Understanding Mantle Edge Pigmentation Through Comprehensive Transcriptomic Profiling of the Chilean Oyster (Ostrea chilensis)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
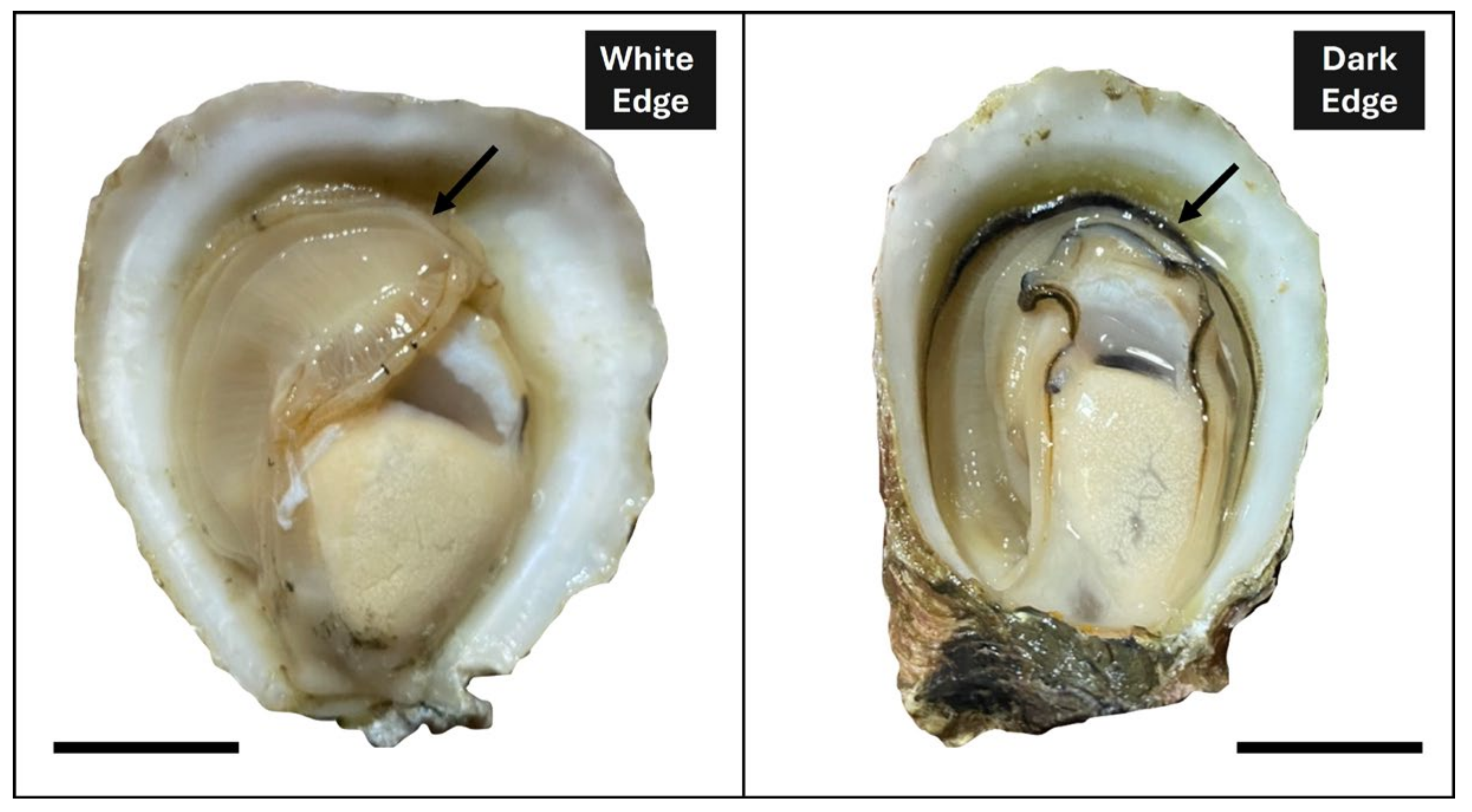
2.1. Oysters Maintenance and Experimental Design
2.2. Library Construction and RNA Sequencing
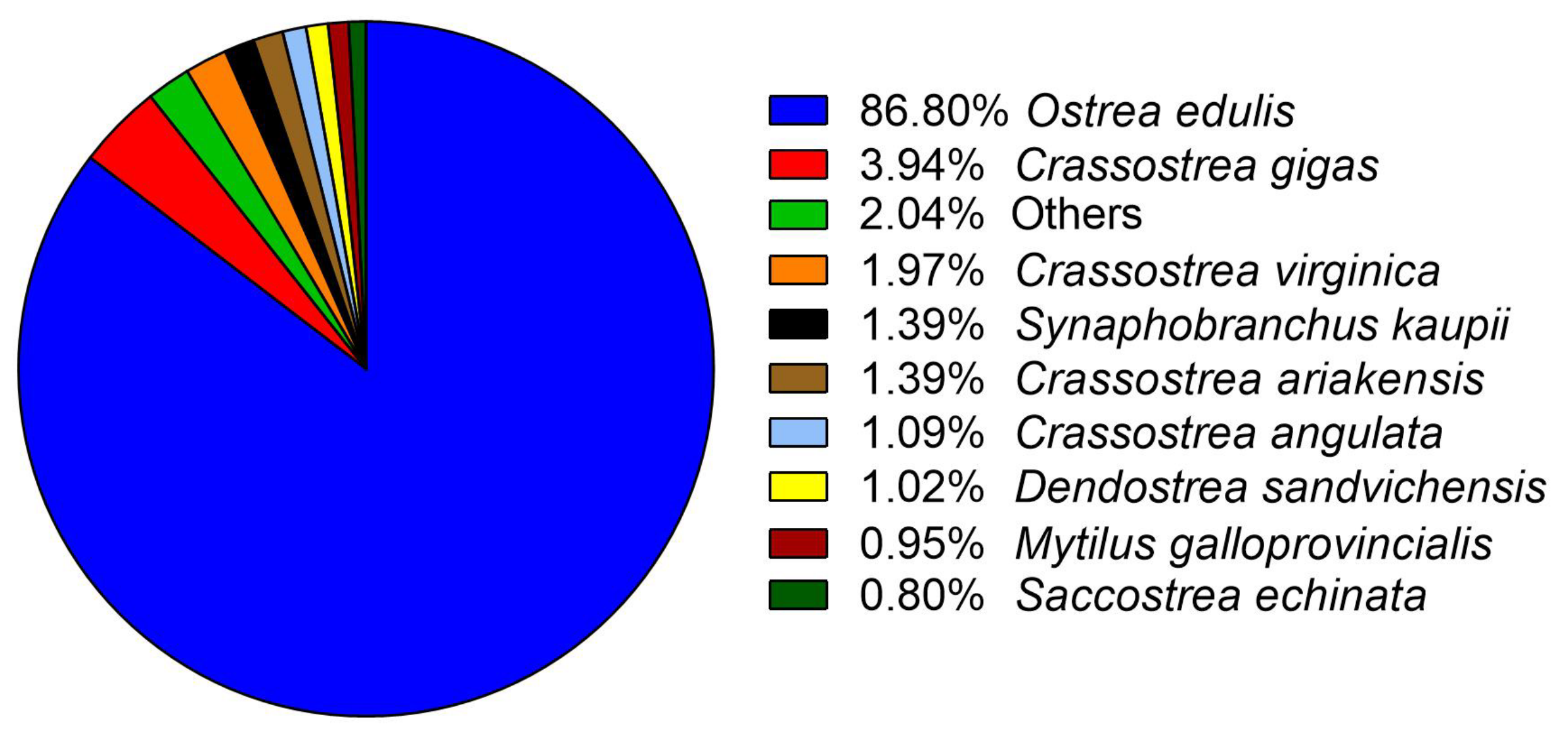
2.3. Transcriptome De Novo Assembly and Annotation
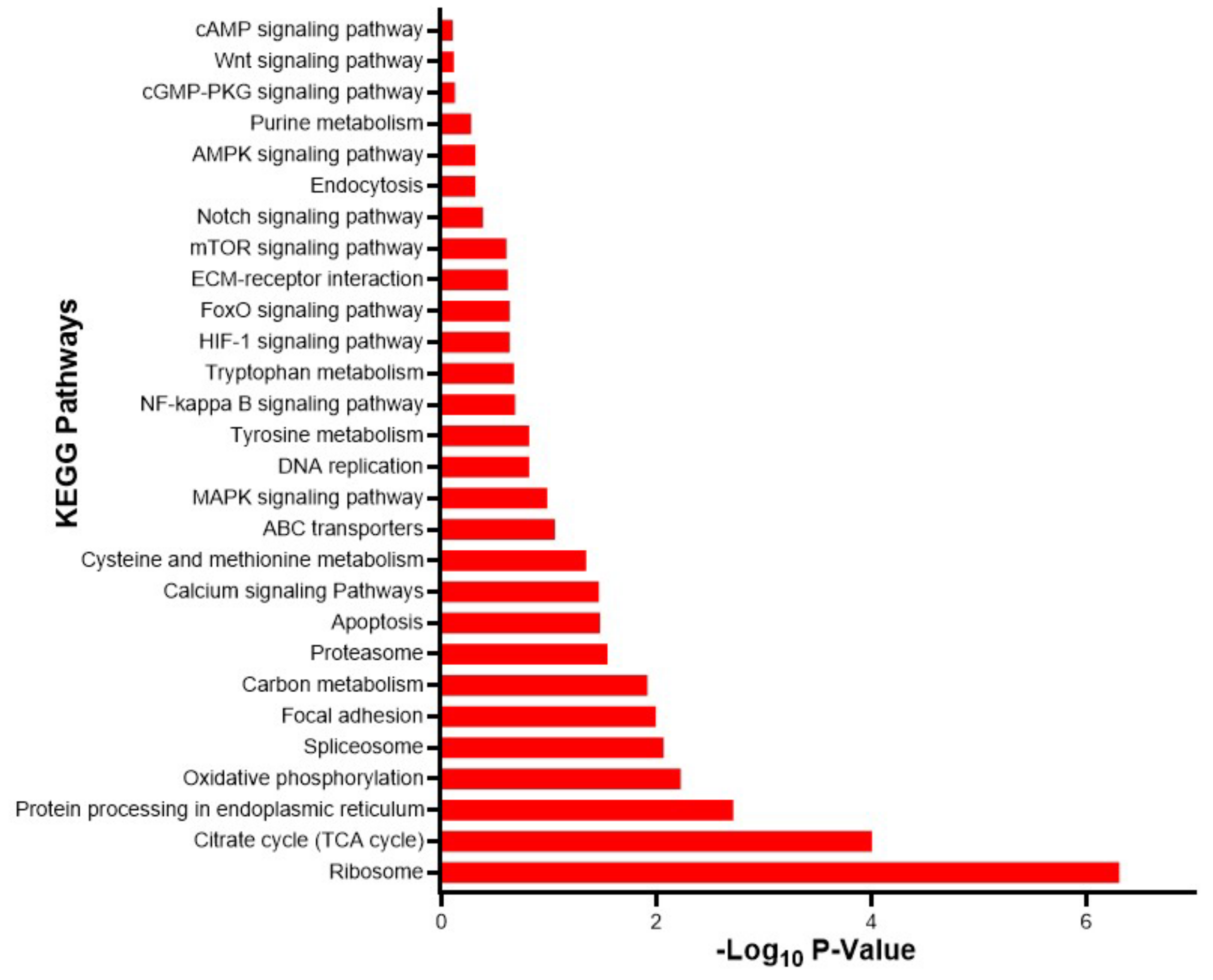
2.4. Differential Expression and GO Enrichment Analyses
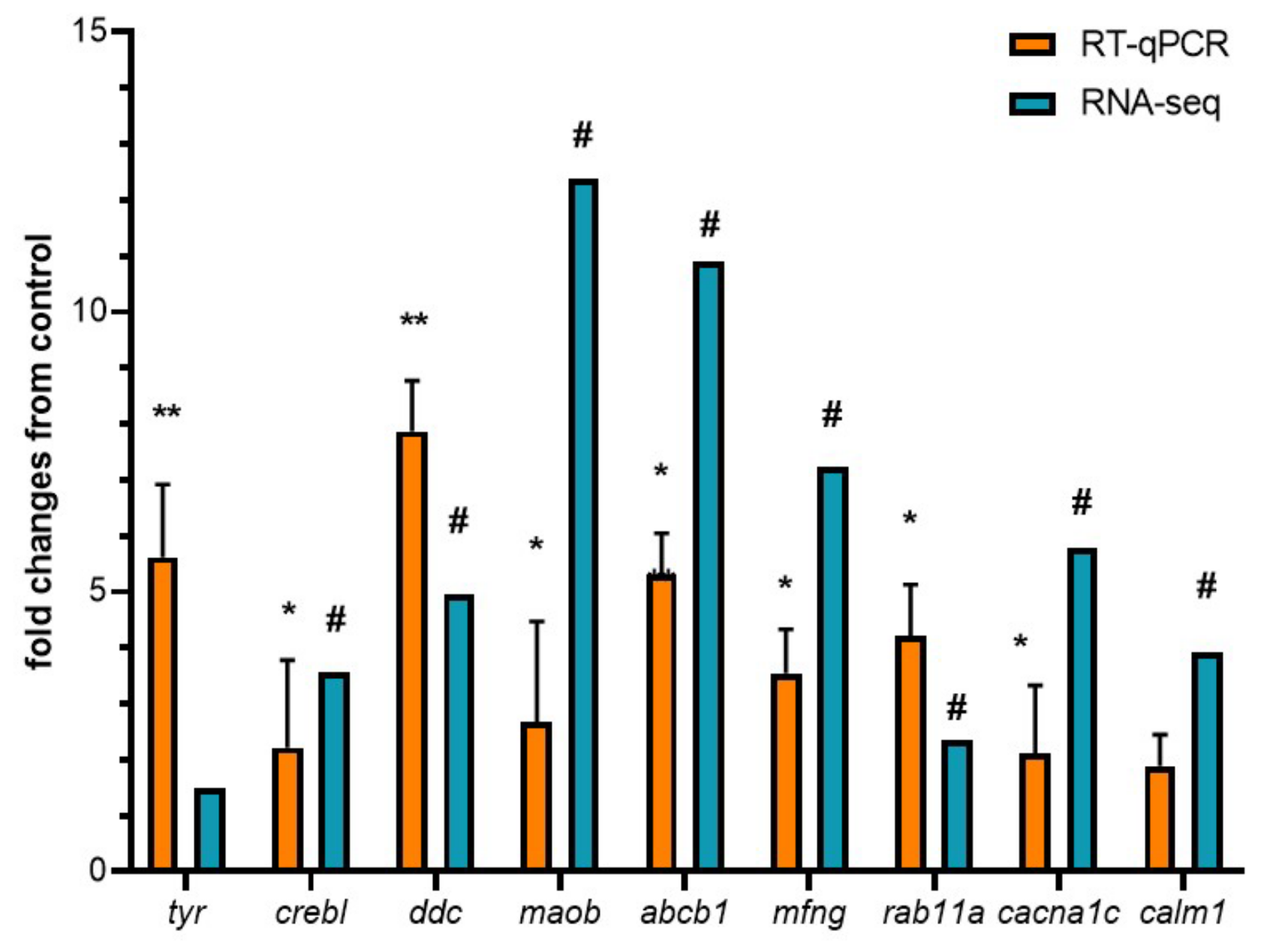
2.5. RNA-Seq Validation by Real-Time PCR
2.6. Statistical Analysis
3. Results
3.1. De Novo Assembly and Annotation of Ostrea Chilensis Transcriptome
