Poor Sympathetic Compensation During Active Standing Increases the Risk of Morbidity–Mortality in the Post-Surgery of Patients with Severe Calcific Aortic Stenosis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Heart Rate Variability (HRV)
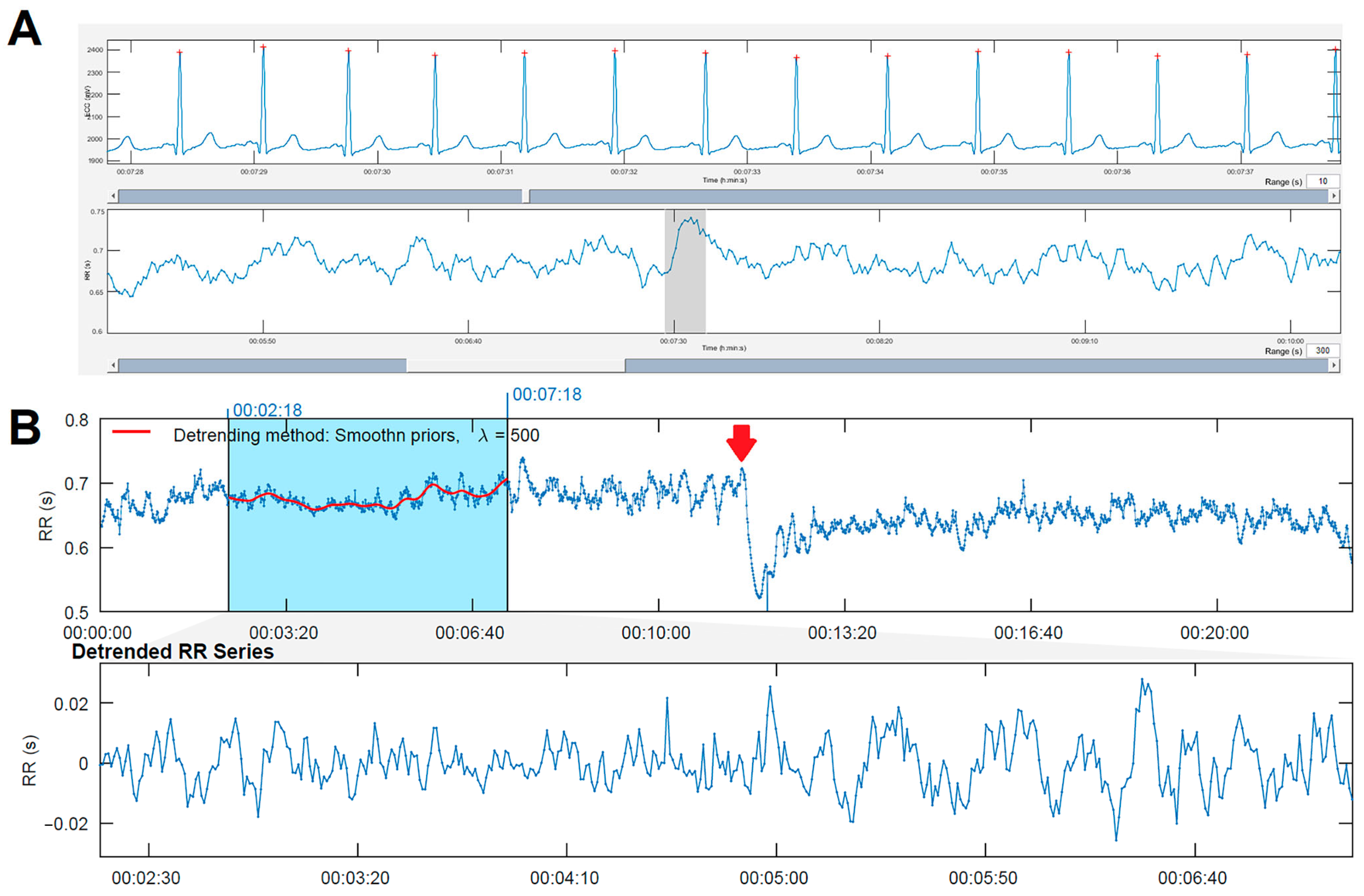
2.2.1. ECG Acquisition Protocol
2.2.2. Computational Analysis of HRV
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Perspectives
4.2. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osnabrugge, R.L.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.; Piazza, N.; Kappetein, A.P. Aortic stenosis in the elderly: Disease prevalence and number of candidates for transcatheter aortic valve replacement: A meta-analysis and modeling study. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lei, H.; Liu, L.; Xu, D. Lipoprotein(a), a Lethal Player in Calcific Aortic Valve Disease. Front. Cell Dev. Biol. 2022, 10, 812368. [Google Scholar] [CrossRef] [PubMed]
- Torres-Arellano, J.M.; Echeverría, J.C.; Ávila-Vanzzini, N.; Springall, R.; Toledo, A.; Infante, O.; Bojalil, R.; Cossío-Aranda, J.E.; Fajardo, E.; Lerma, C. Cardiac Autonomic Response to Active Standing in Calcific Aortic Valve Stenosis. J. Clin. Med. 2021, 10, 2004. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, J.C.; Ávila-Vanzzini, N.; Springall, R.; Torres-Arellano, J.M.; Toledo, A.; Infante, O.; Bojalil, R.; Cossío, J.; Fajardo, E.; Lerma, C. Inflammation and Reduced Parasympathetic Cardiac Modulation in Aortic-Valve Sclerosis. Appl. Sci. 2019, 9, 4020. [Google Scholar] [CrossRef]
- Żebrowski, J.J.; Kowalik, I.; Orłowska-Baranowska, E.; Andrzejewska, M.; Baranowski, R.; Gierałtowski, J. On the risk of aortic valve replacement surgery assessed by heart rate variability parameters. Physiol. Meas. 2015, 36, 163–175. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar]
- Barreto-Filho, J.A.; Wang, Y.; Dodson, J.A.; Desai, M.M.; Sugeng, L.; Geirsson, A.; Krumholz, H.M. Trends in aortic valve replacement for elderly patients in the United States, 1999–2011. JAMA 2013, 310, 2078–2085. [Google Scholar] [CrossRef]
