Physiological Adaptation to Different Heavy Metal Stress in Seedlings of Halophyte Suaeda liaotungensis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Seedling Growth
2.3. Detection of Physiological Indexes
2.4. Statistical Analysis
3. Results
3.1. Effect of Heavy Metals on Seedling Growth
3.2. Effect of Heavy Metals on ROS Levels in Seedlings
3.3. Effect of Heavy Metals on Antioxidant Enzyme Activity in Seedlings
3.4. Effect of Heavy Metals on Osmotic Regulating Substances in Seedlings
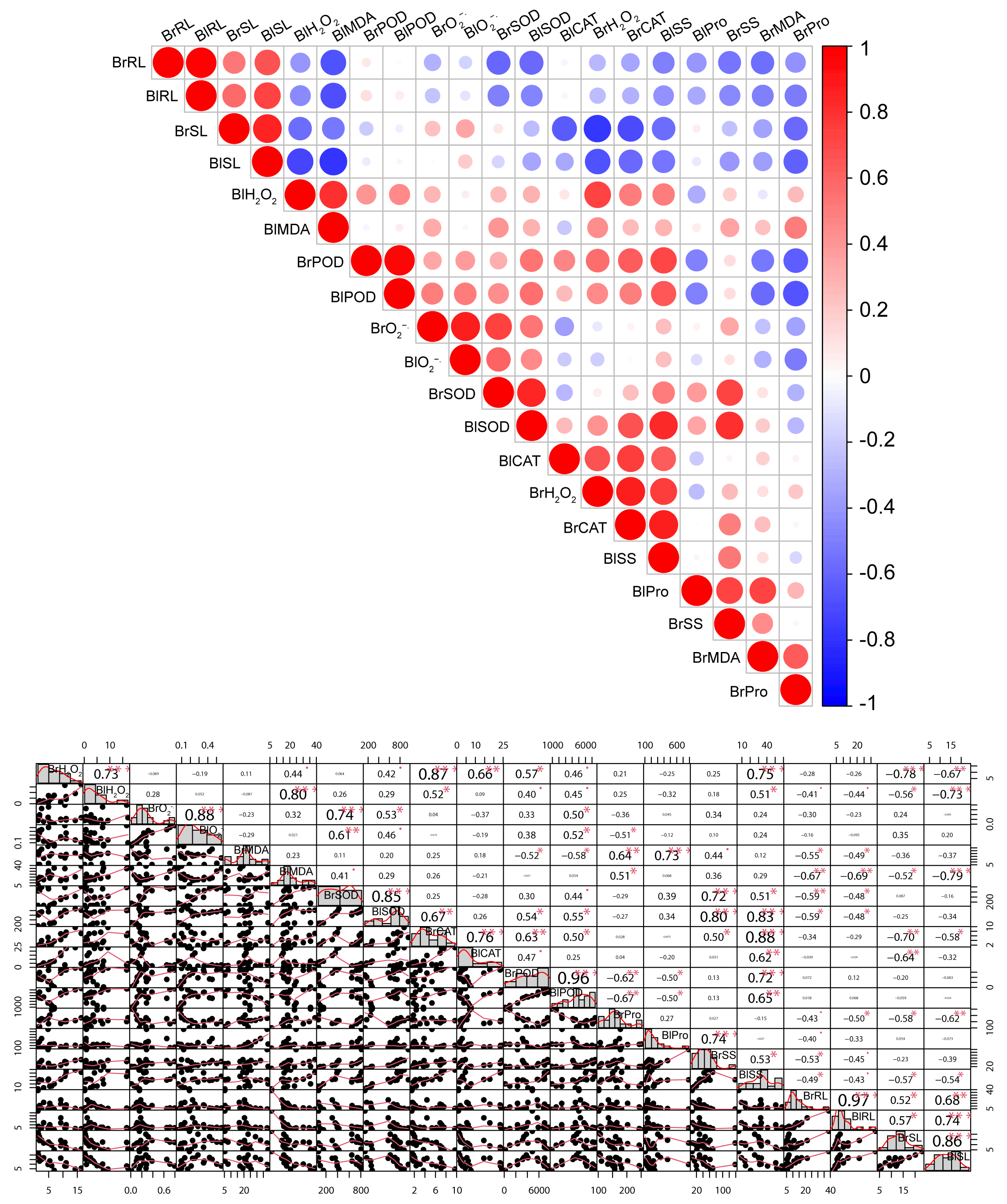
3.5. Principal Component Analysis, Correlation, and Regression Insights Under Heavy Metal Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, X.F.; Pu, L.J.; Zhu, M.; Wu, T.; Xu, Y. Spatio-temporal variability of soil salinity and sodicity in agriculture reclaimed coastal wetlands, Eastern China. Arch. Agron. Soil Sci. 2020, 66, 1639–1650. [Google Scholar] [CrossRef]
- Vilas-Boas, J.A.; Arenas-Súnchez, A.; Vighi, M.; Romo, S.; Van den Brink, P.J.; Pedroso Dias, R.J.; Rico, A. Multiple stressors in Mediterranean coastal wetland ecosystems: Influence of salinity and an insecticide on zooplankton communities under different temperature conditions. Chemosphere 2021, 269, 129381. [Google Scholar] [CrossRef] [PubMed]
- Breda, A.; Saco, P.M.; Sandi, S.G.; Saintilan, N.; Riccardi, G.; Rodríguez, J.F. Accretion, retreat and transgression of coastal wetlands experiencing sealevel rise. Hydrol. Earth Syst. Sci. 2021, 25, 769–786. [Google Scholar] [CrossRef]
- Xiao, R.; Bai, J.H.; Lu, Q.Q.; Zhao, Q.Q.; Gao, Z.Q.; Wen, X.J.; Liu, X.H. Fractionation, transfer, and ecological risks of heavy metals in riparian and ditch wetlands across a 100-year chronosequence of reclamation in an estuary of China. Sci. Total Environ. 2015, 517, 66–75. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Wang, F.F.; Xie, C.J.; Ning, Z.H.; Li, J.; Qin, H.F.; Bai, J.H.; Cui, B.S. Assessing heavy metal pollution in surface sediments of salt marsh in Liaohe Estuary. J. Beijing Norm. Univ. 2018, 54, 144–149. [Google Scholar]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Boyd, R.S.; Rajakaruna, N. Heavy metal tolerance. In Oxford Bibliographies in Ecology; Gibson, D., Ed.; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Kulbat-Warycha, K.; Georgiadou, E.C.; Mańkowska, D.; Smolińska, B.; Fotopoulos, V.; Leszczynska, J. Response to stress and allergen production caused by metal ions (Ni, Cu and Zn) in oregano (Origanum vulgare L.) plants. J. Biotechnol. 2020, 324, 171–182. [Google Scholar] [CrossRef]
- Waalkes, M.P. Cadmium carcinogenesis. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2003, 533, 107–120. [Google Scholar] [CrossRef]
- Zou, M.M.; Zhou, S.L.; Zhou, Y.J.; Jia, Z.Y.; Guo, T.W.; Wang, J.X. Cadmium pollution of soil-rice ecosystems in rice cultivation dominated regions in China: A review. Environ. Pollut. 2021, 280, 116965. [Google Scholar] [CrossRef]