3.2. Assessment of Differentially Expressed Transcripts and Validation of Transcriptomic Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chanley, P.; Dinamani, P. Comparative Descriptions of Some Oyster Larvae from New Zealand and Chile, and a Description of a New Genus of Oyster, Tiostrea. N. Z. J. Mar. Freshw. Res. 1980, 14, 103–120. [Google Scholar] [CrossRef]
- Foighil, D.Ó.; Marshall, B.A.; Hilbish, T.J.; Pino, M.A. Trans-Pacific Range Extension by Rafting Is Inferred for the Flat Oyster Ostrea chilensis. Biol. Bull. 1999, 196, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Oyarzún, P.A.; Hidalgo-Cabrera, A.; Stam, G.; Estrada, J.M.; Ruiz-Tagle, G.; Navarro, J.M.; Toro, J.E. Settlement of Chilean Flat Oysters (Ostrea chilensis) on Ribbed Mussel Shell Collectors for Commercial Grow-Out: Towards Sustainable Management. JMSE 2024, 12, 1148. [Google Scholar] [CrossRef]
- Toro, J.; Chaparro, O. Conocimiento Biológico de Ostrea chilensis Philippi 1845. Impacto y Perspectivas En El Desarrollo de La Ostricultura En Chile. Cultiv. Moluscos América Latina. CIID/CANADA Ed. Guadalupe Bogotá 1990, 0, 231–264. [Google Scholar]
- Toro, J.E.; Oyarzún, P.A.; Toledo, F.E.; Navarro, J.M.; Illesca, A.F.; Gardner, J.P.A. Genetic Structure and Diversity of the Chilean Flat Oyster Ostrea chilensis (Bivalvia: Ostreidae) along Its Natural Distribution from Natural Beds Subject to Different Fishing Histories. Genet. Mol. Biol. 2022, 45, e20210214. [Google Scholar] [CrossRef]
- Toro, J.E.; Sanhueza, M.A.; Winter, J.E.; Senn, C.M.; Aguila, P.; Vergara, A.M. Environmental Effects on the Growth of the Chilean Oyster Ostrea chilensis in Five Mariculture Locations in the Chiloé Island, Southern Chile. Aquaculture 1995, 136, 153–164. [Google Scholar] [CrossRef]
- Toro, J.E.; Aguila, P.R. Genetic Differentiation of Populations of the Oyster Ostrea chilensis in Southern Chile. Aquat. Living Resour. 1996, 9, 75–78. [Google Scholar] [CrossRef]
- Sernapesca (2023) Anuario Estadistico de Pesquera y Acuicultura 2023. Available online: https://www.sernapesca.cl/informacion-utilidad/anuarios-estadisticos-de-pesca-y-acuicultura/ (accessed on 16 May 2024).