- Maganti, M.D.; Rao, V.; Borger, M.A.; Ivanov, J.; David, T.E. Predictors of low cardiac output syndrome after isolated aortic valve surgery. Circulation 2005, 112, I448–I452. [Google Scholar] [CrossRef]
- Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381.
- Benichou, T.; Pereira, B.; Mermillod, M.; Tauveron, I.; Pfabigan, D.; Maqdasy, S.; Dutheil, F. Heart rate variability in type 2 diabetes mellitus: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0195166. [Google Scholar] [CrossRef]
- Dimitrow, P.P.; Sorysz, D. Orthostatic stress echocardiography as a useful test to measure variability of transvalvular pressure gradients in aortic stenosis. Cardiovasc. Ultrasound. 2013, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F.; Otto, C.M. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 372–392. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, M.P.; Niskanen, J.P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Software for advanced HRV analysis. Comput. Methods Programs Biomed. 2004, 76, 73–81. [Google Scholar]
- Barantke, M.; Krauss, T.; Ortak, J.; Lieb, W.; Reppel, M.; Burgdorf, C.; Pramstaller, P.P.; Schunkert, H.; Bonnemeier, H. Effects of gender and aging on differential autonomic responses to orthostatic maneuvers. J. Cardiovasc. Electrophysiol. 2008, 19, 1296–1303. [Google Scholar] [CrossRef]
- Reulecke, S.; Charleston-Villalobos, S.; Voss, A.; González-Camarena, R.; González-Hermosillo, J.; Gaitán-González, M.; Hernández-Pacheco, G.; Schroeder, R.; Aljama-Corrales, T. Dynamics of the cardiovascular autonomic regulation during orthostatic challenge is more relaxed in women. Biomed. Tech. 2018, 63, 139–150. [Google Scholar] [CrossRef]
- Reulecke, S.; Charleston-Villalobos, S.; Voss, A.; González-Camarena, R.; González-Hermosillo, J.; Gaitán-González, M.J.; Hernández-Pacheco, G.; Schroeder, R.; Aljama-Corrales, T. Orthostatic stress causes immediately increased blood pressure variability in women with vasovagal syncope. Comput. Methods Programs Biomed. 2016, 127, 185–196. [Google Scholar] [CrossRef]
- Hynynen, E.; Konttinen, N.; Kinnunen, U.; Kyröläinen, H.; Rusko, H. The incidence of stress symptoms and heart rate variability during sleep and orthostatic test. Eur. J. Appl. Physiol. 2011, 111, 733–741. [Google Scholar] [CrossRef]
