Current and Projected Future Spatial Distribution Patterns of Prunus microcarpa in the Kurdistan Region of Iraq
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
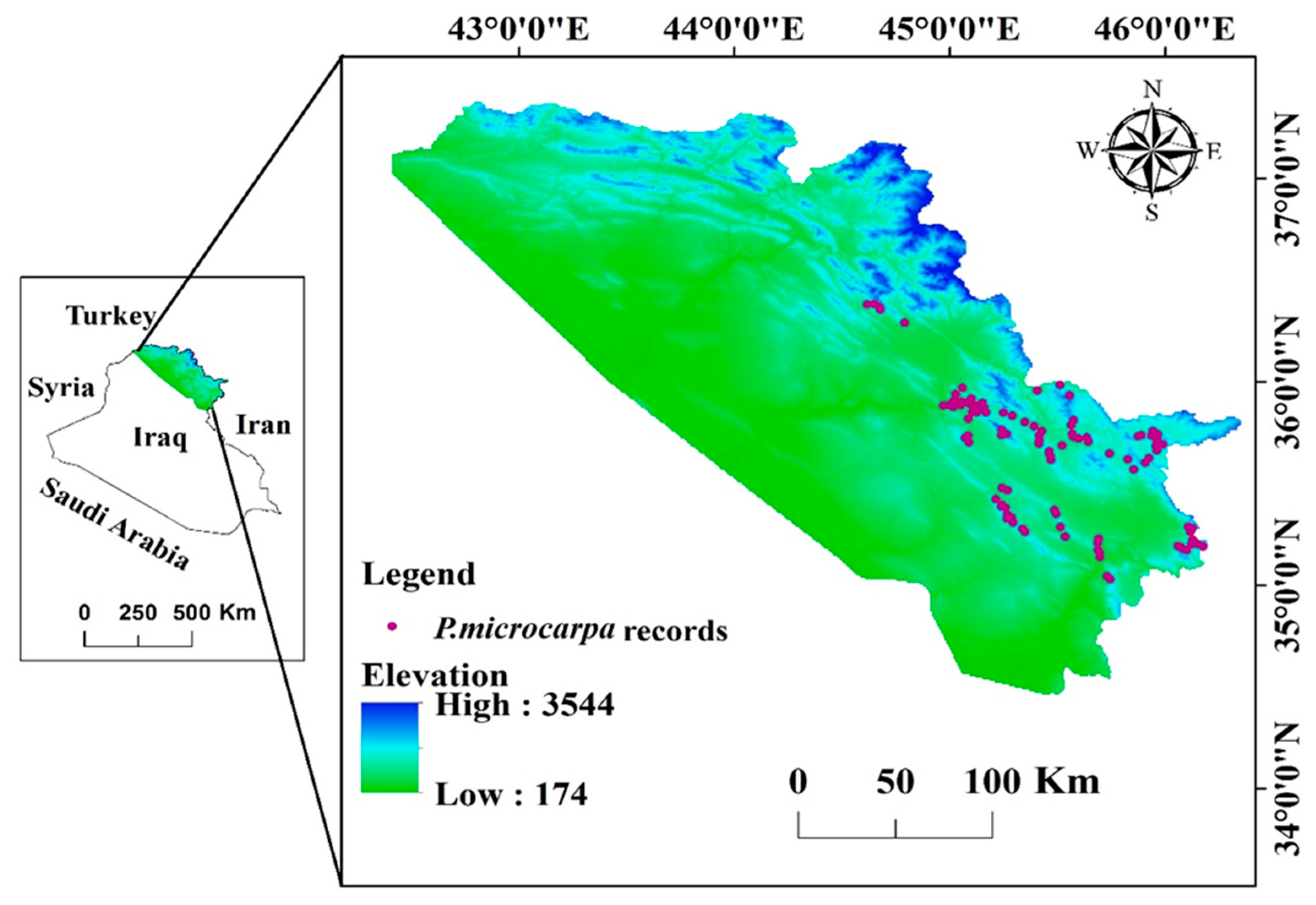
2.1. Study Area
2.2. Species Data
2.3. Environmental Variables
2.4. Model Building
2.5. Model Evaluation
2.6. Current and Future Change Analysis
3. Results
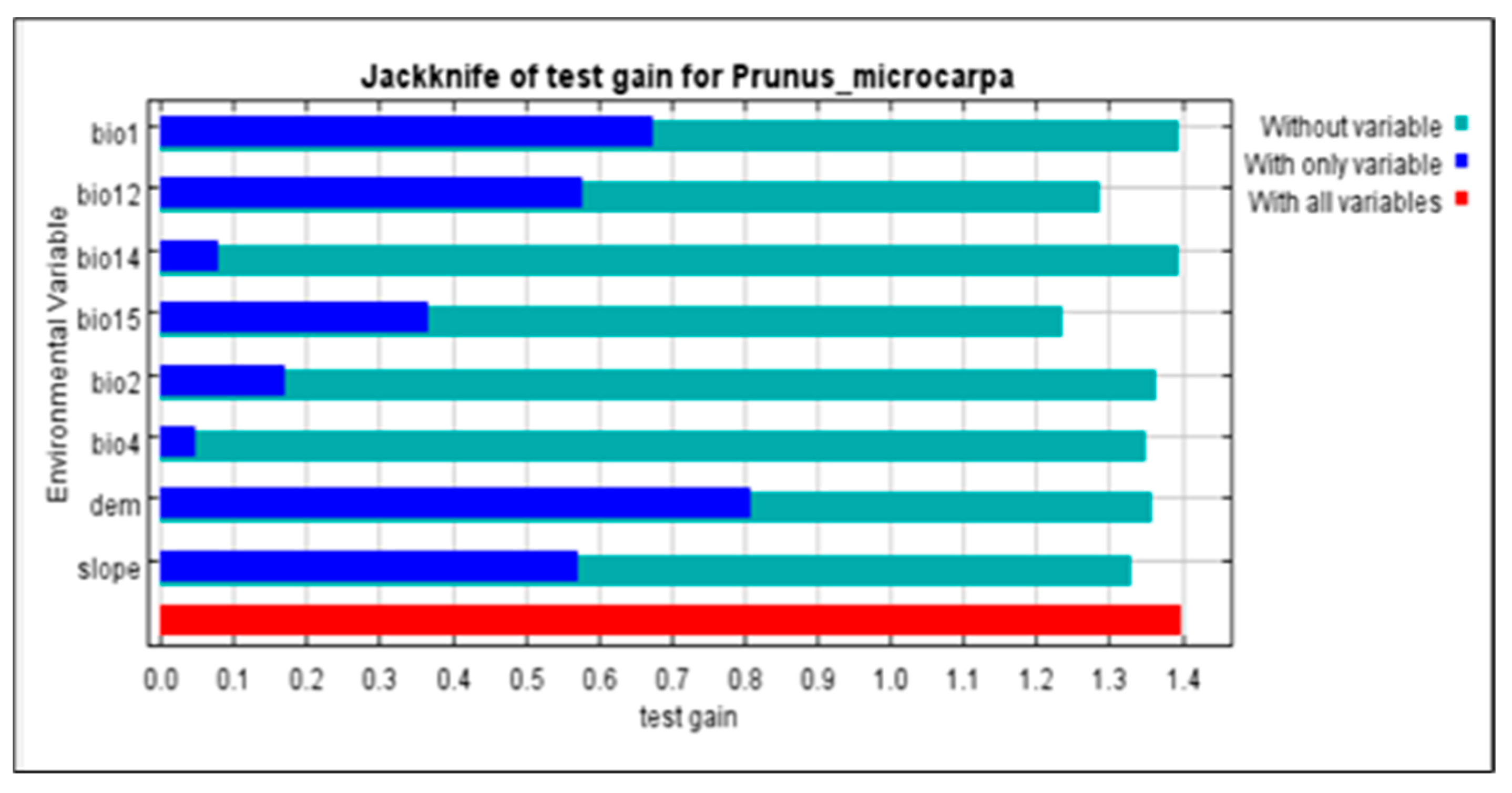
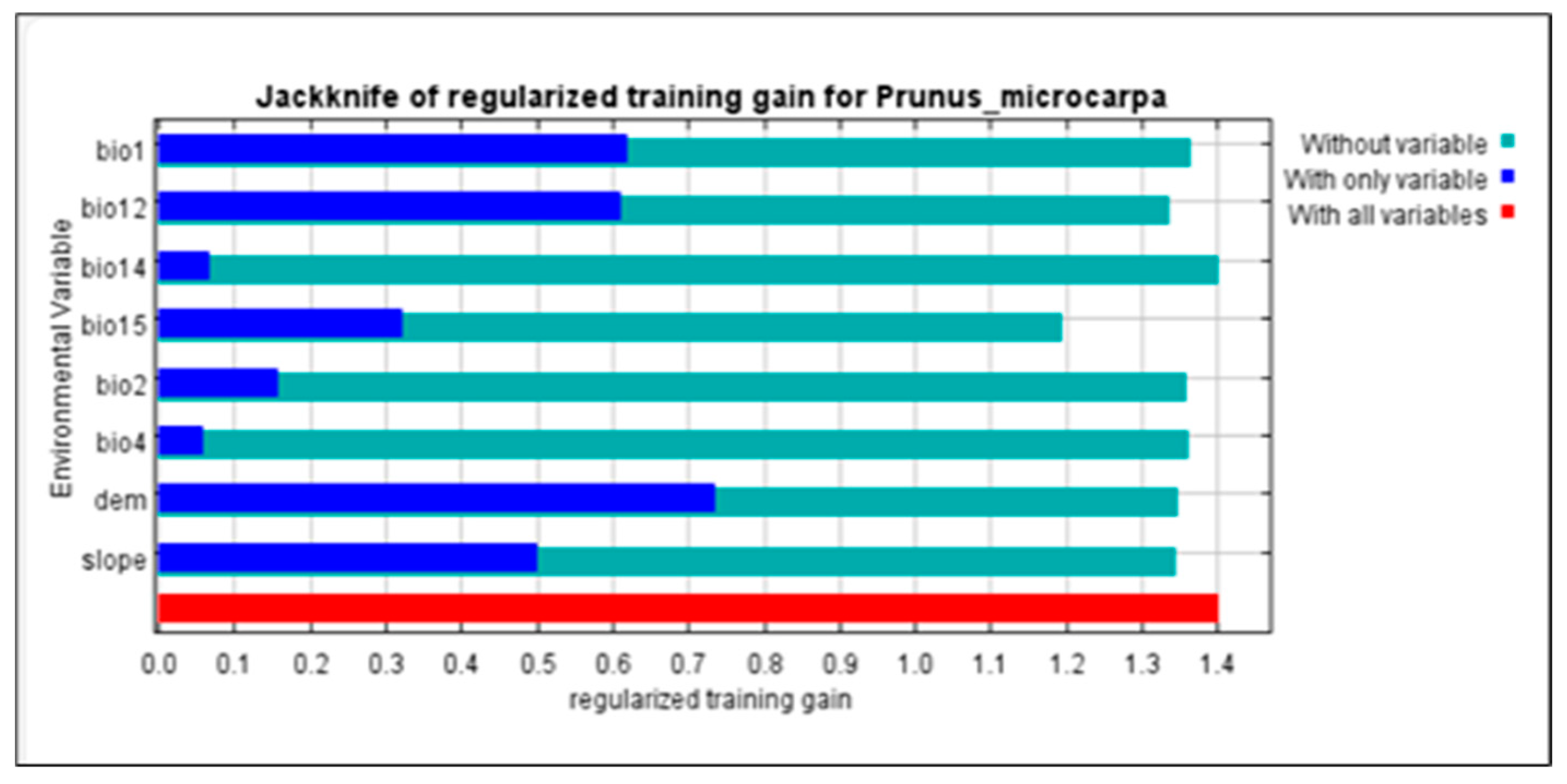
3.1. Distributions and Variable Importance
3.2. The Model’s Performance
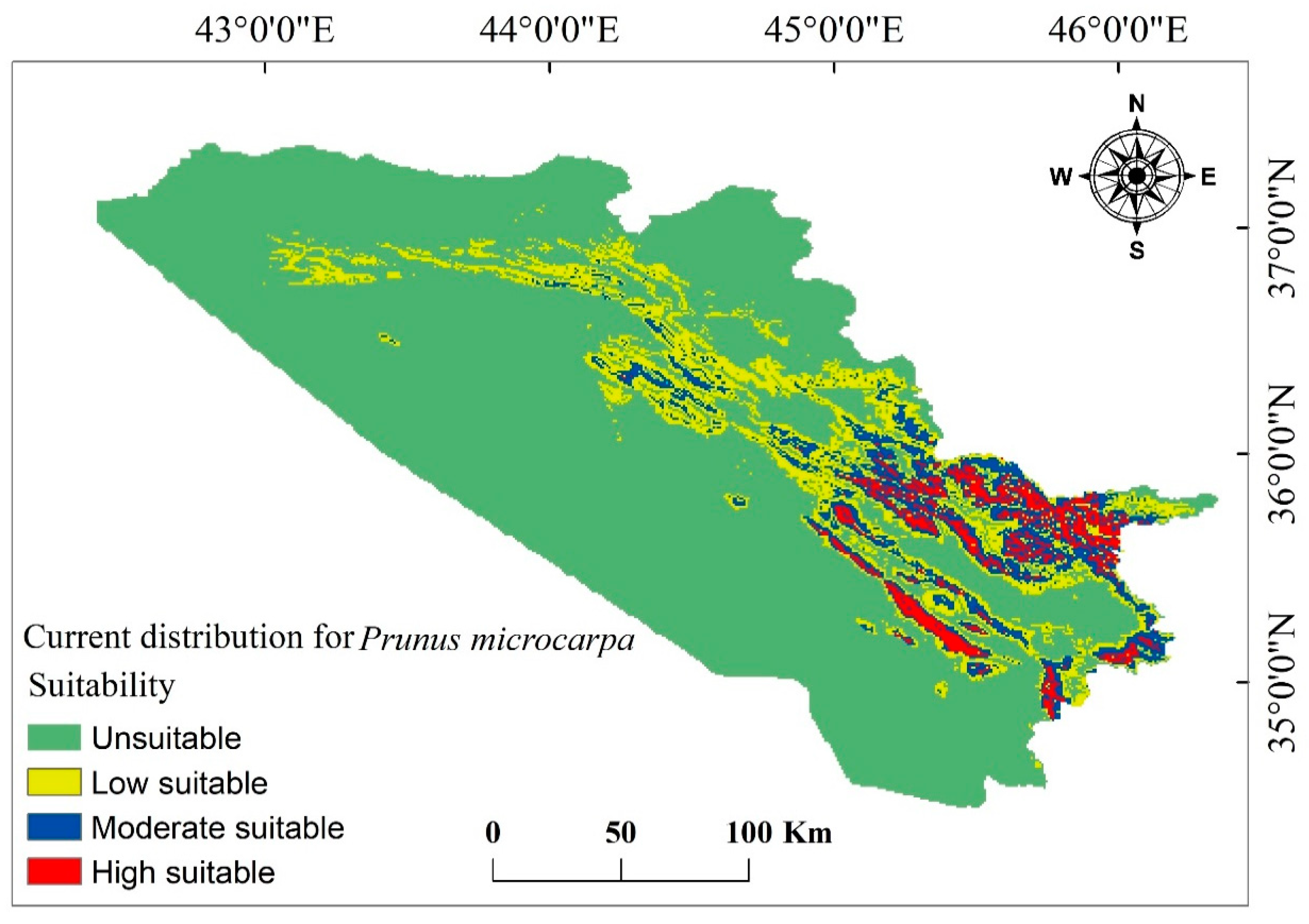
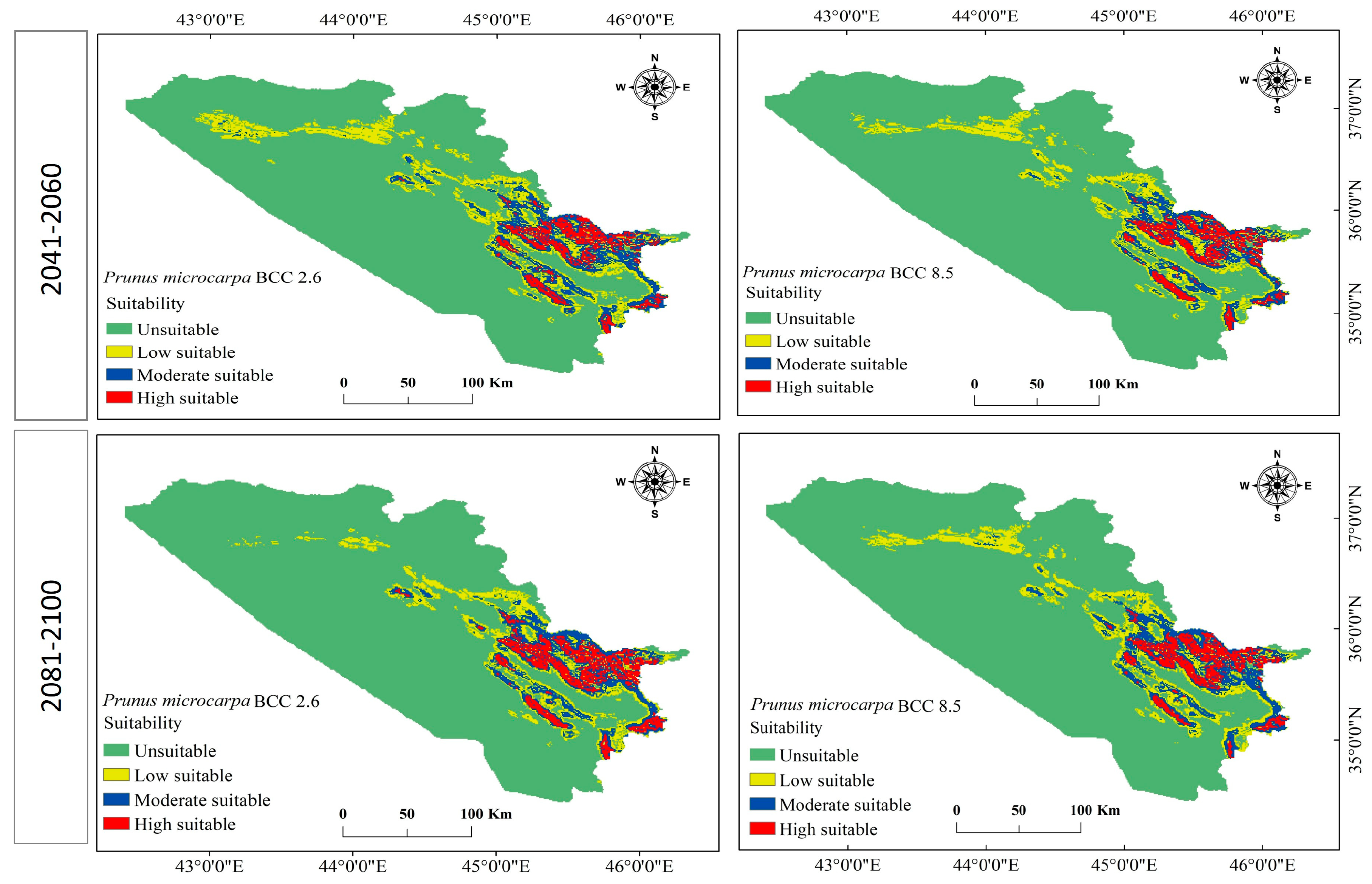
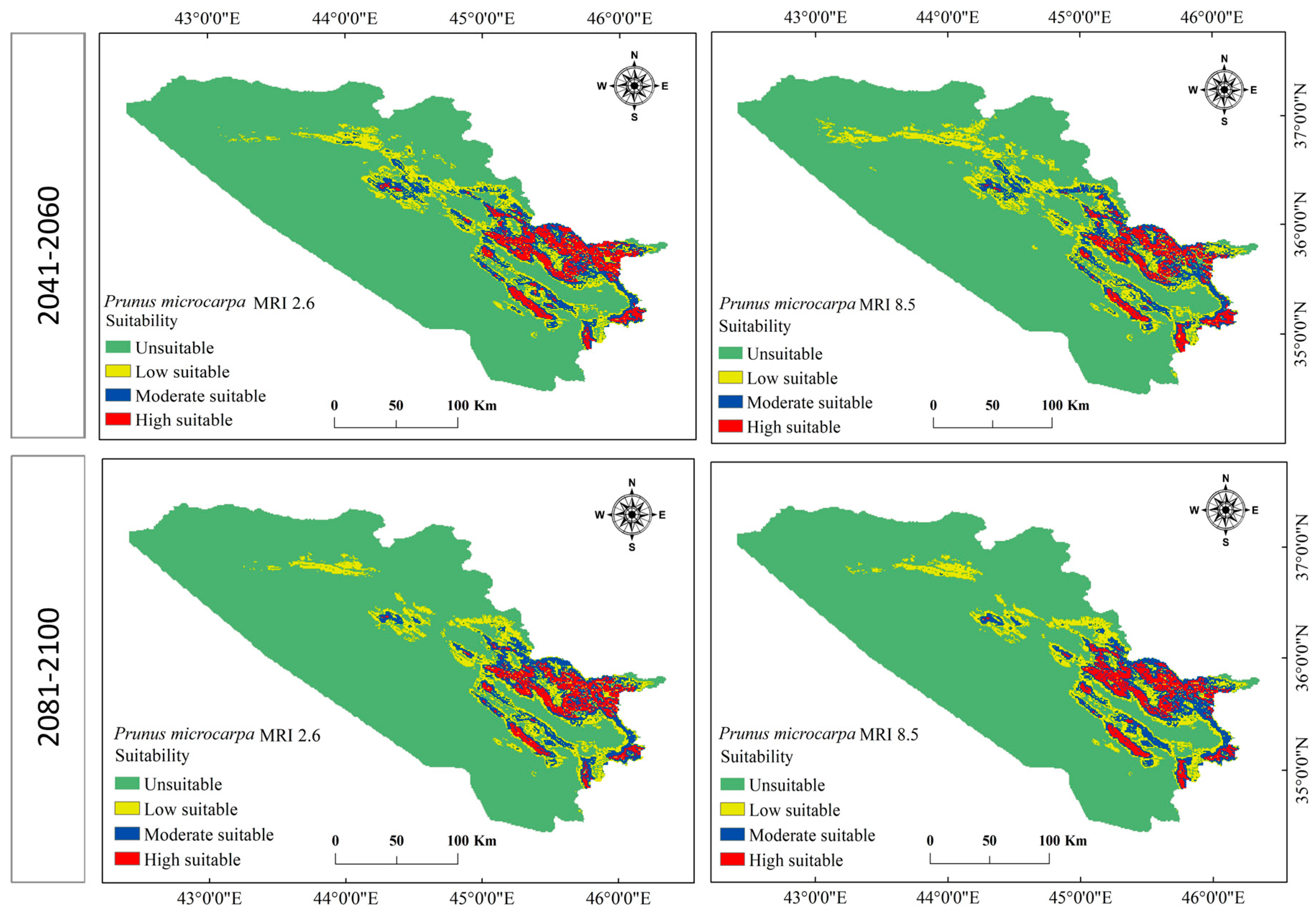
3.3. Current and Future Change Analysis for P. microcarpa
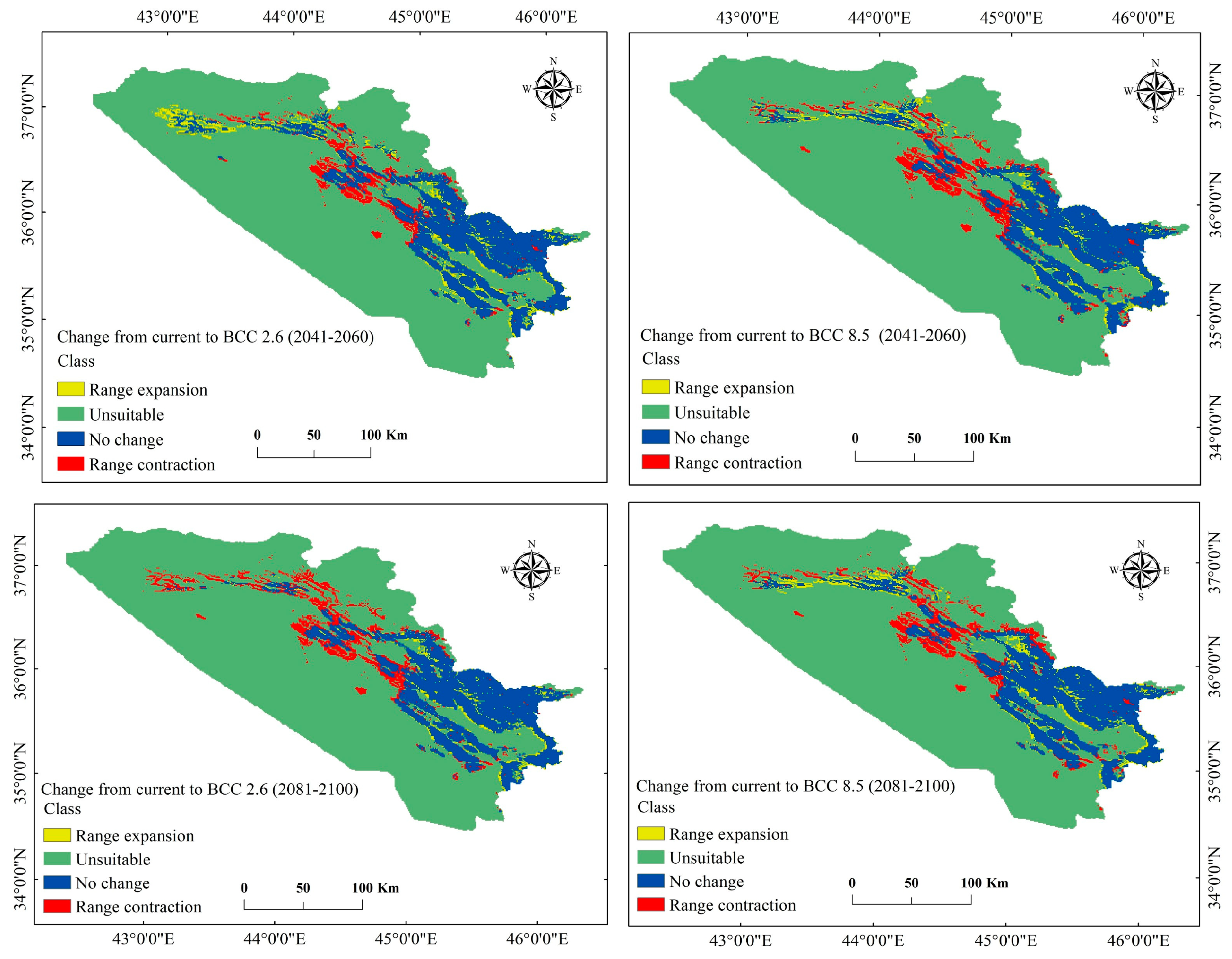
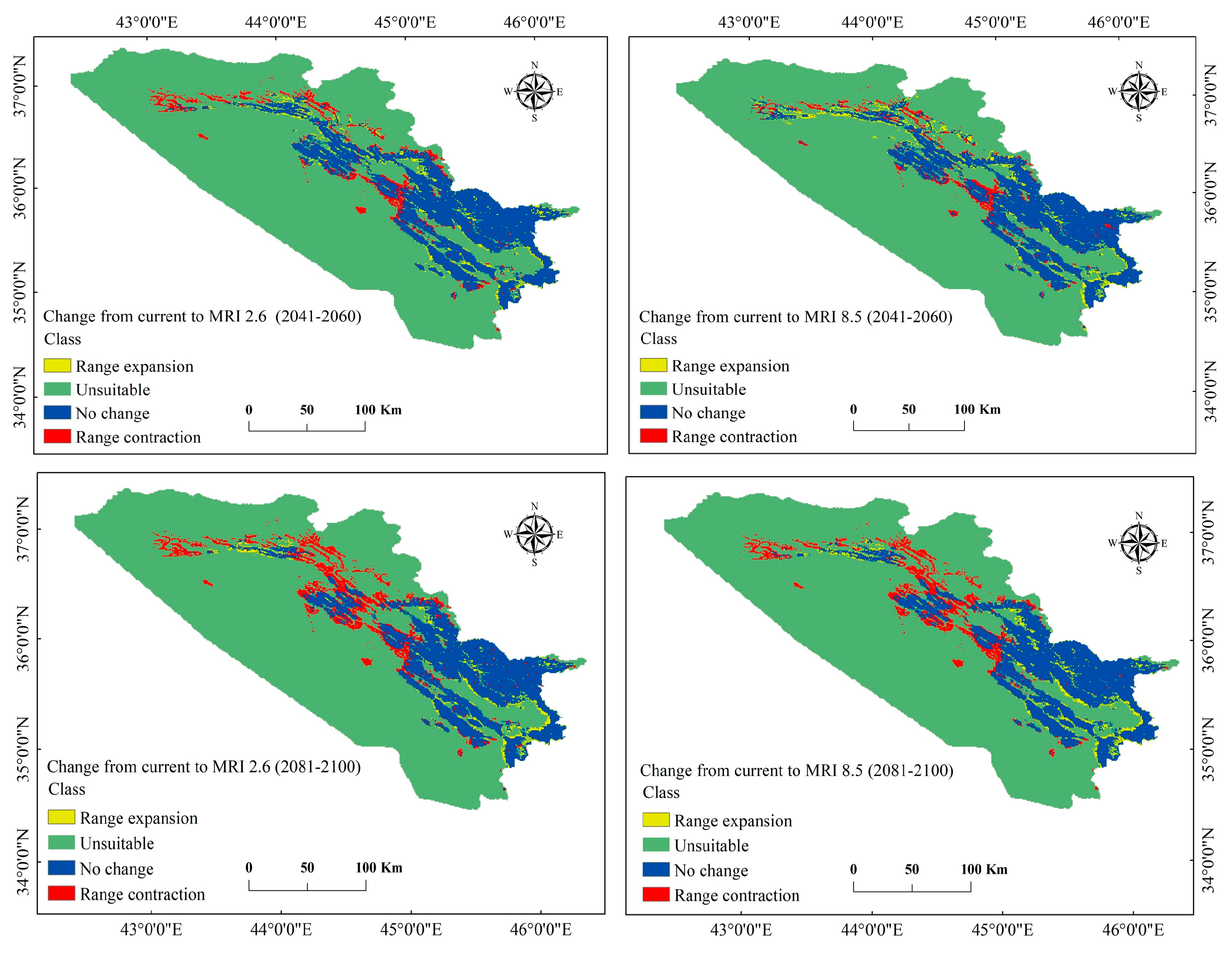
3.4. Current and Future Distribution Changes for P. microcarpa
4. Discussion
4.1. P. microcarpa Distribution Pattern Under Current and Future Climate Conditions
4.2. Variables Controlling the Distribution of P. microcarpa
4.3. Uncertainties of the Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalinina, A.; Brown, D.C. Micropropagation of ornamental Prunus spp. and GF305 peach, a Prunus viral indicator. Plant Cell Rep. 2007, 26, 927–935. [Google Scholar] [PubMed]
- Guest, E.; Townsend, C.C.; Iraq. Wizārat al-Zirāʻah, Iraq. Wizārat al-Zirāʻah wa-al-Iṣlāḥ al-Zirāʻī. In Flora of Iraq; Ministry of Agriculture of the Republic of Iraq, Royal Botanic Gardens, Kew: Baghdad, Iraq; Richmond, VA, USA; Surrey, UK, 1966. [Google Scholar]
- Hooker, W.J.; Prain, D.; Stapf, O.; Trust, B.-M.; Society, R.H.; Royal Botanic Gardens, K.; Trust, S.S.H. Curtis’s Botanical Magazine; Academic Press: Cambridge, MA, USA, 1911. [Google Scholar]
- Browicz, K. Distribution of Woody ‘Rosaceae’ in W. Asia; Polska Akademia Nauk, Zakład Dendrologii i Arboretum Kórnickie w Kórniku: Kórnik, Poland, 1969. [Google Scholar]
- Nas, M.N.; Bolek, Y.; Bardak, A. Genetic diversity and phylogenetic relationships of Prunus microcarpa CA Mey. subsp. tortusa analyzed by simple sequence repeats (SSRs). Sci. Hortic. 2011, 127, 220–227. [Google Scholar]