- ADUANA (2023) Estadistica Comercial Del Servicio Nacional de Aduana de Chile. 2023. Available online: https://www.aduana.cl/aduana/site/edic/base/port/estadisticas.html (accessed on 28 September 2024).
- Fundación Chinquihue. Estudio Para Recuperar e Incrementar La Producción y Mercados de La Ostra Chilena, Ostrea chilensis, Como Una Vía de Diversificación de Las Actividades Productivas de La Pesca Artesanal de La Xa Región; Videla, V., Tilleria, J., Leal, M., Escalona, C., Valencia, J., Eds.; Gobierno Regional X Región de Los Lagos: Los Lagos, Chile, 2010; 342p. [Google Scholar]
- Navarro, J.M.; Oyarzún, P.A.; Haarmann, V.; Toro, J.E.; Garrido, C.; Valenzuela, A.; Pizarro, G. Feeding Response and Dynamic of Intoxication and Detoxification in Two Populations of the Flat Oyster Ostrea chilensis Exposed to Paralytic Shellfish Toxins (PST). Mar. Environ. Res. 2022, 177, 105634. [Google Scholar] [CrossRef]
- Couyoumdjian, J.R. El Mar y El Paladar: El Consumo de Pescados y Mariscos En Chile Desde La Independencia Hasta 1930. Historia 2009, 42, 57–107. [Google Scholar] [CrossRef]
- Kang, J.-H.; Kang, H.-S.; Lee, J.-M.; An, C.-M.; Kim, S.-Y.; Lee, Y.-M.; Kim, J.-J. Characterizations of Shell and Mantle Edge Pigmentation of a Pacific Oyster, Crassostrea gigas, in Korean Peninsula. Asian Australas. J. Anim. Sci 2013, 26, 1659–1664. [Google Scholar] [CrossRef]
- Brake, J.; Evans, F.; Langdon, C. Evidence for Genetic Control of Pigmentation of Shell and Mantle Edge in Selected Families of Pacific Oysters, Crassostrea gigas. Aquaculture 2004, 229, 89–98. [Google Scholar] [CrossRef]
- Jeffs, A.G. Gametogenic Cycle of the Chilean Oyster, Tiostrea chilensis (Philippi, 1845), in North-Eastern New Zealand. Invertebr. Reprod. Dev. 1998, 34, 109–116. [Google Scholar] [CrossRef]
- Jeffs, A.G.; Hickman, R.W. Reproductive Activity in a Pre-Epizootic Wild Population of the Chilean Oyster, Ostrea chilensis, from Southern New Zealand. Aquaculture 2000, 183, 241–253. [Google Scholar] [CrossRef]
- Brown, S.; Handley, S.; Michael, K.; Schiel, D. Annual Pattern of Brooding and Settlement in a Population of the Flat Oyster Ostrea chilensis from Central New Zealand. N. Z. J. Mar. Freshw. Res. 2010, 44, 217–227. [Google Scholar] [CrossRef][Green Version]
- Chaparro, O.R.; Mardones-Toledo, D.A.; Gray, M.W.; Cubillos, V.M.; Navarro, J.M.; Salas-Yanquin, L.P. Female–Embryo Relationships in Ostrea chilensis: Brooding, Embryo Recognition, and Larval Hatching. Mar. Biol. 2019, 166, 10. [Google Scholar] [CrossRef]
- Winter, J.E.; Acevedo, M.A.; Navarro, J.M. Quempillén Estuary, an Experimental Oyster Cultivation Station in Southern Chile. Energy Balance in Ostrea chilensis. Mar. Ecol. Prog. Ser. 1984, 20, 151–164. [Google Scholar] [CrossRef]
- Toro, J.E.; González, C.P. La Estructura Genética de La Ostra Chilena (Ostrea chilensis Philippi, 1845) En Poblaciones Naturales Del Sur de Chile, Basada En Análisis Con Marcadores RAPDs. Rev. Biol. Mar. Ocean. 2009, 44, 467–476. [Google Scholar] [CrossRef]
- Guo, X.-Z.; Wei, K.-J.; Yan, R.-J.; Gardner, J.P.A. Pronounced Mitochondrial DNA Population Genetic Structure in a Brooding Coastal Marine Invertebrate. Malacologia 2022, 65, 113–136. [Google Scholar] [CrossRef]
- Valenzuela, A.; Oyarzún, P.A.; Toro, J.E.; Navarro, J.M.; Ramírez, O.; Farias, A. Proximal and Fatty Acid Analysis in Ostrea chilensis, Crassostrea gigas and Mytilus Chilensis (Bivalvia: Mollusca) from Southern Chile. PLoS ONE 2022, 17, e0270825. [Google Scholar] [CrossRef]
