Role of Rhizophagus intraradices in Mitigating Salt Stress of Sulla carnosa Through Modulating Plant Hormones (ABA, SA, and JA) and Nutrient Profile
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Fungal Inocula
2.2. Experimental Design, Biological Treatments, and Growth Conditions
2.3. Determination of Photosynthetic Pigment Concentrations
2.4. Hydrogen Peroxide Concentration
2.5. Hormone Analysis
2.6. Nutrient Content
2.7. Statistical Analysis
3. Results
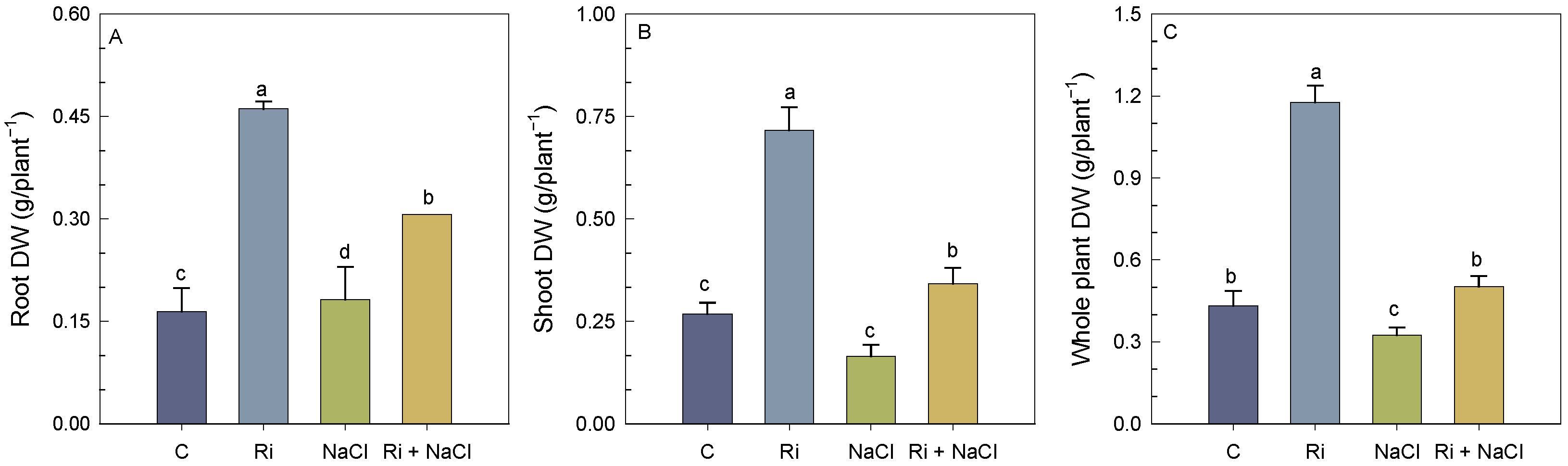
3.1. Biomass Yield
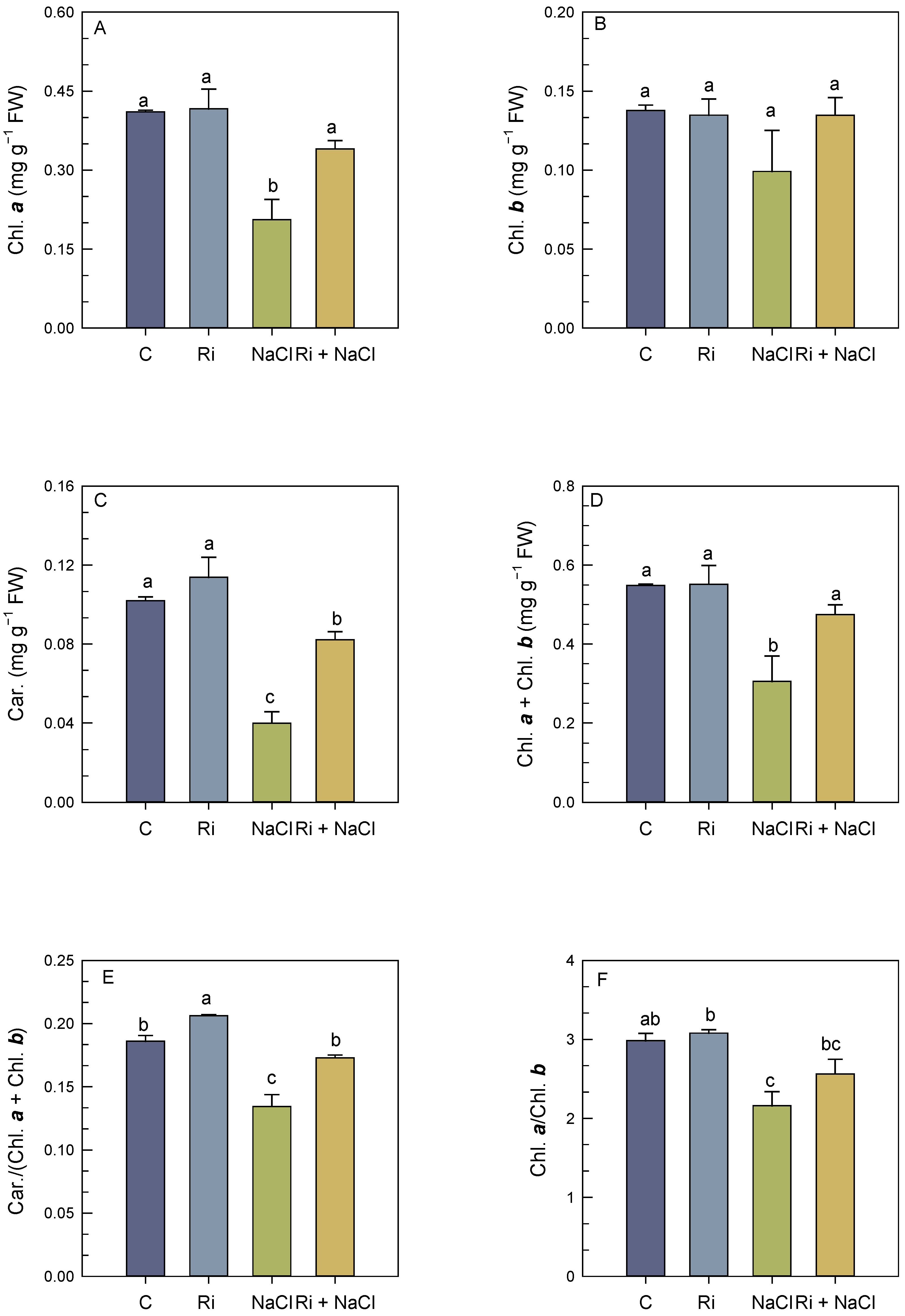
3.2. Photosynthetic Pigment Concentrations
3.3. Contents of Nutrients
3.4. H2O2 Content
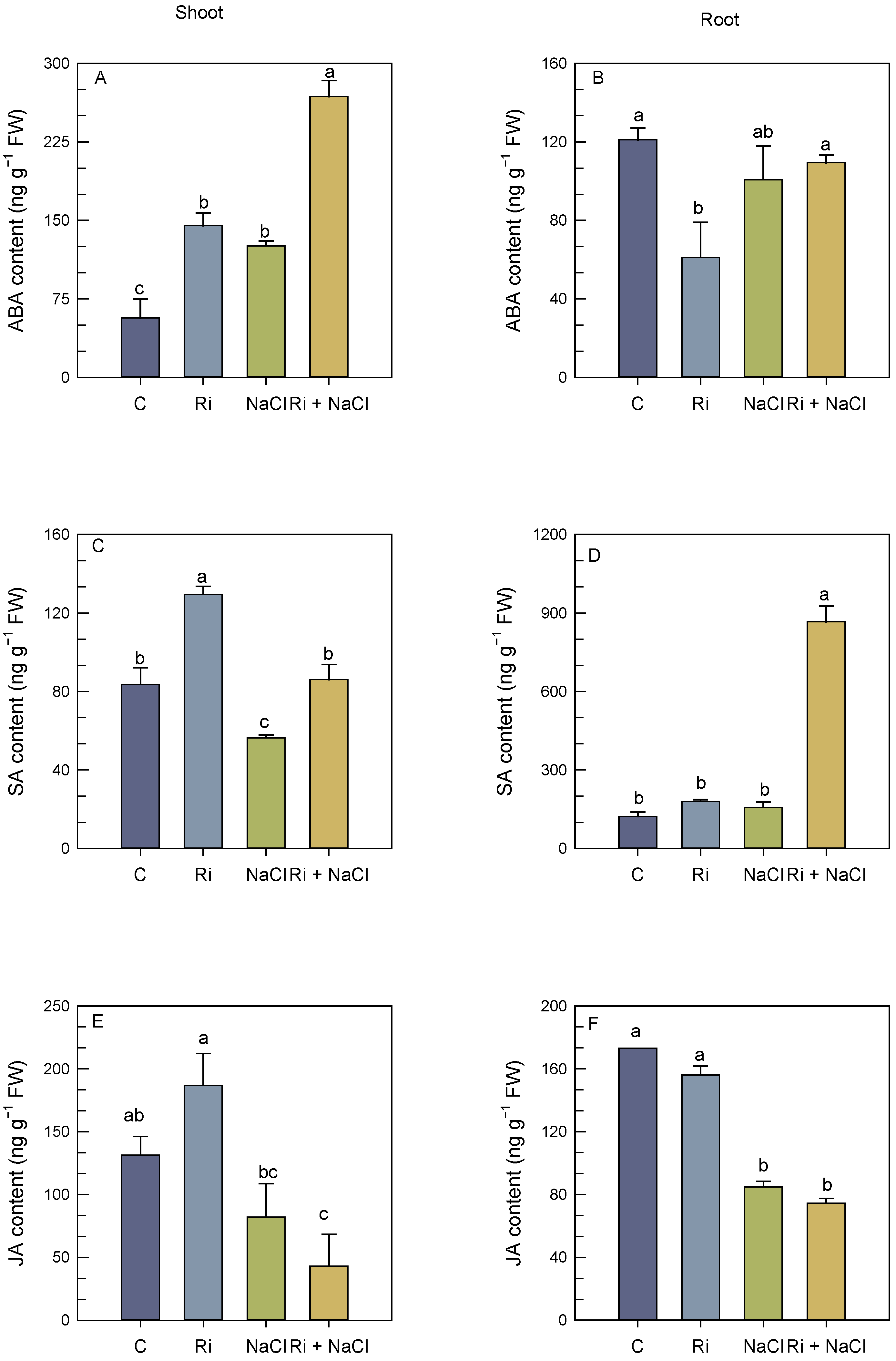
3.5. Endogenous Phytohormone Status
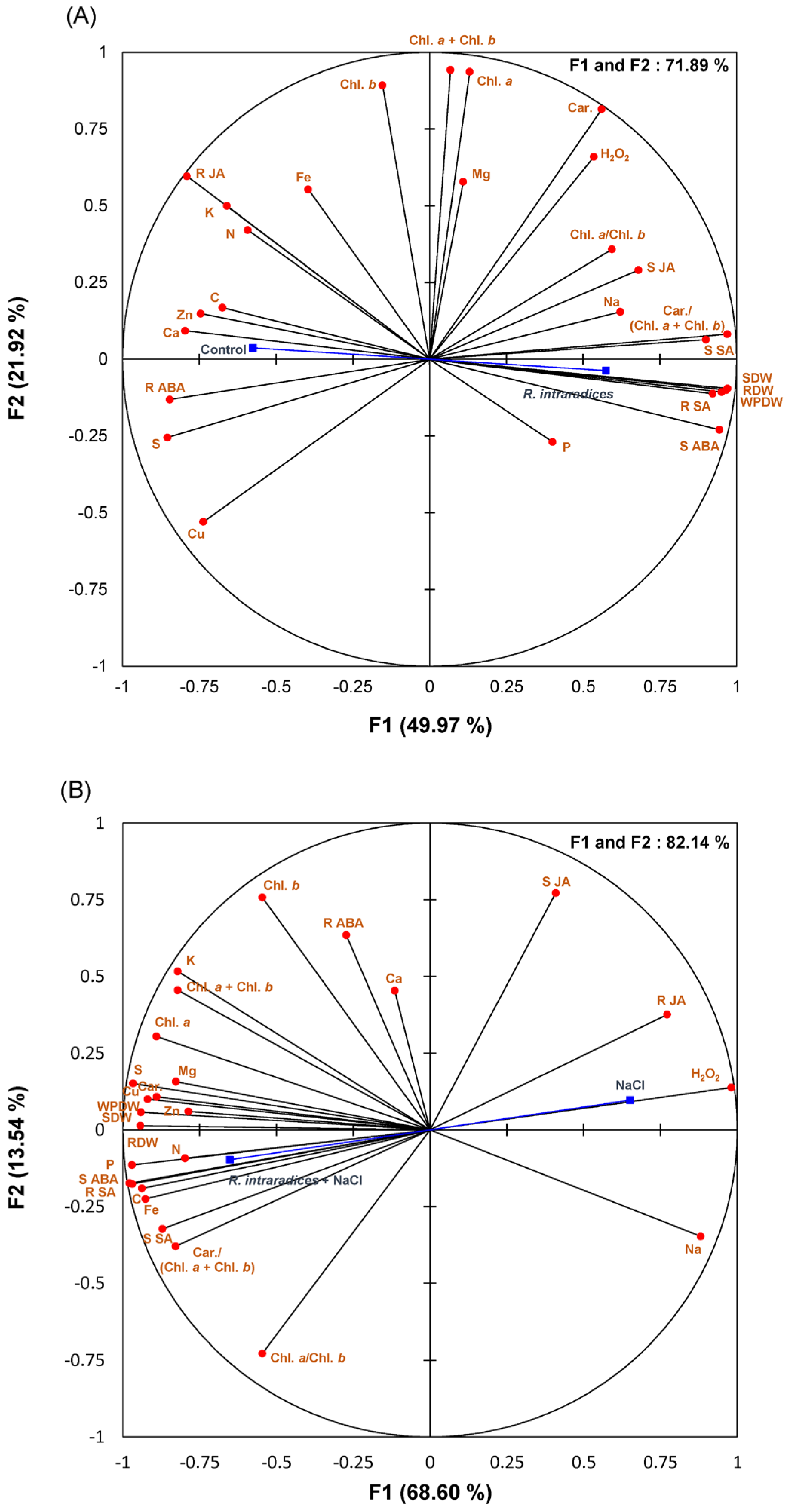
3.6. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muhammad, M.; Waheed, A.; Wahab, A.; Majeed, M.; Nazim, M.; Liu, Y.H.; Li, L.; Li, W.J. Soil salinity and drought tolerance: An evaluation of plant growth, productivity, microbial diversity, and amelioration strategies. Plant Stress 2023, 11, 100319. [Google Scholar] [CrossRef]
- Navarro-Torre, S.; Garcia-Caparrós, P.; Nogales, A.; Abreu, M.M.; Santos, E.; Cortinhas, A.L.; Caperta, A.D. Sustainable agricultural management of saline soils in arid and semi-arid Mediterranean regions through halophytes, microbial and soil-based technologies. Environ. Exp. Bot. 2023, 212, 105397. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Boughattas, S.; Hu, S.; Oh, S.H.; Sa, T. A meta-analysis of arbuscular mycorrhizal effects on plants grown under salt stress. Mycorrhiza 2014, 24, 611–625. [Google Scholar] [CrossRef]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef]
- Pooja, P.; Tallapragada, S.; Lamba, A.; Punia, S. Role played by arbuscular mycorrhizal fungi in amelioration of salinity stress: A review. Plant Soil 2024, 1–16. [Google Scholar] [CrossRef]
- Chandra, P.; Singh, A.; Prajapat, K.; Rai, A.K.; Yadav, R.K. Native arbuscular mycorrhizal fungi improve growth, biomass yield, and phosphorus nutrition of sorghum in saline and sodic soils of the semi–arid region. Environ. Exp. Bot. 2022, 201, 104982. [Google Scholar] [CrossRef]
- Pu, Z.; Hu, R.; Wang, D.; Wang, C.; Chen, Y.; Wang, S.; Zhuge, Y.; Xie, Z. The impact of arbuscular mycorrhizal fungi on soybean growth strategies in response to salt stress. Plant Soil 2024, 1–15. [Google Scholar] [CrossRef]
- Huang, T.; Xie, K.; Zhang, Z.; Zhang, Q.; Li, Y.; Lin, S.; Zhou, J.; Chen, J.; Li, X. The colonization of the arbuscular mycorrhizal fungus Rhizophagus irregularis affects the diversity and network structure of root endophytic bacteria in maize. Sci. Hortic. 2024, 326, 112774. [Google Scholar] [CrossRef]
- Hamzehzadeh, H.; Abbaspour, H.; Safipour Afshar, A.; Hamdi, S.M.M. AMF-mediated salinity adaptation in Pistachio plants: Photosynthetic efficiency and ionic balance. Biologia 2024, 80, 1–17. [Google Scholar] [CrossRef]
- Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol. 2007, 173, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Hammer, E.C.; Rillig, M.C. The influence of different stresses on glomalin levels in an arbuscular mycorrhizal fungus—Salinity increases glomalin content. PLoS ONE 2011, 6, e28426. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Peng, F.; Tedeschi, A.; Xue, X.; Wang, T.; Liao, J.; Zhang, W.; Huang, C. Do halophytes and glycophytes differ in their interactions with arbuscular mycorrhizal fungi under salt stress? A meta-analysis. Bot. Stud. 2020, 61, 13. [Google Scholar] [CrossRef]
- Rillig, M.C.; Steinberg, P.D. Glomalin production by an arbuscular mycorrhizal fungus: A mechanism of habitat modification? Soil Biol. Biochem. 2002, 34, 1371–1374. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Abd_Allah, E.F. Plant hormones as key regulators in plant-microbe interactions under salt stress. In Plant Microbiome: Stress Response; Springer: Singapore, 2018; pp. 165–182. [Google Scholar]
- Kang, S.M.; Khan, A.L.; Waqas, M.; You, Y.H.; Kim, J.H.; Kim, J.G.; Hamayun, M.; Lee, I.J. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef]
- Yoo, S.J.; Weon, H.Y.; Song, J.; Sang, M.K. Induced tolerance to salinity stress by halotolerant bacteria Bacillus aryabhattai H19-1 and B. mesonae H20-5 in tomato plants. J. Microbiol. Biotechnol. 2019, 29, 1124–1136. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Li, X.; Sun, P.; Zhang, Y.; Jin, C.; Guan, C. A novel PGPR strain Kocuria rhizophila Y1 enhances salt stress tolerance in maize by regulating phytohormone levels, nutrient acquisition, redox potential, ion homeostasis, photosynthetic capacity and stress-responsive genes expression. Environ. Exp. Bot. 2020, 174, 104023. [Google Scholar] [CrossRef]
- Ayaz, M.; Ali, Q.; Jiang, Q.; Wang, R.; Wang, Z.; Mu, G.; Khan, S.A.; Khan, A.R.; Manghwar, H.; Wu, H.; et al. Salt tolerant Bacillus strains improve plant growth traits and regulation of phytohormones in wheat under salinity stress. Plants 2022, 11, 2769. [Google Scholar] [CrossRef]
- Hashem, A.; Alqarawi, A.A.; Radhakrishnan, R.; Al-Arjani, A.B.F.; Aldehaish, H.A.; Egamberdieva, D.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi regulate the oxidative system, hormones and ionic equilibrium to trigger salt stress tolerance in Cucumis sativus L. Saudi J. Biol. Sci. 2018, 25, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.J.; Tong, C.L.; Wang, Q.S.; Bie, S. Mycorrhizas Affect Physiological Performance, Antioxidant System, Photosynthesis, Endogenous Hormones, and Water Content in Cotton under Salt Stress. Plants 2024, 13, 805. [Google Scholar] [CrossRef] [PubMed]
- Khalloufi, M.; Martínez-Andújar, C.; Karray-Bouraouib, N.; Pérez-Alfocea, F.; Albacete, A. The crosstalk interaction of ethylene, gibberellins, and arbuscular mycorrhiza improves growth in salinized tomato plants by modulating the hormonal balance. J. Plant Physiol. 2024, 303, 154336. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ugurlar, F.; Ashraf, M.; Ahmad, P. Salicylic acid interacts with other plant growth regulators and signal molecules in response to stressful environments in plants. Plant Physiol. Biochem. 2023, 196, 431–443. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Tuteja, N. Abscisic acid and abiotic stress signaling. Plant Signal. Behav. 2007, 2, 135–138. [Google Scholar] [CrossRef]