- Steinfath, M.; Geertz, B.; Schmitz, W.; Scholz, H.; Haverich, A.; Breil, I.; Hanrath, P.; Reupcke, C.; Sigmund, M.; Lo, H.B. Distinct down-regulation of cardiac beta 1- and beta 2-adrenoceptors in different human heart diseases. Naunyn Schmiedeberg’s Arch. Pharmacol. 1991, 343, 217–220. [Google Scholar] [CrossRef]
- Michel, M.C.; Maisel, A.S.; Brodde, O.E. Mitigation of beta 1- and/or beta 2-adrenoceptor function in human heart failure. Br. J. Clin. Pharmacol. 1990, 30, 37s–42s. [Google Scholar] [CrossRef]
- Guzzetti, S.; Mennini, T.; Cagnotto, A.; Di Biasi, P.; Scrofani, R.; Mezzetti, S.; Cogliati, C.; Paglia, S.; Malliani, A. Myocardial beta-adrenergic and muscarinic receptor density in cardiac pressure or volume overload. J. Mol. Cell Cardio. 1998, 30, 2095–2102. [Google Scholar] [CrossRef]
- Hasking, G.J.; Esler, M.D.; Jennings, G.L.; Burton, D.; Johns, J.A.; Korner, P.I. Norepinephrine spillover to plasma in patients with congestive heart failure: Evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation 1986, 73, 615–621. [Google Scholar] [CrossRef]
- Hammond, H.K.; Roth, D.A.; Insel, P.A.; Ford, C.E.; White, F.C.; Maisel, A.S.; Ziegler, M.G.; Bloor, C.M. Myocardial beta-adrenergic receptor expression and signal transduction after chronic volume-overload hypertrophy and circulatory congestion. Circulation 1992, 85, 269–280. [Google Scholar] [CrossRef]
- Heilbrunn, S.M.; Shah, P.; Bristow, M.R.; Valantine, H.A.; Ginsburg, R.; Fowler, M.B. Increased beta-receptor density and improved hemodynamic response to catecholamine stimulation during long-term metoprolol therapy in heart failure from dilated cardiomyopathy. Circulation 1989, 79, 483–490. [Google Scholar] [CrossRef]
- Lemoine, H.; Schönell, H.; Kaumann, A.J. Contribution of beta 1- and beta 2-adrenoceptors of human atrium and ventricle to the effects of noradrenaline and adrenaline as assessed with (−)-atenolol. Br. J. Pharmacol. 1988, 95, 55–66. [Google Scholar] [CrossRef]
- Werner, B.; Piorecka-Makula, A.; Bobkowski, W. Heart rate variability in children with aortic valve stenosis—A pilot study. Arch. Med. Sci. 2013, 9, 535–539. [Google Scholar] [CrossRef]
