Tethya wilhelma (Porifera) Is Highly Resistant to Radiation Exposure and Possibly Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Lab Cultures
2.2. DNA Damage by X-Ray Irradiation
2.3. Experimental Settings
2.4. Morphological Analysis
2.5. Quantification of DNA Damage
2.6. Molecular Genetic Analysis
2.7. Statistical Analysis
3. Results
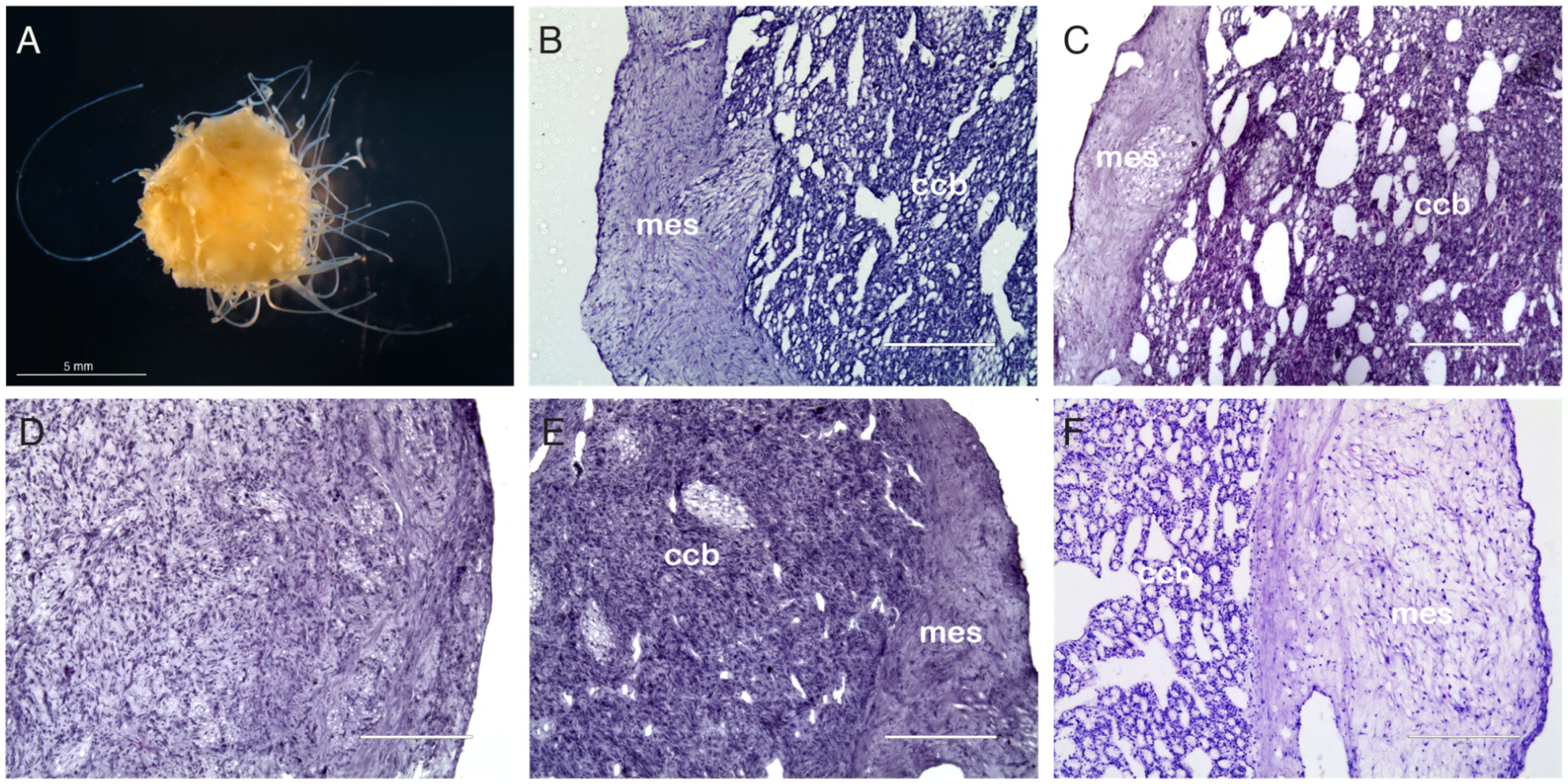
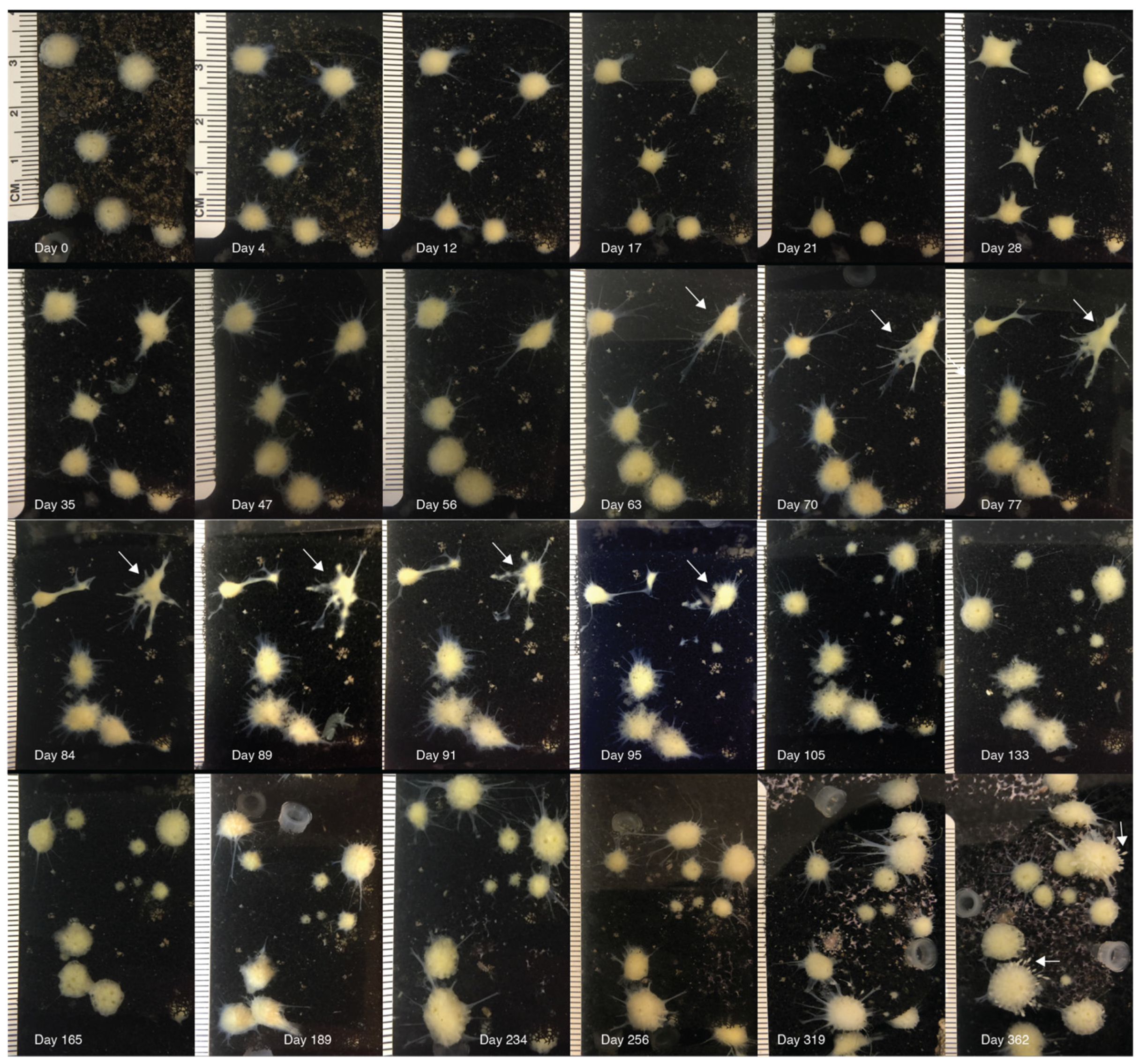
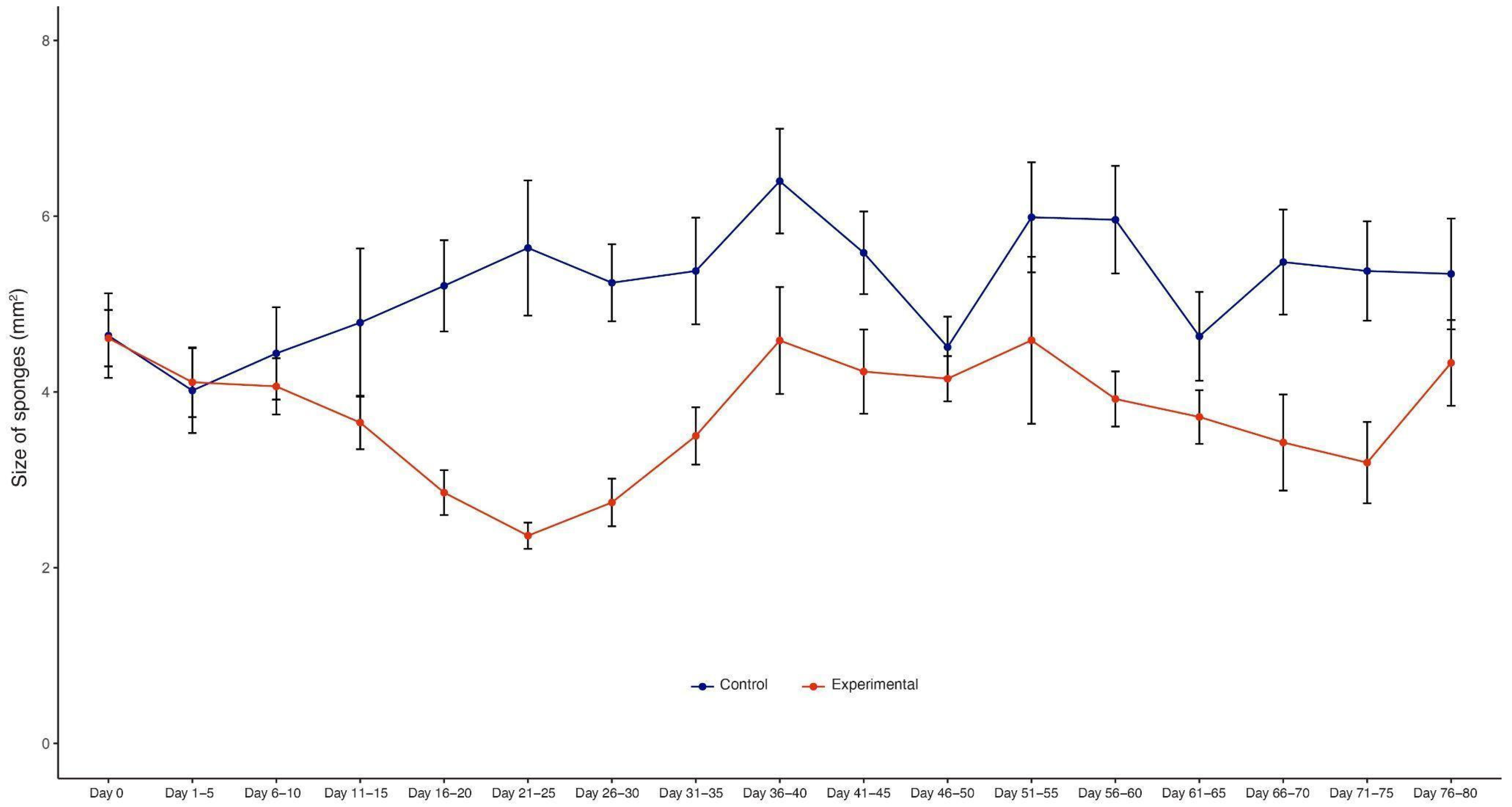
3.1. Morphological Observations
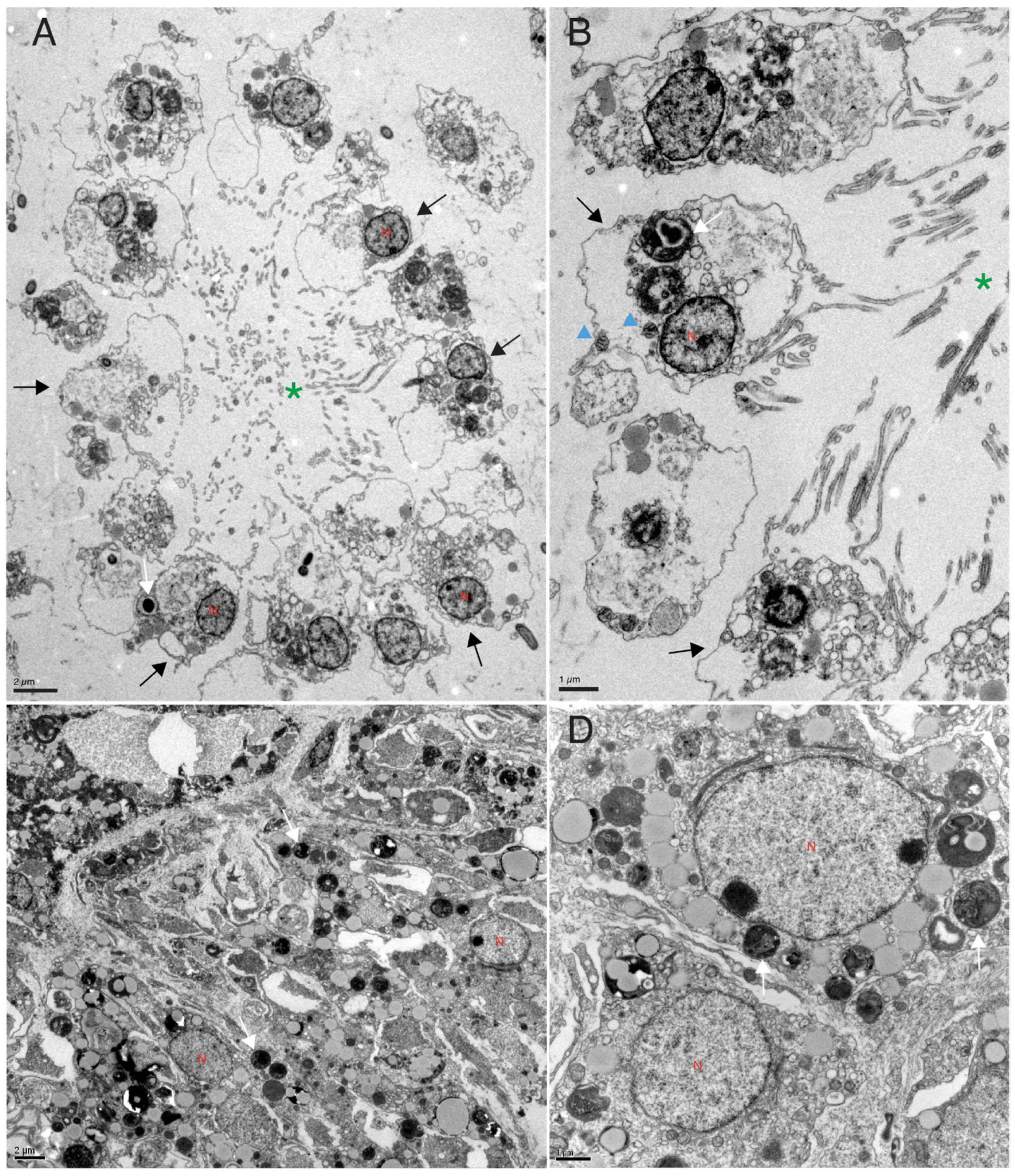
3.2. Histological Analysis
3.3. DNA Damage Analysis