- Bakirdere, S.; Bölücek, C.; Yaman, M. Determination of contamination levels of Pb, Cd, Cu, Ni, and Mn caused by former lead mining gallery. Environ. Monit. Assess. 2016, 188, 132. [Google Scholar] [CrossRef]
- Lv, J.; Liu, Y.; Zhang, Z.; Dai, J. Factorial kriging and stepwise regression approach to identify environmental factors influencing spatial multi-scale variability of heavy metals in soils. J. Hazard. Mater. 2013, 261, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Matayoshi, C.L.; Pena, L.B.; Arbona, V.; Gómez-Cadenas, A.; Gallego, S.M. Early responses of maize seedlings to Cu stress include sharp decreases in gibberellins and jasmonates in the root apex. Protoplasma 2020, 257, 1243–1256. [Google Scholar] [CrossRef]
- Zhu, H.M.; Fang, Y.X.; Ding, Y.S.; Jiang, Y.M.; Chen, J.; Huang, S.T.; Yan, X.J.; Ding, D.W. Seasonal transformation variation of common heavy metals in Suaeda heteroptera-rhizosphere sediment. Ocean. Lakes 2010, 41, 784–790. [Google Scholar]
- Xiao, R.; Bai, J.H.; Wang, Q.G.; Gao, H.F.; Huang, L.B.; Liu, X.H. Assessment of heavy metal contamination of wetland soils from a typical aquatic-terrestrial ecotone in Haihe River Basin, North China. Clean-Soil Air Water 2011, 39, 612–618. [Google Scholar] [CrossRef]
- Alam, M.Z.; Alim, P.; Al-Harbi, N.A. Contamination status of arsenic, lead, and cadmium of different wetland waters. Toxicol. Environ. Chem. 2011, 93, 1934–1945. [Google Scholar] [CrossRef]
- Asare, M.O.; Száková, J.; Tlustoš, P. Mechanisms of As, Cd, Pb, and Zn hyperaccumulation by plants and their effects on soil microbiome in the rhizosphere. Front. Environ. Sci. 2023, 11, 1157415. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Z.W.; Li, S.H.; Chen, J.Y. Bioavailability and toxicity of trace metals (Cd, Cr, Cu, Ni, and Zn) in sediment cores from the Shima River, South China. Chemosphere 2018, 192, 31–42. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A Comprehensive review on the heavy metal toxicity and sequestration in plants. Biomolecules 2021, 12, 43. [Google Scholar] [CrossRef]
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprasanna, P.; Hong, J.C. Comprehensive mechanisms of heavy metal toxicity in plants, detoxiffcation, and remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef]
- Schützendübel, A.; Polle, A. Plant responses to abiotic stresses: Heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 2002, 53, 1351–1365. [Google Scholar] [CrossRef]
- Gall, J.E.; Rajakaruna, N. The physiology, functional genomics, and applied ecology of heavy metal-tolerant Brassicaceae. In Brassicaceae: Characterization, Functional Genomics and Health Benefits; Lang, M., Ed.; Nova: New York, NY, USA, 2013; pp. 121–148. [Google Scholar]
