Recommendations on How to Use Flight Initiation Distance Data in Birds
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
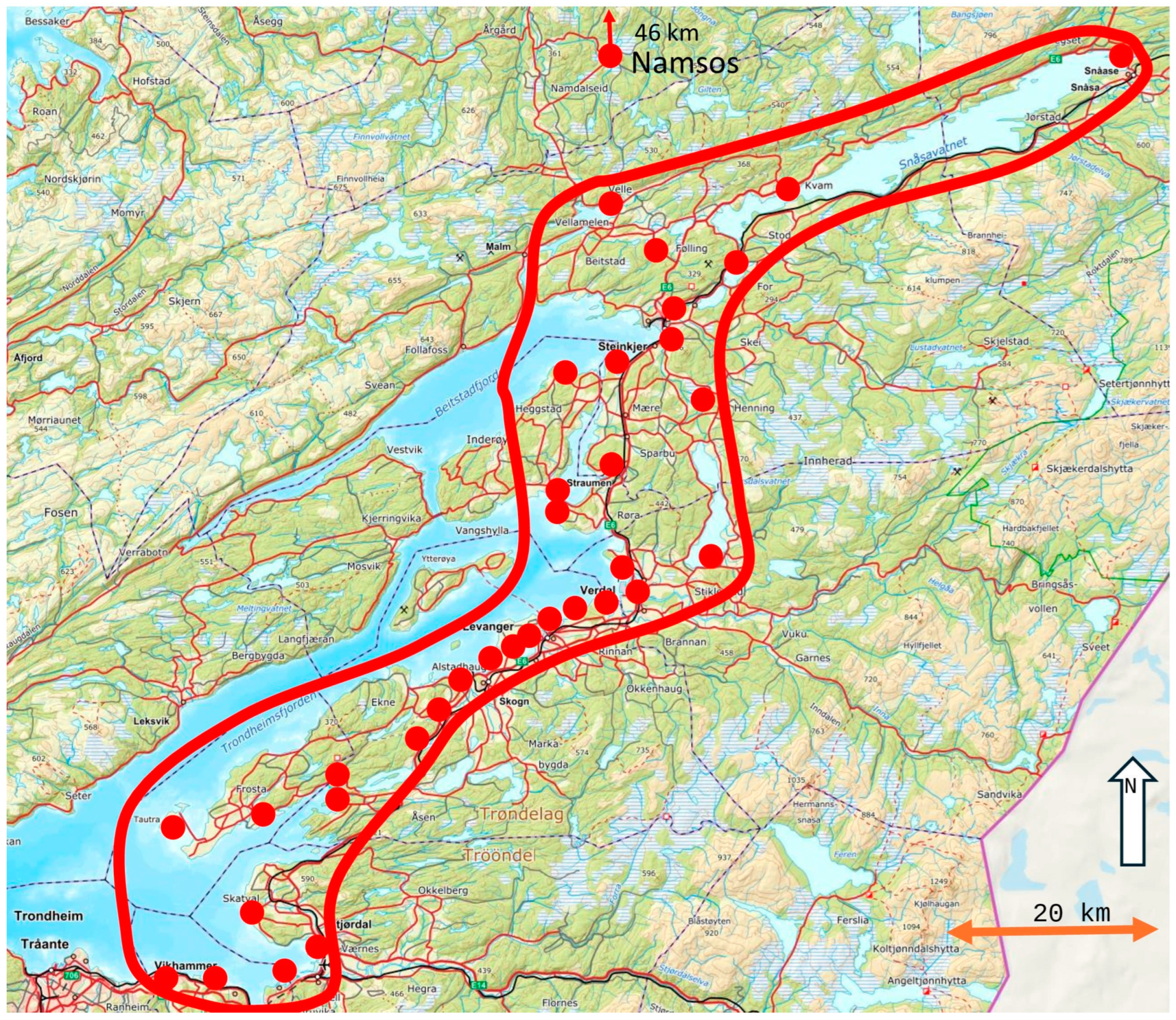
2.1. Field Methods
2.2. Statistics
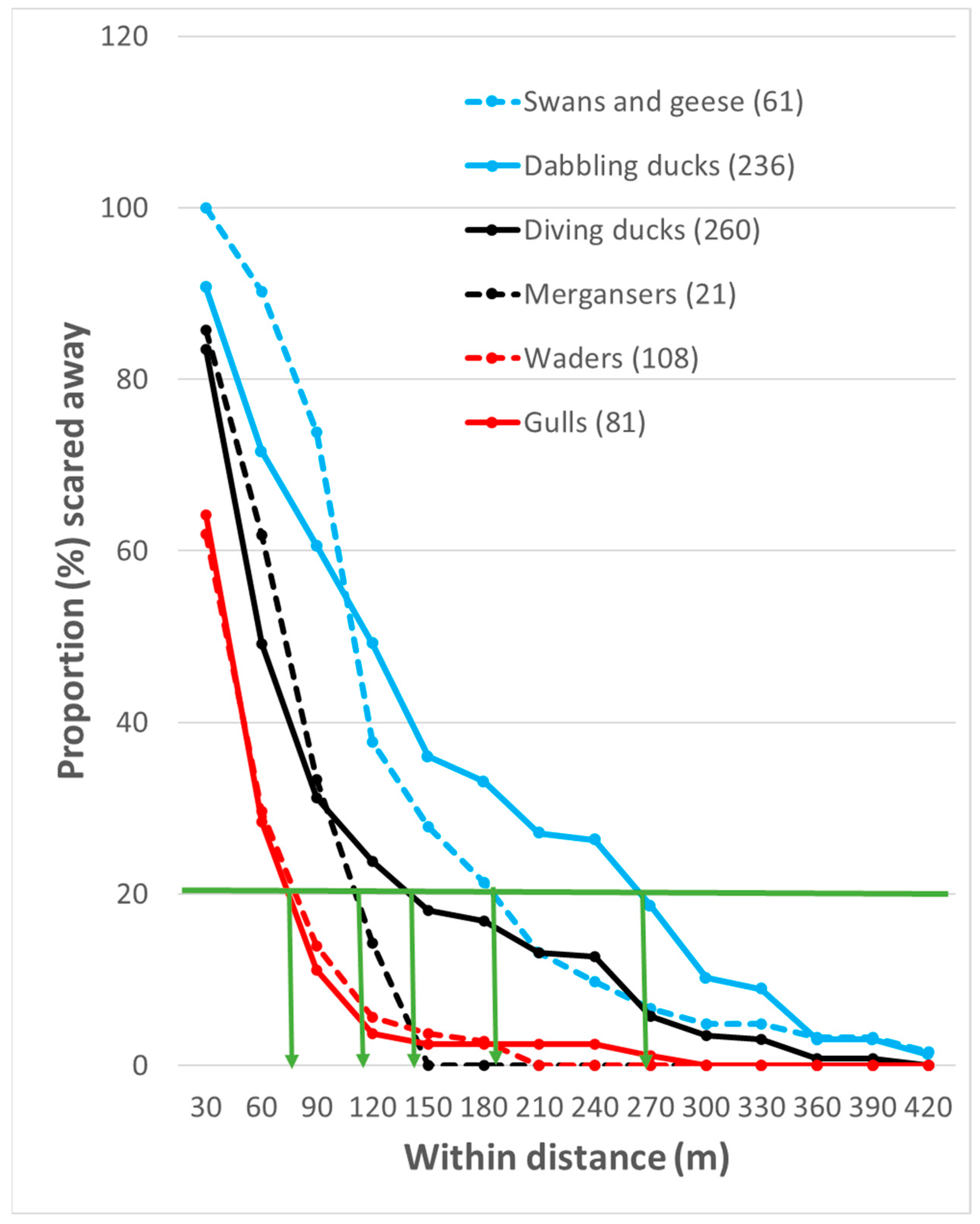
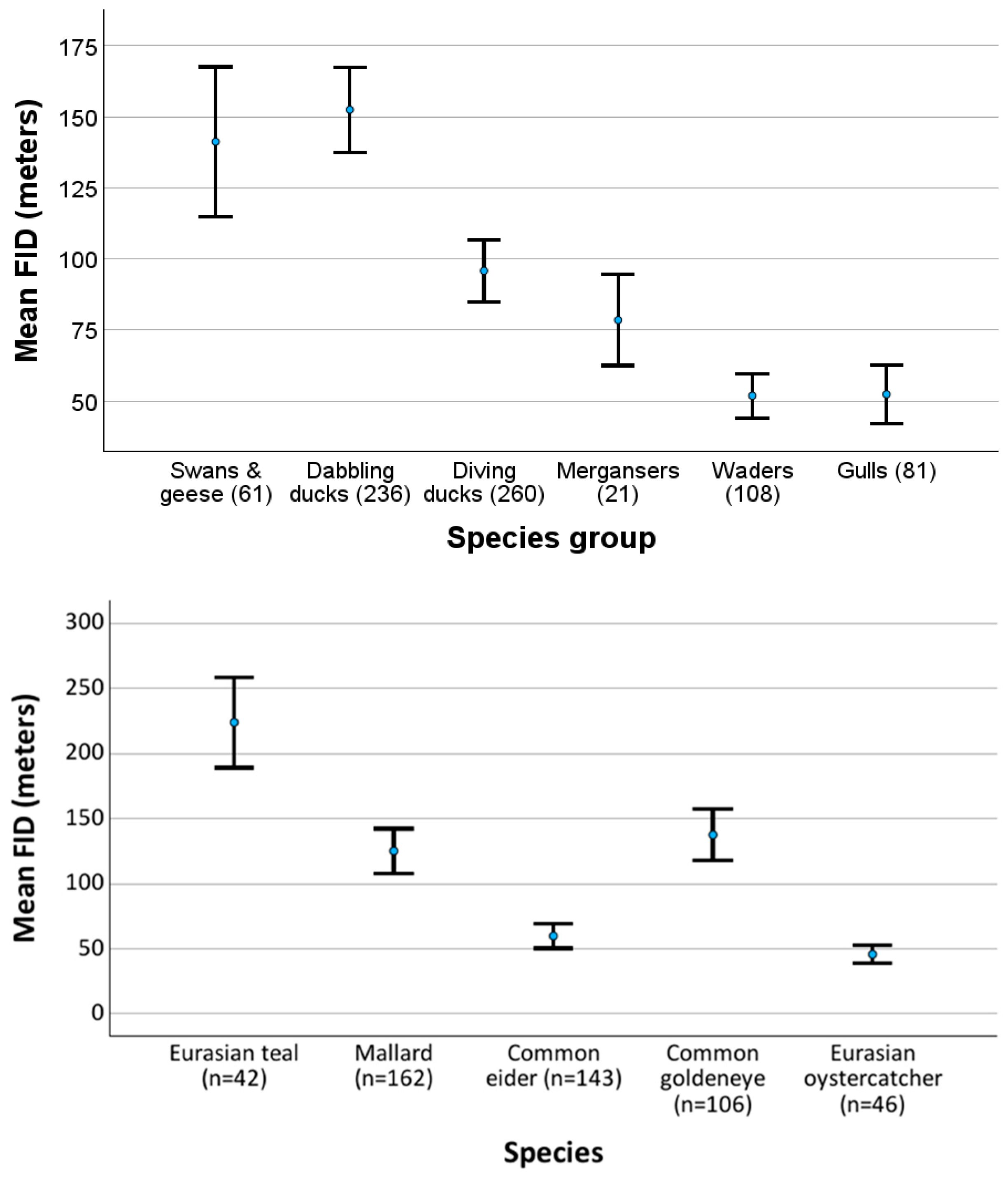
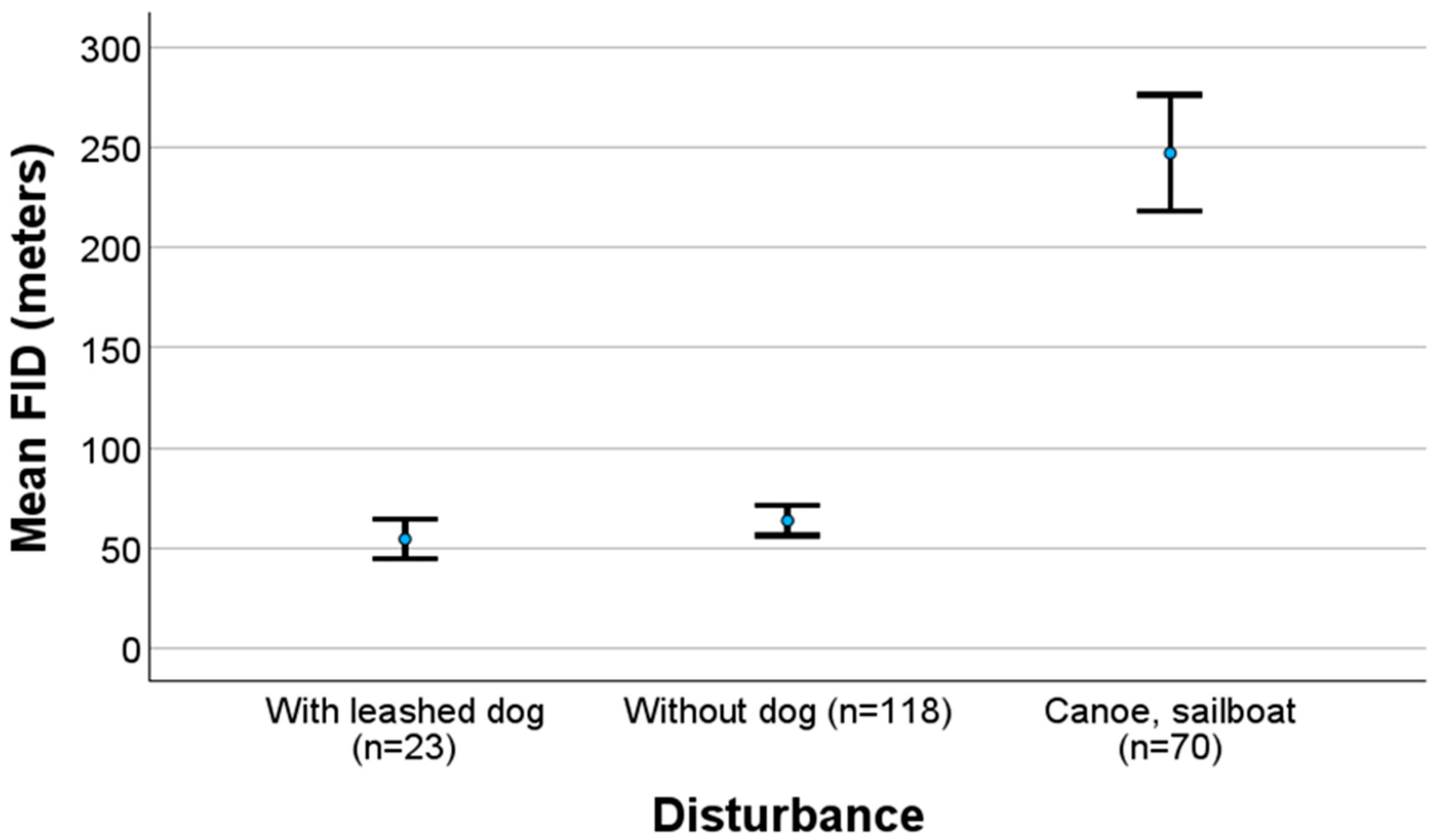
3. Results
3.1. FID Variation Between Different Waterbirds
3.2. Factors Affecting the FID and Fleeing Method
3.3. The Relationship Between FID and Other Behaviors
4. Discussion
4.1. FID Variation Between Different Waterbirds
4.2. Factors Affecting the FID and Fleeing Method
4.3. Effects of Human Disturbance on Population Sizes
4.4. Could a Worldwide FID Database Be Useful?
4.5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sekercioglu, C.H. Increasing awareness of avian ecological function. Trends Ecol. Evol. 2006, 21, 464–471. [Google Scholar] [CrossRef]
- Methorst, J. Positive relationship between bird diversity and human mental health: An analysis of repeated cross-sectional data. Lancet Planet. Health 2024, 8, e285–e296. [Google Scholar] [CrossRef]
- Furness, R.W.; Greenwood, J.J.D. Birds as Monitors of Environmental Change; Chapman & Hall: London, UK, 1993. [Google Scholar]
- Gregory, R.D.; Noble, D.; Field, R.; Marchant, J.; Raven, M.; Gibbons, D.W. Using birds as indicators of biodiversity. Ornis Hung. 2003, 12, 11–24. [Google Scholar]
- Lees, A.C.; Haskell, L.; Allinson, T.; Bezeng, S.B.; Burfield, I.J.; Renjifo, L.M.; Rosenberg, K.V.; Viswanathan, A.; Butchart, S.H.M. State of theWorld’s Birds. Annu. Rev. Environ. Resour. 2022, 47, 231–260. [Google Scholar] [CrossRef]
- Brlík, V.; Šilarová, E.; Škorpilová, J.; Alonso, H.; Anton, M.; Aunins, A.; Benkö, Z.; Biver, G.; Busch, M.; Chodkiewicz, T.; et al. Long-term and large-scale multispecies dataset tracking population changes of common European breeding birds. Sci. Data 2021, 8, 1–9. [Google Scholar] [CrossRef]
- Sauer, J.R.; Pardieck, K.L.; Ziolkowski, D.J.; Smith, A.C.; Hudson, M.A.R.; Rodriguez, V.; Berlanga, H.; Niven, D.K.; Link, W.A. The first 50 years of the North American Breeding Bird Survey. Condor 2017, 119, 576–593. [Google Scholar] [CrossRef]
- Rosenberg, K.V.; Dokter, A.M.; Blancher, P.J.; Sauer, J.R.; Smith, A.C.; Smith, P.A.; Stanton, J.C.; Panjabi, A.; Helft, L.; Parr, M.; et al. Decline of the North American avifauna. Science 2019, 366, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Burns, F.; Eaton, M.A.; Burfield, I.J.; Klvariova, A.; Silarova, E.; Staneva, A.; Gregory, R.D. Abundance decline in the avifauna of the European Union reveals cross-continental similarities in biodiversity change. Ecol. Evol. 2021, 11, 16647–16660. [Google Scholar] [CrossRef]
- BirdLife-International. State of the World’s Birds 2022: Insights and Solutions for the Biodiversity Crisis; BirdLife International: Cambridge, UK, 2022; pp. 1–88. [Google Scholar]
- IPBES. Global Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019; pp. 1–1144. [Google Scholar]
- Gurevitch, J.; Padilla, D.K. Are invasive species a major cause of extinctions? Trends Ecol. Evol. 2004, 19, 470–474. [Google Scholar] [CrossRef]
