Factors Contributing to the High Malignancy Level of Cholangiocarcinoma and Its Epidemiology: Literature Review and Data
Simple Summary
Abstract
1. Etiology and Subtype of CCA
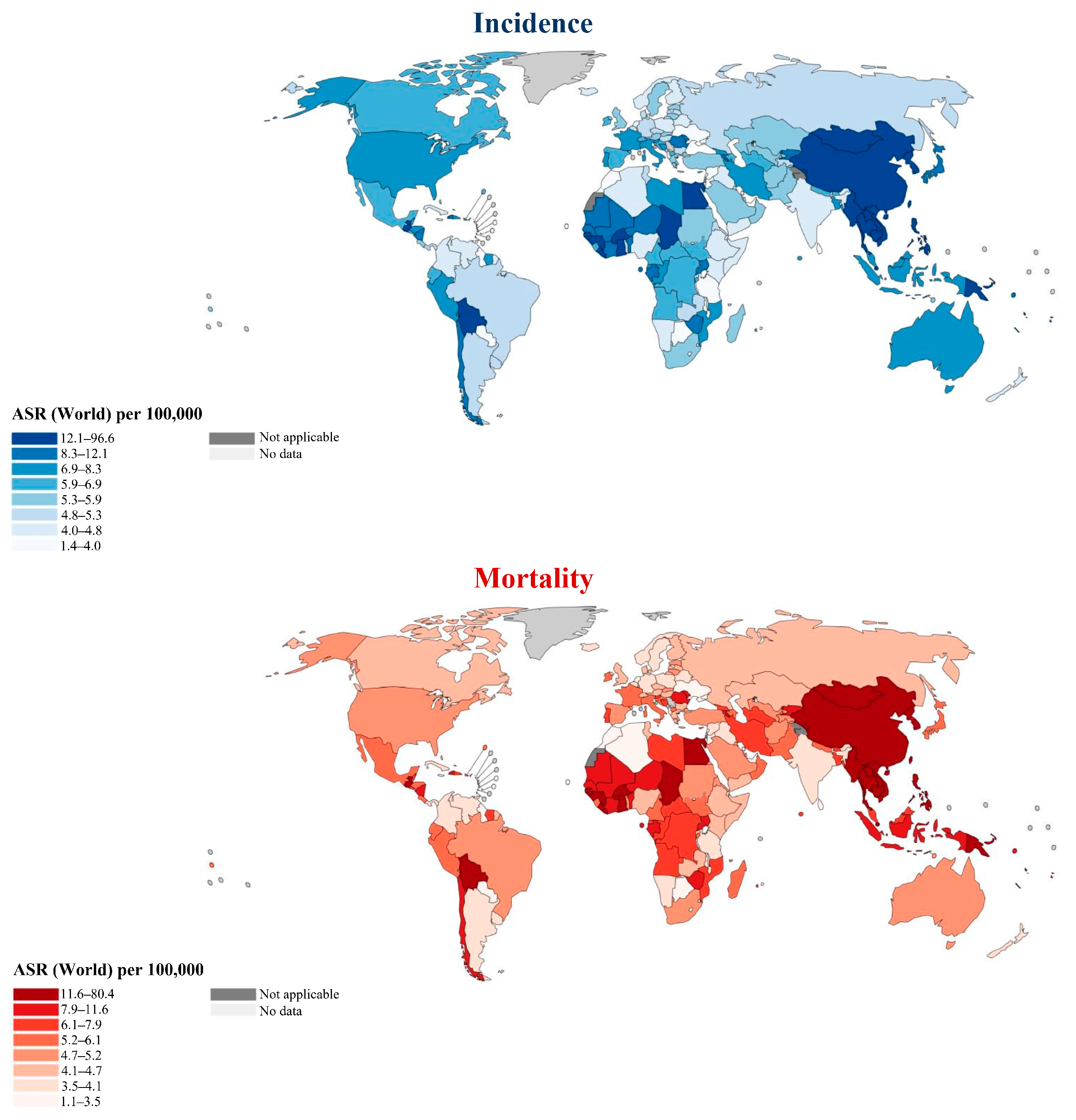
2. Geodemographic Distributions of Disease Incidence and Mortality
3. State of Treatment of the Disease
4. Factors Contributing to the High Malignancy Level of the Disease
4.1. Framework for Cancer Evolution Based on Fenton Reactions and Associated Metabolic Reprogramming
4.2. Hypoxic Stress

4.3. Macrophages and Neutrophils in TIMEs
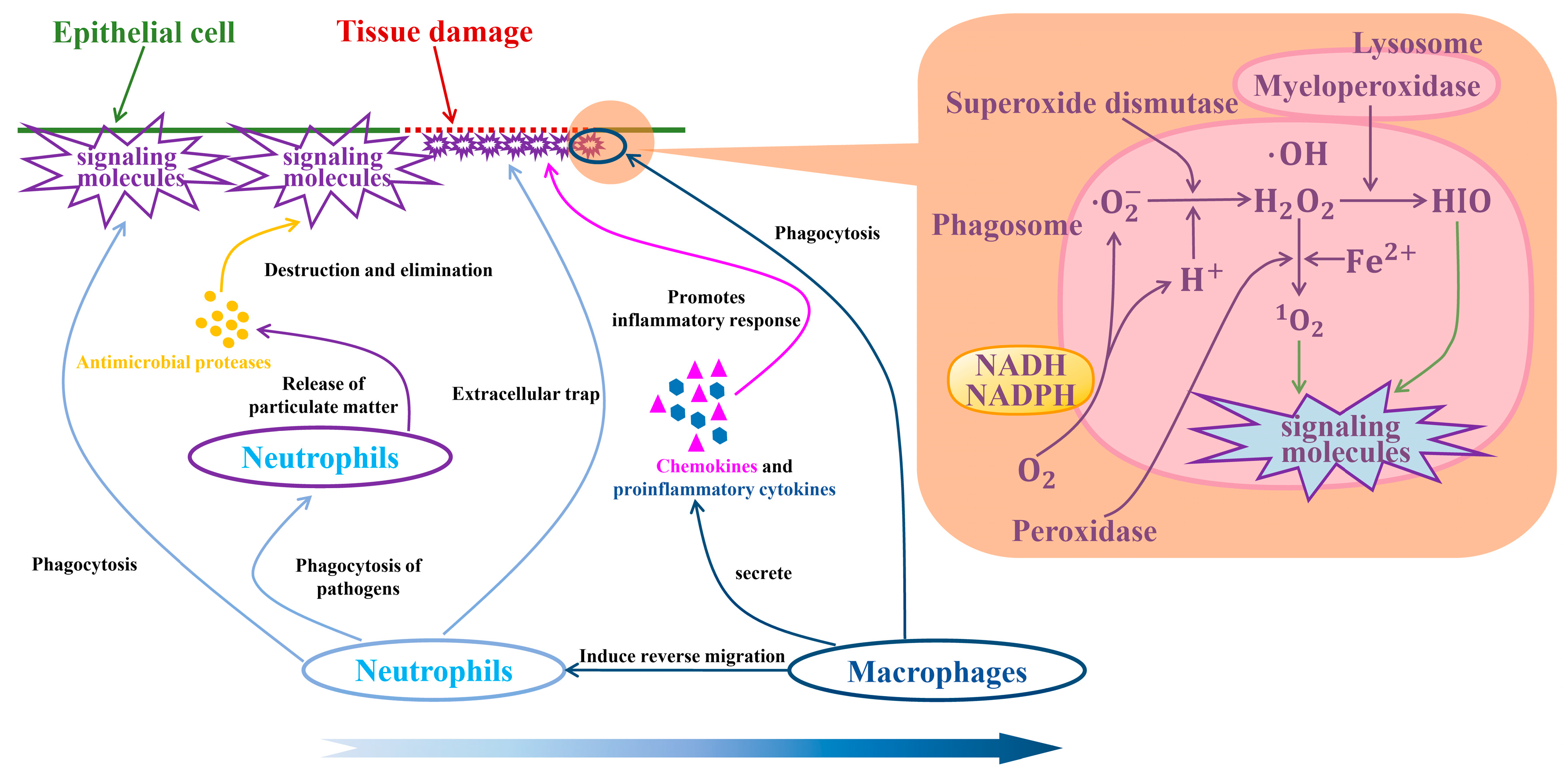
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Ilyas, S.I.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef]
- Munoz-Garrido, P.; Rodrigues, P.M. The jigsaw of dual hepatocellular-intrahepatic cholangiocarcinoma tumours. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.; Aishima, S.; Clavien, P.A.; Fowler, K.; Goodman, Z.; Gores, G.; Gouw, A.; Kagen, A.; Klimstra, D.; Komuta, M.; et al. cHCC-CCA: Consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology 2018, 68, 113–126. [Google Scholar] [CrossRef]
- Rossner, F.; Sinn, B.V.; Horst, D. Pathology of Combined Hepatocellular Carcinoma-Cholangiocarcinoma: An Update. Cancers 2023, 15, 494. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, S.; Navas, M.C.; Restrepo, J.C.; Botero, R.C. Current controversies in cholangiocarcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Tangkawattana, S.; Brindley, P.J. Update on Pathogenesis of Opisthorchiasis and Cholangiocarcinoma. Adv. Parasitol. 2018, 102, 97–113. [Google Scholar] [CrossRef]
- Schwartz, D.A. Helminths in the induction of cancer: Opisthorchis viverrini, Clonorchis sinensis and cholangiocarcinoma. Trop. Geogr. Med. 1980, 32, 95–100. [Google Scholar]
- Qian, M.B.; Utzinger, J.; Keiser, J.; Zhou, X.N. Clonorchiasis. Lancet 2016, 387, 800–810. [Google Scholar] [CrossRef]
- Na, B.K.; Pak, J.H.; Hong, S.J. Clonorchis sinensis and clonorchiasis. Acta Trop. 2020, 203, 105309. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.S.; Zhou, X.N.; Pan, Z.H.; Utzinger, J.; Vounatsou, P. Risk mapping of clonorchiasis in the People’s Republic of China: A systematic review and Bayesian geostatistical analysis. PLoS Negl. Trop. Dis. 2017, 11, e0005239. [Google Scholar] [CrossRef]
- Qian, M.B.; Chen, Y.D.; Liang, S.; Yang, G.J.; Zhou, X.N. The global epidemiology of clonorchiasis and its relation with cholangiocarcinoma. Infect. Dis. Poverty 2012, 1, 4. [Google Scholar] [CrossRef]
- Doanh, P.N.; Nawa, Y. Clonorchis sinensis and Opisthorchis spp. in Vietnam: Current status and prospects. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Lee, K.Y.; Lee, B.C.; Cho, P.Y.; Cheun, H.I.; Hong, S.T.; Sohn, W.M.; Kim, T.S. Prevalence of clonorchiasis in southern endemic areas of Korea in 2006. Korean J. Parasitol. 2008, 46, 133–137. [Google Scholar] [CrossRef]
- Sohn, W.M.; Na, B.K.; Cho, S.H.; Lee, H.I.; Lee, M.R.; Ju, J.W.; Kim, G.O. High Endemicity with Clonorchis sinensis Metacercariae in Fish from Yongjeon-cheon (Stream) in Cheongsong-gun, Gyeongsangbuk-do, Korea. Korean J. Parasitol. 2021, 59, 97–101. [Google Scholar] [CrossRef]
- Sripa, B.; Kaewkes, S.; Intapan, P.M.; Maleewong, W.; Brindley, P.J. Food-borne trematodiases in Southeast Asia epidemiology, pathology, clinical manifestation and control. Adv. Parasitol. 2010, 72, 305–350. [Google Scholar] [CrossRef] [PubMed]
- Sithithaworn, P.; Andrews, R.H.; Nguyen, V.D.; Wongsaroj, T.; Sinuon, M.; Odermatt, P.; Nawa, Y.; Liang, S.; Brindley, P.J.; Sripa, B. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol. Int. 2012, 61, 10–16. [Google Scholar] [CrossRef]
