I.M.; Mohamed, D.A. Obesity as Inducer of Cognitive Function Decline via Dysbiosis of Gut Microbiota in Rats. Brain Sci. 2024, 14, 807. [Google Scholar] [CrossRef]
- Kim, H.H.; Jeong, S.H.; Park, M.Y.; Bhosale, P.B.; Abusaliya, A.; Lee, S.J.; Heo, J.D.; Kim, H.W.; Seong, J.K.; Kim, D.I.; et al. Binding affinity screening of polyphenolic compounds in Stachys affinis extract (SAE) for their potential antioxidant and anti-inflammatory effects. Sci. Rep. 2024, 14, 18095. [Google Scholar] [CrossRef]
- Lachowicz-Wiśniewska, S.; Pratap-Singh, A.; Kapusta, I.; Kruszyńska, A.; Rapak, A.; Ochmian, I.; Cebulak, T.; Żukiewicz-Sobczak, W.; Rubiński, P. Flowers and leaves extracts of Stachys palustris L. exhibit stronger antiproliferative, antioxidant, anti-diabetic, and anti-obesity potencies than stems and roots due to more phenolic compounds as revealed by UPLC-PDA-ESI-TQD-MS/MS. Pharmaceuticals 2022, 15, 785. [Google Scholar] [CrossRef]
- Simonyan, K.; Chavushyan, V.; Avetisyan, L.; Simonyan, R.; Isoyan, A.; Simonyan, G.; Hovhannisyan, L.; Simonyan, M. Regulatory effects of Stevia rebaudiana on NADPH oxidase-related manifestations of oxidative stress in diabetic rats with spinal cord injury. Neurophysiology 2021, 53, 13–21. [Google Scholar] [CrossRef]
- Hernández García, E.; Osnaya Brizuela, N.; Valenzuela Peraza, A.; Calderón Guzmán, D.; Ortiz Herrera, M.; Juárez Olguín, H.; Barragán Mejía, G.; Santamaría Del Ángel, D.; Rojas Ochoa, A. Biochemical and histological changes produced by sweeteners and cytarabine in the brain of young rats. Nutr. Hosp. 2018, 35, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Chavushyan, V.A.; Simonyan, K.V.; Simonyan, R.M.; Isoyan, A.S.; Simonyan, G.M.; Babakhanyan, M.A.; Hovhannisyian, L.E.; Nahapetyan, K.H.; Avetisyan, L.G.; Simonyan, M.A. Effects of Stevia on synaptic plasticity and NADPH oxidase level of CNS in conditions of metabolic disorders caused by fructose. BMC Complement. Altern. Med. 2017, 17, 540. [Google Scholar] [CrossRef]
- Khatun, M.C.S.; Muhit, M.A.; Hossain, M.J.; Al-Mansur, M.A.; Rahman, S.M.A. Isolation of phytochemical constituents from Stevia rebaudiana (Bert.) and evaluation of their anticancer, antimicrobial and antioxidant properties via in vitro and in silico approaches. Heliyon 2021, 7, e08475. [Google Scholar] [CrossRef]
- Wen, K.; Zhang, K.; Gao, W.; Bai, S.; Wang, J.; Song, W.; Zeng, Q.; Peng, H.; Lv, L.; Xuan, Y.; et al. Effects of Stevia extract on production performance, serum biochemistry, antioxidant capacity, and gut health of laying hens. Poult. Sci. 2024, 103, 103188. [Google Scholar] [CrossRef]
- Noreen, A.; Hussain, F.; Rashid, M. Insights on the antioxidant, antidiabetic, antiamnesic, cytotoxic, thrombolytic and antibiofilm activities of Stevia rebaudiana leaves. Prog. Nutr. 2020, 22, 3. [Google Scholar] [CrossRef]
- Carrera-Lanestosa, A.; Acevedo-Fernández, J.J.; Segura-Campos, M.R.; Velázquez-Martínez, R.; Moguel-Ordóñez, Y.B. Efecto antihipertensivo, antihiperglucemiante y antioxidante de los extractos de Stevia rebaudiana Bertoni (variedad criolla INIFAP C01) en ratas Wistar con síndrome metabólico inducido. Nutr. Hosp. 2020, 37, 730–741. [Google Scholar] [CrossRef]
- Papaefthimiou, M.; Kontou, P.I.; Bagos, P.G.; Braliou, G.G. Antioxidant activity of leaf extracts from Stevia rebaudiana Bertoni exerts attenuating effect on diseased experimental rats: A systematic review and meta-analysis. Nutrients 2023, 15, 3325. [Google Scholar] [CrossRef]
- Mostafa, A.F.; Elalfy, M.M.; Shata, A.; Elhadidy, M.G. Prophylactic effect of aquatic extract of stevia on acetic acid induced-ulcerative colitis in male rats: A possible role of Nrf2 and PPARγ. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 1093–1104. [Google Scholar] [CrossRef]
- Izumi, Y.; Kataoka, H.; Takada-Takatori, Y.; Koyama, Y.; Irie, K.; Akaike, A.; Kume, T. Isolation and purification of harpagogenin as an Nrf2-ARE activator from the tubers of chinese artichoke (Stachys sieboldii). Biol. Pharm. Bull. 2023, 46, 1576–1582. [Google Scholar] [CrossRef]