- Robinson, S.K.; Thompson, F.R.; Donovan, T.M.; Whitehead, D.R.; Faaborg, J. Regional forest fragmentation and the nesting success of migratory birds. Science 1995, 267, 1987–1990. [Google Scholar] [CrossRef]
- Husby, M.; Hoset, K.; Butler, S. Non-random sampling along rural–urban gradients may reduce reliability of multi-species farmland bird indicators and their trends. Ibis 2021, 163, 579–592. [Google Scholar] [CrossRef]
- Husby, M. Shoreline translocation during road expansion was successful for most waterbirds but not for waders. Land 2024, 13, 1384. [Google Scholar] [CrossRef]
- Forman, R.T.T.; Alexander, L.E. Roads and their major ecological effects. Annu. Rev. Ecol. Syst. 1998, 29, 207–231. [Google Scholar] [CrossRef]
- Erritzøe, J.; Mazgajski, T.D.; Rejt, L. Bird casualties on European roads—A review. Acta Ornithol. 2003, 38, 77–93. [Google Scholar]
- Burns, F.; Eaton, M.A.; Barlow, K.E.; Beckmann, B.C.; Brereton, T.; Brooks, D.R.; Brown, P.M.J.; Fulaij, N.A.; Gent, T.; Henderson, I.; et al. Agricultural management and climatic change are the major drivers of biodiversity change in the UK. PLoS ONE 2016, 11, e0151595. [Google Scholar] [CrossRef]
- Kleijn, D.; Schekkerman, H.; Dimmers, W.J.; Van Kats, R.J.M.; Melman, D.; Teunissen, W.A. Adverse effects of agricultural intensification and climate change on breeding habitat quality of Black-tailed Godwits Limosa l. limosa in the Netherlands. Ibis 2010, 152, 475–486. [Google Scholar]
- Butler, S.J.; Boccaccio, L.; Gregory, R.D.; Voříšek, P.; Norris, K. Quantifying the impact of land-use change to European farmland bird populations. Agric. Ecosyst. Environ. 2010, 137, 348–357. [Google Scholar] [CrossRef]
- Stoate, C.; Baldi, A.; Beja, P.; Boatman, N.D.; Herzon, I.; van Doorn, A.; de Snoo, G.R.; Rakosy, L.; Ramwell, C. Ecological impacts of early 21st century agricultural change in Europe—A review. J. Environ. Manag. 2009, 91, 22–46. [Google Scholar] [CrossRef]
- Reif, J.; Voříšek, P.; Stastny, K.; Bejcek, V.; Petr, J. Agricultural intensification and farmland birds: New insights from a central European country. Ibis 2008, 150, 596–605. [Google Scholar] [CrossRef]
- Wretenberg, J.; Lindstrom, A.; Svensson, S.; Part, T. Linking agricultural policies to population trends of Swedish farmland birds in different agricultural regions. J. Appl. Ecol. 2007, 44, 933–941. [Google Scholar] [CrossRef]
- Fraixedas, S.; Lindén, A.; Meller, K.; Lindström, Å.; Keišs, O.; Kålås, J.A.; Husby, M.; Leivits, A.; Leivits, M.; Lehikoinen, A. Substantial decline of Northern European peatland bird populations: Consequences of drainage. Biol. Conserv. 2017, 214, 223–232. [Google Scholar]
- Wade, A.S.I.; Barov, B.; Burfield, I.J.; Gregory, R.D.; Norris, K.; Butler, S.J. Quantifying the detrimental impacts of land-use and management change on European forest bird populations. PLoS ONE 2013, 8, e64552. [Google Scholar]
- Newton, I. Population Limitation in Birds; Academic Press Limited: Cambridge, MA, USA, 1998; p. 597. [Google Scholar]