- Parwez, R.; Aftab, T.; Gill, S.S.; Naeem, M. Abscisic acid signaling and crosstalk with phytohormones in regulation of environmental stress responses. Environ. Exp. Bot. 2022, 199, 104885. [Google Scholar] [CrossRef]
- Wilkinson, S.; Davies, W.J. Drought, ozone, ABA and ethylene: New insights from cell to plant to community. Plant Cell Environ. 2010, 33, 510–525. [Google Scholar] [CrossRef]
- Cabot, C.; Sibole, J.V.; Barceló, J.; Poschenrieder, C. Abscisic acid decreases leaf Na+ exclusion in salt-treated Phaseolus vulgaris L. J. Plant Growth Regul. 2009, 28, 187–192. [Google Scholar] [CrossRef]
- Liu, X.; Niu, H.; Li, J.; Jiang, D.; Chen, R.; Zhang, R.; Li, Q. Higher endogenous abscisic acid confers greater tolerance to saline-alkaline stress in Petunia hybrida. Environ. Exp. Bot. 2024, 228, 106035. [Google Scholar] [CrossRef]
- Jayakannan, M.; Bose, J.; Babourina, O.; Rengel, Z.; Shabala, S. Salicylic acid in plant salinity stress signaling and tolerance. Plant Growth Regul. 2015, 76, 25–40. [Google Scholar] [CrossRef]
- Singh, P.K.; Gautam, S. Role of salicylic acid on physiological and biochemical mechanism of salinity stress tolerance in plants. Acta Physiol. Plant 2013, 35, 2345–2353. [Google Scholar] [CrossRef]
- Ilyas, M.; Maqsood, M.F.; Shahbaz, M.; Zulfiqar, U.; Ahmad, K.; Naz, N.; Ali, M.F.; Ahmad, M.; Ali, Q.; Yong, J.W.H.; et al. Alleviating salinity stress in canola (Brassica napus L.) through exogenous application of salicylic acid. BMC Plant Biol. 2024, 24, 611. [Google Scholar] [CrossRef] [PubMed]
- Sultan, I.; Khan, I.; Chattha, M.U.; Hassan, M.U.; Barbanti, L.; Calone, R.; Ali, M.; Majeed, S.; Ghani, M.A.; Batool, M.; et al. Improved salinity tolerance in early growth stage of maize through salicylic acid foliar application. Ital. J. Agron. 2021, 16, 1810. [Google Scholar] [CrossRef]
- Ali, A.; Kant, K.; Kaur, N.; Gupta, S.; Jindal, P.; Gill, S.S.; Naeem, M. Salicylic acid: Homeostasis, signalling and phytohormone crosstalk in plants under environmental challenges. S. Afr. J. Bot. 2024, 169, 314–335. [Google Scholar] [CrossRef]
- Riemann, M.; Dhakarey, R.; Hazman, M.; Miro, B.; Kohli, A.; Nick, P. Exploring jasmonates in the hormonal network of drought and salinity responses. Front. Plant Sci. 2015, 6, 1077. [Google Scholar] [CrossRef]
- Ahmad, B.; Raina, A.; Naikoo, M.I.; Khan, S. Role of methyl jasmonates in salt stress tolerance in crop plants. In Plant Signaling Molecules; Woodhead Publishing: Sawston, UK, 2019; pp. 371–384. [Google Scholar]
- Khan, V.; Jha, A.; Seth, T.; Iqbal, N.; Umar, S. Exploring the role of jasmonic acid in boosting the production of secondary metabolites in medicinal plants: Pathway for future research. Ind. Crops Prod. 2024, 220, 119227. [Google Scholar] [CrossRef]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef]