- Seneviratne, M.; Rajakaruna, N.; Rizwan, M.; Madawala, H.M.S.P.; Ok, Y.S.; Vithanage, M. Heavy metal-induced oxidative stress on seed germination and seedling development: A critical review. Environ. Geochem. Health 2019, 41, 1813–1831. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Wang, Y.J.; Chen, J.B.; Zhao, Y. Effects of combined stress of salt and heavy metals on germination and growth of Suaeda salsa and regulation measures. Acta Ecol. Sin. 2023, 43, 3307–3318. [Google Scholar]
- Lou, T.X.; Lü, S.L.; Li, Y.X. Application potential of Salicornia europaea in remediation of Cd, Pb and Li contaminated saline soil. J. Bioeng. 2020, 36, 481–492. [Google Scholar]
- Dong, J.; Wu, F.B.; Zhang, G.P. Effect of cadmium on growth and photosynthesis of tomato seedlings. J. Zhejiang Univ. Sci. B 2005, 6, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, X.; Borthakur, D.; Ni, H. Photosynthetic activity and antioxidative response of seagrass Thalassia hemprichii to trace metal stress. Acta Oceanol. Sin. 2012, 31, 98–108. [Google Scholar] [CrossRef]
- Shang, C.L.; Wang, L.; Tian, C.Y.; Song, J. Heavy metal tolerance and potential for remediation of heavy metal-contaminated saline soils for the euhalophyte Suaeda salsa. Plant Signal. Behav. 2020, 15, e1805902. [Google Scholar] [CrossRef]
- Lutts, S.; Lefèvre, I. How can we take advantage of halophyte properties to cope with heavy metal toxicity in salt-affected areas? Ann. Bot. 2015, 115, 509–528. [Google Scholar] [CrossRef]
- Curado, G.; Grewell, B.J.; Figueroa, E.; Castillo, J.M. Effectiveness of the aquatic halophyte Sarcocornia perennis spp. perennis as a biotool for ecological restoration of salt marshes. Water Air Soil Pollut. 2014, 225, 2108. [Google Scholar] [CrossRef]
- Song, J.Q.; Liu, X.J.; Li, X.X.; Wang, H.F.; Chu, R.W.; Qu, F.F.; Zhang, S.X.; Li, Q.L. Transcriptome analysis reveals genes and pathways associated with salt tolerance during seed germination in Suaeda liaotungensis. Int. J. Mol. Sci. 2022, 23, 12229. [Google Scholar] [CrossRef]
- Song, J.Q.; Wang, H.F.; Chu, R.W.; Zhao, L.T.; Li, X.X.; An, S.; Qiang, M.K.; Du, W.Y.; Li, Q.L. Differences in physiological characteristics, seed germination, and seedling establishment in response to salt stress between dimorphic seeds in the halophyte Suaeda liaotungensis. Plants 2023, 12, 1408. [Google Scholar] [CrossRef]
- Zhu, M.H.; Ding, Y.S.; Ding, D.W. Seasonal variation about accumulation distribution and transference of heavy metals in Suaeda heteroptera. China Env. Sci. 2006, 26, 110–113. [Google Scholar]
- Gao, Y.F.; Li, X.Q.; Dong, G.C.; Liu, F.; Wang, Y.N.; Ke, H. Purification of several salt marsh plants to the coastal wetlands in the estuary of Yellow River. J. Anhui Agric. Sci. 2010, 38, 19499–19501. [Google Scholar]
- Chen, K.H. Phytoextraction in Cd or Cd-Pb Contaminated Soils by Suaeda salsa. Master’s Thesis, Jinan University, Guangzhou, China, 2017. [Google Scholar]