- Ferns, P. Bird Life of Coasts and Estuaries; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Both, C.; van Turnhout, C.A.M.; Bijlsma, R.G.; Siepel, H.; Van Strien, A.J.; Foppen, R.P.B. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc. R. Soc. B-Biol. Sci. 2010, 277, 1259–1266. [Google Scholar] [CrossRef]
- Visser, M.E.; Gienapp, P.; Husby, A.; Morrisey, M.; de la Hera, I.; Pulido, F.; Both, C. Effects of spring temperatures on the strength of selection on timing of reproduction in a long-distance migratory bird. PLoS Biol. 2015, 13, e1002120. [Google Scholar] [CrossRef]
- Virkkala, R.; Lehikoinen, A. Birds on the move in the face of climate change: High species turnover in northern Europe. Ecol. Evol. 2017, 7, 8201–8209. [Google Scholar] [CrossRef]
- Stephens, P.A.; Mason, L.R.; Green, R.E.; Gregory, R.D.; Sauer, J.R.; Alison, J.; Aunins, A.; Brotons, L.; Butchart, S.H.M.; Campedelli, T.; et al. Consistent response of bird populations to climate change on two continents. Science 2016, 352, 84–87. [Google Scholar]
- Pearce-Higgins, J.W.; Green, R.E. Birds and Climate Change: Impacts and Conservation Responses; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Dunn, P.O.; Møller, A.P. Effects of Climate Change on Birds, 2nd ed.; Oxford University Press: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Watson, R.T.; Kolar, P.S.; Ferrer, M.; Nygard, T.; Johnston, N.; Hunt, W.G.; Smit-Robinson, H.A.; Farmer, C.J.; Huso, M.; Katzner, T.E. Raptor interactions with wind energy: Case studies from around the world. J. Raptor Res. 2018, 52, 1–18. [Google Scholar] [CrossRef]
- Estellés-Domingo, I.; López-López, P. Effects of wind farms on raptors: A systematic review of the current knowledge and the potential solutions to mitigate negative impacts. Anim. Conserv. 2024, 1–19. [Google Scholar] [CrossRef]
- Peschko, V.; Mendel, B.; Muller, S.; Markones, N.; Mercker, M.; Garthe, S. Effects of offshore windfarms on seabird abundance: Strong effects in spring and in the breeding season. Mar. Environ. Res. 2020, 162, 12. [Google Scholar] [CrossRef]
- Perold, V.; Ralston-Paton, S.; Ryan, P. On a collision course? The large diversity of birds killed by wind turbines in South Africa. Ostrich 2020, 91, 228–239. [Google Scholar] [CrossRef]
- Husby, M.; Pearson, M. Wind farms and power lines have negative effects on territory occupancy in Eurasian eagle owls (Bubo bubo). Animals 2022, 12, 1089. [Google Scholar] [CrossRef]
- Husby, M. Wind farms and power lines reduced the territory status and probability of fledgling production in the Eurasian goshawk Accipiter gentilis. Diversity 2024, 16, 128. [Google Scholar] [CrossRef]
- Tolvanen, A.; Routavaara, H.; Jokikokko, M.; Rana, P. How far are birds, bats, and terrestrial mammals displaced from onshore wind power development?—A systematic review. Biol. Conserv. 2023, 288, 11. [Google Scholar] [CrossRef]
- Nebel, C.; Stjernberg, T.; Tikkanen, H.; Laaksonen, T. Reduced survival in a soaring bird breeding in wind turbine proximity along the northern Baltic Sea coast. Biol. Conserv. 2024, 294, 9. [Google Scholar] [CrossRef]