- Mohammadi, R.; Khadivi, A.; Khaleghi, A.; Akramian, M. Morphological characterization of Prunus microcarpa Boiss. germplasm: Implications for conservation and breeding. Sci. Hortic. 2019, 246, 718–725. [Google Scholar]
- Jalili, S.; Arzani, K.; Prudencio, A.S.; Salazar, J.A.; Martínez-García, P.J.; Bouzari, N.; Martínez-Gómez, P. Integrated Morphological, Physiological and Molecular Analysis of the Drought Response in Cultivated and Wild Prunus L. Subgenera Cerasus Species. Plant Mol. Biol. Rep. 2023, 41, 440–453. [Google Scholar] [CrossRef]
- Kawarty, A.M.A.; Behçet, L.; Çakilcioğlu, U. An ethnobotanical survey of medicinal plants in Ballakayati (Erbil, North Iraq). Turk. J. Bot. 2020, 44, 345–357. [Google Scholar] [CrossRef]
- Hussain, F.; Majid, S. Fatty Acids Composition of Seed and Fleshoil of Prunus microcarpa Fruit. J. Zankoy Sulaimani Part A 2003, 6, 51–60. [Google Scholar] [CrossRef]
- López-Tirado, J.; Vessella, F.; Schirone, B.; Hidalgo, P.J. Trends in evergreen oak suitability from assembled species distribution models: Assessing climate change in south-western Europe. New For. 2018, 49, 471–487. [Google Scholar]
- Awchi, T.A.; Kalyana, M.M. Meteorological drought analysis in northern Iraq using SPI and GIS. Sustain. Water Resour. Manag. 2017, 3, 451–463. [Google Scholar]
- Khwarahm, N.R. Modeling forest-shrubland fire susceptibility based on machine learning and geospatial approaches in mountains of Kurdistan Region, Iraq. Arab. J. Geosci. 2022, 15, 1184. [Google Scholar] [CrossRef]
- Khwarahm, N.R. Spatial modeling of land use and land cover change in Sulaimani, Iraq, using multitemporal satellite data. Environ. Monit. Assess. 2021, 193, 148. [Google Scholar] [CrossRef]
- Khwarahm, N.R.; Ararat, K.; Qader, S.; Al-Quraishi, A.M.F. Modelling habitat suitability for the breeding Egyptian vulture (Neophron percnopterus) in the Kurdistan Region of Iraq. Iran. J. Sci. Technol. Trans. A Sci. 2021, 45, 1519–1530. [Google Scholar] [CrossRef]