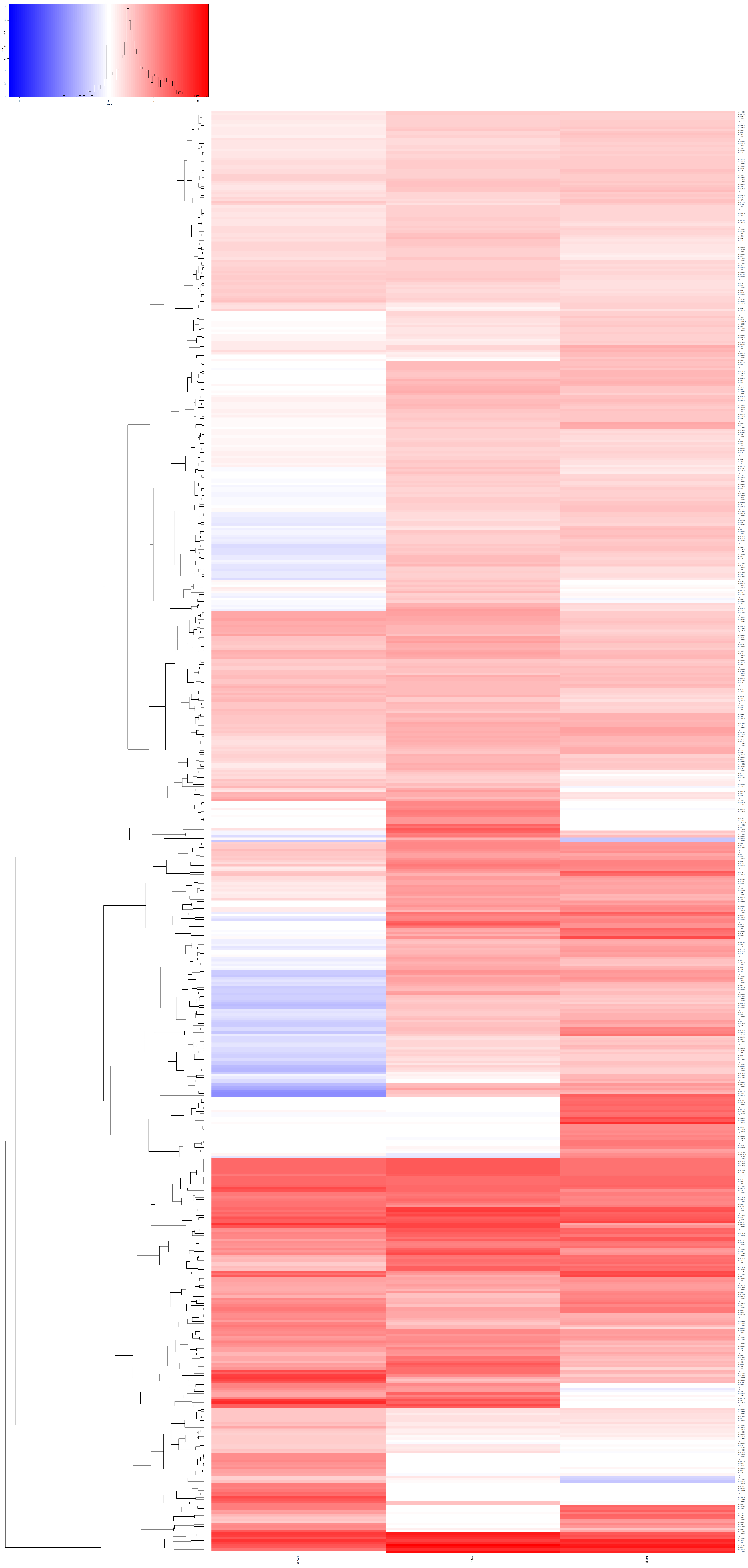
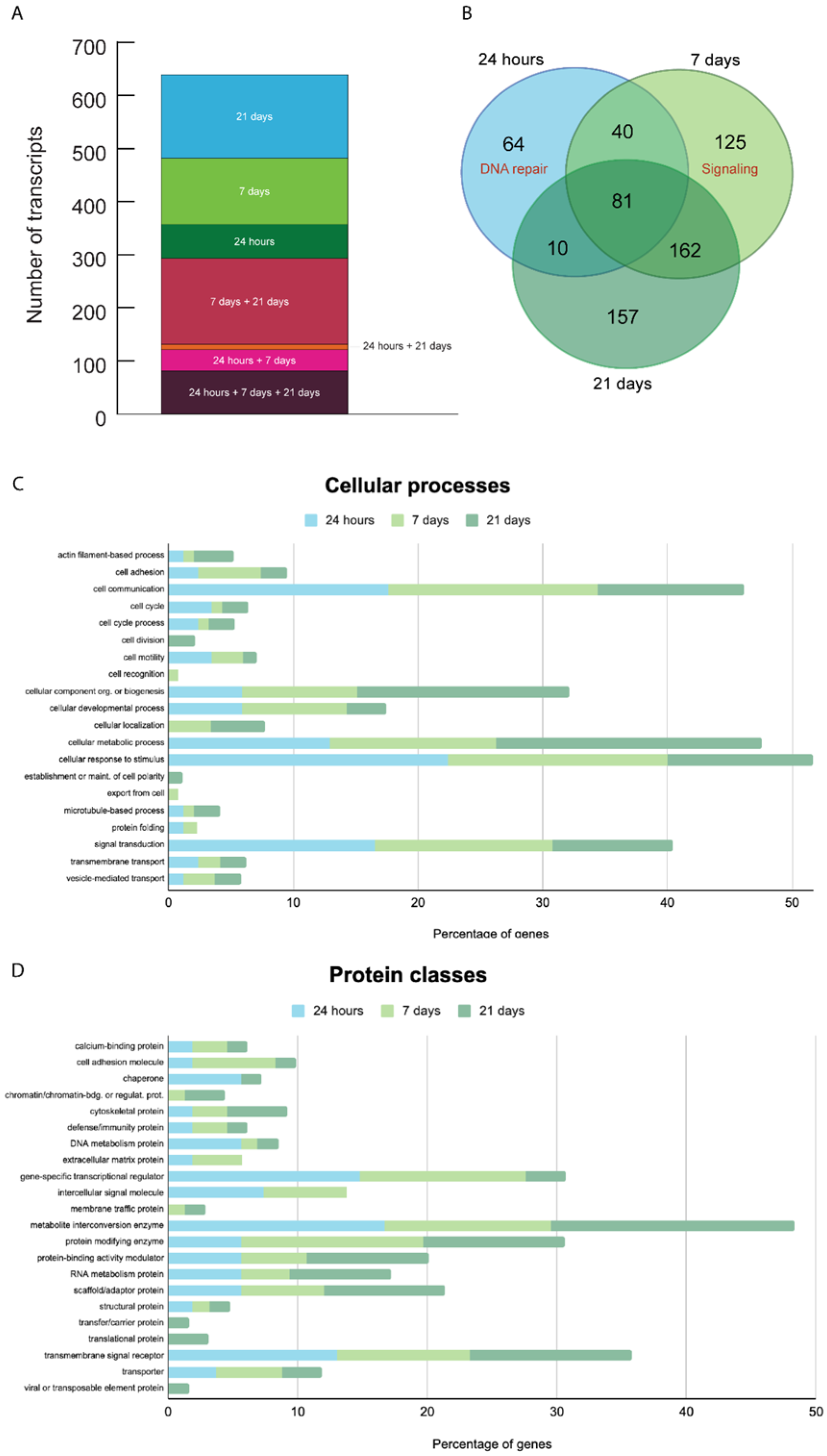
3.4. Gene Expression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Müller, W.E.G. Sponges (Porifera); Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 9783642555190. [Google Scholar]
- Aktipis, C.A.; Boddy, A.M.; Jansen, G.; Hibner, U.; Hochberg, M.E.; Maley, C.C.; Wilkinson, G.S. Cancer across the Tree of Life: Cooperation and Cheating in Multicellularity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140219. [Google Scholar] [CrossRef]
- Domazet-Lošo, T.; Klimovich, A.; Anokhin, B.; Anton-Erxleben, F.; Hamm, M.J.; Lange, C.; Bosch, T.C.G. Naturally Occurring Tumours in the Basal Metazoan Hydra. Nat. Commun. 2014, 5, 4222. [Google Scholar] [CrossRef] [PubMed]
- Tascedda, F.; Ottaviani, E. Tumors in Invertebrates. Available online: https://www.isj.unimore.it/index.php/ISJ/article/download/321/235 (accessed on 1 January 2022).