- Jlassi, A.; Zorrig, W.; Khouni, A.E.; Lakhdar, A.; Smaoui, A.; Abdelly, C.; Rabhi, M. Phytodesalination of a moderately-salt-affected soil by Sulla carnosa. Int. J. Phytoremediation 2013, 15, 398–404. [Google Scholar] [CrossRef]
- Hidri, R.; Barea, J.M.; Mahmoud, O.M.B.; Abdelly, C.; Azcón, R. Impact of microbial inoculation on biomass accumulation by Sulla carnosa provenances, and in regulating nutrition, physiological and antioxidant activities of this species under non-saline and saline conditions. J. Plant Physiol. 2016, 201, 28–41. [Google Scholar] [CrossRef]
- Bejaoui, F.; Salas, J.J.; Nouairi, I.; Smaoui, A.; Abdelly, C.; Martínez-Force, E.; Youssef, N.B. Changes in chloroplast lipid contents and chloroplast ultrastructure in Sulla carnosa and Sulla coronaria leaves under salt stress. J. Plant Physiol. 2016, 198, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Hidri, R.; Metoui-Ben Mahmoud, O.; Debez, A.; Zorrig, W.; Abdelly, C.; Zamarreño, A.M.; García-Mina, J.M.; Azcon, R.; Aroca, R. Dual PGPR-AMF inoculation offsets salinity stress impact on the fodder halophyte Sulla carnosa by concomitantly modulating plant ABA content and leaf antioxidant response. J. Plant Growth Regul. 2024, 1–19. [Google Scholar] [CrossRef]
- Spagnoletti, F.N.; Carmona, M.; Balestrasse, K.; Chiocchio, V.; Giacometti, R.; Lavado, R.S. The arbuscular mycorrhizal fungus Rhizophagus intraradices reduces the root rot caused by Fusarium pseudograminearum in wheat. Rhizosphere 2021, 19, 100369. [Google Scholar] [CrossRef]
- Onyeaka, H.N.; Akinsemolu, A.A.; Siyanbola, K.F.; Adetunji, V.A. Green Microbe Profile: Rhizophagus intraradices—A Review of Benevolent Fungi Promoting Plant Health and Sustainability. Microbiol. Res. 2024, 15, 1028–1049. [Google Scholar] [CrossRef]
- Schenck, N.C.; Smith, G.S. Additional new and unreported species of mycorrhizal fungi (Endogonaceae) from Florida. Mycologia 1982, 74, 77–92. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Aroca, R.; Irigoyen, J.J.; Sánchez-Díaz, M. Drought enhances maize chilling tolerance. II. Photosynthetic traits and protective mechanisms against oxidative stress. Physiol. Plant 2003, 117, 540–549. [Google Scholar] [CrossRef]
- Aroca, R.; Ruiz-Lozano, J.M.; Zamarreño, Á.M.; Paz, J.A.; García-Mina, J.M.; Pozo, M.J.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 2013, 170, 47–55. [Google Scholar] [CrossRef]
- Sánchez-Romera, B.; Ruiz-Lozano, J.M.; Li, G.; Luu, D.T.; Martinez-Ballesta, M.D.; Carvajal, M.; Zamarreno, A.M.; Garcia-Mina, J.M.; Maurel, C.; Aroca, R. Enhancement of root hydraulic conductivity by methyl jasmonate and the role of calcium and abscisic acid in this process. Plant Cell Environ. 2014, 37, 995–1008. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Lugo, M.A.; Negritto, M.A.; Crespo, E.M.; Iriarte, H.J.; Núñez, S.; Espinosa, L.F.; Pagano, M.C. Arbuscular Mycorrhizal Fungi as a Salt Bioaccumulation Mechanism for the Establishment of a Neotropical Halophytic Fern in Saline Soils. Microorganisms 2024, 12, 2587. [Google Scholar] [CrossRef] [PubMed]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Li, W.; Li, Y.; An, Y.; Wu, H.; Guo, Y. Effects of plant nutrient acquisition strategies on biomass allocation patterns in wetlands along successional sequences in the semi-arid upper Yellow River basin. Front. Plant Sci. 2024, 15, 1441567. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under stress: How plants balance growth and the stress response. Dev. Cell. 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Ma, P.; Shi, Z.; Diao, F.; Hao, L.; Zhang, J.; Xu, J.; Wang, L.; Dang, Z.; Guo, W. Effects of arbuscular mycorrhizal fungi on growth and Na+ accumulation of Suaeda glauca (Bunge) grown in salinized wetland soils. Appl. Soil Ecol. 2021, 166, 104065. [Google Scholar] [CrossRef]
- Zhang, H.S.; Qin, F.F.; Qin, P.; Pan, S.M. Evidence that arbuscular mycorrhizal and phosphate-solubilizing fungi alleviate NaCl stress in the halophyte Kosteletzkya virginica: Nutrient uptake and ion distribution within root tissues. Mycorrhiza 2014, 24, 383–395. [Google Scholar] [CrossRef]
- Hajiboland, R.; Dashtebani, F.; Aliasgharzad, N. Physiological responses of halophytic C4 grass Aeluropus littoralis to salinity and arbuscular mycorrhizal fungi colonization. Photosynthetica 2015, 53, 572–584. [Google Scholar] [CrossRef]
- Dashtebani, F.; Hajiboland, R.; Aliasgharzad, N. Characterization of salt-tolerance mechanisms in mycorrhizal (Claroideoglomus etunicatum) halophytic grass, Puccinellia distans. Acta Physiol. Plant 2014, 36, 1713–1726. [Google Scholar] [CrossRef]
- Estrada, B.; Aroca, R.; Azcon-Aguilar, C.; Miguel Barea, J.; Manuel Ruiz-Lozano, J. Importance of native arbuscular mycorrhizal inoculation in the halophyte Asteriscus maritimus for successful establishment and growth under saline conditions. Plant Soil 2013, 370, 175–185. [Google Scholar] [CrossRef]
- Li, T.; Liu, R.; He, X.; Wang, B. Enhancement of superoxide dismutase and catalase activities and salt tolerance of euhalophyte Suaeda salsa L. by mycorrhizal fungus Glomus mosseae. Pedosphere 2012, 22, 217–224. [Google Scholar] [CrossRef]
- Bhupenchandra, I.; Chongtham, S.K.; Devi, A.G.; Dutta, P.; Sahoo, M.R.; Mohanty, S.; Kumar, S.; Choudhary, A.K.; Devi, E.L.; Sinyorita, S.; et al. Unlocking the Potential of Arbuscular Mycorrhizal Fungi: Exploring Role in Plant Growth Promotion, Nutrient Uptake Mechanisms, Biotic Stress Alleviation, and Sustaining Agricultural Production Systems. J. Plant Growth Regul. 2024, 1–39. [Google Scholar] [CrossRef]
- Naz, M.; Ghani, M.I.; Atif, M.J.; Raza, M.A.; Bouzroud, S.; Afzal, M.R.; Riaz, M.; Ali, M.; Tariq, M.; Fan, X. Sodium and abiotic stress tolerance in plants. In Beneficial Chemical Elements of Plants: Recent Developments and Future Prospects; Wiley: Hoboken, NJ, USA, 2023; pp. 307–330. [Google Scholar]