- Khwarahm, N.R. Predicting the spatial distribution of Hyalomma ssp., vector ticks of Crimean–Congo Haemorrhagic fever in Iraq. Sustainability 2023, 15, 13669. [Google Scholar] [CrossRef]
- Anderson, R.P.; Martınez-Meyer, E. Modeling species’ geographic distributions for preliminary conservation assessments: An implementation with the spiny pocket mice (Heteromys) of Ecuador. Biol. Conserv. 2004, 116, 167–179. [Google Scholar] [CrossRef]
- Sinclair, S.J.; White, M.D.; Newell, G.R. How useful are species distribution models for managing biodiversity under future climates? Ecol. Soc. 2010, 15, 8. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Mirhashemi, H.; Ahmadi, K.; Heydari, M.; Karami, O.; Valkó, O.; Khwarahm, N.R. Climatic variables are more effective on the spatial distribution of oak forests than land use change across their historical range. Environ. Monit. Assess. 2024, 196, 289. [Google Scholar] [CrossRef]
- Nasser, M. Forests and forestry in Iraq: Prospects and limitations. Commonw. For. Rev. 1984, 299–304. [Google Scholar]
- Bhatta, K.P.; Chaudhary, R.P.; Vetaas, O.R. A comparison of systematic versus stratified-random sampling design for gradient analyses: A case study in subalpine Himalaya, Nepal. Phytocoenologia 2012, 42, 191–202. [Google Scholar] [CrossRef]
- Brown, J.L. SDM toolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Huang, E.; Chen, Y.; Fang, M.; Zheng, Y.; Yu, S. Environmental drivers of plant distributions at global and regional scales. Glob. Ecol. Biogeogr. 2021, 30, 697–709. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar]
- Tu, W.; Xiong, Q.; Qiu, X.; Zhang, Y. Dynamics of invasive alien plant species in China under climate change scenarios. Ecol. Indic. 2021, 129, 107919. [Google Scholar]
- Hama, A.A.; Khwarahm, N.R. Predictive mapping of two endemic oak tree species under climate change scenarios in a semiarid region: Range overlap and implications for conservation. Ecol. Inform. 2023, 73, 101930. [Google Scholar]