- Liu, Y.; Meng, F.P.; Yao, R.H.; Xie, S. Tolerance and accumulation of Pb, Cd, Cu, Zn by Suaeda salsa seedlings. Environ. Sci. Technol. 2009, 32, 55–59. [Google Scholar]
- Zheng, D.M.; Ma, H.C.; Xin, Y.; Zhang, S.W.; Miao, Y.; Shi, L. Mercury and arsenic pollution in soil of Liaohe Estuary wetland in different years and their risk assessment. J. Shenyang Univ. 2020, 32, 206–212. [Google Scholar]
- Zhang, H.; Hu, M.F.; Ma, H.Y.; Jiang, L.; Zhao, Z.Y.; Ma, J.B.; Wang, L. Differential responses of dimorphic seeds and seedlings to abiotic stresses in the halophyte Suaeda salsa. Front. Plant Sci. 2021, 12, 630338. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, L.H.; Zhao, J.F.; Song, Y.; Zhang, C.J.; Guo, Y. Identification of an apoplastic protein involved in the initial phase of salt stress response in rice root by two-dimensional electrophoresis. Plant Physiol. 2009, 149, 916–928. [Google Scholar] [CrossRef]
- Li, W.; Khan, M.A.; Yamaguchi, S.; Kamiya, Y. Effects of heavy metals on seed germination and early seedling growth of Arabidopsis thaliana. Plant Growth Regul. 2005, 46, 45–50. [Google Scholar] [CrossRef]
- Ahsan, N.; Lee, D.G.; Lee, S.H.; Kang, K.Y.; Lee, J.J.; Kim, P.J.; Yoon, H.S.; Kim, J.S.; Lee, B.H. Excess copper induced physiological and proteomic changes in germinating rice seeds. Chemosphere 2007, 67, 1182–1193. [Google Scholar] [CrossRef]
- Curado, G.; Rubio-Casal, A.E.; Figueroa, E.; Castillo, J.M. Germination and establishment of the invasive cordgrass Spartina densiflora in acidic and metal polluted sediments of the Tinto River. Mar. Pollut. Bull. 2010, 60, 1842–1848. [Google Scholar] [CrossRef]
- Kuriakose, S.V.; Prasad, M.N.V. Cadmium stress affects seed germination and seedling growth in Sorghum bicolor (L.) Moench by changing the activities of hydrolyzing enzymes. Plant Growth Regul. 2008, 54, 143–156. [Google Scholar] [CrossRef]
- Infante-Izquierdoa, M.D.; Polo-Ávilaa, A.; Sanjoséa, I.; Castillob, J.M.; Nievaa, F.J.J.; Grewell, B.J.; Muñoz-Rodríguez, A.F. Effects of heavy metal pollution on germination and early seedling growth in native and invasive Spartina cordgrasses. Mar. Pollut. Bull. 2020, 158, 111376. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.L.; Wu, Y.Y.X.; Sun, Z.; Yu, X.W.; Zhuang, J.L. Effects of Pb2+, Zn2+, and Cd2+ stress on seed germination of Suaeda salsa. J. Zhejiang Agric. Sci. 2018, 59, 2265–2269. [Google Scholar]
- Li, Q.Y.; Yu, C.; Zhou, Y.; Li, H.M.; Xia, Z.; Chen, X.; Huang, Y. Effects of Cr6+ on seed germination and seedling physiological characteristics of Salvia miltiorrhiza Bunge. Seed 2024, 43, 124–129. [Google Scholar]