- Sundaray, J.K.; Dixit, S.; Rather, A.; Rasal, K.D.; Sahoo, L. Aquaculture Omics: An Update on the Current Status of Research and Data Analysis. Mar. Genom. 2022, 64, 100967. [Google Scholar] [CrossRef]
- Chandhini, S.; Rejish Kumar, V.J. Transcriptomics in Aquaculture: Current Status and Applications. Rev. Aquac. 2019, 11, 1379–1397. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Shi, C.; Li, Y.; Li, Q.; Liu, S. Transcriptome Profiling of the Pacific Oyster (Crassostrea gigas) Suggests Distinct Host Immune Strategy in Response to Vibrio Alginolyticus Infection. Aquaculture 2022, 560, 738563. [Google Scholar] [CrossRef]
- Zhang, F.; Hu, B.; Fu, H.; Jiao, Z.; Li, Q.; Liu, S. Comparative Transcriptome Analysis Reveals Molecular Basis Underlying Fast Growth of the Selectively Bred Pacific Oyster, Crassostrea gigas. Front. Genet. 2019, 10, 610. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Li, Q.; Yu, H.; Zhao, X.; Kong, L. Comparative Transcriptome Analysis of the Pacific Oyster Crassostrea gigas Characterized by Shell Colors: Identification of Genetic Bases Potentially Involved in Pigmentation. PLoS ONE 2015, 10, e0145257. [Google Scholar] [CrossRef]
- Jiang, K.; Xu, C.; Yu, H.; Kong, L.; Liu, S.; Li, Q. Transcriptomic and Physiological Analysis Reveal Melanin Synthesis-Related Genes and Pathways in Pacific Oysters (Crassostrea gigas). Mar. Biotechnol. 2024, 26, 364–379. [Google Scholar] [CrossRef]
- Bean, T.P.; Khatir, Z.; Lyons, B.P.; Van Aerle, R.; Minardi, D.; Bignell, J.P.; Smyth, D.; Giraldes, B.W.; Leitão, A. De Novo Transcriptome Assembly of the Qatari Pearl Oyster Pinctada Imbricata Radiata. Mar. Genom. 2020, 51, 100734. [Google Scholar] [CrossRef]
- Gong, J.; Li, Q.; Yu, H.; Liu, S.; Kong, L. First de Novo Transcriptome Assembly of Iwagaki Oyster, Crassostrea nippona, and Comparative Evolutionary Analysis of Salinity-Stress Response Genes in Crassostrea Oysters. Mar. Genom. 2021, 56, 100805. [Google Scholar] [CrossRef]
- Entizne, J.C.; Guo, W.; Calixto, C.P.G.; Spensley, M.; Tzioutziou, N.; Zhang, R.; Brown, J.W.S. TranSuite: A Software Suite for Accurate Translation and Characterization of Transcripts. BioRxiv 2020. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for Clustering the next-Generation Sequencing Data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness with Single-Copy Orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S. Blast2GO: A Comprehensive Suite for Functional Analysis in Plant Genomics. Int. J. Plant Genom. 2008, 2008, 619832. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.C. QGENE: Software for Marker-Based Genomic Analysis and Breeding. Mol. Breed. 1997, 3, 239–245. [Google Scholar] [CrossRef]
- Ferreira, C.P.; Moreira, R.S.; Bastolla, C.L.V.; Saldaña-Serrano, M.; Lima, D.; Gomes, C.H.A.M.; Bainy, A.C.D.; Lüchmann, K.H. Transcriptomic Investigation and Biomarker Discovery for Zinc Response in Oysters Crassostrea Gasar. Mar. Genom. 2024, 75, 101109. [Google Scholar] [CrossRef]
- Lim, H.-J.; Lim, J.-S.; Lee, J.-S.; Choi, B.-S.; Kim, D.-I.; Kim, H.-W.; Rhee, J.-S.; Choi, I.-Y. Transcriptome Profiling of the Pacific Oyster Crassostrea gigas by Illumina RNA-Seq. Genes Genom. 2016, 38, 359–365. [Google Scholar] [CrossRef]
- Jones, D.B.; Jerry, D.R.; Forêt, S.; Konovalov, D.A.; Zenger, K.R. Genome-Wide SNP Validation and Mantle Tissue Transcriptome Analysis in the Silver-Lipped Pearl Oyster, Pinctada Maxima. Mar. Biotechnol. 2013, 15, 647–658. [Google Scholar] [CrossRef]
- Sun, J.; Chen, M.; Fu, Z.; Yu, G.; Ma, Z.; Xing, Y. Transcriptome Analysis of the Mantle Tissue of Pinctada Fucata with Red and Black Shells under Salinity Stress. Gene 2022, 823, 146367. [Google Scholar] [CrossRef]