- Elith, J.; Graham, C.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.; Huettmann, F.; Leathwick, J.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar]
- Moreno, J.S.; Sandoval-Arango, S.; Palacio, R.D.; Alzate, N.F.; Rincón, M.; Gil, K.; Morales, N.G.; Harding, P.; Hazzi, N.A. Distribution models and spatial analyses provide robust assessments of conservation status of orchid species in Colombia: The case of Lephantes mucronata. Harv. Pap. Bot. 2020, 25, 111–121. [Google Scholar]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. A maximum entropy approach to species distribution modeling. In Proceedings of the Twenty-First International Conference on Machine Learning, Banff, AB, Canada, 4–8 July 2004; p. 83. [Google Scholar] [CrossRef]
- Morales, N.S.; Fernández, I.C.; Baca-González, V. MaxEnt’s parameter configuration and small samples: Are we paying attention to recommendations? A systematic review. PeerJ 2017, 5, e3093. [Google Scholar]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar]
- Phillips, S.J. A brief tutorial on Maxent. AT&T Res. 2005, 190, 231–259. [Google Scholar]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Lobo, J.M. Threshold criteria for conversion of probability of species presence to either–or presence–absence. Acta Oecologica 2007, 31, 361–369. [Google Scholar] [CrossRef]
- Jiang, R.; Zou, M.; Qin, Y.; Tan, G.; Huang, S.; Quan, H.; Zhou, J.; Liao, H. Modeling of the potential geographical distribution of three Fritillaria species under climate change. Front. Plant Sci. 2022, 12, 749838. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Mulieri, P.R.; Patitucci, L.D. Using ecological niche models to describe the geographical distribution of the myiasis-causing Cochliomyia hominivorax (Diptera: Calliphoridae) in southern South America. Parasitol. Res. 2019, 118, 1077–1086. [Google Scholar] [CrossRef]
- Arslan, E.S.; Akyol, A.; Örücü, Ö.K.; Sarıkaya, A.G. Distribution of rose hip (Rosa canina L.) under current and future climate conditions. Reg. Environ. Change 2020, 20, 107. [Google Scholar] [CrossRef]