- Nadarajah, K.K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Kamal, M.M.; Erazo, C.; Tanino, K.K.; Kawamura, Y.; Kasuga, J.; Laarveld, B.; Olkowski, A.; Uemura, M. A single seed treatment mediated through reactive oxygen species increases germination, growth performance, and abiotic stress tolerance in Arabidopsis and rice. Biosci. Biotechnol. Biochem. 2020, 84, 2597–2608. [Google Scholar] [CrossRef]
- Nahar, K.; Rhaman, M.S.; Parvin, K.; Bardhan, K.; Marques, D.N.; García-Caparrós, P.; Hasanuzzaman, M. Arsenic-induced oxidative stress and antioxidant defense in plants. Stresses 2022, 2, 179–209. [Google Scholar] [CrossRef]
- Pandian, S.; Rakkammal, K.; Rathinapriya, P.; Rency, A.S.; Satish, L.; Ramesh, M. Physiological and biochemical changes in sorghum under combined heavy metal stress: An adaptive defence against oxidative stress. Biocatal. Agric. Biotechnol. 2020, 29, 101830. [Google Scholar] [CrossRef]
- Lou, Y.H.; Zhao, P.; Wang, D.L.; Amombo, E.; Sun, X.; Wang, H.; Zhuge, Y.P. Germination, physiological responses and gene expression of Tall Fescue (Festuca arundinacea Schreb.) growing under Pb and Cd. PLoS ONE 2017, 12, e0169495. [Google Scholar] [CrossRef]
- Bankaji, I.; Caçador, I.; Sleimi, N. Physiological and biochemical responses of Suaeda fruticosa to cadmium and copper stresses: Growth, nutrient uptake, antioxidant enzymes, phytochelatin, and glutathione levels. Env. Sci. Pollut. Res. Int. 2015, 22, 13058–13069. [Google Scholar] [CrossRef]
- Shackira, A.M.; Puthur, J.T. Enhanced phytostabilization of cadmium by a halophyte-Acanthus ilicifolius L. Int. J. Phytorem. 2017, 19, 319–326. [Google Scholar] [CrossRef]
- Guo, B.H.; Dai, S.X.; Wang, R.G.; Guo, J.K.; Ding, Y.Z.; Xu, Y.M. Combined effects of elevated CO2 and Cd-contaminated soil on the growth, gas exchange, antioxidant defense, and Cd accumulation of poplars and willows. Environ. Exp. Bot. 2015, 115, 1–10. [Google Scholar] [CrossRef]
- Mzoughi, Z.; Souid, G.; Timoumi, R.; LeCerf, D.; Majdoub, H. Partial characterization of the edible Spinacia oleracea polysaccharides: Cytoprotective and antioxidant potentials against Cd induced toxicity in HCT116 and HEK293 cells. Int. J. Biol. Macromol. 2019, 136, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Qiu, W.W.; Chen, Z.Y.; Chen, W.Y.; Li, Y.F.; Zhu, J.L.; Rahman, S.U.; Han, Z.X.; Jiang, Y.; Yang, G.J.; et al. Phosphorus influence Cd phytoextraction in Populus stems via modulating xylem development, cell wall Cd storage and antioxidant defense. Chemosphere 2020, 242, 125154. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Li, K.; Wang, B. Overexpression of SsCHLAPXs confers protection against oxidative stress induced by high light in transgenic Arabidopsis thaliana. Physiol. Plant. 2011, 143, 355–366. [Google Scholar] [CrossRef]
- Ullah, S.; Hadi, F.; Ali, N.; Khan, S. Foliar application of Iron (Fe) improved the antioxidant defense and Cd accumulation potential of Ricinus communis under hydroponic condition. Water Air Soil Pollut. 2018, 229, 284. [Google Scholar] [CrossRef]
