Evolution of CEACAM1 N Domain Biologically Active Sites in Primates
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. N Domain Amino Acid Sequences
2.2. Phylogenetic Analyses
2.3. Identification of Single Nucleotide Polymorphisms (SNPs) in Human Populations
3. Results
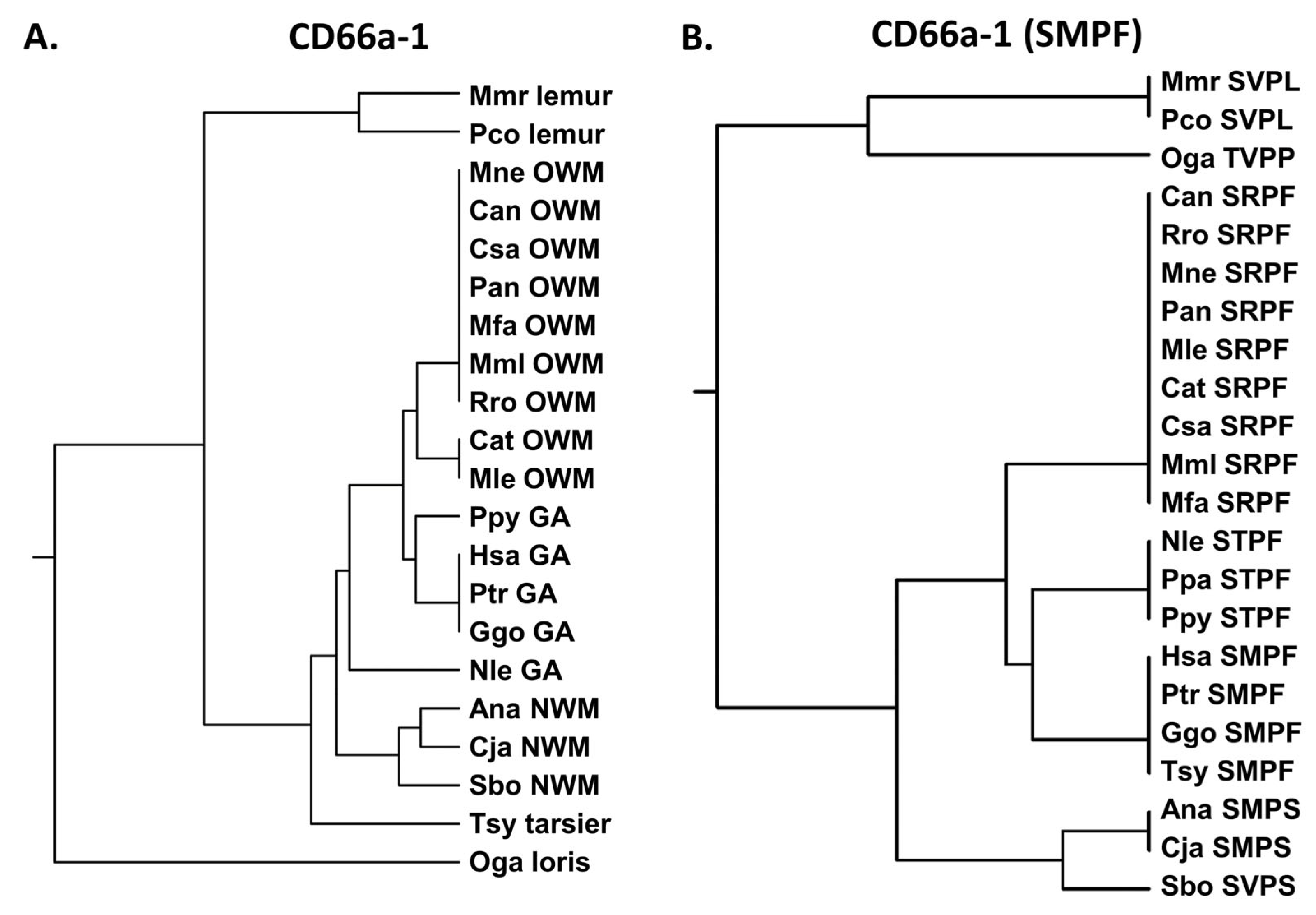
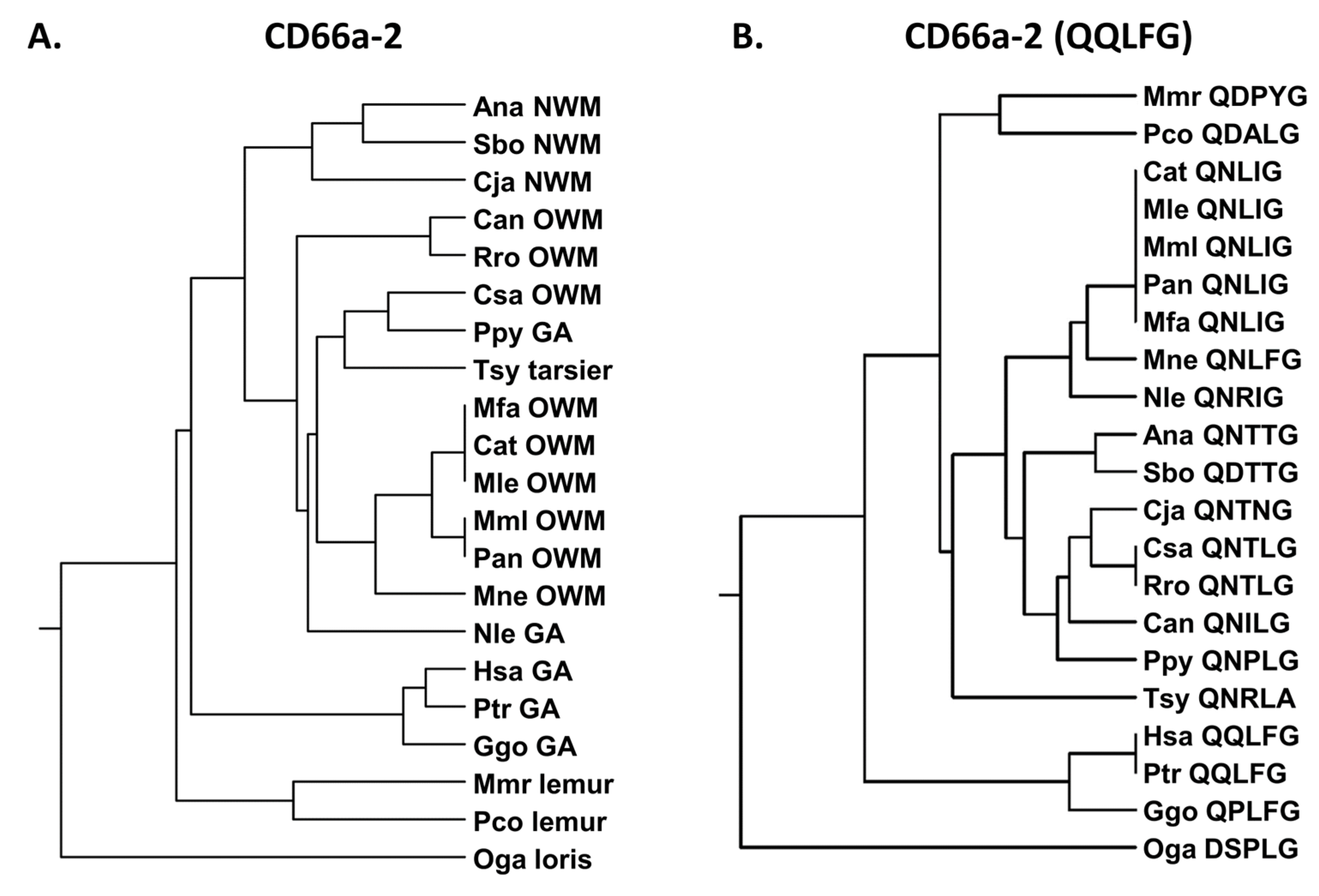
3.1. N Domain Exon Nucleotide Sequences from Pathogen CEACAM Receptors Exhibit a Closer Relationship Within than Between Species in Humans and Other Primates
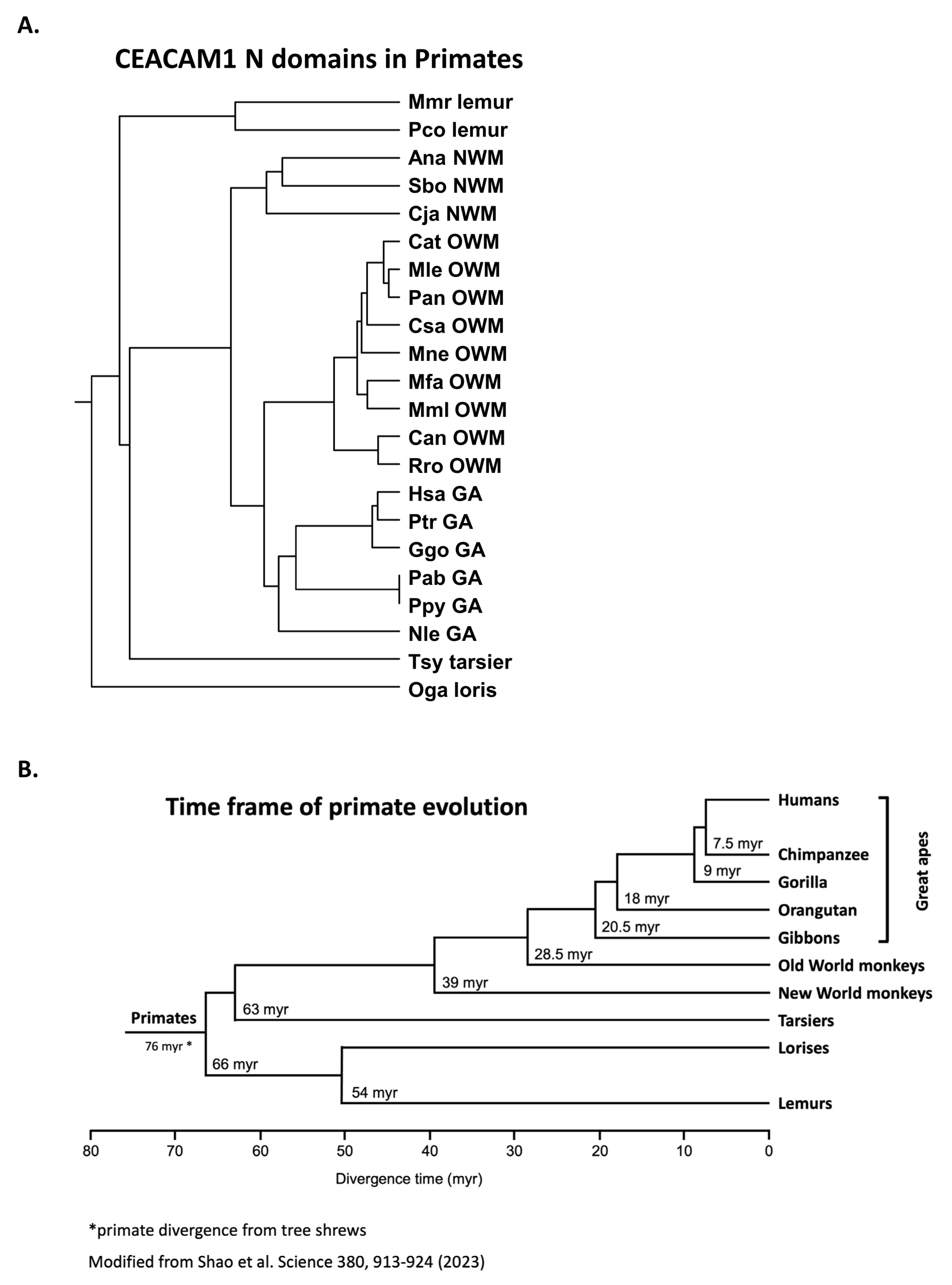
3.2. Phylogeny of Primate Mature CEACAM1 N Amino Acid Sequences Reflect Primate Relationships
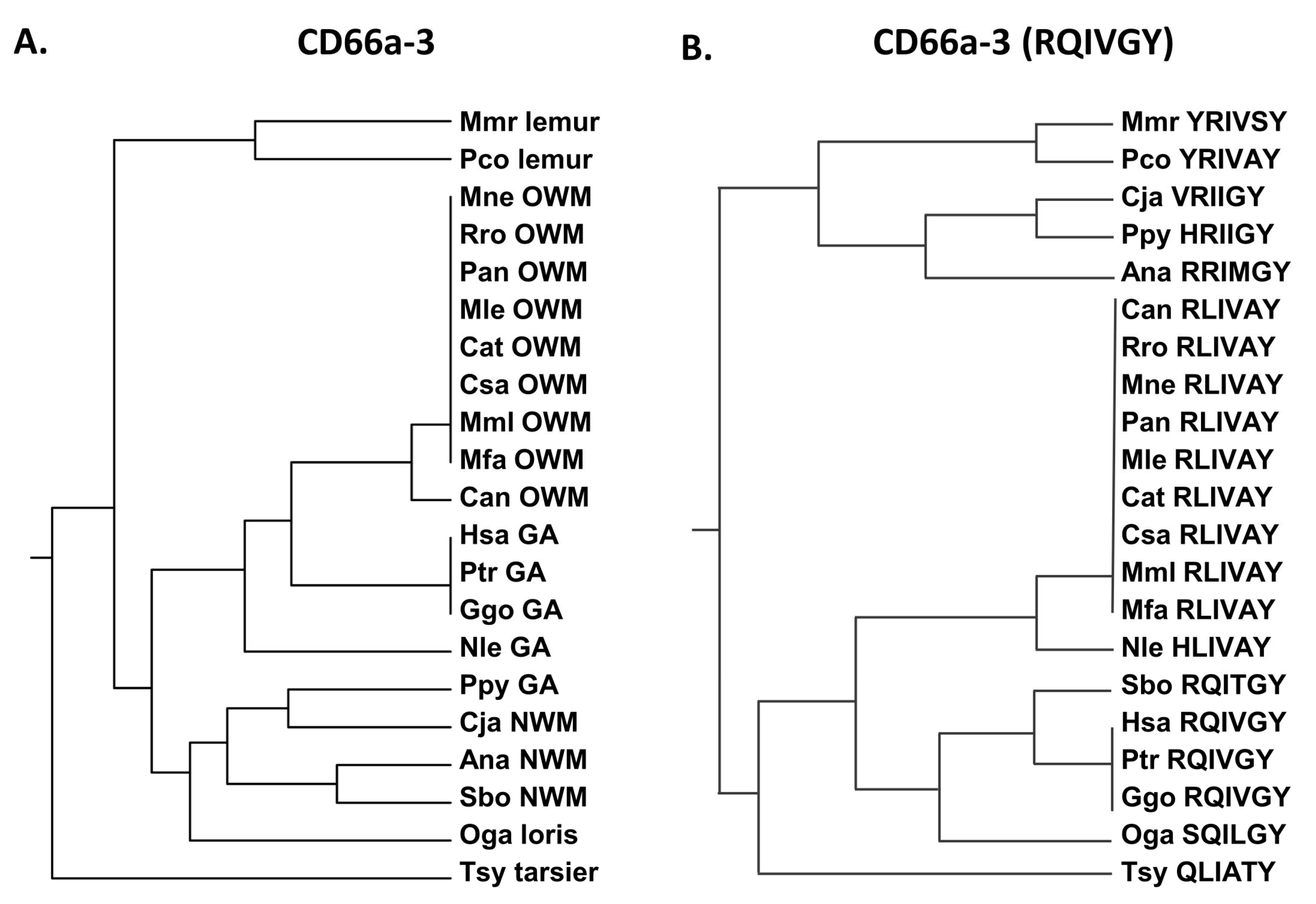
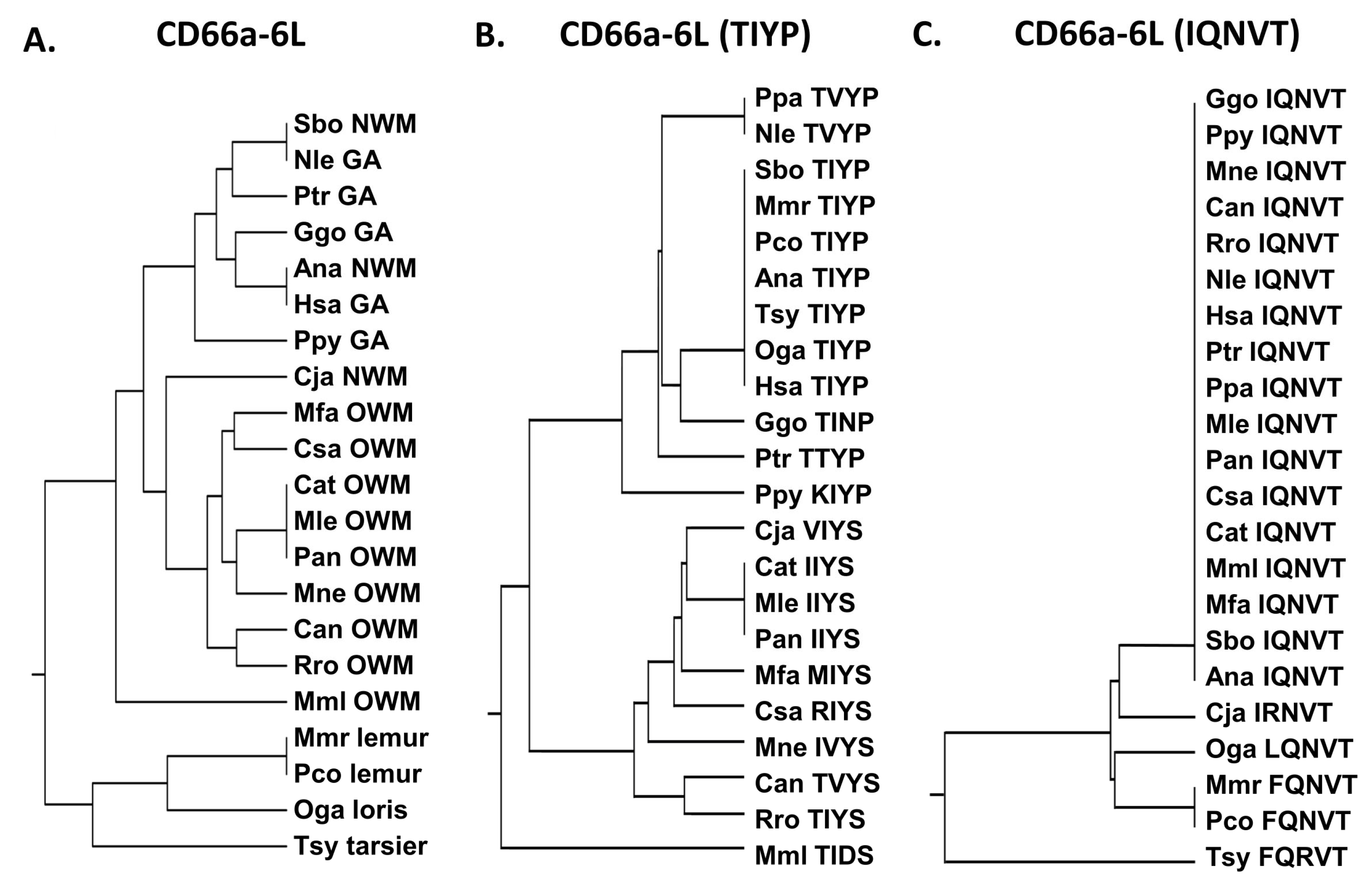
3.3. Activating Peptides and Functionally Critical Subregions from the Human CEACAM1 N Domain Are Generally Better Conserved than the Whole Domain
3.4. Non-Synonymous Single Nucleotide Polymorphisms in Activating Peptides Differ Between Chimpanzees, Bonobos, and Various African Populations
4. Discussion
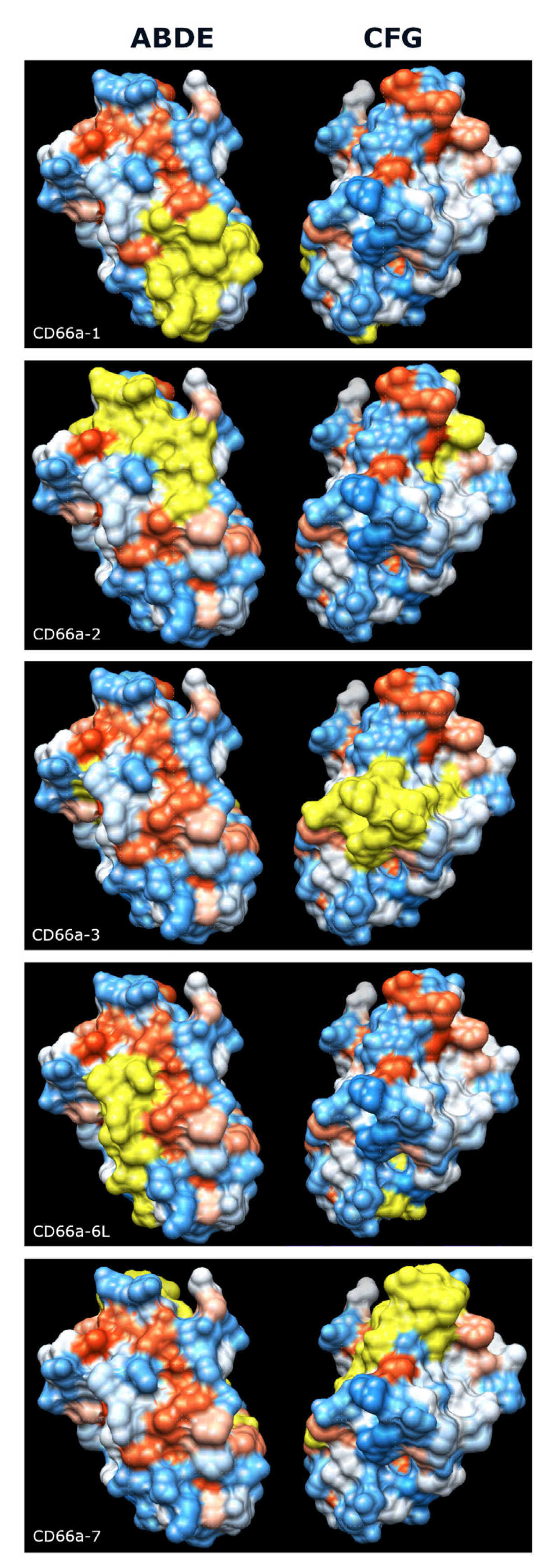
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CEACAM | Carcinoembryonic antigen cell adhesion molecules |
| PSG | Pregnancy-specific glycoprotein |
| SNP | Single nucleotide polymorphism |
| SNV | Single nucleotide variation |
| VAF | Variant allele frequency |
| WGS | Whole genome sequencing |
References
- Thompson, J.A.; Pande, H.; Paxton, R.J.; Shively, L.; Padma, A.; Simmer, R.L.; Todd, C.W.; Riggs, A.D.; Shively, J.E. Molecular cloning of a gene belonging to the carcinoembryonic antigen gene family and discussion of a domain model. Proc. Natl. Acad. Sci. USA 1987, 84, 2965–2969. [Google Scholar] [CrossRef]