- Sohn, W.M.; Jung, B.K.; Hong, S.J.; Lee, K.H.; Park, J.B.; Kim, H.S.; Cho, S.; Htoon, T.T.; Tin, H.H.; Chai, J.Y. Low-Grade Endemicity of Opisthorchiasis, Yangon, Myanmar. Emerg. Infect. Dis. 2019, 25, 1435–1437. [Google Scholar] [CrossRef]
- Namsanor, J.; Kiatsopit, N.; Laha, T.; Andrews, R.H.; Petney, T.N.; Sithithaworn, P. Infection Dynamics of Opisthorchis viverrini Metacercariae in Cyprinid Fishes from Two Endemic Areas in Thailand and Lao PDR. Am. J. Trop. Med. Hyg. 2020, 102, 110–116. [Google Scholar] [CrossRef]
- Lee, T.Y.; Lee, S.S.; Jung, S.W.; Jeon, S.H.; Yun, S.C.; Oh, H.C.; Kwon, S.; Lee, S.K.; Seo, D.W.; Kim, M.H.; et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: A case-control study. Am. J. Gastroenterol. 2008, 103, 1716–1720. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, M.M.; Vogel, A. Epidemiology and Risk Factors of Cholangiocarcinoma. Visc. Med. 2016, 32, 395–400. [Google Scholar] [CrossRef]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39 (Suppl. S1), 19–31. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, K.; Weersma, R.K.; van Erpecum, K.J.; Rauws, E.A.; Spanier, B.W.; Poen, A.C.; van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013, 58, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, K.N.; LaRusso, N.F. Primary Sclerosing Cholangitis. N. Engl. J. Med. 2016, 375, 1161–1170. [Google Scholar] [CrossRef]
- Petrick, J.L.; Yang, B.; Altekruse, S.F.; Van Dyke, A.L.; Koshiol, J.; Graubard, B.I.; McGlynn, K.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based study in SEER-Medicare. PLoS ONE 2017, 12, e0186643. [Google Scholar] [CrossRef]
- Soreide, K.; Korner, H.; Havnen, J.; Soreide, J.A. Bile duct cysts in adults. Br. J. Surg. 2004, 91, 1538–1548. [Google Scholar] [CrossRef]
- Fard-Aghaie, M.H.; Makridis, G.; Reese, T.; Feyerabend, B.; Wagner, K.C.; Schnitzbauer, A.; Bechstein, W.O.; Oldhafer, F.; Kleine, M.; Klempnauer, J.; et al. The rate of cholangiocarcinoma in Caroli Disease A German multicenter study. HPB 2022, 24, 267–276. [Google Scholar] [CrossRef]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2020, 72, 95–103. [Google Scholar] [CrossRef]
- Nordenstedt, H.; Mattsson, F.; El-Serag, H.; Lagergren, J. Gallstones and cholecystectomy in relation to risk of intra- and extrahepatic cholangiocarcinoma. Br. J. Cancer 2012, 106, 1011–1015. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Y.; Li, B.; Huang, J.; Wu, L.; Xu, D.; Yang, J.; He, J. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: Evidence from a meta-analysis. BMC Cancer 2012, 12, 289. [Google Scholar] [CrossRef]
- Ralphs, S.; Khan, S.A. The role of the hepatitis viruses in cholangiocarcinoma. J. Viral. Hepat. 2013, 20, 297–305. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Engels, E.A.; Landgren, O.; Chiao, E.; Henderson, L.; Amaratunge, H.C.; Giordano, T.P. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology 2009, 49, 116–123. [Google Scholar] [CrossRef]
- Wongjarupong, N.; Assavapongpaiboon, B.; Susantitaphong, P.; Cheungpasitporn, W.; Treeprasertsuk, S.; Rerknimitr, R.; Chaiteerakij, R. Non-alcoholic fatty liver disease as a risk factor for cholangiocarcinoma: A systematic review and meta-analysis. BMC Gastroenterol. 2017, 17, 149. [Google Scholar] [CrossRef]
- Chaiteerakij, R.; Yang, J.D.; Harmsen, W.S.; Slettedahl, S.W.; Mettler, T.A.; Fredericksen, Z.S.; Kim, W.R.; Gores, G.J.; Roberts, R.O.; Olson, J.E.; et al. Risk factors for intrahepatic cholangiocarcinoma: Association between metformin use and reduced cancer risk. Hepatology 2013, 57, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Khosla, D.; Misra, S.; Chu, P.L.; Guan, P.; Nada, R.; Gupta, R.; Kaewnarin, K.; Ko, T.K.; Heng, H.L.; Srinivasalu, V.K.; et al. Cholangiocarcinoma: Recent Advances in Molecular Pathobiology and Therapeutic Approaches. Cancers 2024, 16, 801. [Google Scholar] [CrossRef]