- Ashraf, M.; Shahzad, S.M.; Imtiaz, M.; Rizwan, M.S. Salinity effects on nitrogen metabolism in plants–focusing on the activities of nitrogen metabolizing enzymes: A review. J. Plant Nutr. 2018, 41, 1065–1081. [Google Scholar] [CrossRef]
- de Souza Miranda, R.; Gomes-Filho, E.; Prisco, J.T.; Alvarez-Pizarro, J.C. Ammonium improves tolerance to salinity stress in Sorghum bicolor plants. Plant Growth Regul. 2016, 78, 121–131. [Google Scholar] [CrossRef]
- Gupta, S.; Schillaci, M.; Walker, R.; Smith, P.M.; Watt, M.; Roessner, U. Alleviation of salinity stress in plants by endophytic plant-fungal symbiosis: Current knowledge, perspectives and future directions. Plant Soil 2021, 461, 219–244. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ. Exp. Bot. 2014, 98, 20–31. [Google Scholar] [CrossRef]
- Liu, A.; Hamel, C.; Hamilton, R.I.; Ma, B.L.; Smith, D.L. Acquisition of Cu, Zn, Mn and Fe by mycorrhizal maize (Zea mays L.) grown in soil at different P and micronutrient levels. Mycorrhiza 2000, 9, 331–336. [Google Scholar] [CrossRef]
- Wang, H.; An, T.; Huang, D.; Liu, R.; Xu, B.; Zhang, S.; Deng, X.; Siddique, K.H.; Chen, Y. Arbuscular mycorrhizal symbioses alleviating salt stress in maize is associated with a decline in root-to-leaf gradient of Na+/K+ ratio. BMC Plant Biol. 2021, 21, 457. [Google Scholar] [CrossRef]
- Mao, Y.; Chai, X.; Zhong, M.; Zhang, L.; Zhao, P.; Kang, Y.; Guo, J.; Yang, X. Effects of nitrogen and magnesium nutrient on the plant growth, quality, photosynthetic characteristics, antioxidant metabolism, and endogenous hormone of Chinese kale (Brassica albograbra Bailey). Sci. Hortic. 2022, 303, 111243. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Islam, M.S.; Hossain, A.; Iqbal, M.A.; Mubeen, M.; Waleed, M.; Reginato, M.; Battaglia, M.; Ahmed, S.; Rehman, A.; et al. Phytohormones as growth regulators during abiotic stress tolerance in plants. Front. Agron. 2022, 4, 765068. [Google Scholar] [CrossRef]
- Hussain, S.; Hafeez, M.B.; Azam, R.; Mehmood, K.; Aziz, M.; Ercisli, S.; Javed, T.; Raza, A.; Zahra, N.; Hussain, S.; et al. Deciphering the role of phytohormones and osmolytes in plant tolerance against salt stress: Implications, possible cross-talk, and prospects. J. Plant Growth Regul. 2024, 43, 38–59. [Google Scholar] [CrossRef]
- Kumar, P.; Ashique, S.; Kumar, N.; Jain, A.; Sharma, H.; Pandey, S.N.; Singh, A. Regulation of Plant Hormones Under Abiotic Stress Conditions in Plants. In Plant Secondary Metabolites and Abiotic Stress; Wiley: Hoboken, NJ, USA, 2024; pp. 243–276. [Google Scholar]
- Gutjahr, C.; Paszkowski, U. Weights in the balance: Jasmonic acid and salicylic acid signaling in root-biotroph interactions. Mol. Plant-Microbe Interact. 2009, 22, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, M.; Sun, J.; Wen, J.; Mysore, K.S.; Oldroyd, G.E. Abscisic acid promotion of arbuscular mycorrhizal colonization requires a component of the protein phosphatase 2A complex. Plant Physiol. 2014, 166, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.K.; Khanday, D.M.; Choudhary, S.M.; Kumar, P.; Kumari, S.; Martínez-Andújar, C.; Martínez-Melgarejo, P.A.; Rai, P.K.; Pérez-Alfocea, F. Unlocking nature’s stress buster: Abscisic acid’s crucial role in defending plants against abiotic stress. Plant Stress 2024, 11, 100359. [Google Scholar] [CrossRef]