- Ulagesan, S.; Krishnan, S.; Nam, T.-J.; Choi, Y.-H. A Review of Bioactive Compounds in Oyster Shell and Tissues. Front. Bioeng. Biotechnol. 2022, 10, 913839. [Google Scholar] [CrossRef]
- Mizuta, D.D.; Wikfors, G.H. Seeking the Perfect Oyster Shell: A Brief Review of Current Knowledge. Rev. Aquac. 2019, 11, 586–602. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, X.; Xu, M.; Liu, H.; Xu, H.; He, M. The Role of Smad1/5 in Mantle Immunity of the Pearl Oyster Pinctada Fucata Martensii. Fish Shellfish Immunol. 2021, 113, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, O.V.; Skiteva, O.I.; Voronezhskaya, E.E.; Dyachuk, V.A. Nervous System Development in the Pacific Oyster, Crassostrea gigas (Mollusca: Bivalvia). Front. Zool. 2018, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga-Soto, N.; Pinto-Borguero, I.; Quevedo, C.; Aguilera, F. Secretory and Transcriptomic Responses of Mantle Cells to Low pH in the Pacific Oyster (Crassostrea gigas). Front. Mar. Sci. 2023, 10, 1156831. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Q.; Yu, H.; Liu, S. Pigment Distribution and Secretion in the Mantle of the Pacific Oyster (Crassostrea gigas). J. Ocean Univ. China 2023, 22, 813–820. [Google Scholar] [CrossRef]
- Song, X.; Liu, Z.; Wang, L.; Song, L. Recent Advances of Shell Matrix Proteins and Cellular Orchestration in Marine Molluscan Shell Biomineralization. Front. Mar. Sci. 2019, 6, 41. [Google Scholar] [CrossRef]
- Han, Y.; Xie, C.; Fan, N.; Song, H.; Wang, X.; Zheng, Y.; Zhang, M.; Liu, Y.; Huang, B.; Wei, L.; et al. Identification of Melanin in the Mantle of the Pacific Oyster Crassostrea gigas. Front. Mar. Sci. 2022, 9, 880337. [Google Scholar] [CrossRef]
- Xing, D.; Li, Q.; Kong, L.; Yu, H. Heritability Estimate for Mantle Edge Pigmentation and Correlation with Shell Pigmentation in the White-Shell Strain of Pacific Oyster, Crassostrea gigas. Aquaculture 2018, 482, 73–77. [Google Scholar] [CrossRef]
- Feng, D.; Li, Q.; Yu, H.; Kong, L.; Du, S. Transcriptional Profiling of Long Non-Coding RNAs in Mantle of Crassostrea gigas and Their Association with Shell Pigmentation. Sci. Rep. 2018, 8, 1436. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, G.; He, M. LncRNA7467 Participated in Shell Biomineralization in Pearl Oyster Pinctada Fucata Martensii. Aquac. Rep. 2022, 27, 101398. [Google Scholar] [CrossRef]
- Peng, M.; Cardoso, J.C.R.; Pearson, G.; Vm Canário, A.; Power, D.M. Core Genes of Biomineralization and Cis-Regulatory Long Non-Coding RNA Regulate Shell Growth in Bivalves. J. Adv. Res. 2024, 64, 117–129. [Google Scholar] [CrossRef]
- Stenger, P.-L.; Ky, C.-L.; Reisser, C.; Duboisset, J.; Dicko, H.; Durand, P.; Quintric, L.; Planes, S.; Vidal-Dupiol, J. Molecular Pathways and Pigments Underlying the Colors of the Pearl Oyster Pinctada margaritifera Var. Cumingii (Linnaeus 1758). Genes 2021, 12, 421. [Google Scholar] [CrossRef]
- Nagatsu, T.; Nakashima, A.; Watanabe, H.; Ito, S.; Wakamatsu, K. Neuromelanin in Parkinson’s Disease: Tyrosine Hydroxylase and Tyrosinase. IJMS 2022, 23, 4176. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Q.; Yu, H.; Liu, S.; Kong, L. Shell Biosynthesis and Pigmentation as Revealed by the Expression of Tyrosinase and Tyrosinase-like Protein Genes in Pacific Oyster (Crassostrea gigas) with Different Shell Colors. Mar. Biotechnol. 2021, 23, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Cesura, A.M. Monoamine Oxidase B. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–10. ISBN 978-0-08-055232-3. [Google Scholar] [CrossRef]