- Shin, D.W.; Moon, S.H.; Kim, J.H. Diagnosis of Cholangiocarcinoma. Diagnostics 2023, 13, 233. [Google Scholar] [CrossRef]
- Javle, M.; Lee, S.; Azad, N.S.; Borad, M.J.; Kate Kelley, R.; Sivaraman, S.; Teschemaker, A.; Chopra, I.; Janjan, N.; Parasuraman, S.; et al. Temporal Changes in Cholangiocarcinoma Incidence and Mortality in the United States from 2001 to 2017. Oncologist 2022, 27, 874–883. [Google Scholar] [CrossRef]
- Omotoso, O.; Teibo, J.O.; Atiba, F.A.; Oladimeji, T.; Paimo, O.K.; Ataya, F.S.; Batiha, G.E.; Alexiou, A. Addressing cancer care inequities in sub-Saharan Africa: Current challenges and proposed solutions. Int. J. Equity Health 2023, 22, 189. [Google Scholar] [CrossRef]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers 2021, 7, 65. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Palta, M.; Kim, C.; Allen, P.J.; Morse, M.A.; Lidsky, M.E. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA Cancer J. Clin. 2023, 73, 198–222. [Google Scholar] [CrossRef] [PubMed]
- Bragazzi, M.C.; Venere, R.; Ribichini, E.; Covotta, F.; Cardinale, V.; Alvaro, D. Intrahepatic cholangiocarcinoma: Evolving strategies in management and treatment. Dig. Liver Dis. 2024, 56, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Edeline, J.; Goyal, L. How I treat biliary tract cancer. ESMO Open 2022, 7, 100378. [Google Scholar] [CrossRef]
- Zhan, Q.; Shen, B.-Y. Current management of hilar cholangiocarcinoma. World Chin. J. Dig. 2009, 32, 3313–3317. [Google Scholar]
- Sasaki, R.; Takahashi, M.; Funato, O.; Nitta, H.; Murakami, M.; Kawamura, H.; Suto, T.; Kanno, S.; Saito, K. Prognostic significance of lymph node involvement in middle and distal bile duct cancer. Surgery 2001, 129, 677–683. [Google Scholar] [CrossRef]
- Yoshida, T.; Matsumoto, T.; Sasaki, A.; Morii, Y.; Aramaki, M.; Kitano, S. Prognostic factors after pancreatoduodenectomy with extended lymphadenectomy for distal bile duct cancer. Arch. Surg. 2002, 137, 69–73. [Google Scholar] [CrossRef]
- Hong, S.M.; Cho, H.; Lee, O.J.; Ro, J.Y. The number of metastatic lymph nodes in extrahepatic bile duct carcinoma as a prognostic factor. Am. J. Surg. Pathol. 2005, 29, 1177–1183. [Google Scholar] [CrossRef]
- Murakami, Y.; Uemura, K.; Hayashidani, Y.; Sudo, T.; Ohge, H.; Sueda, T. Pancreatoduodenectomy for distal cholangiocarcinoma: Prognostic impact of lymph node metastasis. World J. Surg. 2007, 31, 337–342, discussion 343–334. [Google Scholar] [CrossRef]
- Ito, K.; Ito, H.; Allen, P.J.; Gonen, M.; Klimstra, D.; D’Angelica, M.I.; Fong, Y.; DeMatteo, R.P.; Brennan, M.F.; Blumgart, L.H.; et al. Adequate lymph node assessment for extrahepatic bile duct adenocarcinoma. Ann. Surg. 2010, 251, 675–681. [Google Scholar] [CrossRef]
- Kiriyama, M.; Ebata, T.; Aoba, T.; Kaneoka, Y.; Arai, T.; Shimizu, Y.; Nagino, M.; Nagoya Surgical Oncology, G. Prognostic impact of lymph node metastasis in distal cholangiocarcinoma. Br. J. Surg. 2015, 102, 399–406. [Google Scholar] [CrossRef]
- Kluge, R.; Schmidt, F.; Caca, K.; Barthel, H.; Hesse, S.; Georgi, P.; Seese, A.; Huster, D.; Berr, F. Positron emission tomography with [(18)F]fluoro-2-deoxy-D-glucose for diagnosis and staging of bile duct cancer. Hepatology 2001, 33, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Noji, T.; Kondo, S.; Hirano, S.; Tanaka, E.; Ambo, Y.; Kawarada, Y.; Morikawa, T. CT evaluation of paraaortic lymph node metastasis in patients with biliary cancer. J. Gastroenterol. 2005, 40, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Nakeeb, A.; Pitt, H.A.; Sohn, T.A.; Coleman, J.; Abrams, R.A.; Piantadosi, S.; Hruban, R.H.; Lillemoe, K.D.; Yeo, C.J.; Cameron, J.L. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann. Surg. 1996, 224, 463–473, discussion 473–475. [Google Scholar] [CrossRef]