- Rodriguez, J.; Blaber, A.P.; Kneihsl, M.; Trozic, I.; Ruedl, R.; Green, D.A.; Broadbent, J.; Xu, D.; Rössler, A.; Hinghofer-Szalkay, H.; et al. Poststroke alterations in heart rate variability during orthostatic challenge. Medicine 2017, 96, e5989. [Google Scholar] [CrossRef]
- Avila-Vanzzini, N.; Berrios-Barcenas, E.; Cossio-Aranda, J.; Herrera-Bello, H.; Rodriguez-Chavez, L.L.; Briseño-Diaz, N.M.; Gaspar-Hernandez, J. Body mass index is associated with low postoperative cardiac output in patients undergoing aortic valve replacement. Arch. Cardiol. Mex 2020, 90, 490–497. [Google Scholar] [CrossRef]
- Posada-Martinez, E.L.; Fritche-Salazar, J.F.; Arias-Godinez, J.A.; Ortiz-Leon, X.A.; Balderas-Muñoz, K.; Ruiz-Esparza, M.E.; Sánchez, E.A.; Sandoval, J.P.; Morales, A.K.T.; Rodriguez-Zanella, H. Right Ventricular Longitudinal Strain Predicts Low-Cardiac- Output Syndrome After Surgical Aortic Valve Replacement in Patients with Preserved and Mid-range Ejection Fraction. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1638–1645. [Google Scholar] [CrossRef]
- Orsinelli, D.A.; Aurigemma, G.P.; Battista, S.; Krendel, S.; Gaasch, W.H. Left ventricular hypertrophy and mortality after aortic valve replacement for aortic stenosis. A high-risk subgroup identified by preoperative relative wall thickness. J. Am. Coll. Cardiol. 1993, 22, 1679–1683. [Google Scholar] [CrossRef]
- Laks, M.M.; Morady, F. Norepinephrine—The myocardial hypertrophy hormone? Am. Heart J. 1976, 91, 674–675. [Google Scholar] [CrossRef]
- Andersson, U.; Tracey, K.J. Reflex principles of immunological homeostasis. Annu. Rev. Immunol. 2012, 30, 313–335. [Google Scholar] [CrossRef]
- Andersson, U.; Tracey, K.J. Neural reflexes in inflammation and immunity. J. Exp. Med. 2012, 209, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carbó, J.; Torres-Arellano, J.M.; Ávila-Vanzzini, N.; Springall, R.; Bojalil, R.; Infante, O.; Lerma, C.; Echeverría, J.C. Association of the Heart Rate Variability Response to Active Standing with the Severity of Calcific Aortic Valve Disease: Novel Insights of a Neurocardiovascular Pathology. J. Clin. Med. 2022, 11, 4771. [Google Scholar] [CrossRef] [PubMed]