- Marques, A.T.; Batalha, H.; Bernardino, J. Bird displacement by wind turbines: Assessing current knowledge and recommendations for future studies. Birds 2021, 2, 460–475. [Google Scholar] [CrossRef]
- Grilo, C.; Koroleva, E.; Andrasik, R.; Bil, M.; Gonzalez-Suarez, M. Roadkill risk and population vulnerability in European birds and mammals. Front. Ecol. Environ. 2020, 18, 323–328. [Google Scholar] [CrossRef]
- Husby, M. Factors affecting road mortality in birds. Ornis Fenn. 2016, 93, 212–224. [Google Scholar] [CrossRef]
- Brockie, R.E.; Sadleir, R.M.F.S.; Linklater, W.L. Long-term wildlife road-kill counts in New Zealand. N. Z. J. Zool. 2009, 36, 123–134. [Google Scholar] [CrossRef]
- Forman, R.T.T. Estimate of the area affected ecologically by the road system in the United States. Conserv. Biol. 2000, 14, 31–35. [Google Scholar] [CrossRef]
- Reijnen, R.; Foppen, R.; Terbraak, C.; Thissen, J. The effects of car traffic on breeding bird populations in woodland. 3. Reduction of density in relation to the proximity of main roads. J. Appl. Ecol. 1995, 32, 187–202. [Google Scholar] [CrossRef]
- Bishop, C.A.; Brogan, J.M. Estimates of Avian Mortality Attributed to Vehicle Collisions in Canada. Avian Conserv. Ecol. 2013, 8, 2. [Google Scholar] [CrossRef]
- Loss, S.R.; Will, T.; Marra, P.P. Estimation of bird-vehicle collision mortality on US roads. J. Wildl. Manag. 2014, 78, 763–771. [Google Scholar] [CrossRef]
- Burger, J. The effect of human activity on shorebirds in two coastal bays in northeastern United States. Environ. Conserv. 1986, 13, 123–130. [Google Scholar]
- Burger, J. The effect of human activity on birds at a coastal bay. Biol. Conserv. 1981, 21, 231–241. [Google Scholar] [CrossRef]
- Blumstein, D.T. Attention, habituation, and antipredator behaviour: Implications for urban birds. In Avian Urban Ecology; Gil, D., Brumm, H., Eds.; Oxford University Press: Oxford, UK, 2013; pp. 41–53. [Google Scholar]
- Blumstein, D.T.; Anthony, L.L.; Harcourt, R.; Ross, G. Testing a key assumption of wildlife buffer zones: Is flight initiation distance a species-specific trait? Biol. Conserv. 2003, 110, 97–100. [Google Scholar] [CrossRef]
- Møller, A.P.; Liang, W.; Samia, D.S.M. Flight initiation distance, color and camouflage. Curr. Zool. 2019, 65, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Møller, A.P.; Flensted-Jensen, E.; Grim, T.; Ibanez-Alamo, J.D.; Jokimaki, J.; Marko, G.; Tryjanowski, P. The geography of fear: A latitudinal gradient in anti-predator escape distances of birds across Europe. PLoS ONE 2013, 8, e64634. [Google Scholar] [CrossRef]
- Møller, A.P. Flight distance and blood parasites in birds. Behav. Ecol. 2008, 19, 1305–1313. [Google Scholar] [CrossRef]
- Morelli, F.; Benedetti, Y.; Díaz, M.; Grim, T.; Ibáñez-Alamo, J.D.; Jokimäki, J.; Kaisanlahti-Jokimäki, M.L.; Tätte, K.; Markó, G.; Jiang, Y.T.; et al. Contagious fear: Escape behavior increases with flock size in European gregarious birds. Ecol. Evol. 2019, 9, 6096–6104. [Google Scholar] [CrossRef]