- DeOliveira, M.L.; Cunningham, S.C.; Cameron, J.L.; Kamangar, F.; Winter, J.M.; Lillemoe, K.D.; Choti, M.A.; Yeo, C.J.; Schulick, R.D. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Ann. Surg. 2007, 245, 755–762. [Google Scholar] [CrossRef]
- Minini, M.; Fouassier, L. Cancer-Associated Fibroblasts and Extracellular Matrix: Therapeutical Strategies for Modulating the Cholangiocarcinoma Microenvironment. Curr. Oncol. 2023, 30, 4185–4196. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Huang, T.; Dai, M.; Kong, X.; Liu, H.; Zheng, Z.; Sun, G.; Sun, G.; Rong, D.; Jin, Z.; et al. Tumor Microenvironment and its Implications for Antitumor Immunity in Cholangiocarcinoma: Future Perspectives for Novel Therapies. Int. J. Biol. Sci. 2022, 18, 5369–5390. [Google Scholar] [CrossRef]
- Wang, J.; Ilyas, S. Targeting the tumor microenvironment in cholangiocarcinoma: Implications for therapy. Expert Opin. Investig. Drugs 2021, 30, 429–438. [Google Scholar] [CrossRef]
- Peng, C.; Xu, Y.; Wu, J.; Wu, D.; Zhou, L.; Xia, X. TME-Related Biomimetic Strategies Against Cancer. Int. J. Nanomed. 2024, 19, 109–135. [Google Scholar] [CrossRef]
- Louis, C.; Edeline, J.; Coulouarn, C. Targeting the tumor microenvironment in cholangiocarcinoma: Implications for therapy. Expert Opin. Ther. Targets 2021, 25, 153–162. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, S.; Gao, F.; Zou, Y.; Ren, Z.; Yu, Z. The role of tumor microenvironment reprogramming in primary liver cancer chemotherapy resistance. Front. Oncol. 2022, 12, 1008902. [Google Scholar] [CrossRef]
- Long, C.; Peng, H.; Yang, W.; Wang, M.; Luo, B.; Hao, J.; Dong, Y.; Zuo, W. Targeted Delivery of Gemcitabine for Precision Therapy of Cholangiocarcinoma Using Hyaluronic Acid-Modified Metal-Organic Framework Nanoparticles. ACS Omega 2024, 9, 11998–12005. [Google Scholar] [CrossRef] [PubMed]
- Maphanao, P.; Phothikul, Y.; Choodet, C.; Puangmali, T.; Katewongsa, K.; Pinlaor, S.; Thanan, R.; Yordpratum, U.; Sakonsinsiri, C. Development and in vitro evaluation of ursolic acid-loaded poly(lactic-co-glycolic acid) nanoparticles in cholangiocarcinoma. RSC Adv. 2024, 14, 24828–24837. [Google Scholar] [CrossRef] [PubMed]
- Lapitz, A.; Azkargorta, M.; Milkiewicz, P.; Olaizola, P.; Zhuravleva, E.; Grimsrud, M.M.; Schramm, C.; Arbelaiz, A.; O’Rourke, C.J.; La Casta, A.; et al. Liquid biopsy-based protein biomarkers for risk prediction, early diagnosis, and prognostication of cholangiocarcinoma. J. Hepatol. 2023, 79, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Meng, C.; Liu, B.; Zheng, M.; Qin, J. A Cholangiocarcinoma Prediction Model Based on Random Forest and Artificial Neural Network Algorithm. J. Coll. Physicians Surg. Pak. 2023, 33, 578–586. [Google Scholar] [CrossRef]
- Tan, J.; Shu, M.; Liao, J.; Liang, R.; Liu, S.; Kuang, M.; Peng, S.; Xiao, H.; Zhou, Q. Identification and validation of a plasma metabolomics-based model for risk stratification of intrahepatic cholangiocarcinoma. J. Cancer Res. Clin. Oncol. 2023, 149, 12365–12377. [Google Scholar] [CrossRef]
- Li, J.; Xiong, J.; Wei, L.; Zhang, M.; Yi, J.; Liu, L. Identification of neutrophil-related genes and development of a prognostic model for cholangiocarcinoma. J. Gene Med. 2024, 26, e3569. [Google Scholar] [CrossRef]
- Damaghi, M.; Wojtkowiak, J.W.; Gillies, R.J. pH sensing and regulation in cancer. Front. Physiol. 2013, 4, 370. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, C.; Cao, S.; Sheng, T.; Dong, N.; Xu, Y. Fenton reactions drive nucleotide and ATP syntheses in cancer. J. Mol. Cell Biol. 2018, 10, 448–459. [Google Scholar] [CrossRef]
