PPARγ Expression in Human Spermatozoa and Its Relationship with Seminal F2-Isoprostanes and Resolvin D1 in the Presence of Varicocele and Urogenital Infections
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Semen Analysis and Sample Preparation
2.3. Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) Determination
2.4. F2-Isoprostane (F2-IsoP) Determination
2.5. Resolvin D1 (RvD1) Determination
2.6. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lenzi, A.; Gandini, L.; Maresca, V.; Rago, R.; Sgrò, P.; Dondero, F.; Picardo, M. Fatty acid composition of spermatozoa and immature germ cells. Mol. Hum. Reprod. 2000, 6, 226–231. [Google Scholar] [CrossRef]
- Vallés, A.S.; Aveldaño, M.I.; Furland, N.E. Altered lipid homeostasis in Sertoli cells stressed by mild hyperthermia. PLoS ONE 2014, 9, e91127. [Google Scholar] [CrossRef]
- Mousavi, M.S.; Shahverdi, A.; Drevet, J.; Akbarinejad, V.; Esmaeili, V.; Sayahpour, F.A.; Topraggaleh, T.R.; Rahimizadeh, P.; Alizadeh, A. Peroxisome Proliferator-Activated Receptors (PPARs) levels in spermatozoa of normozoospermic and asthenozoospermic men. Syst. Biol. Reprod. Med. 2019, 65, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, F.; Hajian, M.; Rouhollahi Varnosfaderani, S.; Jafarpour, F.; Nasr Esfahani, M.H. Effect of rosiglitazone on developmental competence of mouse embryos treated with lipopolysaccharide. Theriogenology 2021, 161, 57–64. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Fatty acids, eicosanoids and PPAR gamma. Eur. J. Pharmacol. 2016, 785, 44–49. [Google Scholar] [CrossRef]
- Moretti, E.; Collodel, G.; Salvatici, M.C.; Belmonte, G.; Signorini, C. New insights into sperm with total globozoospermia: Increased fatty acid oxidation and centrin1 alteration. Syst. Biol. Reprod. Med. 2019, 65, 390–399. [Google Scholar] [CrossRef]
- Aquila, S.; Bonofiglio, D.; Gentile, M.; Middea, E.; Gabriele, S.; Belmonte, M.; Catalano, S.; Pellegrino, M.; Andò, S. Peroxisome proliferator-activated receptor (PPAR)γ is expressed by human spermatozoa: Its potential role on the sperm physiology. J. Cell. Physiol. 2006, 209, 977–986. [Google Scholar] [CrossRef]
- Liu, L.L.; Xian, H.; Cao, J.C.; Zhang, C.; Zhang, Y.H.; Chen, M.M.; Qian, Y.; Jiang, M. Peroxisome proliferator-activated receptor gamma signaling in human sperm physiology. Asian J. Androl. 2015, 17, 942–947. [Google Scholar] [CrossRef]
- Shibata, T.; Bhat, S.A.; Cao, D.; Saito, S.; Bernstein, E.A.; Nishi, E.; Medenilla, J.D.; Wang, E.T.; Chan, J.L.; Pisarska, M.D.; et al. Testicular ACE regulates sperm metabolism and fertilization through the transcription factor PPARγ. J. Biol. Chem. 2024, 300, 105486. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Baskaran, S.; Finelli, R.; Agarwal, A.; Henkel, R. Diagnostic value of routine semen analysis in clinical andrology. Andrologia 2021, 53, e13614. [Google Scholar] [CrossRef]