- Thompson, J.A.; Mauch, E.M.; Chen, F.S.; Hinoda, Y.; Schrewe, H.; Berling, B.; Barnert, S.; von Kleist, S.; Shively, J.E.; Zimmermann, W. Analysis of the size of the carcinoembryonic antigen (CEA) gene family: Isolation and sequencing of N-terminal domain exons. Biochem. Biophys. Res. Commun. 1989, 158, 996–1004. [Google Scholar] [CrossRef]
- Moonens, K.; Hamway, Y.; Neddermann, M.; Reschke, M.; Tegtmeyer, N.; Kruse, T.; Kammerer, R.; Mejias-Luque, R.; Singer, B.B.; Backert, S.; et al. Helicobacter pylori adhesin HopQ disrupts trans dimerization in human CEACAMs. EMBO J. 2018, 37, e98665. [Google Scholar] [CrossRef]
- Adrian, J.; Bonsignore, P.; Hammer, S.; Frickey, T.; Hauck, C.R. Adaptation to Host-Specific Bacterial Pathogens Drives Rapid Evolution of a Human Innate Immune Receptor. Curr. Biol. 2019, 29, 616–630. [Google Scholar] [CrossRef]
- Dery, K.J.; Najjar, S.M.; Beauchemin, N.; Shively, J.E.; Kupiec-Weglinski, J.W. Mechanism and function of CEACAM1 splice isoforms. Eur. J. Clin. Investig. 2024, 54 (Suppl. S2), e14350. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.J.; Chambers, A.C.; Hill, D.J. The emerging role of Fusobacteria in carcinogenesis. Eur. J. Clin. Investig. 2024, 54 (Suppl. S2), e14353. [Google Scholar] [CrossRef] [PubMed]
- Gotz, L.; Rueckschloss, U.; Ergun, S.; Kleefeldt, F. CEACAM1 in vascular homeostasis and inflammation. Eur. J. Clin. Investig. 2024, 54 (Suppl. S2), e14345. [Google Scholar] [CrossRef]