- Imlay, J.A.; Chin, S.M.; Linn, S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 1988, 240, 640–642. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, H.; Xu, Y. Metabolic reprogramming in cancer: The bridge that connects intracellular stresses and cancer behaviors. Natl. Sci. Rev. 2020, 7, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.C.; Dean, D.R.; Smith, A.D.; Johnson, M.K. Structure, function, and formation of biological iron-sulfur clusters. Annu. Rev. Biochem. 2005, 74, 247–281. [Google Scholar] [CrossRef]
- Bian, K.; Gao, Z.; Weisbrodt, N.; Murad, F. The nature of heme/iron-induced protein tyrosine nitration. Proc. Natl. Acad. Sci. USA 2003, 100, 5712–5717. [Google Scholar] [CrossRef] [PubMed]
- Kruszewski, M. Labile iron pool: The main determinant of cellular response to oxidative stress. Mutat. Res. 2003, 531, 81–92. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, Y.; Skaro, M.F.; Wu, Y.; Qu, Z.; Mao, F.; Zhao, S.; Xu, Y. Metabolic Reprogramming in Cancer Is Induced to Increase Proton Production. Cancer Res. 2020, 80, 1143–1155. [Google Scholar] [CrossRef]
- Zhou, Y.; Chang, W.; Lu, X.; Wang, J.; Zhang, C.; Xu, Y. Acid-base Homeostasis and Implications to the Phenotypic Behaviors of Cancer. Genom. Proteom. Bioinform. 2023, 21, 1133–1148. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, Y.; Jiang, H.; Xu, Y. Elucidation of Functional Roles of Sialic Acids in Cancer Migration. Front. Oncol. 2020, 10, 401. [Google Scholar] [CrossRef]
- Tse, J.M.; Cheng, G.; Tyrrell, J.A.; Wilcox-Adelman, S.A.; Boucher, Y.; Jain, R.K.; Munn, L.L. Mechanical compression drives cancer cells toward invasive phenotype. Proc. Natl. Acad. Sci. USA 2012, 109, 911–916. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, K.; Li, H.; Xia, D.; He, T. Role of hypoxia in the tumor microenvironment and targeted therapy. Front. Oncol. 2022, 12, 961637. [Google Scholar] [CrossRef]
- Belaiba, R.S.; Bonello, S.; Zahringer, C.; Schmidt, S.; Hess, J.; Kietzmann, T.; Gorlach, A. Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol. Biol. Cell 2007, 18, 4691–4697. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.T.; Scholz, C.C. The effect of HIF on metabolism and immunity. Nat. Rev. Nephrol. 2022, 18, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Akkiz, H. Emerging Role of Cancer-Associated Fibroblasts in Progression and Treatment of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 3941. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Bai, R.; Li, Y.; Jian, L.; Yang, Y.; Zhao, L.; Wei, M. The hypoxia-driven crosstalk between tumor and tumor-associated macrophages: Mechanisms and clinical treatment strategies. Mol. Cancer 2022, 21, 177. [Google Scholar] [CrossRef]
- Zeng, W.; Li, F.; Jin, S.; Ho, P.C.; Liu, P.S.; Xie, X. Functional polarization of tumor-associated macrophages dictated by metabolic reprogramming. J. Exp. Clin. Cancer Res. 2023, 42, 245. [Google Scholar] [CrossRef]
- He, Z.; Zhang, S. Tumor-Associated Macrophages and Their Functional Transformation in the Hypoxic Tumor Microenvironment. Front. Immunol. 2021, 12, 741305. [Google Scholar] [CrossRef]
- Liang, Y.; Bu, Q.; You, W.; Zhang, R.; Xu, Z.; Gan, X.; Zhou, J.; Qiao, L.; Huang, T.; Lu, L. Single-cell analysis reveals hypoxia-induced immunosuppressive microenvironment in intrahepatic cholangiocarcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167276. [Google Scholar] [CrossRef]
- Bhuria, V.; Xing, J.; Scholta, T.; Bui, K.C.; Nguyen, M.L.T.; Malek, N.P.; Bozko, P.; Plentz, R.R. Hypoxia induced Sonic Hedgehog signaling regulates cancer stemness, epithelial-to-mesenchymal transition and invasion in cholangiocarcinoma. Exp. Cell Res. 2019, 385, 111671. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, X.; Wang, Y.; Zhang, Y.; Zhou, T.; Jiang, W.; Wang, Z.; Chang, J.; Liu, S.; Chen, R.; et al. Hypoxia-induced SKA3 promoted cholangiocarcinoma progression and chemoresistance by enhancing fatty acid synthesis via the regulation of PAR-dependent HIF-1a deubiquitylation. J. Exp. Clin. Cancer Res. 2023, 42, 265. [Google Scholar] [CrossRef]