- Zörb, C.; Geilfus, C.M.; Mühling, K.H.; Ludwig-Müller, J. The influence of salt stress on ABA and auxin concentrations in two maize cultivars differing in salt resistance. J. Plant Physiol. 2013, 170, 220–224. [Google Scholar] [CrossRef]
- Ren, C.G.; Kong, C.C.; Xie, Z.H. Role of abscisic acid in strigolactone-induced salt stress tolerance in arbuscular mycorrhizal Sesbania cannabina seedlings. BMC Plant Biol. 2018, 18, 74. [Google Scholar] [CrossRef]
- Lidoy, J.; López-García, Á.; Amate, C.; García, J.M.; Flors, V.; García-Garrido, J.M.; Azcón-Aguilar, C.; López-Raez, J.A.; Pozo, M.J. Regulation of mycorrhizal colonization under stress in tomato depends on symbiotic efficiency. Environ. Exp. Bot. 2023, 215, 105479. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Syeed, S.; Khan, N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2011, 168, 807–815. [Google Scholar] [CrossRef]
- Syeed, S.; Anjum, N.A.; Nazar, R.; Iqbal, N.; Masood, A.; Khan, N.A. Salicylic acid-mediated changes in photosynthesis, nutrients content and antioxidant metabolism in two mustard (Brassica juncea L.) cultivars differing in salt tolerance. Acta Physiol. Plant 2011, 33, 877–886. [Google Scholar] [CrossRef]
- Khan, N.; Syeed, S.; Masood, A.; Nazar, R.; Iqbal, N. Application of salicylic acid increases contents of nutrients and antioxidative metabolism in mungbean and alleviates adverse effects of salinity stress. Int. J. Plant Biol. 2010, 1, e1. [Google Scholar] [CrossRef]
- Wang, L.J.; Li, S.H. Salicylic acid-induced heat or cold tolerance in relation to Ca2+ homeostasis and antioxidant systems in young grape plants. Plant Sci. 2006, 170, 685–694. [Google Scholar] [CrossRef]
- Nie, W.; Gong, B.; Chen, Y.; Wang, J.; Wei, M.; Shi, Q. Photosynthetic capacity, ion homeostasis and reactive oxygen metabolism were involved in exogenous salicylic acid increasing cucumber seedlings tolerance to alkaline stress. Sci. Hortic. 2018, 235, 413–423. [Google Scholar] [CrossRef]
- Bukhat, S.; Manzoor, H.; Athar, H.U.R.; Zafar, Z.U.; Azeem, F.; Rasul, S. Salicylic acid induced photosynthetic adaptability of Raphanus sativus to salt stress is associated with antioxidant capacity. J. Plant Growth Regul. 2020, 39, 809–822. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Lawlor, D.W.; Lemaire, G.; Gastal, F. Nitrogen, plant growth and crop yield. In Plant Nitrogen; Springer: Berlin/Heidelberg, Germany, 2001; pp. 343–367. [Google Scholar]
- Pai, R.; Sharma, P.K. Exogenous supplementation of salicylic acid ameliorates salt-induced membrane leakage, ion homeostasis and oxidative damage in Sorghum seedlings. Biologia 2024, 79, 23–43. [Google Scholar] [CrossRef]