- Boeri, L.; Belladelli, F.; Capogrosso, P.; Cazzaniga, W.; Candela, L.; Pozzi, E.; Valsecchi, L.; Papaleo, E.; Viganò, P.; Abbate, C.; et al. Normal sperm parameters per se do not reliably account for fertility: A case-control study in the real-life setting. Andrologia 2021, 53, e13861. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.F.S.; Østergren, P.; Dupree, J.M.; Ohl, D.A.; Sønksen, J.; Fode, M. Varicocele and male infertility. Nat. Rev. Urol. 2017, 14, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Signorini, C.; Moretti, E.; Collodel, G. Role of isoprostanes in human male infertility. Syst. Biol. Reprod. Med. 2020, 66, 291–299. [Google Scholar] [CrossRef]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Aitken, R.J.; Drevet, J.R.; Moazamian, A.; Gharagozloo, P. Male Infertility and Oxidative Stress: A Focus on the Underlying Mechanisms. Antioxidants 2022, 11, 306. [Google Scholar] [CrossRef]
- Aitken, R.J.; Drevet, J.R. The Importance of Oxidative Stress in Determining the Functionality of Mammalian Spermatozoa: A Two-Edged Sword. Antioxidants 2020, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Bdeir, R.; Aljabali, S.M.; Banihani, S.A. Role of pyridoxine and oxidative stress in asthenozoospermia. Heliyon 2024, 10, e34799. [Google Scholar] [CrossRef] [PubMed]
- O’flaherty, C. Peroxiredoxin 6: The Protector of Male Fertility. Antioxidants 2018, 7, 173. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.L.; Dai, Q.; Roberts, L.J., 2nd. The isoprostanes—25 years later. Biochim. Biophys. Acta 2015, 1851, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Collodel, G.; Moretti, E.; Noto, D.; Corsaro, R.; Signorini, C. Oxidation of Polyunsaturated Fatty Acids as a Promising Area of Research in Infertility. Antioxidants 2022, 11, 1002. [Google Scholar] [CrossRef]
- Recchiuti, A. Resolvin D1 and its GPCRs in resolution circuits of inflammation. Prostaglandins Other Lipid Mediat. 2013, 107, 64–76. [Google Scholar] [CrossRef]
- Signorini, C.; Moretti, E.; Noto, D.; Micheli, L.; Ponchia, R.; Collodel, G. Fatty Acid Oxidation and Pro-Resolving Lipid Mediators Are Related to Male Infertility. Antioxidants 2022, 11, 107. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Micheli, L.; Collodel, G.; Moretti, E.; Noto, D.; Menchiari, A.; Cerretani, D.; Crispino, S.; Signorini, C. Redox imbalance induced by docetaxel in the neuroblastoma SH-SY5Y cells: A study of docetaxel-induced neuronal damage. Redox Rep. 2021, 26, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Signorini, C.; Ferretti, F.; Noto, D.; Collodel, G. A Study to Validate the Relevance of Semen F2-Isoprostanes on Human Male Infertility. Int. J. Environ. Res. Public Health 2022, 19, 1642. [Google Scholar] [CrossRef]
- Polvani, S.; Tarocchi, M.; Galli, A. PPARγ and Oxidative Stress: Con(β) Catenating NRF2 and FOXO. PPAR Res. 2012, 2012, 641087. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Wang, J.; Sun, S.; Wang, F.; Yang, Y.; Chen, L.; Sun, Z.; Yao, S. Resolvin D1 Alleviates Ventilator-Induced Lung Injury in Mice by Activating PPARγ/NF-κB Signaling Pathway. BioMed Res. Int. 2019, 2019, 6254587. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta 2011, 1812, 1007–1022. [Google Scholar] [CrossRef]