- Kleczyński, P.; Dimitrow, P.P.; Dziewierz, A.; Wiktorowicz, A.; Rakowski, T.; Surdacki, A.; Dudek, D. Predictors of syncope in patients with severe aortic stenosis: The role of orthostatic unload test. Cardiol. J. 2020, 27, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Higashihara, T.; Fukuda, Y.; Nakano, T.; Takeda, A.; Morita, Y.; Ono, M.; Watanabe, N.; Sada, Y.; Ikenaga, H.; Utsunomiya, H.; et al. Left-atrial volume reduction reflects improvement of cardiac sympathetic nervous function in patients with severe aortic stenosis after transcatheter aortic valve replacement. Heart Vessels 2023, 38, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.R.; Astudillo, L.; Elmariah, S.; Purushothaman, K.R.; Purushothaman, M.; Lento, P.A.; Sharma, S.K.; Fuster, V.; Adams, D.H. Increased macrophage infiltration and neovascularization in congenital bicuspid aortic valve stenosis. J. Thorac. Cardiovasc. Surg. 2011, 142, 895–901. [Google Scholar] [CrossRef]
- Chan, K.L.; Ghani, M.; Woodend, K.; Burwash, I.G. Case-controlled study to assess risk factors for aortic stenosis in congenitally bicuspid aortic valve. Am. J. Cardiol. 2001, 88, 690–693. [Google Scholar] [CrossRef]
- Shimura, T.; Yamamoto, M.; Yamaguchi, R.; Adachi, Y.; Sago, M.; Tsunaki, T.; Kagase, A.; Koyama, Y.; Otsuka, T.; Yashima, F.; et al. OCEAN-TAVI investigators. Calculated plasma volume status and outcomes in patients undergoing transcatheter aortic valve replacement. ESC Heart Fail. 2021, 8, 1990–2001. [Google Scholar] [CrossRef]
| Variable | Description | Autonomic Interpretation |
|---|---|---|
| Time domain | ||
| SDNN (ms) | Standard deviation of RR intervals | Sympathetic and parasympathetic |
| RMSSD (ms) | Square root of the mean of the sum of the squares of differences between adjacent RR intervals | Parasympathetic |
| pNN50 (%) | Percentage of adjacent RR intervals differing by more than 50 ms | Parasympathetic |
| Frequency domain | ||
| LF (n.u.) | Mean power in the low-frequency range (0.04 to 0.15 Hz) | Sympathetic |
| HF (n.u.) | Mean power in the high-frequency range (0.15 to 0.4 Hz) | Parasympathetic and respiratory rate |
| LF/HF | LF-to-HF ratio | Sympathetic/parasympathetic ratio |