- Ravichandran, V.A.; Kim, M.; Han, S.K.; Cha, Y.S. Stachys sieboldii Extract supplementation attenuates memory deficits by modulating bdnf-creb and its downstream molecules, in animal models of memory impairment. Nutrients 2018, 10, 917. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Robea, M.A.; Balmus, I.M.; Ciobica, A.; Strungaru, S.; Plavan, G.; Gorgan, L.D.; Savuca, A.; Nicoara, M. Parkinson’s disease-induced zebrafish models: Focusing on oxidative stress implications and sleep processes. Oxid. Med. Cell. Longev. 2020, 2020, 1370837. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Park, M.; Lee, H.J. Stevia rebaudiana extract ameliorates insulin resistance by regulating mitochondrial function and oxidative stress in the skeletal muscle of db/db mice. BMC Complement. Med. Ther. 2023, 23, 264. [Google Scholar] [CrossRef]
- Deenadayalan, A.; Subramanian, V.; Paramasivan, V.; Veeraraghavan, V.P.; Rengasamy, G.; Coiambatore Sadagopan, J.; Rajagopal, P.; Jayaraman, S. Stevioside attenuates insulin resistance in skeletal muscle by facilitating IR/IRS-1/Akt/GLUT 4 signaling pathways: An In Vivo and In Silico Approach. Molecules 2021, 26, 7689. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, J.J.; Kim, Y.K.; Lee, Y.; Ha, J.H. Stachys sieboldii Miq. root attenuates weight gain and dyslipidemia in rats on a high-fat and high-cholesterol diet. Nutrients 2020, 12, 2063. [Google Scholar] [CrossRef]
- Rahzani, K.; Malekirad, A.A.; Zeraatpishe, A.; Hosseini, N.; Seify, S.M.; Abdollahi, M. Anti-oxidative stress activity of Stachys lavandulifolia aqueous extract in human. Cell J. 2013, 14, 314–317. [Google Scholar]

| Vertical Activity | Horizontal Activity | Grooming | Defecation | MDA | DK | KD | SHO | |
|---|---|---|---|---|---|---|---|---|
| Vertical Activity | 1.000 | 0.925 | 0.988 | 0.800 | 0.692 | 0.766 | 0.793 | 0.859 |
| Horizontal Activity | 0.925 | 1.000 | 0.972 | 0.968 | 0.914 | 0.953 | 0.965 | 0.989 |
| Grooming | 0.988 | 0.972 | 1.000 | 0.882 | 0.794 | 0.855 | 0.877 | 0.927 |
| Defecation | 0.800 | 0.968 | 0.882 | 1.000 | 0.987 | 0.999 | 0.999 | 0.994 |
| MDA | 0.692 | 0.914 | 0.794 | 0.987 | 1.000 | 0.994 | 0.989 | 0.964 |
| DK | 0.766 | 0.953 | 0.855 | 0.999 | 0.994 | 1.000 | 0.999 | 0.987 |
| KD | 0.793 | 0.965 | 0.877 | 0.999 | 0.989 | 0.999 | 1.000 | 0.993 |
| SHO | 0.859 | 0.989 | 0.927 | 0.994 | 0.964 | 0.987 | 0.993 | 1.000 |
| Vertical Activity | Horizontal Activity | Grooming | Defecation | MDA | DK | KD | SHO | |
|---|---|---|---|---|---|---|---|---|
| Vertical Activity | 1.000 | 0.954 | 0.998 | 0.996 | 0.963 | 0.969 | 0.999 | 0.986 |
| Horizontal Activity | 0.954 | 1.000 | 0.970 | 0.970 | 0.968 | 0.999 | 0.968 | 0.991 |
| Grooming | 0.998 | 0.970 | 1.000 | 0.945 | 0.977 | 0.981 | 1.000 | 0.994 |
| Defecation | 0.996 | 0.970 | 0.945 | 1.000 | 0.993 | 0.990 | 0.942 | 0.975 |
| MDA | 0.963 | 0.968 | 0.977 | 0.993 | 1.000 | 0.975 | 0.980 | 0.994 |
| DK | 0.969 | 0.999 | 0.981 | 0.990 | 0.975 | 1.000 | 0.980 | 0.997 |
| KD | 0.999 | 0.968 | 1.000 | 0.942 | 0.980 | 0.980 | 1.000 | 0.993 |
| SHO | 0.986 | 0.991 | 0.994 | 0.975 | 0.994 | 0.997 | 0.993 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozdnyakova, Y.; Murzatayeva, A. Neuroprotective Potential of Stevia rebaudiana and Stachys sieboldii: Effects on Oxidative Stress and Locomotor Activity in Male Rats Fed a High-Fat, High-Sucrose Diet. Biology 2025, 14, 359. https://doi.org/10.3390/biology14040359
Pozdnyakova Y, Murzatayeva A. Neuroprotective Potential of Stevia rebaudiana and Stachys sieboldii: Effects on Oxidative Stress and Locomotor Activity in Male Rats Fed a High-Fat, High-Sucrose Diet. Biology. 2025; 14(4):359. https://doi.org/10.3390/biology14040359
Chicago/Turabian StylePozdnyakova, Yelena, and Aigul Murzatayeva. 2025. "Neuroprotective Potential of Stevia rebaudiana and Stachys sieboldii: Effects on Oxidative Stress and Locomotor Activity in Male Rats Fed a High-Fat, High-Sucrose Diet" Biology 14, no. 4: 359. https://doi.org/10.3390/biology14040359
APA StylePozdnyakova, Y., & Murzatayeva, A. (2025). Neuroprotective Potential of Stevia rebaudiana and Stachys sieboldii: Effects on Oxidative Stress and Locomotor Activity in Male Rats Fed a High-Fat, High-Sucrose Diet. Biology, 14(4), 359. https://doi.org/10.3390/biology14040359




