Bacterial Pathogens of Bovine Mastitis: Prevalence, Antimicrobial Susceptibility, and Sensitivity to Caesalpinia sappan Both In Vitro and In Vivo Studies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
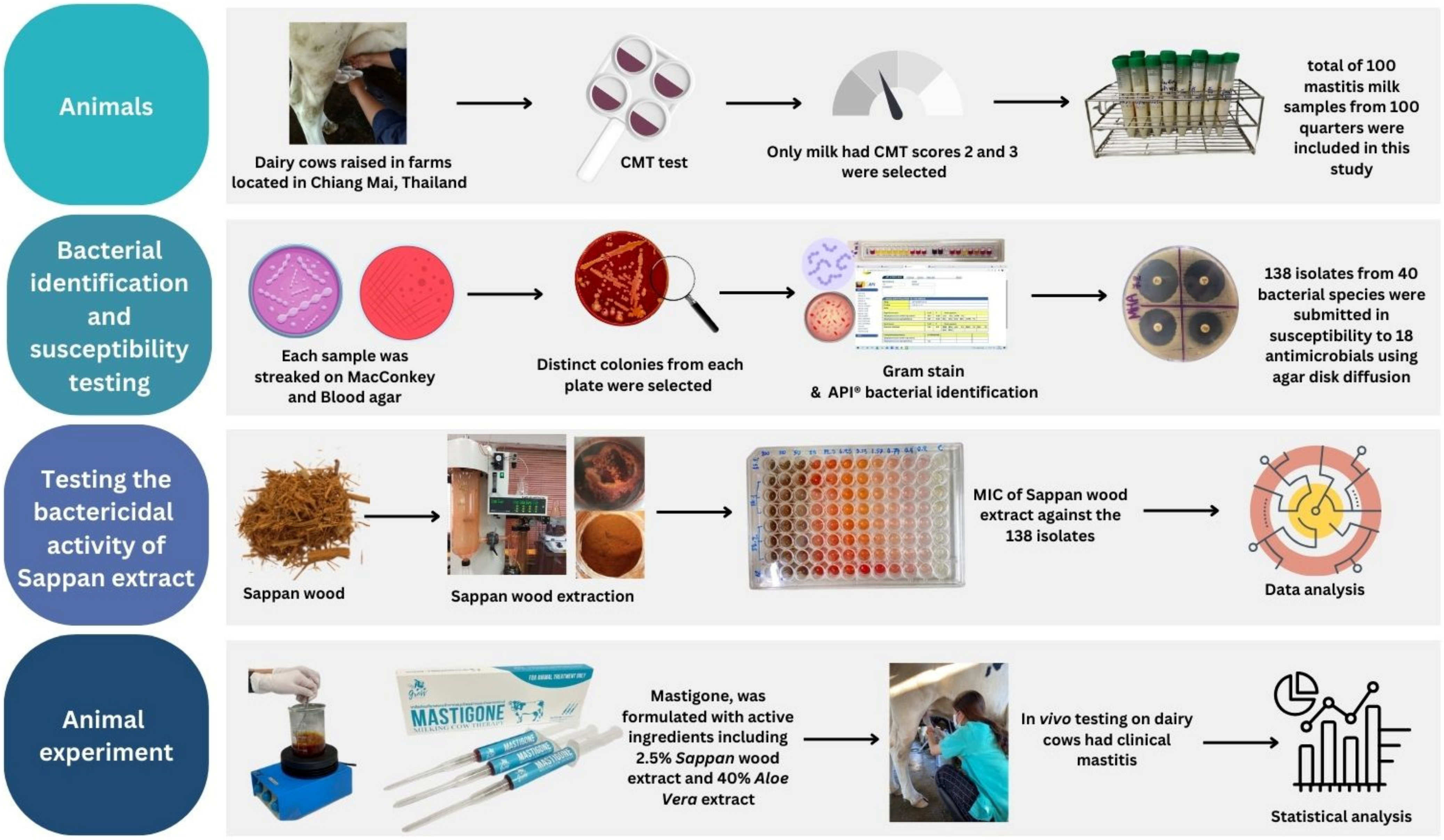
2.1. Sample Collection
2.2. Microbial Identification and Susceptibility Testing
2.3. Testing the Bactericidal Activity of Herbal Preparations
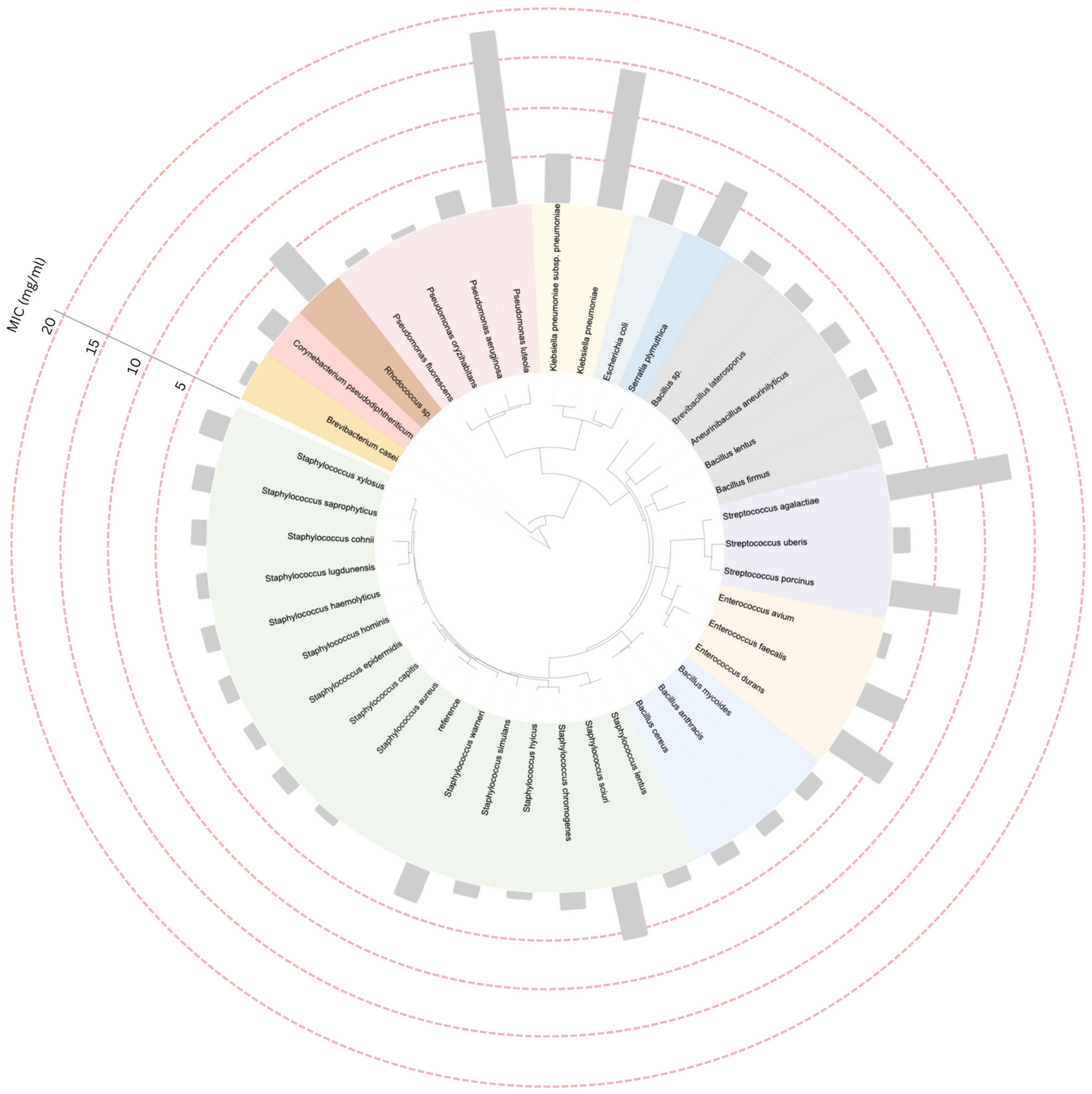
2.4. Construction of Phylogenetic Tree and Data Annotation
2.5. Animal Experiment
2.6. Statistical Analysis