- Morelli, F.; Mikula, P.; Blumstein, D.T.; Diaz, M.; Marko, G.; Jokimaki, J.; Kaisanlahti-Jokimaeki, M.L.; Floigl, K.; Abou Zeid, F.; Siretckaia, A.; et al. Flight initiation distance and refuge in urban birds. Sci. Total Environ. 2022, 842, 8. [Google Scholar] [CrossRef]
- Møller, A.P.; Tryjanowski, P. Direction of approach by predators and flight initiation distance of urban and rural populations of birds. Behav. Ecol. 2014, 25, 960–966. [Google Scholar] [CrossRef]
- Swarthout, E.C.H.; Steidl, R.J. Flush responses of Mexican spotted owls to recreationists. J. Wildl. Manag. 2001, 65, 312–317. [Google Scholar] [CrossRef]
- Price, M.L. The impact of human disturbance on birds: A selective review. Aust. Zool. 2008, 8, 1–17. [Google Scholar] [CrossRef]
- Burger, J. Foraging behavior and the effect of human disturbance on the piping plover (Charadrius melodus). J. Coast. Res. 1991, 7, 39–52. [Google Scholar]
- Burger, J.; Carlucci, S.A.; Jeitner, C.W.; Niles, L. Habitat choice, disturbance, and management of foraging shorebirds and gulls at a migratory stopover. J. Coast. Res. 2007, 23, 1159–1166. [Google Scholar] [CrossRef]
- Palacios, E.; Vargas, J.; Fernández, G.; Reiter, M.E. Impact of human disturbance on the abundance of non-breeding shorebirds in a subtropical wetland. Biotropica 2022, 54, 1160–1169. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Discrimination of the threat of direct versus tangential approach to the nest by incubating herring and great black-backed gulls. J. Comp. Physiol. Psychol. 1981, 95, 676–684. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Risk discrimination of direct versus tangential approach by basking black iguanas (Ctenosaura similis): Variation as a function of human exposure. J. Comp. Psychol. 1990, 104, 388–394. [Google Scholar] [CrossRef]
- Blumstein, D.T. Flight-initiation distance in birds is dependent on intruder starting distance. J. Wildl. Manag. 2003, 67, 852–857. [Google Scholar] [CrossRef]
- Mayer, M.; Natusch, D.; Frank, S. Water body type and group size affect the flight initiation distance of European waterbirds. PLoS ONE 2019, 14, e0219845. [Google Scholar] [CrossRef]
- Samia, D.S.M.; Nomura, F.; Blumstein, D.T. Do animals generally flush early and avoid the rush? A meta-analysis. Biol. Lett. 2013, 9, 4. [Google Scholar] [CrossRef]
- Møller, A.P. The value of a mouthful: Flight initiation distance as an opportunity cost. Eur. J. Ecol. 2015, 1, 1–7. [Google Scholar] [CrossRef]
- de Resende, N.C.; Teixeira, C.P.; de Azevedo, C.S. Flight initiation distance in an urban bird: Influence of the number of people, gaze orientation, and bird behavior. Birds 2024, 5, 255–264. [Google Scholar] [CrossRef]
- Radkovic, A.Z.; Van Dongen, W.F.D.; Kirao, L.; Guay, P.J.; Weston, M.A. Birdwatchers evoke longer escape distances than pedestrians in some African birds. J. Ecotourism 2019, 18, 100–106. [Google Scholar] [CrossRef]
- Butler, P.J. Exercise in birds. J. Exp. Biol. 1991, 160, 233–262. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carre, G.; Marquez, J.R.G.; Gruber, B.; Lafourcade, B.; Leitao, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- IBM. IBM SPSS Statistics, version 27.0.1.0; IBM Corp: Armonk, NY, USA, 2021.