- Gotz, L.; Rueckschloss, U.; Najjar, S.M.; Ergun, S.; Kleefeldt, F. Carcinoembryonic antigen-related cell adhesion molecule 1 in cancer: Blessing or curse? Eur. J. Clin. Investig. 2024, 54 (Suppl. S2), e14337. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.M.; Abdolahipour, R.; Ghadieh, H.E.; Jahromi, M.S.; Najjar, J.A.; Abuamreh, B.A.M.; Zaidi, S.; Kumarasamy, S.; Muturi, H.T. Regulation of Insulin Clearance by Non-Esterified Fatty Acids. Biomedicines 2022, 10, 1899. [Google Scholar] [CrossRef]
- Najjar, S.M.; Caprio, S.; Gastaldelli, A. Insulin Clearance in Health and Disease. Annu. Rev. Physiol. 2023, 85, 363–381. [Google Scholar] [CrossRef]
- Najjar, S.M.; Shively, J.E. Regulation of lipid storage and inflammation in the liver by CEACAM1. Eur. J. Clin. Investig. 2024, 54 (Suppl. S2), e14338. [Google Scholar] [CrossRef] [PubMed]
- Popp, A.; Dehio, C.; Grunert, F.; Meyer, T.F.; Gray-Owen, S.D. Molecular analysis of neisserial Opa protein interactions with the CEA family of receptors: Identification of determinants contributing to the differential specificities of binding. Cell Microbiol. 1999, 1, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Skubitz, K.M. The role of CEACAMs in neutrophil function. Eur. J. Clin. Investig. 2024, 54 (Suppl. S2), e14349. [Google Scholar] [CrossRef] [PubMed]
- Tchoupa, A.K.; Schuhmacher, T.; Hauck, C.R. Signaling by epithelial members of the CEACAM family—Mucosal docking sites for pathogenic bacteria. Cell Commun. Signal 2014, 12, 27. [Google Scholar] [CrossRef]