- Gupta, A.P. Immunology of Invertebrates: Cellular. Encycl. Life Sci. 2001. [Google Scholar] [CrossRef]
- Melillo, D.; Marino, R.; Italiani, P.; Boraschi, D. Innate Immune Memory in Invertebrate Metazoans: A Critical Appraisal. Front. Immunol. 2018, 9, 1915. [Google Scholar] [CrossRef]
- Alexander, B.E.; Liebrand, K.; Osinga, R.; van der Geest, H.G.; Admiraal, W.; Cleutjens, J.P.M.; Schutte, B.; Verheyen, F.; Ribes, M.; van Loon, E.; et al. Cell Turnover and Detritus Production in Marine Sponges from Tropical and Temperate Benthic Ecosystems. PLoS ONE 2014, 9, e109486. [Google Scholar] [CrossRef]
- Nickel, M.; Donath, T.; Schweikert, M.; Beckmann, F. Functional Morphology of Tethya Species (Porifera): 1. Quantitative 3D-Analysis of Tethya Wilhelma by Synchrotron Radiation Based X-Ray Microtomography. Zoomorphology 2006, 125, 209–223. [Google Scholar] [CrossRef]
- Leys, S.P.; Hill, A. The Physiology and Molecular Biology of Sponge Tissues. Adv. Mar. Biol. 2012, 62, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Leys, S.P.; Nichols, S.A.; Adams, E.D.M. Epithelia and Integration in Sponges. Integr. Comp. Biol. 2009, 49, 167–177. [Google Scholar] [CrossRef]
- Mitchell, J.M.; Nichols, S.A. Diverse Cell Junctions with Unique Molecular Composition in Tissues of a Sponge (Porifera). Evodevo 2019, 10, 26. [Google Scholar] [CrossRef]
- Hammel, J.U.; Herzen, J.; Beckmann, F.; Nickel, M. Sponge Budding Is a Spatiotemporal Morphological Patterning Process: Insights from Synchrotron Radiation-Based X-Ray Microtomography into the Asexual Reproduction of Tethya Wilhelma. Front. Zool. 2009, 6, 19. [Google Scholar] [CrossRef]
- Nickel, M. Body Extension Types of Tethya Wilhelma: Cellular Organisation and Their Locomotory Function. In Proceedings of the Sponge Science in the new Millennium “Papers contributed to the VI International Sponge Conference”, Rapallo, Italy, 29 September–5 October 2002; Volume 68. [Google Scholar]
- Wörheide, G.; Francis, W.R.; Deister, F.; Krebs, S.; Erpenbeck, D.; Vargas, S. The Genomes of the Aquarium Sponges Tethya Wilhelma and Tethya Minuta (Porifera: Demospongiae). F1000Research 2024, 13, 679. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C. Early Animal Evolution: A Morphologist’s View. R. Soc. Open Sci. 2019, 6, 190638. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Dhawan, A.; Bajpayee, M.; Parmar, D. Comet Assay: A Reliable Tool for the Assessment of DNA Damage in Different Models. Cell Biol. Toxicol. 2009, 25, 5–32. [Google Scholar] [CrossRef] [PubMed]
- Bajpayee, M.; Kumar, A.; Dhawan, A. The Comet Assay: Assessment of In Vitro and In Vivo DNA Damage. In Genotoxicity Assessment; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; pp. 325–345. ISBN 9781627035286. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Yates, A.D.; Achuthan, P.; Akanni, W.; Allen, J.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Azov, A.G.; Bennett, R.; et al. Ensembl 2020. Nucleic Acids Res. 2020, 48, D682–D688. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Muruganujan, A.; Huang, X.; Ebert, D.; Mills, C.; Guo, X.; Thomas, P.D. Protocol Update for large-scale genomeand gene function analysis with the PANTHER classification system (v140). Nat. Protoc. 2019, 14, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Nickel, M.; Scheer, C.; Hammel, J.U.; Herzen, J.; Beckmann, F. The Contractile Sponge Epithelium Sensu Lato–Body Contraction of the Demosponge Tethya Wilhelma Is Mediated by the Pinacoderm. J. Exp. Biol. 2011, 214, 1692–1698. [Google Scholar] [CrossRef]
- Coutinho, C.C.; Rosa, I.; de Andrade Rosa, I.; de Oliveira Teixeira, J.D.; Andrade, L.R.; Costa, M.L.; Mermelstein, C. Cellular Migration, Transition and Interaction during Regeneration of the Sponge Hymeniacidon heliophila. PLoS ONE 2017, 12, e0178350. [Google Scholar] [CrossRef]
- Christensen, E.I.; Nielsen, R.; Birn, H. From Bowel to Kidneys: The Role of Cubilin in Physiology and Disease. Nephrol. Dial. Transplant. 2013, 28, 274–281. [Google Scholar] [CrossRef]