- Lo, S.; Andrews, S. To transform or not to transform: Using generalized linear mixed models to analyse reaction time data. Front. Psychol. 2015, 6, 1–16. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference. A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; p. 488. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Mundry, R. Issues in information theory-based statistical inference-a commentary from a frequentist’s perspective. Behav. Ecol. Sociobiol. 2011, 65, 57–68. [Google Scholar] [CrossRef]
- Blumstein, D.T. Developing an evolutionary ecology of fear: How life history and natural history traits affect disturbance tolerance in birds. Anim. Behav. 2006, 71, 389–399. [Google Scholar] [CrossRef]
- Collop, C.; Stillman, R.A.; Garbutt, A.; Yates, M.G.; Rispin, E.; Yates, T. Variability in the area, energy and time costs of wintering waders responding to disturbance. Ibis 2016, 158, 711–725. [Google Scholar] [CrossRef]
- Chiatante, G.; Carere, C. Flight initiation distance in waterbirds of two coastal wetlands with different protection regimes. Rend. Lincei.-Sci. Fis. Nat. 2024, 35, 293–303. [Google Scholar] [CrossRef]
- Ekanayake, K.B.; Gnanapragasam, J.J.; Ranawana, K.; Vidanapathirana, D.R.; Abeyawardhana, U.T.; Fernando, C.; McQueen, A.; Weston, M.A.; Symonds, M.R.E. Ecological and environmental predictors of escape among birds on a large tropical island. Behav. Ecol. Sociobiol. 2022, 76, 13. [Google Scholar] [CrossRef]
- Reynolds, C.; Henry, D.A.W.; Tye, D.R.C.; Tye, N.D. Defining separation zones for coastal birds at a wetland of global importance. Wildl. Res. 2021, 48, 134–141. [Google Scholar] [CrossRef]
- Weston, M.A.; McLeod, E.M.; Blumstein, D.T.; Guay, P.J. A review of flight-initiation distances and their application to managing disturbance to Australian birds. Emu-Austral Ornithol. 2012, 112, 269–286. [Google Scholar] [CrossRef]
- Samia, D.S.M.; Nakagawa, S.; Nomura, F.; Rangel, T.F.; Blumstein, D.T. Increased tolerance to humans among disturbed wildlife. Nat. Commun. 2015, 6, 8. [Google Scholar] [CrossRef]
- Samia, D.S.M.; Møller, A.P.; Blumstein, D.T. Brain size as a driver of avian escape strategy. Sci. Rep. 2015, 5, 8. [Google Scholar] [CrossRef]
- Madsen, J.; Cracknell, G.; Fox, T. Goose Populations of the Western Palearctic. A Review of Status and Distribution; Wetlands International: Wageningen, The Netherlands; National Environmental Research Institute: Rönde, Denmark, 1999; p. 344. [Google Scholar]
- Kersten, M.; Piersma, T. High levels of energy expenditure in shorebirds—Metabolic adaptations to an energetically expensive way of life. Ardea 1987, 75, 175–187. [Google Scholar]
- Burger, J.; Gochfeld, M. Effects of ecotourists on bird behaviour at Loxahatchee national wildlife refuge, Florida. Environ. Conserv. 1998, 25, 13–21. [Google Scholar] [CrossRef]
- Shuai, L.Y.; Morelli, F.; Mikula, P.; Benedetti, Y.; Weston, M.A.; Ncube, E.; Tarakini, T.; Díaz, M.; Markó, G.; Jokimäki, J.; et al. A meta-analysis of the relationship between flock size and flight initiation distance in birds. Anim. Behav. 2024, 210, 1–9. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Human activity influence and diurnal and nocturnal foraging of sanderlings (Calidris alba). Condor 1991, 93, 259–265. [Google Scholar] [CrossRef]
- Elafri, A.; Halassi, I.; Boutabia, L.; Telailia, S. Responses of shorebirds to human disturbance at exposed sandy beaches of north-eastern Algeria. Int. J. Ecol. Dev. 2022, 37, 1–8. [Google Scholar]
- Glover, H.K.; Weston, M.A.; Maguire, G.S.; Miller, K.K.; Christie, B.A. Towards ecologically meaningful and socially acceptable buffers: Response distances of shorebirds in Victoria, Australia, to human disturbance. Landsc. Urban Plan. 2011, 103, 326–334. [Google Scholar] [CrossRef]
- Scarton, F. Flight initiation distances in relation to pedestrian and boat disturbance in five species of waders breeding in a Mediterranean lagoon. Rev. Ecol.-Terre Vie 2018, 73, 375–384. [Google Scholar] [CrossRef]
- Miller, S.G.; Knight, R.L.; Miller, C.K. Wildlife responses to pedestrians and dogs. Wildl. Soc. Bull. 2001, 29, 124–132. [Google Scholar]
- Novcic, I. Behavioural responses of grey herons Ardea cinerea and great egrets Ardea alba to human-caused disturbance. J. Vertebr. Biol. 2022, 71, 9. [Google Scholar] [CrossRef]
- Lafferty, K.D. Disturbance to wintering western snowy plovers. Biol. Conserv. 2001, 101, 315–325. [Google Scholar] [CrossRef]
- Møller, A.P. Flight distance of urban birds, predation, and selection for urban life. Behav. Ecol. Sociobiol. 2008, 63, 63–75. [Google Scholar] [CrossRef]
- Mikula, P. Pedestrian density influences flight distances of urban birds. Ardea 2014, 102, 53–60. [Google Scholar] [CrossRef]
- Yin, L.Q.; Wang, C.; Han, W.J.; Zhang, C. Birds’ flight initiation distance in residential areas of Beijing are lower than in pristine environments: Implications for the conservation of urban bird diversity. Sustainability 2023, 15, 4994. [Google Scholar] [CrossRef]