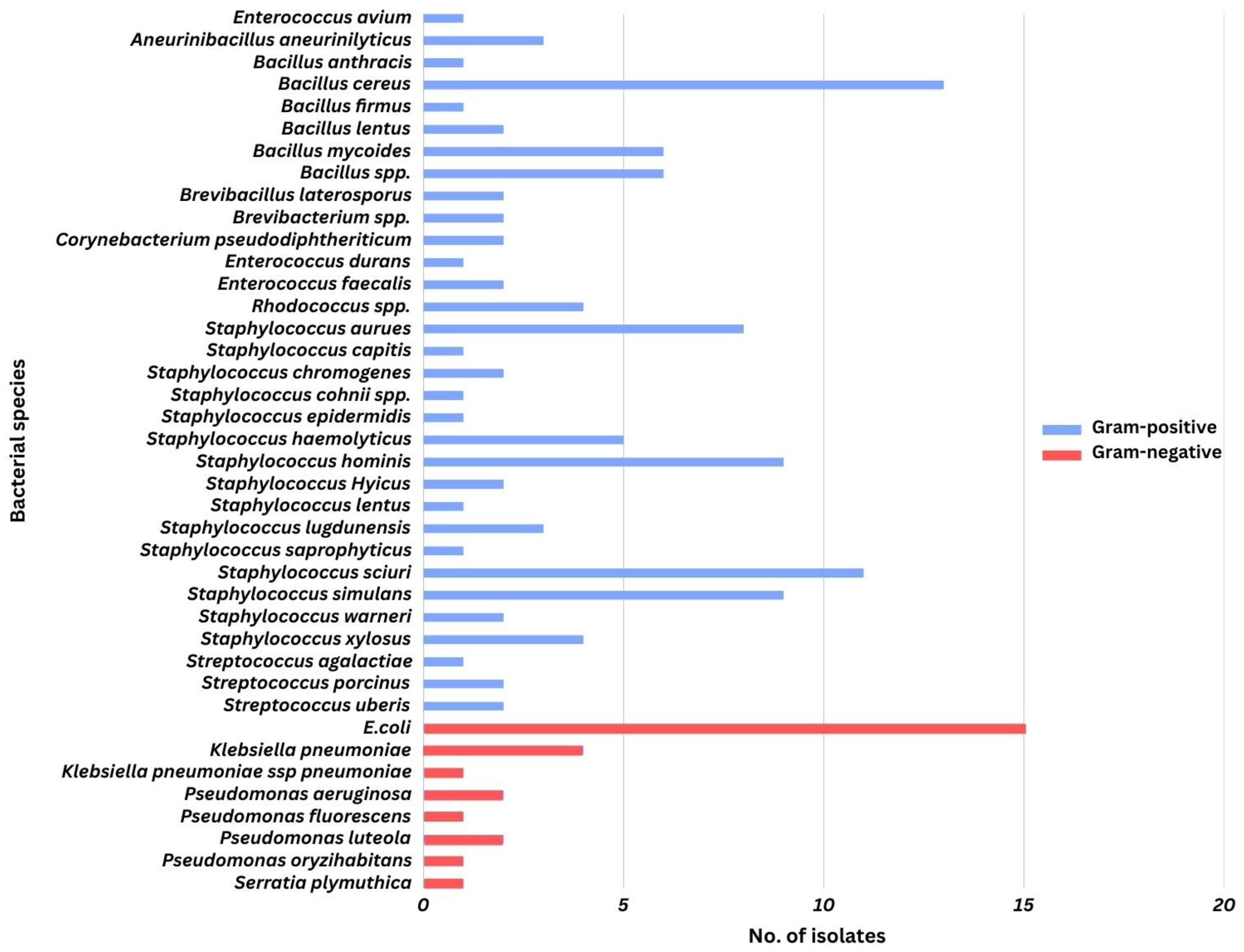
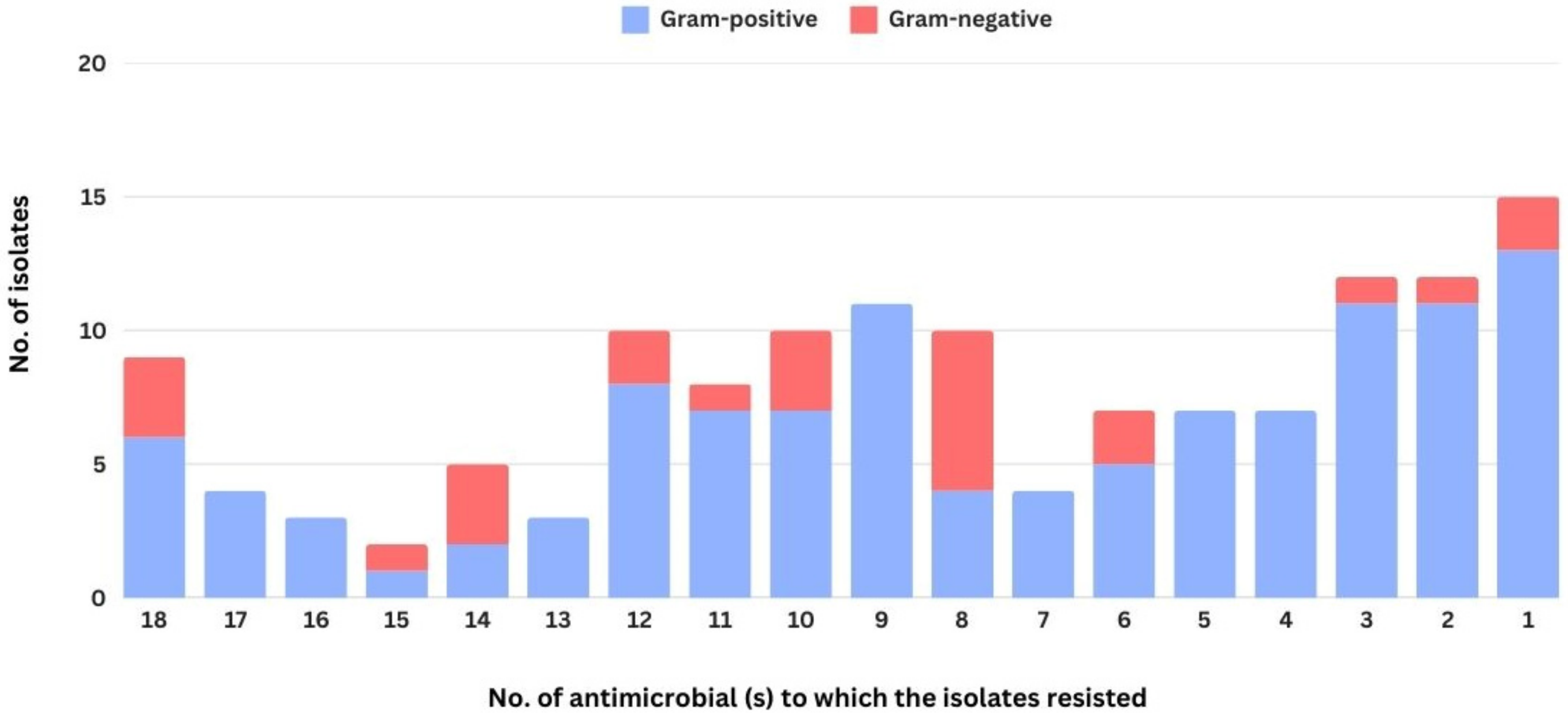
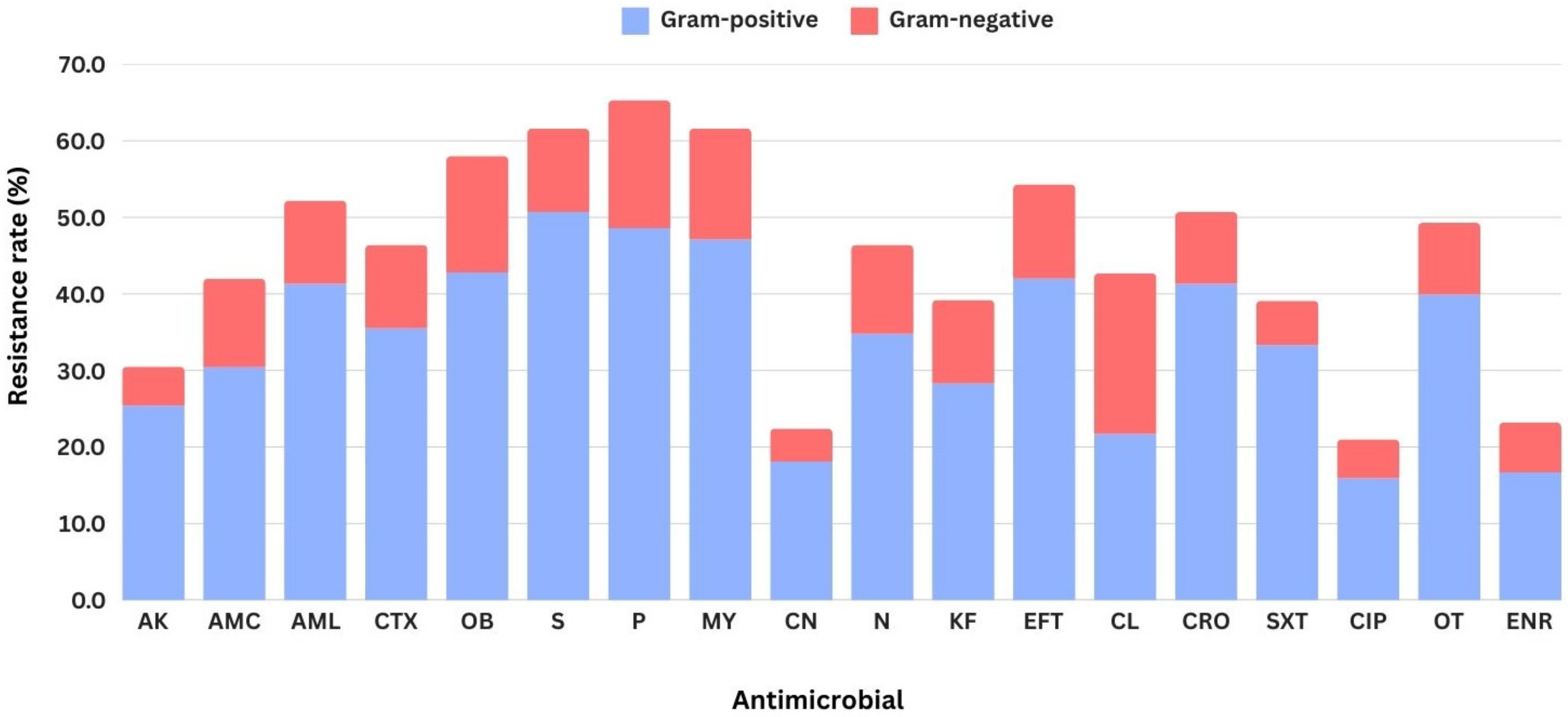
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kossaibati, M.; Esslemont, R. The costs of production diseases in dairy herds in England. Vet. J. 1997, 154, 41–51. [Google Scholar] [PubMed]
- Bradley, A.J. Bovine mastitis: An evolving disease. Vet. J. 2002, 164, 116–128. [Google Scholar] [PubMed]
- Pașca, C.; Mărghitaș, L.A.; Dezmirean, D.S.; Matei, I.A.; Bonta, V.; Pașca, I.; Chirilă, F.; Cîmpean, A.; Fiț, N.I. Efficacy