- Ashrafzadeh, M.R.; Naghipour, A.A.; Haidarian, M.; Kusza, S.; Pilliod, D.S. Effects of climate change on habitat and connectivity for populations of a vulnerable, endemic salamander in Iran. Glob. Ecol. Conserv. 2019, 19, e00637. [Google Scholar] [CrossRef]
- Khwarahm, N.R. Mapping current and potential future distributions of the oak tree (Quercus aegilops) in the Kurdistan Region, Iraq. Ecol. Process. 2020, 9, 56. [Google Scholar] [CrossRef]
- Çoban, H.O.; Örücü, Ö.K.; Arslan, E.S. MaxEnt modeling for predicting the current and future potential geographical distribution of Quercus libani Olivier. Sustainability 2020, 12, 2671. [Google Scholar] [CrossRef]
- Dimobe, K.; Ouédraogo, A.; Ouédraogo, K.; Goetze, D.; Stein, K.; Schmidt, M.; Nacoulma, B.M.I.; Gnoumou, A.; Traoré, L.; Porembski, S. Climate change reduces the distribution area of the shea tree (Vitellaria paradoxa CF Gaertn.) in Burkina Faso. J. Arid Environ. 2020, 181, 104237. [Google Scholar] [CrossRef]
- Sommer, J.H.; Kreft, H.; Kier, G.; Jetz, W.; Mutke, J.; Barthlott, W. Projected impacts of climate change on regional capacities for global plant species richness. Proc. R. Soc. B Biol. Sci. 2010, 277, 2271–2280. [Google Scholar]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Naghipour, A.A.; Asl, S.T.; Ashrafzadeh, M.R.; Haidarian, M. Predicting the potential distribution of Crataegus azarolus L. under climate change in central Zagros, Iran. J. Wildl. Biodivers. 2021, 5, 28–43. [Google Scholar]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Hassan, W.H.; Nile, B.K. Climate change and predicting future temperature in Iraq using CanESM2 and HadCM3 modeling. Model. Earth Syst. Environ. 2021, 7, 737–748. [Google Scholar]
- Taleshi, H.; Jalali, S.G.; Alavi, S.J.; Hosseini, S.M.; Naimi, B.; Zimmermann, N.E. Climate change impacts on the distribution and diversity of major tree species in the temperate forests of Northern Iran. Reg. Environ. Change 2019, 19, 2711–2728. [Google Scholar]