- Hou, P.; Kang, Y.; Luo, J. Hypoxia-mediated miR-212-3p downregulation enhances progression of intrahepatic cholangiocarcinoma through upregulation of Rab1a. Cancer Biol. Ther. 2018, 19, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Jiang, D. Research Progress of Small Molecule Anti-angiogenic Drugs in Non-small Cell Lung Cancer. Zhongguo Fei Ai Za Zhi 2021, 24, 56–62. [Google Scholar] [CrossRef]
- Langer, C.; Soria, J.C. The role of anti-epidermal growth factor receptor and anti-vascular endothelial growth factor therapies in the treatment of non-small-cell lung cancer. Clin. Lung Cancer 2010, 11, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Bu, J.; Chen, J.; Ni, B.; Fu, B.; Zhou, F.; Pang, S.; Zhang, J.; Xu, S.; He, C. PIGF and Flt-1 on the surface of macrophages induces the production of TGF-beta1 by polarized tumor-associated macrophages to promote lung cancer angiogenesis. Eur. J. Pharmacol. 2021, 912, 174550. [Google Scholar] [CrossRef]
- Aziz, S.N.; Badawy, A.A.; Nessem, D.I.; Abd El Malak, N.S.; Naguib, M.J. Chitosan-coated alginate (CCA) nanoparticles for augmentation of topical antihistaminic activity of diphenhydramine: In-vitro optimization, skin histopathology and pharmacodynamic studies with in vitro/in vivo correlation. Drug Dev. Ind. Pharm. 2023, 49, 316–327. [Google Scholar] [CrossRef]
- Liu, C.; Wu, K.; Li, J.; Mu, X.; Gao, H.; Xu, X. Nanoparticle-mediated therapeutic management in cholangiocarcinoma drug targeting: Current progress and future prospects. Biomed. Pharmacother. 2023, 158, 114135. [Google Scholar] [CrossRef]
- Alpuim Costa, D.; Goncalves-Nobre, J.G.; Sampaio-Alves, M.; Guerra, N.; Arana Ribeiro, J.; Espiney Amaro, C. Hyperbaric oxygen therapy as a complementary treatment in neuroblastoma—A narrative review. Front. Oncol. 2023, 13, 1254322. [Google Scholar] [CrossRef]
- Yuen, C.M.; Tsai, H.P.; Tseng, T.T.; Tseng, Y.L.; Lieu, A.S.; Kwan, A.L.; Chang, A.Y.W. Hyperbaric Oxygen Therapy Adjuvant Chemotherapy and Radiotherapy through Inhibiting Stemness in Glioblastoma. Curr. Issues Mol. Biol. 2023, 45, 8309–8320. [Google Scholar] [CrossRef]
- Cui, Y.; Li, Y.Y.; Li, J.; Zhang, H.Y.; Wang, F.; Bai, X.; Li, S.S. STAT3 regulates hypoxia-induced epithelial mesenchymal transition in oesophageal squamous cell cancer. Oncol. Rep. 2016, 36, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Y.; Ma, X.; Hu, H. The Influence of Cell Cycle Regulation on Chemotherapy. Int. J. Mol. Sci. 2021, 22, 6923. [Google Scholar] [CrossRef]
- Khizar, H.; Hu, Y.; Wu, Y.; Yang, J. The role and implication of autophagy in cholangiocarcinoma. Cell Death Discov. 2023, 9, 332. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Packer, M. Autophagy-dependent and -independent modulation of oxidative and organellar stress in the diabetic heart by glucose-lowering drugs. Cardiovasc. Diabetol. 2020, 19, 62. [Google Scholar] [CrossRef] [PubMed]
- Tinganelli, W.; Durante, M. Tumor Hypoxia and Circulating Tumor Cells. Int. J. Mol. Sci. 2020, 21, 9592. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F.X.C.; Mu, J.; Li, J.; Yao, H.; Chen, K. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023, 12, 11149–11165. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- van Tienderen, G.S.; Groot Koerkamp, B.; JNM, I.J.; van der Laan, L.J.W.; Verstegen, M.M.A. Recreating Tumour Complexity in a Dish: Organoid Models to Study Liver Cancer Cells and their Extracellular Environment. Cancers 2019, 11, 1706. [Google Scholar] [CrossRef]