of natural formulations in bovine mastitis pathology: Alternative solution to antimicrobial treatment. J. Vet. Res. 2020, 64, 523–529. [Google Scholar] [PubMed]
- Dufour, S.; Labrie, J.; Jacques, M. The mastitis pathogens culture collection. Microbiol. Res. Announc. 2019, 8, e00133-19. [Google Scholar] [CrossRef]
- Oliver, S.P.; Murinda, S.E. Antimicrobial resistance of mastitis pathogens. Vet. Clin. Food Anim. Pract. 2012, 28, 165–185. [Google Scholar]
- Molla, B.; Miko, A.; Pries, K.; Hildebrandt, G.; Kleer, J.; Schroeter, A.; Helmuth, R. Class 1 integrons and resistance gene cassettes among multidrug resistant Salmonella serovars isolated from slaughter animals and foods of animal origin in Ethiopia. Acta Trop. 2007, 103, 142–149. [Google Scholar]
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments—A review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699. [Google Scholar]
- Kimestri, A. Microbiological and physicochemical quality of pasteurized milk supplemented with sappan wood extract (Caesalpinia sappan L.). Int. Food Res. J. 2018, 25, 392–398. [Google Scholar]
- Teplicki, E.; Ma, Q.; Castillo, D.E.; Zarei, M.; Hustad, A.P.; Chen, J.; Li, J. The Effects of Aloe vera on Wound Healing in Cell Proliferation, Migration, and Viability. Wound 2018, 30, 263–268. [Google Scholar]
- Philpot, W.N.; Nickerson, S.C. Mastitis: Counter Attack; Babson Bros. Co.: Oak Brook, IL, USA, 1992. [Google Scholar]
- University of Zaragoza. Win Epi: Sample Size: Estimate Percentage. 2006. Available online: http://www.winepi.net/uk/index.htm (accessed on 25 May 2021).