- Bazrmanesh, A.; Tarkesh, M.; Bashari, H.; Poormanafi, S. Effect of climate change on the ecological niches of the climate of Bromus tomentellus Boiss using Maxent in Isfahan province. J. Range Watershed Manag. 2019, 71, 857–867. [Google Scholar]
- Mahmoodi, S.; Heydari, M.; Ahmadi, K.; Khwarahm, N.R.; Karami, O.; Almasieh, K.; Naderi, B.; Bernard, P.; Mosavi, A. The current and future potential geographical distribution of Nepeta crispa Willd., an endemic, rare and threatened aromatic plant of Iran: Implications for ecological conservation and restoration. Ecol. Indic. 2022, 137, 108752. [Google Scholar]
- Becerra-López, J.L.; Ramírez-Bautista, A.; Romero-Méndez, U.; Pavón, N.P.; Sánchez-Rojas, G. Effect of climate change on halophytic grasslands loss and its impact in the viability of Gopherus flavomarginatus. Nat. Conserv. 2017, 21, 39. [Google Scholar] [CrossRef]
| Environmental Variable (Abbreviation) | Environmental Variable (Abbreviation) |
|---|---|
| Annual Mean Temperature (bio 1) | Precipitation of Driest Month (bio 14) |
| Mean Diurnal Range (bio 2) | Precipitation of Seasonality (bio 15) |
| Isothermality (bio 3) | Precipitation of Wettest Quarter (bio 16) |
| Temperature Seasonality (bio 4) | Precipitation of Driest Quarter (bio 17) |
| Max Temperature of Warmest Month (bio 5) | Precipitation of Warmest Quarter (bio 18) |
| Min Temperature of Coldest Month (bio 6) | Precipitation of Coldest Quarter (bio 19) |
| Temperature Annual Range (bio 7) | Digital Elevation Model (m) DEM |
| Mean Temperature of Wettest Quarter (bio 8) | Slope (degree) |
| Mean Temperature of Driest Quarter (bio 9) | Aspect (degree) |
| Mean Temperature of Warmest Quarter (bio 10) | |
| Mean Temperature of Coldest Quarter (bio11) | |
| Annual Precipitation (bio12) | |
| Precipitation of Wettest Month (bio13) |
| Variable | Contribution % | Permutation Importance % |
|---|---|---|
| dem | 31.8 | 10.3 |
| bio15 | 23 | 41.6 |
| slope | 21.6 | 5.6 |
| bio12 | 10 | 19.7 |
| bio2 | 4.9 | 4.3 |
| bio14 | 4.6 | 0 |
| bio4 | 3.2 | 4.4 |