- Lu, J.; Ma, Y.L.; Xing, G.L.; Li, W.L.; Kong, X.X.; Li, J.Y.; Wang, L.J.; Yuan, H.L.; Yang, J.S. Revelation of microalgae’s lipid production and resistance mechanism to ultra-high Cd stress by integrated transcriptome and physiochemical analyses. Environ. Pollut. 2019, 250, 186–195. [Google Scholar] [CrossRef]
- Huang, H.L.; Rizwan, M.; Li, M.; Song, F.; Zhou, S.J.; He, X.; Ding, R.; Dai, Z.H.; Yuan, Y.; Cao, M.H.; et al. Comparative efficacy of organic and inorganic silicon fertilizers on antioxidant response, Cd/Pb accumulation and health risk assessment in wheat (Triticum aestivum L.). Environ. Pollut. 2019, 255, 113146. [Google Scholar] [CrossRef]
- Wu, H.F.; Liu, X.L.; Zhao, J.M.; Yu, J.B. Regulation of metabolites, gene expression, and antioxidant enzymes to environmentally relevant lead and zinc in the halophyte Suaeda salsa. J. Plant Growth Regul. 2013, 32, 353–361. [Google Scholar] [CrossRef]
- Kumari, A.; Sheokand, S.; Kumar, A.; Mann, A.; Kumar, N.; Devi, S.; Rani, B.; Kumar, A.; Meena, B.L. Halophyte growth and physiology under metal toxicity. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Springer: Singapore, 2019; pp. 83–113. [Google Scholar]
- Khalilzadeh, R.; Pirzad, A.; Sepehr, E.; Khan, S.; Anwar, S. Long-term effect of heavy metal–polluted wastewater irrigation on physiological and ecological parameters of Salicornia europaea L. J. Soil Sci. Plant Nutr. 2020, 20, 1574–1587. [Google Scholar] [CrossRef]
- Ran, C.; Gulaqa, A.; Zhu, J.; Wang, X.W.; Zhang, S.Q.; Geng, Y.Q.; Guo, L.Y.; Jin, F.; Shao, X.W. Benefits of biochar for improving ion contents, cell membrane permeability, leaf water status and yield of rice under saline-sodic paddy field condition. J. Plant Growth Regul. 2020, 39, 370–377. [Google Scholar] [CrossRef]
- Banu, N.A.; Hoque, A.; Watanabe-Sugimoto, M.; Matsuoka, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycine-betaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J. Plant Physiol. 2009, 166, 146–156. [Google Scholar] [CrossRef]
- Zhao, Y.R.; Cai, H.J.; Zhang, J.F.; Chen, W.H.; Liu, Y.; Chen, M. Effects of Cu2+ and Zn2+ on seed germination, osmotic adjustment substances in seedling of Suaeda heteroptera Kitagawa. J. Anhui Agric. Sci. 2019, 47, 45–47. [Google Scholar]
- Nikalje, G.C.; Suprasanna, P. Coping with metal toxicity-cues from halophytes. Front. Plant Sci. 2018, 9, 777. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, P.; Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxiffcation of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef]
- Martinoia, E.; Maeshima, M.; Neuhaus, H.E. Vacuolar transporters and their essential role in plant metabolism. J. Exp. Bot. 2006, 58, 83–102. [Google Scholar] [CrossRef]