- Zimmermann, W.; Kammerer, R. The immune-modulating pregnancy-specific glycoproteins evolve rapidly and their presence correlates with hemochorial placentation in primates. BMC Genom. 2021, 22, 128. [Google Scholar] [CrossRef]
- Zimmermann, W.; Kammerer, R. Evolution of CEACAM pathogen decoy receptors in primates. Eur. J. Clin. Investig. 2024, 54 (Suppl. S2), e14356. [Google Scholar] [CrossRef]
- Boulton, I.C.; Gray-Owen, S.D. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat. Immunol. 2002, 3, 229–236. [Google Scholar] [CrossRef]
- Chang, C.L.; Semyonov, J.; Cheng, P.J.; Huang, S.Y.; Park, J.I.; Tsai, H.J.; Lin, C.Y.; Grutzner, F.; Soong, Y.K.; Cai, J.J.; et al. Widespread divergence of the CEACAM/PSG genes in vertebrates and humans suggests sensitivity to selection. PLoS ONE 2013, 8, e61701. [Google Scholar] [CrossRef]
- Zimmermann, W.; Kammerer, R. Coevolution of paired receptors in Xenopus carcinoembryonic antigen-related cell adhesion molecule families suggests appropriation as pathogen receptors. BMC Genom. 2016, 17, 928. [Google Scholar] [CrossRef]
- Kammerer, R.; Zimmermann, W. Coevolution of activating and inhibitory receptors within mammalian carcinoembryonic antigen families. BMC Biol. 2010, 8, 12. [Google Scholar] [CrossRef]
- Pan, H.; Shively, J.E. Carcinoembryonic antigen-related cell adhesion molecule-1 regulates granulopoiesis by inhibition of granulocyte colony-stimulating factor receptor. Immunity 2010, 33, 620–631. [Google Scholar] [CrossRef]