| bio1 | 0.8 | 13.9 |
| Category | Current Distribution | Future Distribution | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BCC CSM2 MR | ||||||||||
| 2041–2060 | 2081–2100 | |||||||||
| SSP1 2.6 | SSP5 8.5 | SSP1 2.6 | SSP5 8.5 | |||||||
| Area (km2) | % | Area (km2) | % | Area (km2) | % | Area (km2) | % | Area (km2) | % | |
| Unsuitable | 40,955.612 | 79.4 | 41,119.084 | 79.8 | 42,351.731 | 82.1 | 42,834.495 | 83.1 | 42,001.136 | 81.5 |
| Low suitability | 5759.774 | 11.2 | 5055.802 | 9.8 | 4385.219 | 8.5 | 3507.341 | 6.8 | 4360.177 | 8.5 |
| Moderate suitability | 3373.781 | 6.5 | 3540.731 | 6.9 | 2877.105 | 5.6 | 2939.711 | 5.7 | 3195.005 | 6.2 |
| High suitability | 1469.160 | 2.8 | 1842.710 | 3.6 | 1944.272 | 3.8 | 2276.780 | 4.4 | 2002.008 | 3.9 |
| Total | 51,558.327 | 100 | 51,558.327 | 100 | 51,558.327 | 100 | 51,558.327 | 100 | 51,558.327 | 100 |
| Category | Current Distribution | Future Distribution | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MRI-ESM2.0 | ||||||||||
| 2041–2060 | 2081–2100 | |||||||||
| SSP1 2.6 | SSP5 8.5 | SSP1 2.6 | SSP5 8.5 | |||||||
| Area (km2) | % | Area (km2) | % | Area (km2) | % | Area (km2) | % | Area (km2) | % | |
| Unsuitable | 40,955.612 | 79.4 | 41,470.375 | 80.4 | 40,820.661 | 79.2 | 42,677.979 | 82.8 | 42,156.956 | 81.8 |
| Low suitability | 5759.774 | 11.2 | 4361.568 | 8.5 | 5345.877 | 10.4 | 4114.621 | 8.0 | 4196.009 | 8.1 |
| Moderate suitability | 3373.781 | 6.5 | 3416.910 | 6.6 | 3475.342 | 6.7 | 2987.709 | 5.8 | 3222.830 | 6.3 |
| High suitability | 1469.160 | 2.8 | 2309.475 | 4.5 | 1916.447 | 3.7 | 1778.017 | 3.4 | 1982.531 | 3.8 |
| Total | 51,558.327 | 100 | 51,558.327 | 100 | 51,558.327 | 100 | 51,558.327 | 100 | 51,558.327 | 100 |
| Change Status | BCC CSM2 MR | |||||||
|---|---|---|---|---|---|---|---|---|
| 2041–2060 | 2081–2100 | |||||||
| Current-SSP1 2.6 | Current-SSP5 8.5 | Current-SSP1 2.6 | Current-SSP5 8.5 | |||||
| Area (km2) | % | Area (km2) | % | Area (km2) | % | Area (km2) | % | |