- Song, J.; Wang, B.S. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann. Bot. 2015, 115, 541–553. [Google Scholar] [CrossRef]
- Zhang, X.; Lia, M.; Yang, H.; Lia, X.; Cui, Z. Physiological responses of Suaeda glauca and Arabidopsis thaliana in phytoremediation of heavy metals. J. Environ. Manag. 2018, 223, 132–139. [Google Scholar] [CrossRef]
- Panda, A.; Rangani, J.; Kumari, A.; Parida, A.K. Efficient regulation of Arsenic translocation to shoot tissue and modulation of phytochelatin levels and antioxidative defense system confers salinity and arsenic tolerance in the halophyte Suaeda maritima. Environ. Exp. Bot. 2017, 143, 149–171. [Google Scholar] [CrossRef]
- Pedro, C.A.; Santos, M.S.; Ferreira, S.M.; Gonçalves, S.C. The influence of cadmium contamination and salinity on the survival growth and phytoremediation capacity of the saltmarsh plant Salicornia ramosissima. Mar. Environ. Res. 2013, 92, 197–205. [Google Scholar] [CrossRef]
- Shu, W.S.; Liu, W.; Lan, C.Y. Viola, baoshanensis Shu, Liu et Lan, a new species of Violaceae from Hunan Province, China. Acta Sci. Nat. Univ. Sunyatseni. 2003, 42, 118–119. [Google Scholar]






| Heavy Metal Concentration (mg/L) | Root Length (mm) | Shoot Length (mm) | ||
|---|---|---|---|---|
| Seedlings from Brown Seeds | Seedlings from Black Seeds | Seedlings from Brown Seeds | Seedlings from Black Seeds | |
| Control | 38.69 ± 4.33 Aa | 33.24 ± 0.38 Ba | 22.98 ± 0.73 Aa | 23.66 ± 2.42 Aa |
| Pb | ||||
| 100 | 21.46 ± 1.81 Ab | 21.53 ± 0.42 Ab | 14.08 ± 0.93 Bb | 16.39 ± 1.54 Ab |
| 200 | 13.53 ± 1.06 Ac | 13.35 ± 1.23 Ac | 8.97 ± 0.21 Bc | 16.67 ± 0.58 Ab |
| 400 | 12.03 ± 1.47 Acd | 10.02 ± 0.79 Ad | 7.49 ± 0.53 Bd | 11.53 ± 0.40 Ac |
| 800 | 8.53 ± 1.70 Ade | 6.15 ± 0.46 Be | 5.36 ± 0.90 Be | 8.01 ± 1.10 Ad |
| 1000 | 5.60 ± 0.20 Ae | 3.89 ± 0.63 Bf | 4.78 ± 0.64 Ae | 4.59 ± 0.77 Ae |
| Cd | ||||
| 5 | 16.94 ± 2.79 Ab | 13.53 ± 2.15 Ab | 11.69 ± 1.62 Bb | 16.87 ± 2.59 Ab |
| 10 | 14.81 ± 2.10 Ab | 10.11 ± 1.33 Bc | 11.09 ± 0.66 Bb | 13.52 ± 0.51 Ac |
| 20 | 8.36 ± 2.49 Ac | 4.07 ± 0.83 Bd | 9.41 ± 2.19 Ab | 8.49 ± 1.28 Ad |
| 50 | 4.13 ± 0.42 Ac | 2.00 ± 0.18 Bd | 5.19 ± 0.68 Ac | 3.17 ± 0.37 Be |
| Cu | ||||
| 20 | 10.91 ± 0.43 Ab | 6.02 ± 0.36 Bb | 13.92 ± 0.94 Ab | 13.33 ± 0.79 Ab |
| 50 | 6.64 ± 0.23 Ac | 5.94 ± 0.58 Ab | 12.49 ± 1.43 Abc | 10.18 ± 0.90 Bc |
| 100 | 6.37 ± 0.54 Ac | 5.23 ± 0.79 Ab | 12.07 ± 1.07 Ac | 8.11 ± 0.27 Bc |
| 200 | 4.35 ± 0.88 Ac | 3.63 ± 0.17 Ac | 5.72 ± 0.19 Ad | 5.50 ± 0.45 Ad |
| Zn | ||||
| 50 | 6.81 ± 0.37 Bb | 7.68 ± 0.46 Ab | 20.14 ± 1.43 Ab | 19.86 ± 1.52 Ab |
| 100 | 6.22 ± 0.40 Ab | 6.18 ± 0.08 Ac | 17.53 ± 0.65 Ac | 17.25 ± 0.76 Ac |
| 200 | 5.45 ± 0.12 Ab | 5.52 ± 0.67 Acd | 14.39 ± 0.75 Ad | 15.14 ± 0.94 Ac |
| 500 | 4.53 ± 0.44 Ab | 4.96 ± 0.15 Ad | 11.51 ± 0.17 Ae | 9.83 ± 0.46 Bd |
| Heavy Metal Concentration (mg/L) | Root Tolerance Index | Shoot Tolerance Index | ||