- Skubitz, K.M.; Campbell, K.D.; Skubitz, A.P. Synthetic peptides of CD66a stimulate neutrophil adhesion to endothelial cells. J. Immunol. 2000, 164, 4257–4264. [Google Scholar] [CrossRef]
- Skubitz, K.M.; Campbell, K.D.; Skubitz, A.P. Synthetic peptides from the N-domains of CEACAMs activate neutrophils. J. Pept. Res. 2001, 58, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Skubitz, K.M.; Skubitz, A.P. Two new synthetic peptides from the N-domain of CEACAM1 (CD66a) stimulate neutrophil adhesion to endothelial cells. Biopolymers 2011, 96, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Perelman, P.; Johnson, W.E.; Roos, C.; Seuanez, H.N.; Horvath, J.E.; Moreira, M.A.; Kessing, B.; Pontius, J.; Roelke, M.; Rumpler, Y.; et al. A molecular phylogeny of living primates. PLoS Genet. 2011, 7, e1001342. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, L.; Li, F.; Zhao, L.; Zhang, B.L.; Shao, F.; Chen, J.W.; Chen, C.Y.; Bi, X.; Zhuang, X.L.; et al. Phylogenomic analyses provide insights into primate evolution. Science 2023, 380, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Saragovi, U.; Fuks, A.; Makkerh, J.; Mort, J.; Stanners, C.P. Self recognition in the Ig superfamily. Identification of precise subdomains in carcinoembryonic antigen required for intercellular adhesion. J. Biol. Chem. 2000, 275, 26935–26943. [Google Scholar] [CrossRef]
- Baker, E.P.; Sayegh, R.; Kohler, K.M.; Borman, W.; Goodfellow, C.K.; Brush, E.R.; Barber, M.F. Evolution of host-microbe cell adherence by receptor domain shuffling. Elife 2022, 11, e73330. [Google Scholar] [CrossRef]