- Gremel, G.; Djureinovic, D.; Niinivirta, M.; Laird, A.; Ljungqvist, O.; Johannesson, H.; Bergman, J.; Edqvist, P.-H.; Navani, S.; Khan, N.; et al. A Systematic Search Strategy Identifies Cubilin as Independent Prognostic Marker for Renal Cell Carcinoma. BMC Cancer 2017, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Hong, S.; Lee, M.H.; Koo, H.-S. A PHF8 Homolog in C. Elegans Promotes DNA Repair via Homologous Recombination. PLoS ONE 2015, 10, e0123865. [Google Scholar] [CrossRef]
- Słabicki, M.; Theis, M.; Krastev, D.B.; Samsonov, S.; Mundwiller, E.; Junqueira, M.; Paszkowski-Rogacz, M.; Teyra, J.; Heninger, A.-K.; Poser, I.; et al. A Genome-Scale DNA Repair RNAi Screen Identifies SPG48 as a Novel Gene Associated with Hereditary Spastic Paraplegia. PLoS Biol. 2010, 8, e1000408. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.-F.; Su, Y.-H.; Huang, Y.-N.; Tsai, M.-T.; Li, L.-T.; Chen, Y.-L.; Cheng, C.-J.; Dai, D.-F.; Yang, R.-B. Localization and Characterization of a Novel Secreted Protein SCUBE1 in Human Platelets. Cardiovasc. Res. 2006, 71, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.P.; Brown, S.L.; Georges, G.E.; Hauer-Jensen, M.; Hill, R.P.; Huser, A.K.; Kirsch, D.G.; Macvittie, T.J.; Mason, K.A.; Medhora, M.M.; et al. Animal Models for Medical Countermeasures to Radiation Exposure. Radiat. Res. 2010, 173, 557–578. [Google Scholar] [CrossRef] [PubMed]
- Plett, P.A.; Sampson, C.H.; Chua, H.L.; Joshi, M.; Booth, C.; Gough, A.; Johnson, C.S.; Katz, B.P.; Farese, A.M.; Parker, J.; et al. Establishing a Murine Model of the Hematopoietic Syndrome of the Acute Radiation Syndrome. Health Phys. 2012, 103, 343. [Google Scholar] [CrossRef]
- Wagner, R.H.; Boles, M.A.; Henkin, R.E. Treatment of Radiation Exposure and Contamination. Radiographics 1994, 14, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Karam, P.A.; Leslie, S.A. Changes in Terrestrial Natural Radiation Levels over the History of Life. In Radioactivity in the Environment; Elsevier: Amsterdam, The Netherlands, 2005; Volume 7, pp. 107–117. [Google Scholar]
- Genta-Jouve, G.; Cachet, N.; Oberhänsli, F.; Noyer, C.; Teyssié, J.-L.; Thomas, O.P.; Lacoue-Labarthe, T. Comparative Bioaccumulation Kinetics of Trace Elements in Mediterranean Marine Sponges. Chemosphere 2012, 89, 340–349. [Google Scholar] [CrossRef]
- Maloubier, M.; Shuh, D.K.; Minasian, S.G.; Pacold, J.I.; Solari, P.-L.; Michel, H.; Oberhaensli, F.R.; Bottein, Y.; Monfort, M.; Moulin, C.; et al. How Do Radionuclides Accumulate in Marine Organisms? A Case Study of Europium with Aplysina Cavernicola. Environ. Sci. Technol. 2016, 50, 10730–10738. [Google Scholar] [CrossRef]
- Poinssot, C.; Geckeis, H. Radionuclide Behaviour in the Natural Environment: Science, Implications and Lessons for the Nuclear Industry; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 9780857097194. [Google Scholar]
- Krisko, A.; Radman, M. Biology of Extreme Radiation Resistance: The Way of Deinococcus Radiodurans. Cold Spring Harb. Perspect. Biol. 2013, 5, a012765. [Google Scholar] [CrossRef] [PubMed]
- Gladyshev, E.; Meselson, M. Extreme Resistance of Bdelloid Rotifers to Ionizing Radiation. Proc. Natl. Acad. Sci. USA 2008, 105, 5139–5144. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, K.I.; Ingemar Jönsson, K.; Harms-Ringdahl, M.; Torudd, J. Radiation Tolerance in the eutardigradeRichtersius Coronifer. Int. J. Radiat. Biol. 2005, 81, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Reuner, A.; Brümmer, F.; Schill, R.O. DNA Damage in Storage Cells of Anhydrobiotic Tardigrades. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 153, 425–429. [Google Scholar] [CrossRef]
- Altiero, T.; Guidetti, R.; Caselli, V.; Cesari, M.; Rebecchi, L. Ultraviolet Radiation Tolerance in Hydrated and Desiccated Eutardigrades. J. Zool. Syst. Evol. Res. 2011, 49, 104–110. [Google Scholar] [CrossRef]
- Chavez, C.; Cruz-Becerra, G.; Fei, J.; Kassavetis, G.A.; Kadonaga, J.T. The Tardigrade Damage Suppressor Protein Binds to Nucleosomes and Protects DNA from Hydroxyl Radicals. eLife 2019, 8, e47682. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.C. Life History of Milnesium Tardigradum Doyère (tardigrada) under a Rearing Environment. Zool. Sci. 2003, 20, 49–57. [Google Scholar] [CrossRef]