- Zhou, B.; Liang, W. Avian escape responses to observers wearing clothing of different colors: A comparison of urban and rural populations. Glob. Ecol. Conserv. 2020, 22, 8. [Google Scholar] [CrossRef]
- Samia, D.S.M.; Blumstein, D.T.; Díaz, M.; Grim, T.; Ibáñez-Alamo, J.D.; Jokimäki, J.; Tätte, K.; Markó, G.; Tryjanowski, P.; Møller, A.P. Rural-urban differences in escape behavior of European birds across a latitudinal gradient. Front. Ecol. Evol. 2017, 5, 13. [Google Scholar] [CrossRef]
- Azaki, B.D.A.; Cresswell, W. Level of local human disturbance and feeding state determines escape behaviour in Eurasian Oystercatchers. Ethology 2021, 127, 986–994. [Google Scholar] [CrossRef]
- Xu, W.Y.; Gong, Y.; Wang, H.T. Alert time reflects the negative impacts of human disturbance on an endangered bird species in Changbai Mountain, China. Glob. Ecol. Conserv. 2021, 28, 8. [Google Scholar] [CrossRef]
- Nepali, A.; Katuwal, H.B.; Kc, S.; Regmi, S.; Sharma, H.P. Flight initiation distance and bird tolerance to humans in rural and urban habitats. R. Soc. Open Sci. 2024, 11, 12. [Google Scholar] [CrossRef]
- Trulio, L.A.; Sokale, J. Foraging shorebird response to trail use around San Francisco bay. J. Wildl. Manag. 2008, 72, 1775–1780. [Google Scholar] [CrossRef]
- Glover, H.K.; Guay, P.J.; Weston, M.A. Up the creek with a paddle; avian flight distances from canoes versus walkers. Wetl. Ecol. Manag. 2015, 23, 775–778. [Google Scholar] [CrossRef]
- Mo, M.; Bonatakis, K. An examination of trends in the growing scientific literature on approaching wildlife with drones. Drone Syst. Appl. 2022, 10, 111–139. [Google Scholar] [CrossRef]
- Howell, L.G.; Allan, B.M.; Driscoll, D.A.; Ierodiaconou, D.; Doran, T.A.; Weston, M.A. Attenuation of responses of waterbirds to repeat drone surveys involving a sequence of altitudes and drone types: A case study. Drones 2023, 7, 497. [Google Scholar] [CrossRef]
- Francis, R.J.; Lyons, M.B.; Kingsford, R.T.; Brandis, K.J. Counting Mixed Breeding Aggregations of Animal Species Using Drones: Lessons from Waterbirds on Semi-Automation. Remote Sens. 2020, 12, 1185. [Google Scholar] [CrossRef]
- Corregidor-Castro, A.; Scarton, F.; Panzarin, L.; Verza, E.; Valle, R.G. Faster and better: Comparison between traditional and drone monitoring in a cryptic species, the Purple Heron Ardea purpurea. Acta Ornithol. 2022, 57, 134–142. [Google Scholar] [CrossRef]
- Jarrett, D.; Calladine, J.; Cotton, A.; Wilson, M.W.; Humphreys, E. Behavioural responses of non-breeding waterbirds to drone approach are associated with flock size and habitat. Bird Stud. 2020, 67, 190–196. [Google Scholar] [CrossRef]
- Møller, A.P. Flight distance and population trends in European breeding birds. Behav. Ecol. 2008, 19, 1095–1102. [Google Scholar] [CrossRef]
- Lilleyman, A.; Franklin, D.C.; Szabo, J.K.; Lawes, M.J. Behavioural responses of migratory shorebirds to disturbance at a high-tide roost. Emu-Austral Ornithol. 2016, 116, 111–118. [Google Scholar] [CrossRef]
- Goss-Custard, J.D.; Jenyon, R.A.; Jones, R.E.; Newbery, P.E.; Williams, R.L.B. Ecology of the Wash 2. Seasonal variation in feeding conditions of wading birds (Charadrii). J. Appl. Ecol. 1977, 14, 701–719. [Google Scholar] [CrossRef]
- Urfi, A.J.; Goss-Custard, J.D.; Durell, S. The ability of oystercatchers Haematopus ostralegus to compensate for lost feeding time: Field studies on individually marked birds. J. Appl. Ecol. 1996, 33, 873–883. [Google Scholar] [CrossRef]
- Tätte, K.; Møller, A.P.; Mänd, R. Towards an integrated view of escape decisions in birds: Relation between flight initiation distance and distance fled. Anim. Behav. 2018, 136, 75–86. [Google Scholar] [CrossRef]
- Husby, M. On the adaptive value of brood reduction in birds: Experiments with the magpie Pica pica. J. Anim. Ecol. 1986, 55, 75–83. [Google Scholar]
- Colwell, M.A. Shorebird Ecology, Conservation, and Management; University of California Press: Berkley, CA, USA, 2010. [Google Scholar]
- Livezey, K.B.; Fernandez-Juricic, E.; Blumstein, D.T. Database of bird flight initiation distances to assist in estimating effects from human disturbance and delineating buffer areas. J. Fish Wildl. Manag. 2016, 7, 181–191. [Google Scholar] [CrossRef]
- Mikula, P.; Tomásek, O.; Romportl, D.; Aikins, T.K.; Avendano, J.E.; Braimoh-Azaki, B.D.A.; Chaskda, A.; Cresswell, W.; Cunningham, S.J.; Dale, S.; et al. Bird tolerance to humans in open tropical ecosystems. Nat. Commun. 2023, 14, 10. [Google Scholar] [CrossRef]
- Jiang, Y.; Møller, A.P. Escape from predators and genetic variance in birds. J. Evol. Biol. 2017, 30, 2059–2067. [Google Scholar] [CrossRef]
- Møller, A.P.; Erritzøe, J. Flight distance and eye size in birds. Ethology 2010, 116, 458–465. [Google Scholar] [CrossRef]