- Sayed, R.H.; Soliman, R.T.; Elsaady, S.A. Development of a lateral flow device for rapid simultaneous multiple detections of some common bacterial causes of bovine mastitis. J. Adv. Vet. Anim. Res. 2023, 10, 292–300. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Archive of EUCAST Tables and Documents: Breakpoint Table. 2019. Available online: https://www.eucast.org/ast_of_bacteria/previous_versions_of_documents (accessed on 20 October 2021).
- Escherichia coli ATCC® 25922. Available online: https://www.atcc.org/products/25922 (accessed on 13 March 2025).
- Golus, J.; Sawicki, R.; Widelski, J.; Ginalska, G. The agar microdilution method—A new method for antimicrobial susceptibility testing for essential oils and plant extracts. J. Appl. Microbiol. 2016, 121, 1291–1299. [Google Scholar] [PubMed]
- Clinical & Laboratory Standards Institute. CLSI Guidelines. In Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Aiumlamai, S. Dairy management, health and production in Thailand. Int. Dairy. Top. 2009, 8, 11–13. [Google Scholar]
- Gonçalves, J.; Kamphuis, C.; Martins, C.; Barreiro, J.; Tomazi, T.; Gameiro, A.H.; Hogeveen, H.; Dos Santos, M. Bovine subclinical mastitis reduces milk yield and economic return. Livest. Sci. 2018, 210, 25–32. [Google Scholar]
- Prasomsri, P. Effect of lameness on daily milk yield in dairy cow. Thai J. Vet. Med. 2022, 52, 679–687. [Google Scholar] [CrossRef]
- Dingwell, R.T.; Leslie, K.E.; Schukken, Y.H.; Sargeant, J.M.; Timms, L.L. Evaluation of the California mastitis test to detect an intramammary infection with a major pathogen in early lactation dairy cows. Can. Vet. J. 2003, 44, 413. [Google Scholar]
- Kala, S.R.; Rani, N.L.; Rao, V.V.; Subramanyam, K.V. Comparison of different diagnostic tests for the detection of subclinical mastitis in buffaloes. Buffalo. Bull. 2021, 40, 653–659. [Google Scholar]
- Ramuada, M.; Tyasi, T.L.; Gumede, L.; Chitura, T. A practical guide to diagnosing bovine mastitis: A review. Front. Anim. Sci. 2024, 5, 1504873. [Google Scholar]
- Rifatbegović, M.; Nicholas, R.A.; Mutevelić, T.; Hadžiomerović, M.; Maksimović, Z. Pathogens Associated with Bovine Mastitis: The Experience of Bosnia and Herzegovina. Vet Sci. 2024, 11, 63. [Google Scholar]
- Abebe, R.; Hatiya, H.; Abera, M.; Megersa, B.; Asmare, K. Bovine mastitis: Prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet. Res. 2016, 12, 270. [Google Scholar]
- Jayarao, B.M.; Wolfgang, D.R. Bulk-tank milk analysis: A useful tool for improving milk quality and herd udder health. Vet. Clin. Food Anim. Pract. 2003, 19, 75–92. [Google Scholar]
- Zigo, F.; Vasil’, M.; Ondrašovičová, S.; Výrostková, J.; Bujok, J.; Pecka-Kielb, E. Maintaining optimal mammary gland health and prevention of mastitis. Front. Vet. Sci. 2021, 8, 607311. [Google Scholar] [CrossRef] [PubMed]
- Saed, H.A.E.-M.R.; Ibrahim, H.M.M. Antimicrobial profile of multidrug-resistant Streptococcus spp. isolated from dairy cows with clinical mastitis. J. Adv. Vet. Anim. Res. 2020, 7, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Klaas, I.; Zadoks, R. An update on environmental mastitis: Challenging perceptions. Transbound. Emerg. Dis. 2018, 65, 166–185. [Google Scholar]