| Range expansion | 1674.369 | 3.2 | 1046.915 | 2.0 | 706.755 | 1.4 | 1306.384 | 2.5 |
| Unsuitable | 39,281.243 | 76.2 | 39,908.697 | 77.4 | 40,248.857 | 78.1 | 39,649.229 | 76.9 |
| No change | 8764.874 | 17.0 | 8159.680 | 15.8 | 8017.077 | 15.5 | 8250.807 | 16.0 |
| Range contraction | 1837.841 | 3.6 | 2443.035 | 4.7 | 2585.638 | 5.0 | 2351.908 | 4.6 |
| Total | 51,558.327 | 100 | 51,558.327 | 100 | 51,558.327 | 100 | 51,558.327 | 100 |
| Change Status | MRI-ESM2.0 | |||||||
|---|---|---|---|---|---|---|---|---|
| 2041–2060 | 2081–2100 | |||||||
| Current-SSP1 2.6 | Current-SSP5 8.5 | Current-SSP1 2.6 | Current-SSP5 8.5 | |||||
| Area (km2) | % | Area (km2) | % | Area (km2) | % | Area (km2) | % | |
| Range expansion | 991.961 | 1.9 | 1350.208 | 2.6 | 800.664 | 1.6 | 1015.612 | 2.0 |
| Unsuitable | 39,963.651 | 77.5 | 39,605.404 | 76.8 | 40,154.948 | 77.9 | 39,940.000 | 77.5 |
| No change | 9095.991 | 17.6 | 9387.458 | 18.2 | 8079.683 | 15.7 | 8385.758 | 16.3 |
| Range contraction | 1506.724 | 2.9 | 1215.257 | 2.4 | 2523.032 | 4.9 | 2216.957 | 4.3 |
| Total | 51,558.327 | 100 | 51,558.327 | 100 | 51,558.327 | 100 | 51,558.327 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qadir, R.Y.; Khwarahm, N.R. Current and Projected Future Spatial Distribution Patterns of Prunus microcarpa in the Kurdistan Region of Iraq. Biology 2025, 14, 358. https://doi.org/10.3390/biology14040358
Qadir RY, Khwarahm NR. Current and Projected Future Spatial Distribution Patterns of Prunus microcarpa in the Kurdistan Region of Iraq. Biology. 2025; 14(4):358. https://doi.org/10.3390/biology14040358
Chicago/Turabian StyleQadir, Renas Y., and Nabaz R. Khwarahm. 2025. "Current and Projected Future Spatial Distribution Patterns of Prunus microcarpa in the Kurdistan Region of Iraq" Biology 14, no. 4: 358. https://doi.org/10.3390/biology14040358
APA StyleQadir, R. Y., & Khwarahm, N. R. (2025). Current and Projected Future Spatial Distribution Patterns of Prunus microcarpa in the Kurdistan Region of Iraq. Biology, 14(4), 358. https://doi.org/10.3390/biology14040358