|---|---|---|---|---|
| Seedlings from Brown Seeds | Seedlings from Black Seeds | Seedlings from Brown Seeds | Seedlings from Black Seeds | |
| Control | 100 ± 0.0 Aa | 100 ± 0.0 Aa | 100 ± 0.0 Aa | 100 ± 0.0 Aa |
| Pb | ||||
| 100 | 57.99 ± 5.90 Bb | 68.47 ± 0.51 Ab | 63.60 ± 2.38 Bb | 70.71 ± 2.02 Ab |
| 200 | 36.43 ± 3.83 Ac | 41.90 ± 4.69 Ac | 40.55 ± 0.67 Bc | 72.09 ± 6.55 Ab |
| 400 | 33.14 ± 7.60 Ac | 32.63 ± 4.10 Ad | 32.75 ± 2.62 Bd | 50.16 ± 7.16 Ac |
| 800 | 23.52 ± 7.37 Ad | 19.32 ± 0.94 Ae | 23.85 ± 3.92 Be | 34.69 ± 1.79 Ad |
| 1000 | 15.06 ± 1.43 Ad | 12.46 ± 1.82 Af | 21.08 ± 3.35 Ae | 20.21 ± 5.14 Ae |
| Cd | ||||
| 5 | 46.36 ± 8.15 Ab | 43.01 ± 8.36 Ab | 52.81 ± 4.25 Bb | 72.80 ± 6.45 Ab |
| 10 | 39.06 ± 4.87 Ab | 30.99 ± 4.67 Ac | 50.25 ± 3.87 Ab | 58.96 ± 8.03 Ac |
| 20 | 22.23 ± 5.84 Ac | 13.16 ± 3.07 Bd | 41.40 ± 8.54 Ac | 37.51 ± 9.36 Ad |
| 50 | 11.33 ± 1.81 Ad | 6.38 ± 0.57 Bd | 23.06 ± 3.11 Ad | 13.95 ± 3.11 Be |
| Cu | ||||
| 20 | 30.06 ± 2.88 Ab | 19.33 ± 1.90 Bb | 62.49 ± 2.55 Ab | 57.67 ± 8.22 Ab |
| 50 | 18.28 ± 2.02 Ac | 18.70 ± 1.91 Ab | 56.25 ± 6.16 Ac | 44.47 ± 8.32 Ac |
| 100 | 17.34 ± 2.19 Ac | 16.97 ± 3.15 Ab | 53.38 ± 2.81 Ac | 35.38 ± 2.73 Bc |
| 200 | 12.02 ± 3.88 Ad | 11.68 ± 0.78 Ac | 25.55 ± 2.26 Ad | 24.28 ± 4.42 Ad |
| Zn | ||||
| 50 | 18.49 ± 1.31 Bb | 24.45 ± 0.75 Ab | 90.29 ± 5.73 Ab | 86.83 ± 15.54 Aab |
| 100 | 17.05 ± 1.75 Bbc | 19.72 ± 0.61 Ac | 78.25 ± 5.40 Ac | 75.08 ± 10.60 Abc |
| 200 | 14.85 ± 1.77 Acd | 17.61 ± 1.53 Ad | 64.18 ± 1.31 Ad | 65.94 ± 9.84 Ac |
| 500 | 12.43 ± 2.81 Ad | 15.66 ± 0.87 Ae | 51.64 ± 1.55 Ae | 42.60 ± 2.44 Bd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Cao, X.; An, R.; Ding, H.; Wang, W.; Zhou, Y.; Wu, C.; Cao, Y.; Wang, H.; Li, C.; et al. Physiological Adaptation to Different Heavy Metal Stress in Seedlings of Halophyte Suaeda liaotungensis. Biology 2025, 14, 260. https://doi.org/10.3390/biology14030260
Song J, Cao X, An R, Ding H, Wang W, Zhou Y, Wu C, Cao Y, Wang H, Li C, et al. Physiological Adaptation to Different Heavy Metal Stress in Seedlings of Halophyte Suaeda liaotungensis. Biology. 2025; 14(3):260. https://doi.org/10.3390/biology14030260
Chicago/Turabian StyleSong, Jieqiong, Xiaoqi Cao, Ruixuan An, Haoran Ding, Wen Wang, Yahan Zhou, Chunyan Wu, Yizihan Cao, Hongfei Wang, Changping Li, and et al. 2025. "Physiological Adaptation to Different Heavy Metal Stress in Seedlings of Halophyte Suaeda liaotungensis" Biology 14, no. 3: 260. https://doi.org/10.3390/biology14030260
APA StyleSong, J., Cao, X., An, R., Ding, H., Wang, W., Zhou, Y., Wu, C., Cao, Y., Wang, H., Li, C., & Li, Q. (2025). Physiological Adaptation to Different Heavy Metal Stress in Seedlings of Halophyte Suaeda liaotungensis. Biology, 14(3), 260. https://doi.org/10.3390/biology14030260