- Angulo, F.J.; Collignon, P.; Powers, J.H.; Chiller, T.M.; Aidara-Kane, A.; Aarestrup, F.M. World Health Organization ranking of antimicrobials according to their importance in human medicine: A critical step for developing risk management strategies for the use of antimicrobials in food production animals. Clin. Infect. Dis. 2009, 49, 132–141. [Google Scholar]
- Thomas, V.; de Jong, A.; Moyaert, H.; Simjee, S.; El Garch, F.; Morrissey, I.; Marion, H.; Vallé, M. Antimicrobial susceptibility monitoring of mastitis pathogens isolated from acute cases of clinical mastitis in dairy cows across Europe: VetPath results. Int. J. Antimicrob. Agents 2015, 46, 13–20. [Google Scholar]
- Timonen, A.; Sammul, M.; Taponen, S.; Kaart, T.; Mõtus, K.; Kalmus, P. Antimicrobial selection for the treatment of clinical mastitis and the efficacy of penicillin treatment protocols in large Estonian dairy herds. Antimicrobials 2021, 11, 44. [Google Scholar]
- Somrup, S.; Mitsuwan, W.; Bhumibhamon, T.; Pereira, M.L.; Paul, A.K.; Nissapatorn, V.; Saengsawang, P. Antibiograms, multidrug resistance, and milk-related parameters of bacteria isolated from milk of dairy cattle in Phatthalung, Thailand. Vet. World 2024, 17, 735–743. [Google Scholar]
- Haq, I.U.; Kamal, M.; Swelum, A.A.; Khan, S.; Ríos-Escalante, P.R.L.; Usman, T. Alarming multidrug resistance in Staphylococcus aureus isolated from raw milk of cows with subclinical mastitis: Antibiotic resistance patterns and occurrence of selected resistance genes. PLoS ONE 2024, 19, e0301200. [Google Scholar]
- Cheng, J.; Qu, W.; Barkema, H.W.; Nobrega, D.B.; Gao, J.; Liu, G.; De Buck, J.; Kastelic, J.P.; Sun, H.; Han, B. Antimicrobial resistance profiles of 5 common bovine mastitis pathogens in large Chinese dairy herds. J. Dairy Sci. 2019, 102, 2416–2426. [Google Scholar]
- Emon, A.A.; Hossain, H.; Chowdhury, M.S.R.; Rahman, M.A.; Tanni, F.Y.; Asha, M.N.; Akter, H.; Hossain, M.M.; Islam, M.R.; Rahman, M.M. Prevalence, antimicrobial susceptibility profiles and resistant gene identification of bovine subclinical mastitis pathogens in Bangladesh. Heliyon 2024, 10, e34567. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, F.M. The livestock reservoir for antimicrobial resistance: A personal view on changing patterns of risks, effects of interventions and the way forward. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140085. [Google Scholar] [CrossRef] [PubMed]
- Argudín, M.A.; Deplano, A.; Meghraoui, A.; Dodémont, M.; Heinrichs, A.; Denis, O.; Nonhoff, C.; Roisin, S. Bacteria from animals as a pool of antimicrobial resistance genes. Antimicrobials 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Karthik, S.; Mathivanan, K.; Baskaran, R.; Karthikeyan, M.; Gopi, M.; Govindasamy, C. In vitro antimicrobial activity of Caesalpinia sappan L. Asian Pac. J. Trop. Biomed. 2012, 2, S136–S139. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Panichayupakaranant, P. Antioxidant, antibacterial, and anti-inflammatory activities of standardized brazilin-rich Caesalpinia sappan extract. Pharm. Biol. 2015, 53, 1339–1343. [Google Scholar] [CrossRef]
- Pattananandecha, T.; Apichai, S.; Julsrigival, J.; Ogata, F.; Kawasaki, N.; Saenjum, C. Antibacterial activity against foodborne pathogens and inhibitory effect on anti-inflammatory mediators’ production of Brazilin-enriched extract from Caesalpinia sappan Linn. Plants 2022, 11, 1698. [Google Scholar] [CrossRef]
- Martinez, J.; Baquero, F. Mutation frequencies and antimicrobial resistance. Antimicrob. Agents. Chemother. 2000, 44, 1771–1777. [Google Scholar] [CrossRef]