- Bonsor, D.A.; Gunther, S.; Beadenkopf, R.; Beckett, D.; Sundberg, E.J. Diverse oligomeric states of CEACAM IgV domains. Proc. Natl. Acad. Sci. USA 2015, 112, 13561–13566. [Google Scholar] [CrossRef]
- Skubitz, K.M.; Skubitz, A.P. Interdependency of CEACAM-1, -3, -6, and -8 induced human neutrophil adhesion to endothelial cells. J. Transl. Med. 2008, 6, 78. [Google Scholar] [CrossRef]
- Muenzner, P.; Bachmann, V.; Zimmermann, W.; Hentschel, J.; Hauck, C.R. Human-restricted bacterial pathogens block shedding of epithelial cells by stimulating integrin activation. Science 2010, 329, 1197–1201. [Google Scholar] [CrossRef]
- Schmitter, T.; Agerer, F.; Peterson, L.; Munzner, P.; Hauck, C.R. Granulocyte CEACAM3 is a phagocytic receptor of the innate immune system that mediates recognition and elimination of human-specific pathogens. J. Exp. Med. 2004, 199, 35–46. [Google Scholar] [CrossRef]
- Sintsova, A.; Wong, H.; MacDonald, K.S.; Kaul, R.; Virji, M.; Gray-Owen, S.D. Selection for a CEACAM receptor-specific binding phenotype during Neisseria gonorrhoeae infection of the human genital tract. Infect. Immun. 2015, 83, 1372–1383. [Google Scholar] [CrossRef]
- Gandhi, A.K.; Sun, Z.J.; Kim, W.M.; Huang, Y.H.; Kondo, Y.; Bonsor, D.A.; Sundberg, E.J.; Wagner, G.; Kuchroo, V.K.; Petsko, G.A.; et al. Structural basis of the dynamic human CEACAM1 monomer-dimer equilibrium. Commun. Biol. 2021, 4, 360. [Google Scholar] [CrossRef]
- Caswell, J.L.; Mallick, S.; Richter, D.J.; Neubauer, J.; Schirmer, C.; Gnerre, S.; Reich, D. Analysis of chimpanzee history based on genome sequence alignments. PLoS Genet. 2008, 4, e1000057. [Google Scholar] [CrossRef]