- Muzio, G.; Barrera, G.; Pizzimenti, S. Peroxisome Proliferator-Activated Receptors (PPARs) and Oxidative Stress in Physiological Conditions and in Cancer. Antioxidants 2021, 10, 1734. [Google Scholar] [CrossRef]
- Lee, C. Collaborative Power of Nrf2 and PPARγ Activators against Metabolic and Drug-Induced Oxidative Injury. Oxid. Med. Cell. Longev. 2017, 2017, 1378175. [Google Scholar] [CrossRef]
- Villapol, S. Roles of Peroxisome Prolif-erator-Activated Receptor Gamma on Brain and Peripheral Inflammation. Cell. Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef]
- Santoro, M.; Guido, C.; De Amicis, F.; Sisci, D.; Vizza, D.; Gervasi, S.; Carpino, A.; Aquila, S. Sperm metabolism in pigs: A role for peroxisome proliferator-activated receptor gamma (PPARγ). J. Exp. Biol. 2013, 216, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Antonuccio, P.; Marini, H.R.; Micali, A.; Romeo, C.; Granese, R.; Retto, A.; Martino, A.; Benvenga, S.; Cuzzocrea, S.; Impellizzeri, D.; et al. The Nutraceutical N-Palmitoylethanolamide (PEA) Reveals Widespread Molecular Effects Unmasking New Therapeutic Targets in Murine Varicocele. Nutrients 2021, 13, 734. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, I.; Aquila, S. Steroid receptors in human ejaculated sperm as molecular markers of the detrimental effects related to the pathophysiology of testicular varicocele. Histol. Histopathol. 2016, 31, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Agarwal, A.; Lewis-Jones, I.; Sharma, R.K.; Thomas, A.J., Jr. Novel associations between specific sperm morphological defects and leukocytospermia. Fertil. Steril. 2004, 82, 621–627. [Google Scholar] [CrossRef]
- Franken, D.R. How accurate is sperm morphology as an indicator of sperm function? Andrologia 2015, 47, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Björndahl, L.; Kirkman Brown, J.; other Editorial Board Members of the WHO Laboratory Manual for the Examination and Processing of Human Semen. The sixth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen: Ensuring quality and standardization in basic examination of human ejaculates. Fertil. Steril. 2022, 117, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Osama, H.M.; Khadrawy, S.M.; El-Nahass, E.S.; Othman, S.I.; Mohamed, H.M. Eltroxin and Hesperidin mitigate testicular and renal damage in hypothyroid rats: Amelioration of oxidative stress through PPARγ and Nrf2/HO-1 signaling pathway. Lab. Anim. Res. 2024, 40, 19. [Google Scholar] [CrossRef] [PubMed]
- El Midaoui, A.; Wu, L.; Wang, R.; de Champlain, J. Modulation of cardiac and aortic peroxisome proliferator-activated receptor-γ expression by oxidative stress in chronically glucose-fed rats. Am. J. Hypertens. 2006, 19, 407–412. [Google Scholar] [CrossRef][Green Version]
- Blanquicett, C.; Kang, B.Y.; Ritzenthaler, J.D.; Jones, D.P.; Hart, C.M. Oxidative stress modulates PPAR γ in vascular endothelial cells. Free Radic. Biol. Med. 2010, 48, 1618–1625. [Google Scholar] [CrossRef]
- Cai, R.; Yu, T.; Huang, C.; Xia, X.; Liu, X.; Gu, J.; Xue, S.; Yeh, E.T.; Cheng, J. SUMO-specific protease 1 regulates mitochondrial biogenesis through PGC-1α. J. Biol. Chem. 2012, 287, 44464–44470. [Google Scholar] [CrossRef]