- Normark, B.H.; Normark, S. Evolution and spread of antimicrobial resistance. J. Intern. Med. 2002, 252, 91–106. [Google Scholar] [CrossRef]
- Fischer, E.; Braun, V. Die Penetrationsbarriere der Bakterienzellwand als Ursache der Antibiotikaresistenz [Permeability barrier of bacterial cell envelopes as cause of resistance to antibiotics (author’s transl)]. Immun. Infekt. 1981, 9, 78–87. [Google Scholar]
- Sarkar, D.; Dutta, A.; Das, M.; Sarkar, K.; Mandal, C.; Chatterjee, M. Effect of Aloe vera on nitric oxide production by macrophages during inflammation. Indian J. Pharmacol. 2005, 37, 371–375. [Google Scholar]
- Serdar, C.C.; Cihan, M.; Yücel, D.; Serdar, M.A. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem. Med. 2021, 31, 010502. [Google Scholar]
| Milk Quality | Reaction | Characteristics of Reaction |
|---|---|---|
| Normal, Very Good | 0 | A homogeneous mixture with a pale purple color that moves quickly when the CMT paddle is rotated. |
| Normal, Good | T | The mixture becomes mucus-like, forms a thread, and then disappears. It exhibits a pale purple color and moves quickly when the CMT paddle is rotated. |
| Normal, Fair | 1 | The mixture is viscous and mucus-like, with the purple color intensifying and moving more slowly when the CMT paddle is rotated. |
| Subclinical Mastitis | 2 | The mixture is viscous and mucus-like, with the purple color intensifying. It moves very slowly when the CMT paddle is rotated, and the udders still appear visually normal. |
| Clinical Mastitis | 3 | The mixture is thick and mucus-like, making it easily observable. |
| Parameters | Before | After | p-Value |
|---|---|---|---|
| Total bacterial count (log10CFU/mL) | 6.875 ± 1.72 | 2.704 ± 2.91 | <0.01 |
| CMT score | 3 ± 0 | 1 ± 0 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadee, P.; Pattanawong, W.; Manwicha, A.; Khaodang, P.; Amornlerdpison, D.; Chansakaow, S.; Tipduangta, P.; Chukiatsiri, K.; Tadee, P. Bacterial Pathogens of Bovine Mastitis: Prevalence, Antimicrobial Susceptibility, and Sensitivity to Caesalpinia sappan Both In Vitro and In Vivo Studies. Biology 2025, 14, 350. https://doi.org/10.3390/biology14040350
Tadee P, Pattanawong W, Manwicha A, Khaodang P, Amornlerdpison D, Chansakaow S, Tipduangta P, Chukiatsiri K, Tadee P. Bacterial Pathogens of Bovine Mastitis: Prevalence, Antimicrobial Susceptibility, and Sensitivity to Caesalpinia sappan Both In Vitro and In Vivo Studies. Biology. 2025; 14(4):350. https://doi.org/10.3390/biology14040350
Chicago/Turabian StyleTadee, Phacharaporn, Wiwat Pattanawong, Apichart Manwicha, Pakasinee Khaodang, Doungporn Amornlerdpison, Sunee Chansakaow, Pramote Tipduangta, Kridda Chukiatsiri, and Pakpoom Tadee. 2025. "Bacterial Pathogens of Bovine Mastitis: Prevalence, Antimicrobial Susceptibility, and Sensitivity to Caesalpinia sappan Both In Vitro and In Vivo Studies" Biology 14, no. 4: 350. https://doi.org/10.3390/biology14040350
APA StyleTadee, P., Pattanawong, W., Manwicha, A., Khaodang, P., Amornlerdpison, D., Chansakaow, S., Tipduangta, P., Chukiatsiri, K., & Tadee, P. (2025). Bacterial Pathogens of Bovine Mastitis: Prevalence, Antimicrobial Susceptibility, and Sensitivity to Caesalpinia sappan Both In Vitro and In Vivo Studies. Biology, 14(4), 350. https://doi.org/10.3390/biology14040350