- Pedranzani, H.; Racagni, G.; Alemano, S.; Miersch, O.; Ramírez, I.; Peña-Cortés, H.; Taleisnik, E.; Machado-Domenech, E.; Abdala, G. Salt tolerant tomato plants show increased levels of jasmonic acid. Plant Growth Regul. 2003, 41, 149–158. [Google Scholar] [CrossRef]
- Gupta, V.; Willits, M.G.; Glazebrook, J. Arabidopsis thaliana EDS4 contributes to salicylic acid (SA)-dependent expression of defense responses: Evidence for inhibition of jasmonic acid signaling by SA. Mol. Plant-Microbe Interact. 2000, 13, 503–511. [Google Scholar] [CrossRef]
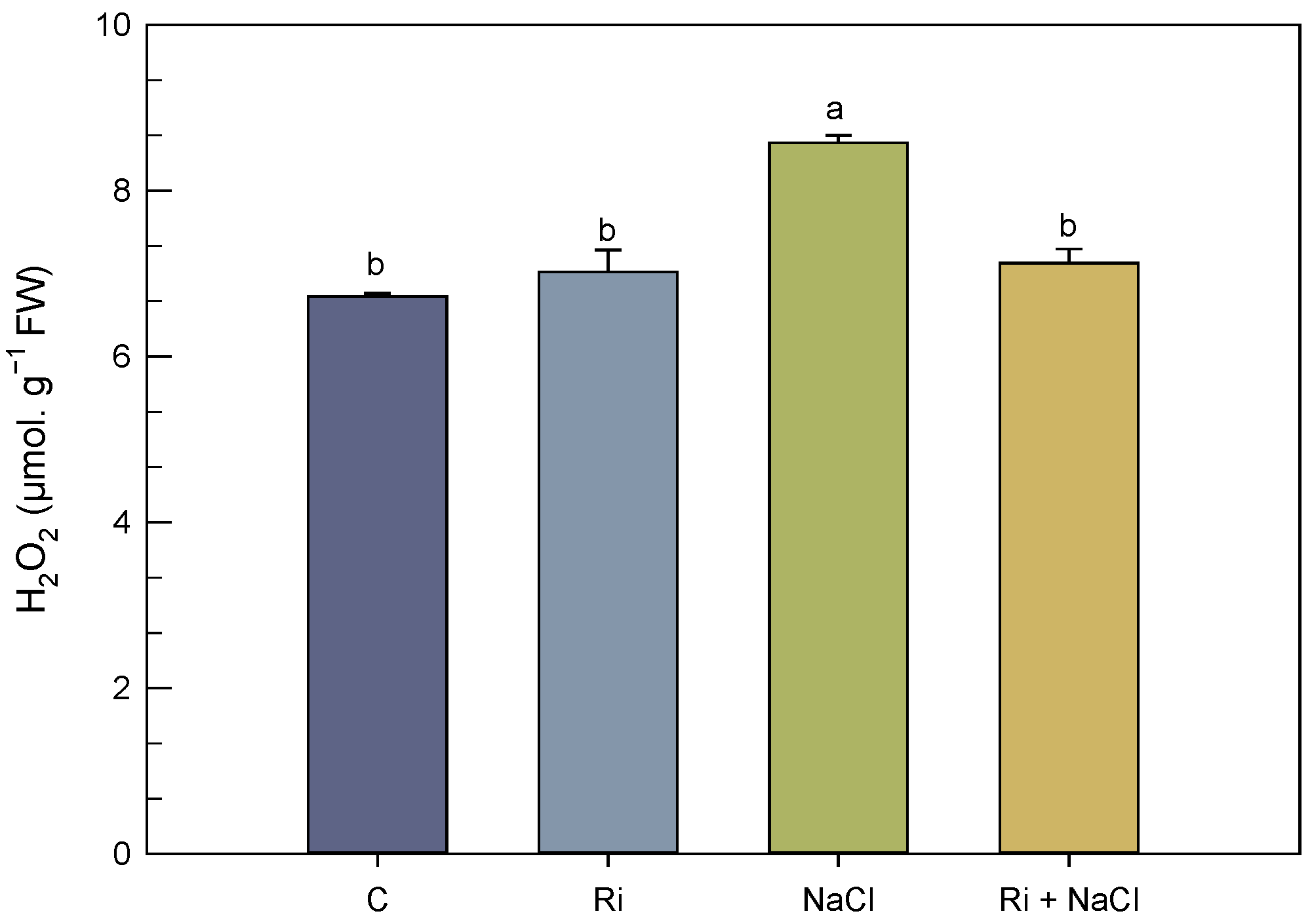
| 0 mM NaCl | 200 mM NaCl | |||
|---|---|---|---|---|
| Control | R. intraradices | Stressed | R. intraradices | |
| Macroelements (mmol/g) | ||||
| C | 28.3 ± 0.57 ab | 27.0 ± 0.45 bc | 25.7 ± 0.16 c | 29.4 ± 0.7 a |
| N | 2.39 ± 0.10 a | 2.11 ± 0.08 ab | 1.86 ± 0.04 b | 2.28 ± 0.14 a |
| Ca | 0.6 ± 0.03 a | 0.6 ± 0.08 a | 0.51 ± 0.01 a | 0.53 ± 0.05 a |
| K | 1.28 ± 0.03 a | 1.19 ± 0.02 b | 0.68 ± 0.03 d | 0.77 ±0.02 c |
| Mg | 0.49 ± 0.032 a | 0.49 ± 0.026 a | 0.40 ± 0.002 b | 0.43 ±0.009 ab |
| Na | 0.10 ± 0.01 c | 0.22 ± 0.08 c | 2.73 ± 0.11 b | 2.41 ± 0.01 a |
| P | 0.048 ± 0.001bc | 0.053 ± 0.005 ab | 0.043 ± 0.001 c | 0.061 ± 0.001 a |
| Microelements (µmol/g) | ||||
| S | 0.80 ± 0.043 a | 0.68 ± 0.032 b | 0.40 ± 0.019 c | 0.47 ± 0.014 c |
| Cu | 0.14 ± 0.007 a | 0.13 ± 0.011 a | 0.10 ± 0.005 b | 0.12 ± 0.004 a |
| Fe | 3.72 ± 0.66 bc | 3.02 ± 0.28 c | 4.7 ± 0 ab | 5.57 ± 0.19 a |
| Zn | 0.89 ± 0.02 a | 0.64 ± 0.07 b | 0.67 ± 0.03 b | 0.84 ± 0.04 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidri, R.; Zorrig, W.; Debez, A.; Mahmoud, O.M.-B.; Zamarreño, A.M.; García-Mina, J.M.; Nait Mohamed, S.; Abdelly, C.; Azcon, R.; Aroca, R. Role of Rhizophagus intraradices in Mitigating Salt Stress of Sulla carnosa Through Modulating Plant Hormones (ABA, SA, and JA) and Nutrient Profile. Biology 2025, 14, 341. https://doi.org/10.3390/biology14040341
Hidri R, Zorrig W, Debez A, Mahmoud OM-B, Zamarreño AM, García-Mina JM, Nait Mohamed S, Abdelly C, Azcon R, Aroca R. Role of Rhizophagus intraradices in Mitigating Salt Stress of Sulla carnosa Through Modulating Plant Hormones (ABA, SA, and JA) and Nutrient Profile. Biology. 2025; 14(4):341. https://doi.org/10.3390/biology14040341
Chicago/Turabian StyleHidri, Rabaa, Walid Zorrig, Ahmed Debez, Ouissal Metoui-Ben Mahmoud, Angel María Zamarreño, José María García-Mina, Salma Nait Mohamed, Chedly Abdelly, Rosario Azcon, and Ricardo Aroca. 2025. "Role of Rhizophagus intraradices in Mitigating Salt Stress of Sulla carnosa Through Modulating Plant Hormones (ABA, SA, and JA) and Nutrient Profile" Biology 14, no. 4: 341. https://doi.org/10.3390/biology14040341
APA StyleHidri, R., Zorrig, W., Debez, A., Mahmoud, O. M.-B., Zamarreño, A. M., García-Mina, J. M., Nait Mohamed, S., Abdelly, C., Azcon, R., & Aroca, R. (2025). Role of Rhizophagus intraradices in Mitigating Salt Stress of Sulla carnosa Through Modulating Plant Hormones (ABA, SA, and JA) and Nutrient Profile. Biology, 14(4), 341. https://doi.org/10.3390/biology14040341