- Yu, Y.; Correll, P.H.; Vanden Heuvel, J.P. Conjugated linoleic acid decreases production of pro-inflammatory products in macrophages: Evidence for a PPAR γ-dependent mechanism. Biochim. Biophys. Acta 2002, 1581, 89–99. [Google Scholar] [CrossRef] [PubMed]
- McSwiggin, H.M.; O’Doherty, A.M. Epigenetic reprogramming during spermatogenesis and male factor infertility. Reproduction 2018, 156, R9–R21. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, X.; Wang, Z.; Wang, D. Is transcription in sperm stationary or dynamic? J. Reprod. Dev. 2017, 63, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.; Silva, J.V.; Howl, J.; Santos, M.A.S.; Fardilha, M. All you need to know about sperm RNAs. Hum. Reprod. Update 2021, 28, 67–91. [Google Scholar] [CrossRef]
- Deng, S.L.; Sun, T.C.; Yu, K.; Wang, Z.P.; Zhang, B.L.; Zhang, Y.; Wang, X.X.; Lian, Z.X.; Liu, Y.X. Melatonin reduces oxidative damage and upregulates heat shock protein 90 expression in cryopreserved human semen. Free Radic. Biol. Med. 2017, 113, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Santonastaso, M.; Mottola, F.; Iovine, C.; Colacurci, N.; Rocco, L. Protective Effects of Curcumin on the Outcome of Cryopreservation in Human Sperm. Reprod. Sci. 2021, 28, 2895–2905. [Google Scholar] [CrossRef] [PubMed]
- Passaponti, S.; Signorini, C.; Collodel, G.; Moretti, E.; Mattioli, S.; Belmonte, G.; Noto, D. Expression and immunolocalisation of PPARγ in reproductive tissues of rabbits fed n-3 PUFA-enriched diets. J. Men’s Health 2022, 18, 72. [Google Scholar] [CrossRef]
- Liao, Z.; Dong, J.; Wu, W.; Yang, T.; Wang, T.; Guo, L.; Chen, L.; Xu, D.; Wen, F. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARγ/NF-κB pathway. Respir. Res. 2012, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, L.; Li, J.; Fang, Z.; Li, S.; Peng, Y.; Zhang, M.; Wang, X. Effect of RvD1/FPR2 on inflammatory response in chorioamnionitis. J. Cell. Mol. Med. 2020, 24, 13397–13407. [Google Scholar] [CrossRef]
- Mattioli, S.; Moretti, E.; Castellini, C.; Signorini, C.; Corsaro, R.; Angelucci, E.; Collodel, G. Can Dietary n-3 Polyunsaturated Fatty Acids Affect Apelin and Resolvin in Testis and Sperm of Male Rabbits? Molecules 2023, 28, 6188. [Google Scholar] [CrossRef]
- Santoro, M.; De Amicis, F.; Aquila, S.; Bonofiglio, D. Peroxisome proliferator-activated receptor gamma expression along the male genital system and its role in male fertility. Hum. Reprod. 2020, 35, 2072–2085. [Google Scholar] [CrossRef]
| Variables | Median (25°–75° percentiles) |
|---|---|
| Semen volume (mL) | 3.50 (2.70–4.50) |
| Sperm concentration (106 per mL) | 75.00 (44.00–17.80) |
| Sperm progressive motility (PR, %) | 47.50 (42.00–56.60) |
| Normal sperm morphology (%) | 11.00 (6.75–14.25) |
| Sperm vitality (%) | 77.00 (70.00–85.25) |
| Seminal F2-Isoprostanes (F2-IsoPs, ng/mL) | 48.87 (16.05–82.01) |
| Seminal Resolvin-D1 (RvD1, pg/mL) | 40.65 (31.63–83.32) |
| PPARγ (relative mRNA expression) | 0.61 (0.29–1.10) |
| Normal Sperm Morphology (%) | Sperm Vitality (%) | Seminal F2-Isoprostanes (F2-IsoPs, ng/mL) | Seminal Resolvin-D1 (RvD1, pg/mL) | |