- Ducoff, H.S. Causes of Death in Irradiated Adult Insects. Biol. Rev. Camb. Philos. Soc. 1972, 47, 211–240. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, A.; Fleming, A.; Aktipis, A.; Maley, C.C. Upregulation of DNA repair genes and cell extrusion underpin the remarkable radiation resistance of Trichoplax adhaerens. PLoS Biol. 2021, 19, e3001471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McMurray, S.E.; Blum, J.E.; Pawlik, J.R. Redwood of the Reef: Growth and Age of the Giant Barrel Sponge Xestospongia Muta in the Florida Keys. Mar. Biol. 2008, 155, 159–171. [Google Scholar] [CrossRef]
- Jochum, K.P.; Wang, X.; Vennemann, T.W.; Sinha, B.; Müller, W.E.G. Siliceous Deep-Sea Sponge Monorhaphis Chuni: A Potential Paleoclimate Archive in Ancient Animals. Chem. Geol. 2012, 300–301, 143–151. [Google Scholar] [CrossRef]
- Fallon, S.J.; James, K.; Norman, R.; Kelly, M.; Ellwood, M.J. A Simple Radiocarbon Dating Method for Determining the Age and Growth Rate of Deep-Sea Sponges. Nucl. Instrum. Methods Phys. Res. B 2010, 268, 1241–1243. [Google Scholar] [CrossRef]
- Van Soest, R.W.M.; Boury-Esnault, N.; Vacelet, J.; Dohrmann, M.; Erpenbeck, D.; De Voogd, N.J.; Santodomingo, N.; Vanhoorne, B.; Kelly, M.; Hooper, J.N.A. Global Diversity of Sponges (Porifera). PLoS ONE 2012, 7, e35105. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Wuu, C.-S. Radiation-Induced Second Cancers: The Impact of 3D-CRT and IMRT. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Rivina, L.; Davoren, M.J.; Schiestl, R.H. Mouse Models for Radiation-Induced Cancers. Mutagenesis 2016, 31, 491–509. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Zhao, W.; Zhou, X.; Miao, G.; Sun, C.; Zhang, H. NADPH Oxidase Activation Contributes to Heavy Ion Irradiation-Induced Cell Death. Dose Response 2017, 15, 1559325817699697. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Funayama, N. The Cellular and Molecular Bases of the Sponge Stem Cell Systems Underlying Reproduction, Homeostasis and Regeneration. Int. J. Dev. Biol. 2018, 62, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Sogabe, S.; Hatleberg, W.L.; Kocot, K.M.; Say, T.E.; Stoupin, D.; Roper, K.E.; Fernandez-Valverde, S.L.; Degnan, S.M.; Degnan, B.M. Pluripotency and the Origin of Animal Multicellularity. Nature 2019, 570, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Puck, T.T.; Marcus, P.I. Action of X-Rays on Mammalian Cells. J. Exp. Med. 1956, 103, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Graham, P.H.; Hao, J.; Ni, J.; Bucci, J.; Cozzi, P.J.; Kearsley, J.H.; Li, Y. Acquisition of Epithelial–mesenchymal Transition and Cancer Stem Cell Phenotypes Is Associated with Activation of the PI3K/Akt/mTOR Pathway in Prostate Cancer Radioresistance. Cell Death Dis. 2013, 4, e875. [Google Scholar] [CrossRef]
- Li, D.; Qu, C.; Ning, Z.; Wang, H.; Zang, K.; Zhuang, L.; Chen, L.; Wang, P.; Meng, Z. Radiation Promotes Epithelial-to-Mesenchymal Transition and Invasion of Pancreatic Cancer Cell by Activating Carcinoma-Associated Fibroblasts. Am. J. Cancer Res. 2016, 6, 2192–2206. [Google Scholar] [PubMed]
- Nagarajan, D.; Melo, T.; Deng, Z.; Almeida, C.; Zhao, W. ERK/GSK3β/Snail Signaling Mediates Radiation-Induced Alveolar Epithelial-to-Mesenchymal Transition. Free Radic. Biol. Med. 2012, 52, 983–992. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortunato, A.; Taylor, J.; Scirone, J.; Seyedi, S.; Aktipis, A.; Maley, C.C. Tethya wilhelma (Porifera) Is Highly Resistant to Radiation Exposure and Possibly Cancer. Biology 2025, 14, 171. https://doi.org/10.3390/biology14020171
Fortunato A, Taylor J, Scirone J, Seyedi S, Aktipis A, Maley CC. Tethya wilhelma (Porifera) Is Highly Resistant to Radiation Exposure and Possibly Cancer. Biology. 2025; 14(2):171. https://doi.org/10.3390/biology14020171
Chicago/Turabian StyleFortunato, Angelo, Jake Taylor, Jonathan Scirone, Sareh Seyedi, Athena Aktipis, and Carlo C. Maley. 2025. "Tethya wilhelma (Porifera) Is Highly Resistant to Radiation Exposure and Possibly Cancer" Biology 14, no. 2: 171. https://doi.org/10.3390/biology14020171
APA StyleFortunato, A., Taylor, J., Scirone, J., Seyedi, S., Aktipis, A., & Maley, C. C. (2025). Tethya wilhelma (Porifera) Is Highly Resistant to Radiation Exposure and Possibly Cancer. Biology, 14(2), 171. https://doi.org/10.3390/biology14020171