- Dawkins, R. The Ancestor’s Tale: A Pilgrimage to the Dawn of Evolution; Houghton Mifflin: Boston, MA, USA, 2004. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Fedarovich, A.; Tomberg, J.; Nicholas, R.A.; Davies, C. Structure of the N-terminal domain of human CEACAM1: Binding target of the opacity proteins during invasion of Neisseria meningitidis and N. gonorrhoeae. Acta Crystallogr. D Biol. Crystallogr. 2006, 62 Pt 9, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Klaile, E.; Vorontsova, O.; Sigmundsson, K.; Muller, M.M.; Singer, B.B.; Ofverstedt, L.G.; Svensson, S.; Skoglund, U.; Obrink, B. The CEACAM1 N-terminal Ig domain mediates cis- and trans-binding and is essential for allosteric rearrangements of CEACAM1 microclusters. J. Cell Biol. 2009, 187, 553–567. [Google Scholar] [CrossRef]
- Muller, M.M.; Klaile, E.; Vorontsova, O.; Singer, B.B.; Obrink, B. Homophilic adhesion and CEACAM1-S regulate dimerization of CEACAM1-L and recruitment of SHP-2 and c-Src. J. Cell Biol. 2009, 187, 569–581. [Google Scholar] [CrossRef] [PubMed]




| Abbreviation | Latin Name | Common Name | Classification |
|---|---|---|---|
| Ana | Aotus nancymaae | Ma’s night monkey | NWM |
| Can | Colobus angolensis palliatus | Black and white colobus monkey | OWM |
| Cat | Cerocebus atys | Sooty mangabey | OWM |
| Cja | Callithrix jacchus | Marmoset | NWM |
| Csa | Chlorocebus sabaeus | Green monkey | OWM |
| Ggo | Gorilla gorilla | Gorilla | GA |
| Hsa | Homo sapiens | Man | GA |
| Mfa | Macaca fascicularis | Crab-eating macaque | OWM |