| Variables | All Patients (N = 49) | Group with Surgical Complications (N = 24) | Group Without Surgical Complications (N = 25) | p |
|---|---|---|---|---|
| Age (years) | 55 ± 12 | 57 ± 12 | 55 ± 14 | 0.582 |
| Male sex (%) | 30 (61) | 15 (62) | 15 (60) | 0.858 |
| Body mass index (kg/m2) | 27.9 ± 3.8 | 27.6 ± 4.2 | 28.4 ± 3.4 | 0.458 |
| Body surface area (m2) | 1.76 ± 0.15 | 1.76 ± 0.17 | 1.78 ± 0.15 | 0.700 |
| Body fat (%) | 30 ± 10 | 29 ± 11 | 32 ± 11 | 0.315 |
| Muscle mass (%) | 31 ± 5 | 32 ± 6 | 30 ± 5 | 0.216 |
| Visceral fat (%) | 10 (7, 13) | 10 (7, 12) | 11 (8, 14) | 0.309 |
| Obesity | 6 (12%) | 2 (8%) | 4 (16%) | 0.354 |
| Type 2 diabetes mellitus | 9 (18%) | 5 (21%) | 4 (16%) | 0.725 |
| Hypertension | 22 (44%) | 10 (41%) | 12 (48%) | 0.656 |
| Smoking | 17 (34%) | 8 (33%) | 9 (36%) | 0.845 |
| Dyslipidemia | 17 (34%) | 10 (41%) | 7 (28%) | 0.315 |
| Hypothyroidism | 3 (6%) | 1 (4%) | 2 (8%) | 0.516 |
| Supine position SBP (mmHg) | 110 (110, 130) | 110 (110, 130) | 120 (110, 130) | 0.887 |
| Active standing SBP (mmHg) | 110 (110, 130) | 110 (105, 125) | 120 (110, 130) | 0.383 |
| ACEI or ARB | 21 (43%) | 10 (42%) | 11 (44%) | 0.549 |
| Calcium antagonist | 4 (8%) | 2 (8%) | 2 (8%) | 1.000 |
| Statins | 19 (39%) | 7(29%) | 12 (48%) | 0.052 |
| Diuretics | 12 (24%) | 8 (33%) | 4 (16%) | 0.288 |
| Other | 25 (51%) | 13 (54%) | 12 (48%) | 0.310 |
| Euroscore scale | 0.64 ± 0.21 | 0.72 ± 0.27 | 0.57 ± 0.05 | 0.011 |
| Bivalve aorta | 21 (43%) | 10 (42%) | 11 (44%) | 0.869 |
| Variables | Morbidity–Mortality After Surgery | p | ||
|---|---|---|---|---|
| Total (N = 49) | Yes (N = 24) | No (N = 25) | ||
| Diastolic diameter (mm) | 44 ± 6 | 44 ± 7 | 44 ± 5 | 0.699 |
| Systolic diameter (mm) | 28 ± 6 | 27 ± 6 | 28 ± 6 | 0.388 |
| Left ventricle mass (gr/m2) | 113 ± 35 | 129 ± 36 | 98 ± 27 | 0.001 |
| End-diastolic volume (mL) | 80 (57, 101) | 80 (60, 119) | 71 (57, 94) | 0.555 |
| End-systolic volume (mL) | 23 (20, 36) | 22 (20, 36) | 25 (22, 36) | 0.342 |
| Left ventricle ejection fraction (%) | 61 ± 6 | 60 ± 7 | 62 ± 5 | 0.210 |
| Diastology | ||||
| Grade 1 Grade 2 Grade 3 | 35 (71%) 12 (24%) 2 (4%) | 15 (62%) 7 (29%) 2 (8%) | 20 (80%) 5 (20%) 0 (0%) | 0.144 |