- Segura-Aguilar, J. Dopamine Synthesis. In Clinical Studies and Therapies in Parkinson’s Disease; Elsevier: Amsterdam, The Netherlands, 2021; pp. 187–193. ISBN 978-0-12-822120-4. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Cross, P.J.; Dobson, R.C.J.; Adams, L.E.; Savka, M.A.; Hudson, A.O. A Three-Ring Circus: Metabolism of the Three Proteogenic Aromatic Amino Acids and Their Role in the Health of Plants and Animals. Front. Mol. Biosci. 2018, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Steven, A.; Friedrich, M.; Jank, P.; Heimer, N.; Budczies, J.; Denkert, C.; Seliger, B. What Turns CREB on? And off? And Why Does It Matter? Cell. Mol. Life Sci. 2020, 77, 4049–4067. [Google Scholar] [CrossRef]
- Jiang, K.; Yu, H.; Kong, L.; Liu, S.; Li, Q. Molecular Characterization of Transcription Factor CREB3L2 and CREB3L3 and Their Role in Melanogenesis in Pacific Oysters (Crassostrea gigas). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2024, 273, 110970. [Google Scholar] [CrossRef]
- Auffret, P.; Le Luyer, J.; Sham Koua, M.; Quillien, V.; Ky, C.-L. Tracing Key Genes Associated with the Pinctada Margaritifera Albino Phenotype from Juvenile to Cultured Pearl Harvest Stages Using Multiple Whole Transcriptome Sequencing. BMC Genom. 2020, 21, 662. [Google Scholar] [CrossRef]
- Sun, X.; Yang, A.; Wu, B.; Zhou, L.; Liu, Z. Characterization of the Mantle Transcriptome of Yesso Scallop (Patinopecten Yessoensis): Identification of Genes Potentially Involved in Biomineralization and Pigmentation. PLoS ONE 2015, 10, e0122967. [Google Scholar] [CrossRef]

| Category | Go Term | Gene Number | p-Value |
|---|---|---|---|
| Biological Process | translation | 19 | 1.19 × 10−10 |
| protein folding | 14 | 2.69 × 10−10 | |
| tricarboxylic acid cycle | 7 | 4.08 × 10−06 | |
| mRNA splicing, via spliceosome | 9 | 2.96 × 10−04 | |
| cytoplasmic translation | 5 | 4.68 × 10−04 | |
| Cellular Component | cytosolic large ribosomal subunit | 12 | 2.79 × 10−11 |
| ribosome | 18 | 2.97 × 10−10 | |
| ribonucleoprotein complex | 20 | 4.98 × 10−10 | |
| cytosol | 33 | 6.36 × 10−10 | |
| cytosolic small ribosomal subunit | 8 | 5.68 × 10−07 | |
| Molecular Function | ATP binding | 68 | 1.11 × 10−15 |
| RNA binding | 41 | 1.42 × 10−15 | |
| ATPase activity | 31 | 3.82 × 10−15 | |
| structural constituent of ribosome | 19 | 3.73 × 10−11 | |
| unfolded protein binding | 13 | 9.33 × 10−10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godoy-Diaz, C.; Llanos-Azócar, K.; Ruiz-Tagle, G.J.; Toro, J.E.; Oyarzún, P.A.; Valdés, J.A. Understanding Mantle Edge Pigmentation Through Comprehensive Transcriptomic Profiling of the Chilean Oyster (Ostrea chilensis). Biology 2025, 14, 145. https://doi.org/10.3390/biology14020145
Godoy-Diaz C, Llanos-Azócar K, Ruiz-Tagle GJ, Toro JE, Oyarzún PA, Valdés JA. Understanding Mantle Edge Pigmentation Through Comprehensive Transcriptomic Profiling of the Chilean Oyster (Ostrea chilensis). Biology. 2025; 14(2):145. https://doi.org/10.3390/biology14020145
Chicago/Turabian StyleGodoy-Diaz, Camila, Katalina Llanos-Azócar, Gonzalo J. Ruiz-Tagle, Jorge E. Toro, Pablo A. Oyarzún, and Juan A. Valdés. 2025. "Understanding Mantle Edge Pigmentation Through Comprehensive Transcriptomic Profiling of the Chilean Oyster (Ostrea chilensis)" Biology 14, no. 2: 145. https://doi.org/10.3390/biology14020145
APA StyleGodoy-Diaz, C., Llanos-Azócar, K., Ruiz-Tagle, G. J., Toro, J. E., Oyarzún, P. A., & Valdés, J. A. (2025). Understanding Mantle Edge Pigmentation Through Comprehensive Transcriptomic Profiling of the Chilean Oyster (Ostrea chilensis). Biology, 14(2), 145. https://doi.org/10.3390/biology14020145