| Mle | Madrillus leucophaeus | Drill | OWM |
| Mml | Macaca mulatta | Rhesus macaque | OWM |
| Mmr | Microcebus murinus | Gray mouse lemur | lemur |
| Mne | Macaca nemestrina | Pig-tailed macaque | OWM |
| Nle | Nomascus leucogenys | Northern white-cheeked gibbon | GA |
| Oga | Otolemur garnettii | Bush baby | loris |
| Pab | Pongo abelii | Sumatran orangutan | GA |
| Pan | Papioanubis | Olive baboon | OWM |
| Pco | Propithecus coquereli | Coquerel’s sifaka | lemur |
| Ppa | Pan paniscus | Bonobo | GA |
| Ppy | Pongo pygmaeus | Bornean orangutan | GA |
| Ptr | Pan troglodytes | Chimpanzee | GA |
| Rro | Rhinopithecus roxellana | Golden snub-nosed monkey | OWM |
| Sbo | Saimiri boliviensis | Bolivian squirrel monkey | NWM |
| Tsy | Tarsius syrichta | Tarsier | tarsier |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skubitz, K.M.; Zimmermann, W. Evolution of CEACAM1 N Domain Biologically Active Sites in Primates. Biology 2025, 14, 1744. https://doi.org/10.3390/biology14121744
Skubitz KM, Zimmermann W. Evolution of CEACAM1 N Domain Biologically Active Sites in Primates. Biology. 2025; 14(12):1744. https://doi.org/10.3390/biology14121744
Chicago/Turabian StyleSkubitz, Keith M., and Wolfgang Zimmermann. 2025. "Evolution of CEACAM1 N Domain Biologically Active Sites in Primates" Biology 14, no. 12: 1744. https://doi.org/10.3390/biology14121744
APA StyleSkubitz, K. M., & Zimmermann, W. (2025). Evolution of CEACAM1 N Domain Biologically Active Sites in Primates. Biology, 14(12), 1744. https://doi.org/10.3390/biology14121744