| Left atrium vol (mL/m2) | 39 ± 11 | 40 ± 12 | 38 ± 11 | 0.642 |
| E/E′ | 11 (9, 14) | 12 (10, 15) | 10 (9, 13) | 0.189 |
| Right ventricle base (mm) | 36 ± 5 | 36 ± 5 | 36 ± 5 | 0.849 |
| Fractional shortening area (%) | 48 ± 8 | 47 ± 8 | 49 ± 9 | 0.582 |
| TASE (mm) | 22 (20, 25) | 23 (21, 28) | 22 (20, 24) | 0.340 |
| PSAP (mmHg) | 31 ± 9 | 32 ± 11 | 30 ± 6 | 0.447 |
| Vmax (m/s) | 5.0 (4.5, 5.5) | 5.1 (4.65, 5.8) | 4.9 (4.4, 5.2) | 0.279 |
| Aortic valve area (cm2) | 0.6 (0.5, 0.7) | 0.6 (0.4, 0.7) | 0.6 (0.6, 0.8) | 0.110 |
| Morbi-Mortality After Surgery | p | |||
|---|---|---|---|---|
| Variables | Total (N = 49) | Yes (N = 24) | No (N = 25) | |
| Extracorporeal circulation time (min) | 126 (104, 149) | 132 (99, 159) | 122 (105, 146) | 0.638 |
| Aortic clamping time (min) | 98 ± 25 | 101 ± 28 | 95 ± 22 | 0.397 |
| Extubation (h) | 12 (6, 24) | 24 (13, 40) | 7 (6, 12) | <0.001 |
| Complication or Death (N = 24) | Without Complication or Death (N = 25) | |||||
|---|---|---|---|---|---|---|
| Variable | Supine Position | Active Standing | Change (Δ) | Supine Position | Active Standing | Change (Δ) |
| Mean RR (ms) | 875 ± 139 | 823 ± 124 ** | 52.9 ± 60.9 | 908 ± 161 | 798 ± 171 ** | 110.2 ± 80.7 & |
| SDNN (ms) | 24.3 ± 15.9 | 204 ± 10.0 | 3.9 ± 8.9 | 29.0 ± 34.0 | 28.8 ± 46.4 | 0.2 ± 14.3 |
| RMSSD (ms) | 19 (14, 26) | 13 (11, 22) ** | 3.4 (0.9, 7.5) | 21 (15, 32) | 14 (9, 26) * | 3.8 (−0.41, 11.6) |
| PNN50 (%) | 1.0 (0.0, 4.1) | 0.3(0.0, 2.0) * | 0.50 (0.0, 2.4) | 1.0 (0.0, 6.5) | 0.0 (0.0, 6.5) * | 0.2 (0.0, 3.2) |
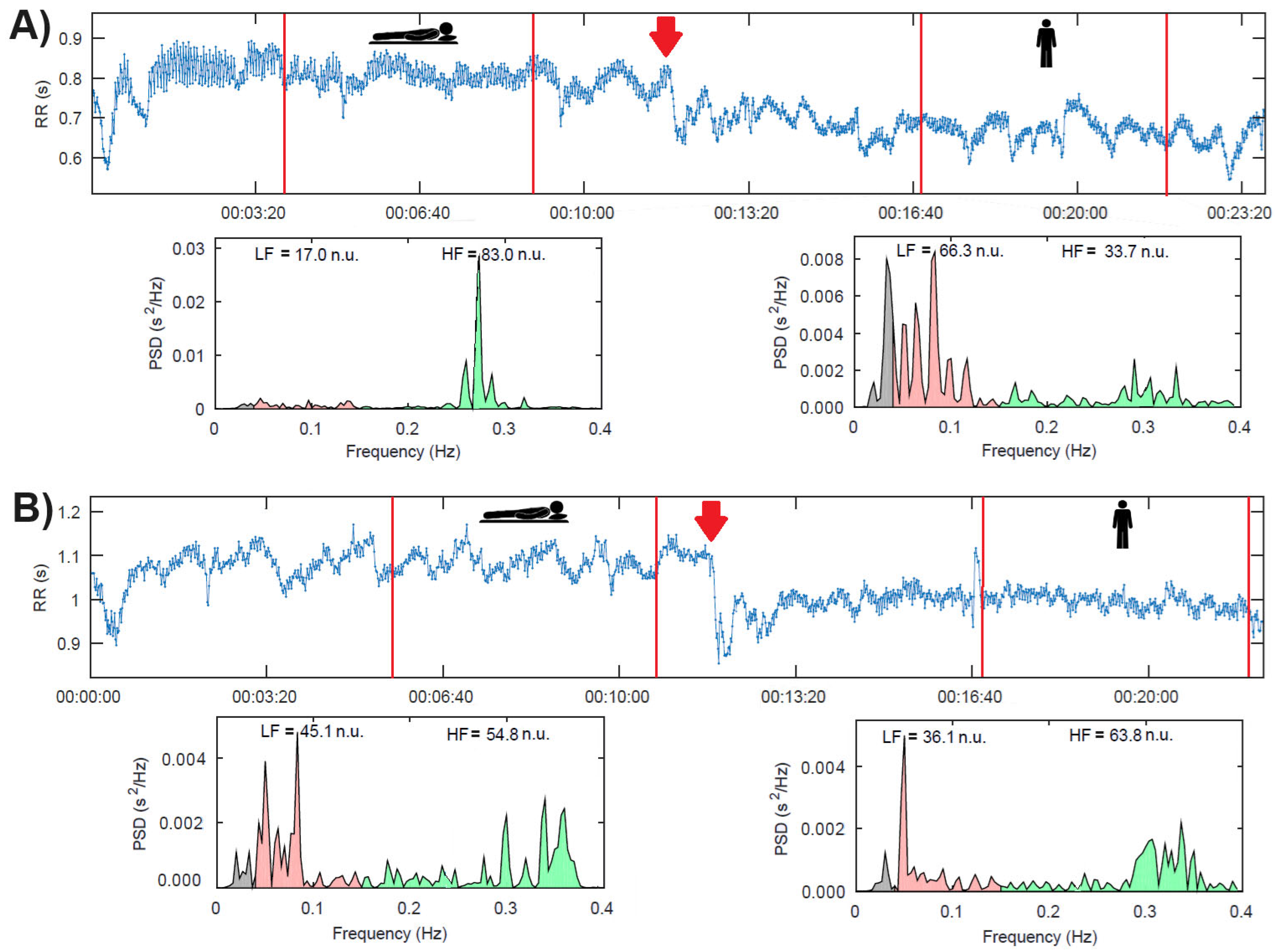
| LF (n.u.) | 59 (39, 71) | 60 (43, 79) | −4.4 (−18.8, 6.20) | 60 (47, 67) | 70 (59, 72) * | −6.60 (−28.3, 5.0) |
| HF (n.u.) | 39 (27, 60) | 38 (20, 56) | 4.2 (−6.1, 18.4) | 39 (32, 52) | 29 (27, 39) * | 6.5 (−5.0, 28.0) |
| LF/HF ratio | 1.51 (0.66, 2.5) | 1.58 (0.77, 3.81) | −0.39 (−1.07, 0.24) | 1.54 (0.89, 2.0) | 2.34 (1.50, 2.68) | −0.87 (−1.65, 0.37) |
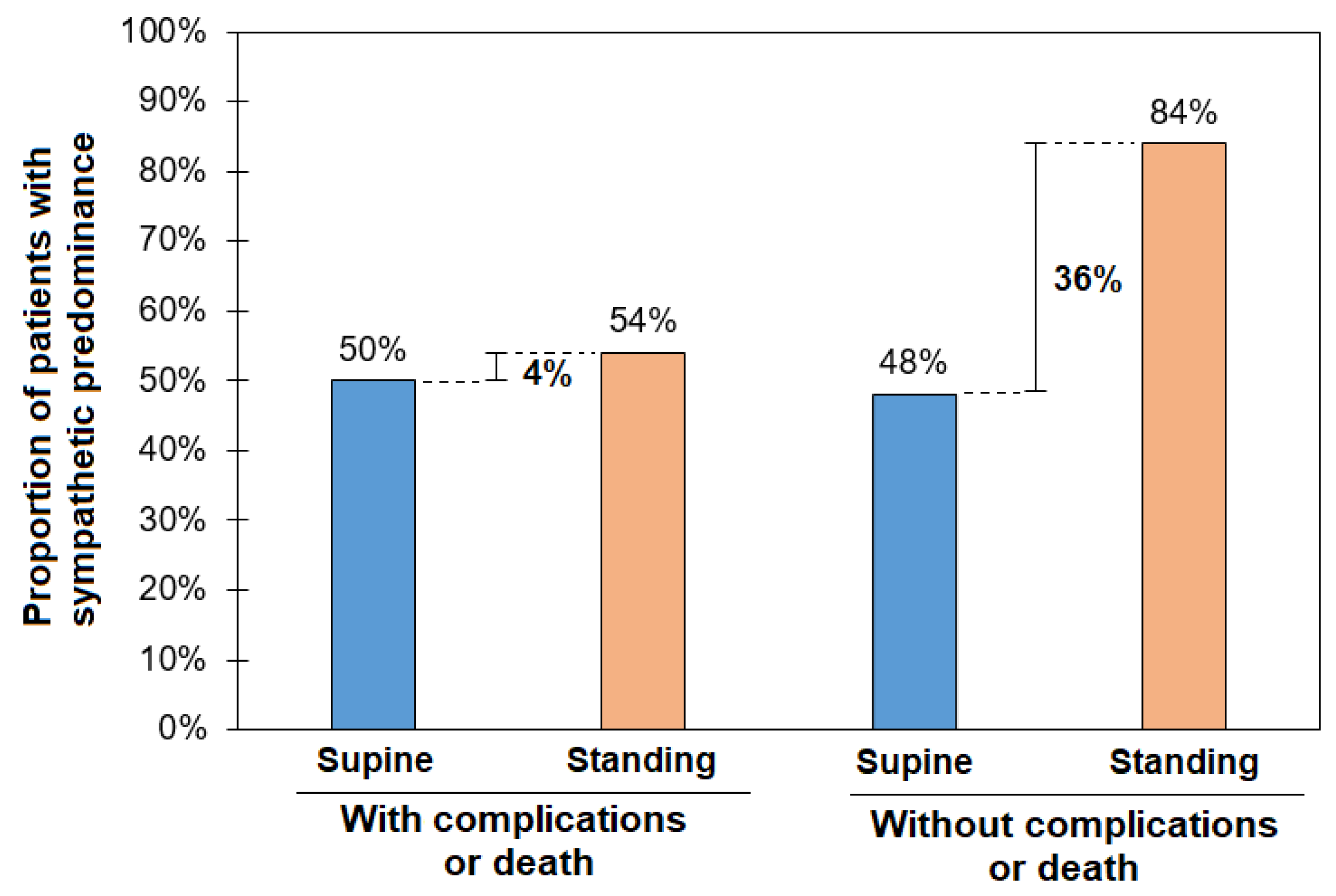
| SP (LF > 60 nu) | 12 (50%) | 13 (54%) | 1 (4.1%) | 12 (48%) | 21 (84%) ** | 9 (36%) & |
| Bivariate Regression | Multivariate Regression Model 1 | Multivariate Regression Model 2 | ||||