|---|---|---|---|---|
| Sperm vitality (%) | r = 0.54 ** 95% C. I. = 0.19 to 0.78 | |||
| Seminal F2-Isoprostanes (F2-IsoPs, ng/mL) | r = −0.40 * 95% C. I. = −0.69 to −0.01 | |||
| Seminal Resolvin-D1 (RvD1, pg/mL) | r = −0.46 * 95% C. I. = −0.73 to −0.08 | r = −0.48 * 95% C. I. = −0.74 to −0.11 | ||
| PPARγ (relative mRNA expression) | r = 0.45 * 95% C. I. = 0.06 to 0.72 | r = 0.58 ** 95% C. I. = 0.24 to 0.80 | r = −0.54 ** 95% C. I. = −0.77 to −0.18 | r = −0.60 ** 95% C. I. = −0.81 to −0.27 |
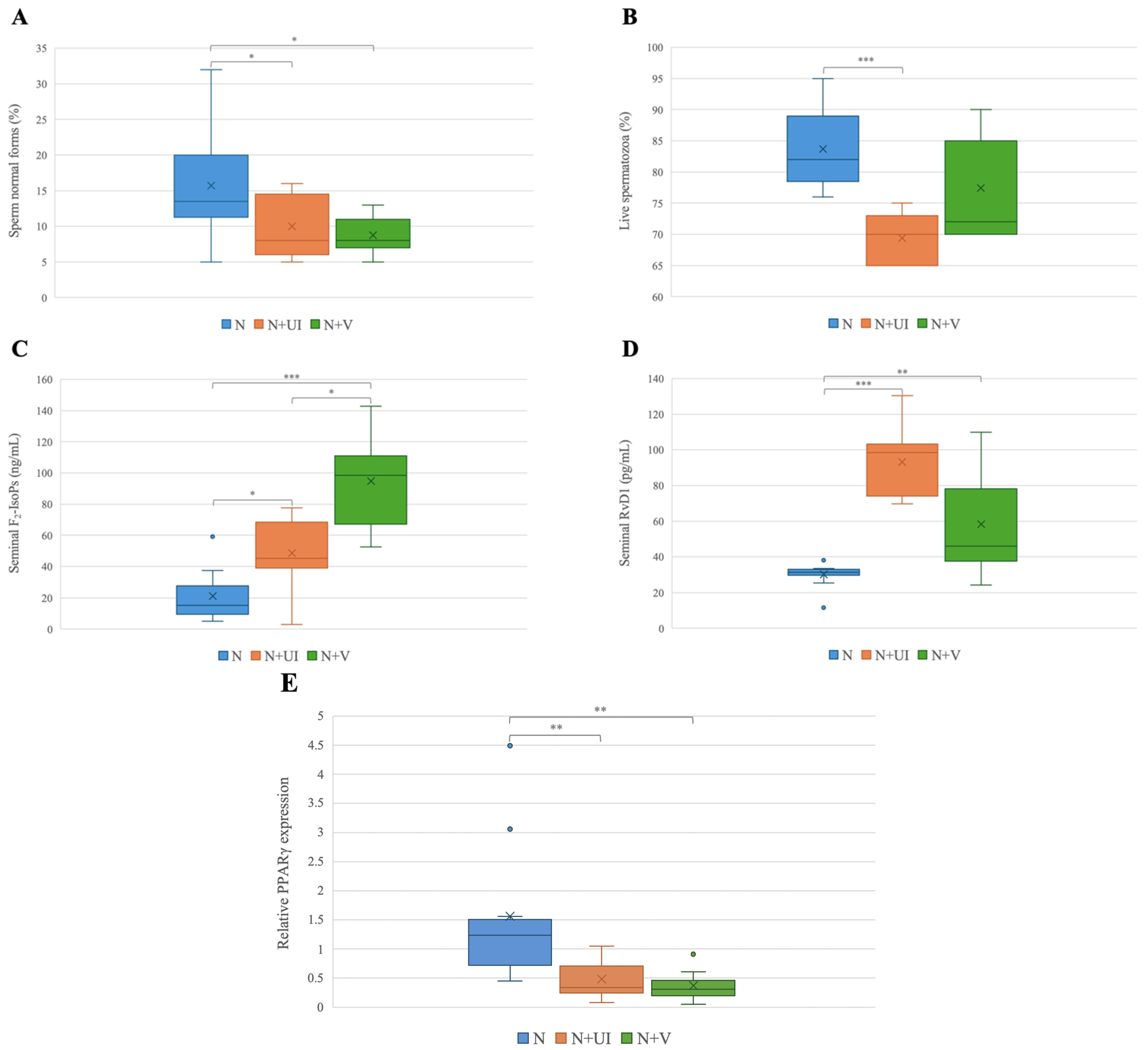
| Variables | N | N + UI | N + V | Kruskal–Wallis Test | Dunnet’s Post Hoc Test |
|---|---|---|---|---|---|
| Semen volume (mL) | 3.70 (2.60–4.75) | 3.50 (3.25–5.75) | 2.80 (2.40–3.80) | NS | / |
| Sperm concentration (106 per mL) | 54.50 (44.25–112.25) | 86.00 (54.00–135.00) | 82.00 (43.00–110.00) | NS | / |
| Sperm progressive motility (PR, %) | 50.00 (47.25–61.00) | 42.00 (40.00–54.50) | 46.00 (44.00–48.00) | NS | / |
| Normal sperm morphology (%) | 13.50 (11.25–20.00) | 8.00 (6.00–14.50) | 8.00 (7.00–11.00) | p < 0.05 | N + V vs. N * N + UI vs. N * |
| Sperm vitality (%) | 82.00 (78.50–89.00) | 70.00 (65.00–73.00) | 72.00 (70.00–85.00) | p < 0.001 | N + UI vs. N *** |
| Seminal F2-IsoPs (ng/mL) | 15.14 (9.42–27.87) | 45.27 (38.94–68.49) | 98.49 (67.11–111.05) | p < 0.01 | N + UI vs. N * N + V vs. N *** N + V vs. N + UI * |
| Seminal RvD1 (pg/mL) | 31.38 (29.85–33.01) | 98.49 (74.07–103.25) | 45.96 (37.65–78.26) | p < 0.001 | N + UI vs. N *** N + V vs. N ** |
| PPARγ (relative mRNA expression) | 1.24 (0.72–1.51) | 0.34 (0.25–0.71) | 0.31 (0.20–0.46) | p < 0.01 | N + UI vs. N ** N + V vs. N ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collodel, G.; Moretti, E.; Marcucci, C.; Liguori, L.; Marchini, D.; Corsaro, R.; Centini, G.; Signorini, C. PPARγ Expression in Human Spermatozoa and Its Relationship with Seminal F2-Isoprostanes and Resolvin D1 in the Presence of Varicocele and Urogenital Infections. Biology 2025, 14, 137. https://doi.org/10.3390/biology14020137
Collodel G, Moretti E, Marcucci C, Liguori L, Marchini D, Corsaro R, Centini G, Signorini C. PPARγ Expression in Human Spermatozoa and Its Relationship with Seminal F2-Isoprostanes and Resolvin D1 in the Presence of Varicocele and Urogenital Infections. Biology. 2025; 14(2):137. https://doi.org/10.3390/biology14020137
Chicago/Turabian StyleCollodel, Giulia, Elena Moretti, Caterina Marcucci, Laura Liguori, Daniela Marchini, Roberta Corsaro, Gabriele Centini, and Cinzia Signorini. 2025. "PPARγ Expression in Human Spermatozoa and Its Relationship with Seminal F2-Isoprostanes and Resolvin D1 in the Presence of Varicocele and Urogenital Infections" Biology 14, no. 2: 137. https://doi.org/10.3390/biology14020137
APA StyleCollodel, G., Moretti, E., Marcucci, C., Liguori, L., Marchini, D., Corsaro, R., Centini, G., & Signorini, C. (2025). PPARγ Expression in Human Spermatozoa and Its Relationship with Seminal F2-Isoprostanes and Resolvin D1 in the Presence of Varicocele and Urogenital Infections. Biology, 14(2), 137. https://doi.org/10.3390/biology14020137