- Meager, J.J.; Schlacher, T.A.; Nielsen, T. Humans alter habitat selection of birds on ocean-exposed sandy beaches. Divers. Distrib. 2012, 18, 294–306. [Google Scholar] [CrossRef]
- Lafferty, K.D.; Goodman, D.; Sandoval, C.P. Restoration of breeding by snowy plovers following protection from disturbance. Biodivers. Conserv. 2006, 15, 2217–2230. [Google Scholar] [CrossRef]
- Ma, A.T.H.; Ng, S.L.; Cheung, L.T.O.; Lam, T.W.L. The effectiveness of bird hides in mitigating recreational disturbances of birdwatchers. J. Nat. Conserv. 2022, 67, 9. [Google Scholar] [CrossRef]
- Clausen, K.K.; Bregnballe, T. Mapping important roost sites for waders to alleviate human-waterbird conflicts in the Danish Wadden Sea. Ocean. Coast. Manag. 2022, 223, 9. [Google Scholar] [CrossRef]
- Mahar, N.; Dobriyal, P.; Badola, R.; Hussain, S.A. Tourism on the roof of the world: Socio-ecological impacts of tourism on the Indian Trans-Himalaya. Land Use Pol. 2024, 138, 14. [Google Scholar] [CrossRef]
| Variable | Value | Comments | N |
|---|---|---|---|
| Target Variable | |||
| FID | Continuous | Log10FID | 907 |
| Fleeing method | 1–2 | 1 = swimming or walking, 2 = flying | 227, 353 |
| Explanatory variable | |||
| N of researchers | 1–2 | 1 = one person, 2 = two people | 720, 187 |
| Open-narrow water | 1–2 | 1 = open area, 2 = narrow (<50 m to the opposite shore) | 664, 243 |
| Flock size | 1–7 | 1–4 = exact number of birds, 5 = 5–10 birds, 6 = 11–30, 7 = 31–700 | 327, 174, 78, 49, 126, 86, 57 |
| Disturbance by dog | 1–2 | 1 = with leashed dog, 2 = without dog, | 140, 767 |
| Rural–urban | 1–2 | 1 = rural, 2 = urban | 368, 539 |
| Random effect | |||
| Period | 1–2 | Winter and early spring, autumn | 568, 507 |
| Variable | Swans and Geese | Dabbling Ducks | Diving Ducks | Waders | Gulls |
|---|---|---|---|---|---|
| Corrected model | 0.898 | +<0.001 | +<0.001 | 0.587 | 0.144 |
| Intercept | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| N of researchers (=2) | 0.795 | −<0.001 *** | 0.395 | 0.817 | 0.055 |
| Open/narrow (=2) | 0.257 | −0.014 * | −<0.001 *** | 0.510 | +0.014 * |
| Flock size (2–7) | Min0.693 | Min0.068 | Min+0.002 (a)** | Min0.165 | Min+0.032 * |
| Disturbance (=2) | 0.420 | +0.006 ** | +<0.001 *** | 0.270 | −0.027 * |
| Rural–urban (=2) | 0.607 | 0.449 | −0.003 ** | 0.927 | 0.566 |
| Random effect | 0.997 | 0.494 | 0.487 | 0.573 | 0.716 |
| N= | 67 | 277 | 306 | 127 | 93 |
| Variable | Eurasian Teal | Mallard | Common Eider | Common Goldeneye | Eurasian Oystercatcher |
|---|---|---|---|---|---|
| Corrected model | +0.002 | +<0.001 | +<0.001 | +<0.001 | +0.004 |
| Intercept | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| N of researchers (>1) | −<0.001 *** | −<0.001 *** | 0.951 | 0.121 | 0.708 |
| Open-narrow (=2) | 0.464 | −<0.001 *** | −0.011 * | 0.939 | −<0.001 *** |
| Flock size (2–7) | Min0.181 | Min0.075 | 0.319 | Min+<0.001 (a)*** | Min−0.019 (b)* |
| Disturbance (=2) | 0.441 | 0.169 | 0.236 | +<0.001 *** | 0.068 |
| Rural–urban (=2) | 0.335 | 0.380 | −<0.001 *** | 0.264 | 0.273 |
| Random effect | 0.911 | 0.597 | 0.604 | 0.512 | 0.710 |
| N= | 45 | 201 | 163 | 130 | 55 |
| Bird Group | z | n | p |
|---|---|---|---|
| Swans and geese | −2.064 | 50 | +0.039 * |
| Dabbling ducks | −5.859 | 190 | +<0.001 *** |
| Diving ducks | −2.970 | 100 | +0.003 ** |
| Mergansers | −0.215 | 12 | 0.830 |
| Waders | −0.393 | 77 | 0.695 |
| Gulls | −0.881 | 71 | 0.378 |
| Stopped Normal Activity | Distance Moved | Time to Return | |
|---|---|---|---|
| Swans and geese | 0.818, n = 17 *** | 0.082, n = 44, ns | No data |
| Dabbling ducks | 0.974, n = 54 *** | 0.463, n = 117 *** | 0.488, n = 15, p = 0.065 |
| Diving ducks | 0.938, n = 94 *** | 0.269, n = 177 *** | −0.181, n = 64, ns |
| Mergansers | Lack of data | 0.208, n = 19 | No data |
| Waders | 0.916, n = 29 *** | 0.159, n = 64, ns | No data |
| Gulls | No data | −0.084, n = 54, ns | No data |
| Eurasian teal | No data | 0.394, n = 10, ns | No data |
| Mallard | 0.973, n = 52 *** | 0.479, n = 100 *** | 0.488, n = 15, p = 0.065 |
| Common eider | 0.967, n = 51 *** | 0.243, n = 94 * | −0.274, n = 49, p = 0.056 |
| Common goldeneye | 0.898, n = 41 *** | 195, n = 72, ns | −0.082, n = 14, ns |
| Eurasian oystercatcher | 0.797, n = 11 ** | 0.033, n = 28, ns | No data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husby, M. Recommendations on How to Use Flight Initiation Distance Data in Birds. Biology 2025, 14, 329. https://doi.org/10.3390/biology14040329
Husby M. Recommendations on How to Use Flight Initiation Distance Data in Birds. Biology. 2025; 14(4):329. https://doi.org/10.3390/biology14040329
Chicago/Turabian StyleHusby, Magne. 2025. "Recommendations on How to Use Flight Initiation Distance Data in Birds" Biology 14, no. 4: 329. https://doi.org/10.3390/biology14040329
APA StyleHusby, M. (2025). Recommendations on How to Use Flight Initiation Distance Data in Birds. Biology, 14(4), 329. https://doi.org/10.3390/biology14040329