- Job, S.; Rapoud, D.; Dos Santos, A.; Gonzalez, P.; Desterke, C.; Pascal, G.; Elarouci, N.; Ayadi, M.; Adam, R.; Azoulay, D.; et al. Identification of Four Immune Subtypes Characterized by Distinct Composition and Functions of Tumor Microenvironment in Intrahepatic Cholangiocarcinoma. Hepatology 2020, 72, 965–981. [Google Scholar] [CrossRef] [PubMed]
- Vonderheide, R.H. The Immune Revolution: A Case for Priming, Not Checkpoint. Cancer Cell 2018, 33, 563–569. [Google Scholar] [CrossRef]
- Bao, X.; Li, Q.; Chen, J.; Chen, D.; Ye, C.; Dai, X.; Wang, Y.; Li, X.; Rong, X.; Cheng, F.; et al. Molecular Subgroups of Intrahepatic Cholangiocarcinoma Discovered by Single-Cell RNA Sequencing-Assisted Multiomics Analysis. Cancer Immunol. Res. 2022, 10, 811–828. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xie, Y.; Cai, Y.; Hu, H.; He, M.; Liu, L.; Liao, C.; Wang, Y.; Wang, J.; Ren, X.; et al. Multiomic Analysis Reveals Comprehensive Tumor Heterogeneity and Distinct Immune Subtypes in Multifocal Intrahepatic Cholangiocarcinoma. Clin. Cancer Res. 2022, 28, 1896–1910. [Google Scholar] [CrossRef] [PubMed]
- Wirta, E.V.; Szeto, S.; Koppatz, H.; Nordin, A.; Makisalo, H.; Arola, J.; Siren, J.; Ahtiainen, M.; Bohm, J.; Mecklin, J.P.; et al. High immune cell infiltration predicts improved survival in cholangiocarcinoma. Front. Oncol. 2024, 14, 1333926. [Google Scholar] [CrossRef]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 macrophages and their overlaps—Myth or reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef]
- Ohms, M.; Moller, S.; Laskay, T. An Attempt to Polarize Human Neutrophils Toward N1 and N2 Phenotypes in vitro. Front. Immunol. 2020, 11, 532. [Google Scholar] [CrossRef]
- Cho, S.Y.; Hwang, H.; Kim, Y.H.; Yoo, B.C.; Han, N.; Kong, S.Y.; Baek, M.J.; Kim, K.H.; Lee, M.R.; Park, J.G.; et al. Refining Classification of Cholangiocarcinoma Subtypes via Proteogenomic Integration Reveals New Therapeutic Prospects. Gastroenterology 2023, 164, 1293–1309. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Dubey, M.; Nagarkoti, S.; Awasthi, D.; Singh, A.K.; Chandra, T.; Kumaravelu, J.; Barthwal, M.K.; Dikshit, M. Nitric oxide-mediated apoptosis of neutrophils through caspase-8 and caspase-3-dependent mechanism. Cell Death Dis. 2016, 7, e2348. [Google Scholar] [CrossRef]
- Herman, K.D.; Wright, C.G.; Marriott, H.M.; McCaughran, S.C.; Bowden, K.A.; Collins, M.O.; Renshaw, S.A.; Prince, L.R. The EGFR/ErbB inhibitor neratinib modifies the neutrophil phosphoproteome and promotes apoptosis and clearance by airway macrophages. Front. Immunol. 2022, 13, 956991. [Google Scholar] [CrossRef]
- Dionisio, F.; Tomas, L.; Schulz, C. Glycolytic side pathways regulating macrophage inflammatory phenotypes and functions. Am. J. Physiol. Cell Physiol. 2023, 324, C558–C564. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, M.; Sun, J.; Li, X.; Shi, H.; Wang, X.; Liu, B.; Zhang, T.; Jiang, X.; Lin, L.; et al. Glycolytic neutrophils accrued in the spleen compromise anti-tumour T cell immunity in breast cancer. Nat. Metab. 2023, 5, 1408–1422. [Google Scholar] [CrossRef] [PubMed]
- Degotte, G.; Frederich, M.; Francotte, P.; Franck, T.; Colson, T.; Serteyn, D.; Mouithys-Mickalad, A. Targeting Myeloperoxidase Activity and Neutrophil ROS Production to Modulate Redox Process: Effect of Ellagic Acid and Analogues. Molecules 2023, 28, 4516. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Murray, H.W.; Juangbhanich, C.W.; Nathan, C.F.; Cohn, Z.A. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J. Exp. Med. 1979, 150, 950–964. [Google Scholar] [CrossRef]
- Murray, H.W.; Cohn, Z.A. Macrophage oxygen-dependent antimicrobial activity. I. Susceptibility of Toxoplasma gondii to oxygen intermediates. J. Exp. Med. 1979, 150, 938–949. [Google Scholar] [CrossRef]