|---|---|---|---|---|---|---|
| Variable | OR (CI, 95%) | p | OR (CI, 95%) | p | OR (CI, 95%) | p |
| Mean RR (delta) | 0.987 (0.976, 0.997) | 0.01 | 0.99 (0.98, 1.00) | 0.061 | ||
| RMSSD delta | 1.03 (0.97, 1.09) | 0.30 | ||||
| pNN50 delta | 0.981 (0.919, 1.04) | 0.55 | ||||
| LF delta | 1.01 (0.987, 1.04) | 0.32 | ||||
| HF delta | 0.987 (0.962, 1.01) | 0.32 | ||||
| LF/HF delta | 1.005 (0.783, 1.29) | 0.96 | ||||
| SP standing (LF > 60 nu) | 5.25 (1.38, 19.9) | 0.02 | 4.82 (1.066, 21.8) | 0.04 | ||
| Left ventricular mass (gr/m2) | 1.03 (1.01, 1.05) | <0.01 | 1.03 (1.007, 1.06) | 0.01 | 1.03 (1.01, 1.06) | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila-Vanzzini, N.; Santana-Ortiz, A.; Sánchez-Estrada, D.; Springall, R.; Lerma, A.; Herrera-Bello, H.; Calderón-Juárez, M.; Lerma, C. Poor Sympathetic Compensation During Active Standing Increases the Risk of Morbidity–Mortality in the Post-Surgery of Patients with Severe Calcific Aortic Stenosis. Biology 2025, 14, 146. https://doi.org/10.3390/biology14020146
Avila-Vanzzini N, Santana-Ortiz A, Sánchez-Estrada D, Springall R, Lerma A, Herrera-Bello H, Calderón-Juárez M, Lerma C. Poor Sympathetic Compensation During Active Standing Increases the Risk of Morbidity–Mortality in the Post-Surgery of Patients with Severe Calcific Aortic Stenosis. Biology. 2025; 14(2):146. https://doi.org/10.3390/biology14020146
Chicago/Turabian StyleAvila-Vanzzini, Nydia, Anayanci Santana-Ortiz, Daniela Sánchez-Estrada, Rashidi Springall, Abel Lerma, Héctor Herrera-Bello, Martín Calderón-Juárez, and Claudia Lerma. 2025. "Poor Sympathetic Compensation During Active Standing Increases the Risk of Morbidity–Mortality in the Post-Surgery of Patients with Severe Calcific Aortic Stenosis" Biology 14, no. 2: 146. https://doi.org/10.3390/biology14020146
APA StyleAvila-Vanzzini, N., Santana-Ortiz, A., Sánchez-Estrada, D., Springall, R., Lerma, A., Herrera-Bello, H., Calderón-Juárez, M., & Lerma, C. (2025). Poor Sympathetic Compensation During Active Standing Increases the Risk of Morbidity–Mortality in the Post-Surgery of Patients with Severe Calcific Aortic Stenosis. Biology, 14(2), 146. https://doi.org/10.3390/biology14020146