| East Asian Countries | Western Countries |
|---|---|
| C. sinensis | Primary sclerosing cholangitis |
| O. viverrini | Choledochal cysts |
| Hepatitis B virus | Caroli disease |
| Caroli syndrome | |
| Liver cirrhosis | |
| Cholelithiasis | |
| Choledocholithiasis | |
| Hepatitis C virus | |
| Non-alcoholic fatty liver disease | |
| Type 2 diabetes mellitus | |
| Inflammatory bowel disease | |
| Alcohol consumption | |
| Smoking | |
| Obesity | |
| Hypertension |
| Continent | Both Sexes | Males | Females |
|---|---|---|---|
| Globe | 988,627 | 644,214 | 344,413 |
| Europe | 101,541|10.27% | 62,160|9.65% | 39,381|11.43% |
| Asia | 695,473|70.35% | 468,793|72.77% | 226,680|65.82% |
| Northern America | 53,714|5.43% | 36,007|5.59% | 17,707|5.14% |
| LAC | 53,203|5.38% | 25,936|4.02% | 27,267|7.92% |
| Africa | 79,356|8.03% | 47,715|7.41% | 31,641|9.19% |
| Oceania | 5340|0.54% | 3603|0.56% | 1737|0.50% |
| Continents | Both Sexes | Males | Females |
|---|---|---|---|
| Globe | 847,780 | 553,232 | 294,548 |
| Europe | 87,294|10.30% | 54,060|9.77% | 33,234|11.28% |
| Asia | 597,749|70.51% | 403,784|72.98% | 193,965|65.85% |
| Northern America | 37,528|4.42% | 24,346|4.40% | 13,182|4.48% |
| LAC | 46,398|5.47% | 23,215|4.20% | 23,183|7.87% |
| Africa | 74,513|8.79% | 45,022|8.14% | 29,491|10.01% |
| Oceania | 4298|0.51% | 2805|0.51% | 1493|0.51% |
| Treatment | Applicable Situation | Effect | Challenge |
|---|---|---|---|
| Surgery | Early-stage CCA; No distant metastasis | 5-year survival rate: intrahepatic 44–63%, perihilar 11–30%, distal 27–28% | Most patients are already in the advanced stage when diagnosed, and the chances of surgery are limited. |
| Liver transplantation | Early-stage iCCA | Possible treatment options | Applicability in advanced or vascular invasion tumors is controversial |
| Chemotherapy | Advanced CCA | Gemcitabine combined with cisplatin is the main treatment option, but the effect is limited | Limited efficacy in advanced iCCA; The role of pCCA in treatment is still unclear |
| Radiotherapy | Postoperative adjuvant therapy | No significant improvement in survival rate | The role of pCCA in treatment is still unclear |
| Immunotherapy | Postoperative adjuvant therapy | Reduce the risk of recurrence | Limited penetration of drugs and T cells due to TME restrictions |
| TME-based therapy | Targeting TME | Some clinical trials have achieved results, but their universality and effectiveness need further verification | Development and validation of therapy strategies is ongoing |
| Machine (deep) learning | Adjunctive therapy | Constructed risk prediction, early diagnosis, prognosis and survival prediction models | The universality and accuracy of the model needs to be improved |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Guan, R.; Zhang, S. Factors Contributing to the High Malignancy Level of Cholangiocarcinoma and Its Epidemiology: Literature Review and Data. Biology 2025, 14, 351. https://doi.org/10.3390/biology14040351
Li X, Guan R, Zhang S. Factors Contributing to the High Malignancy Level of Cholangiocarcinoma and Its Epidemiology: Literature Review and Data. Biology. 2025; 14(4):351. https://doi.org/10.3390/biology14040351
Chicago/Turabian StyleLi, Xuan, Renchu Guan, and Shuangquan Zhang. 2025. "Factors Contributing to the High Malignancy Level of Cholangiocarcinoma and Its Epidemiology: Literature Review and Data" Biology 14, no. 4: 351. https://doi.org/10.3390/biology14040351
APA StyleLi, X., Guan, R., & Zhang, S. (2025). Factors Contributing to the High Malignancy Level of Cholangiocarcinoma and Its Epidemiology: Literature Review and Data. Biology, 14(4), 351. https://doi.org/10.3390/biology14040351






