Unlocking New Horizons: Antibody–Drug Conjugates in Small Cell Lung Cancer
Simple Summary
Abstract
1. Introduction
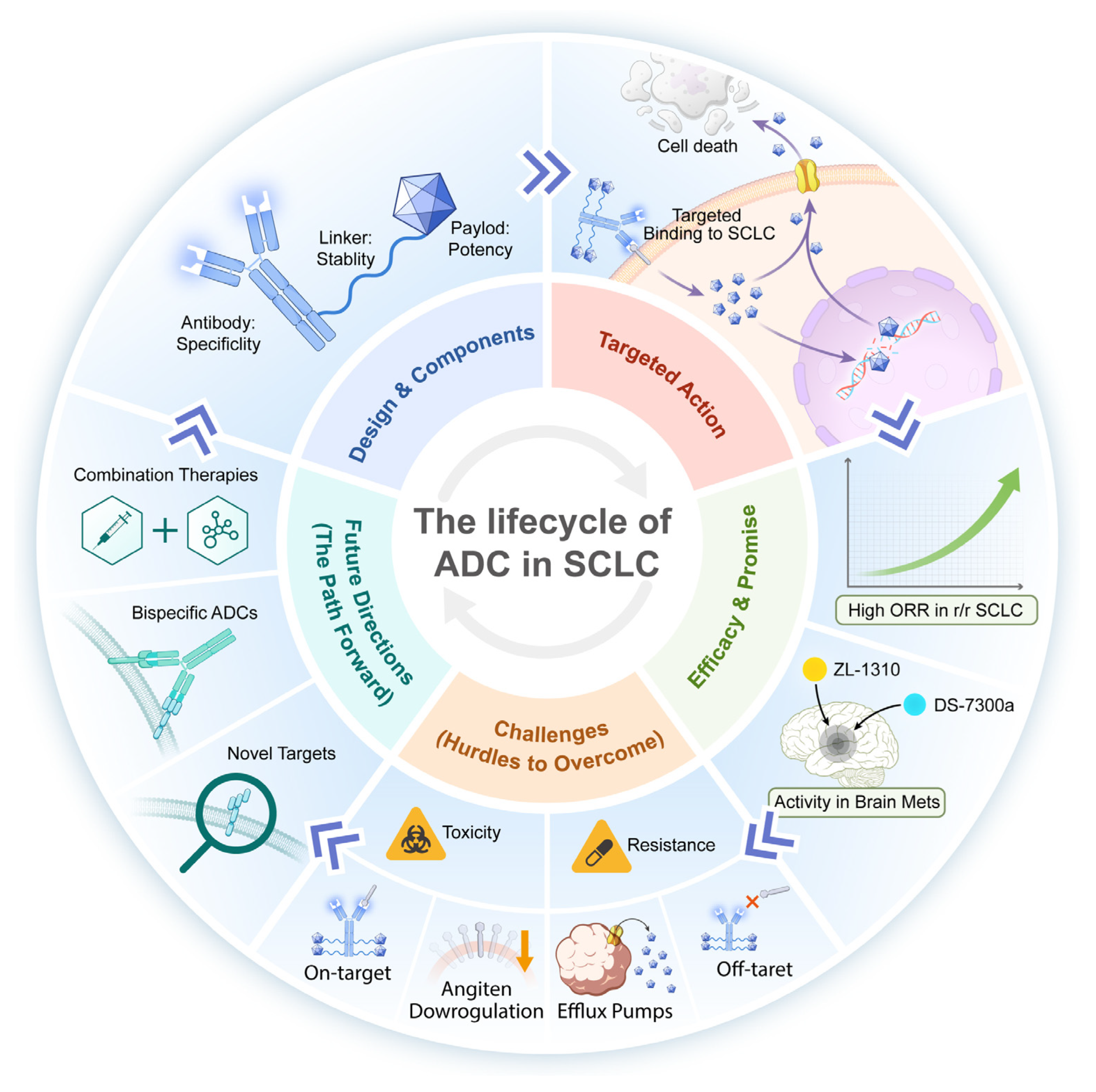
2. Fundamentals and Mechanism of ADC Drugs
2.1. The Fundamentals of ADCs
2.1.1. Payload
2.1.2. Antibody
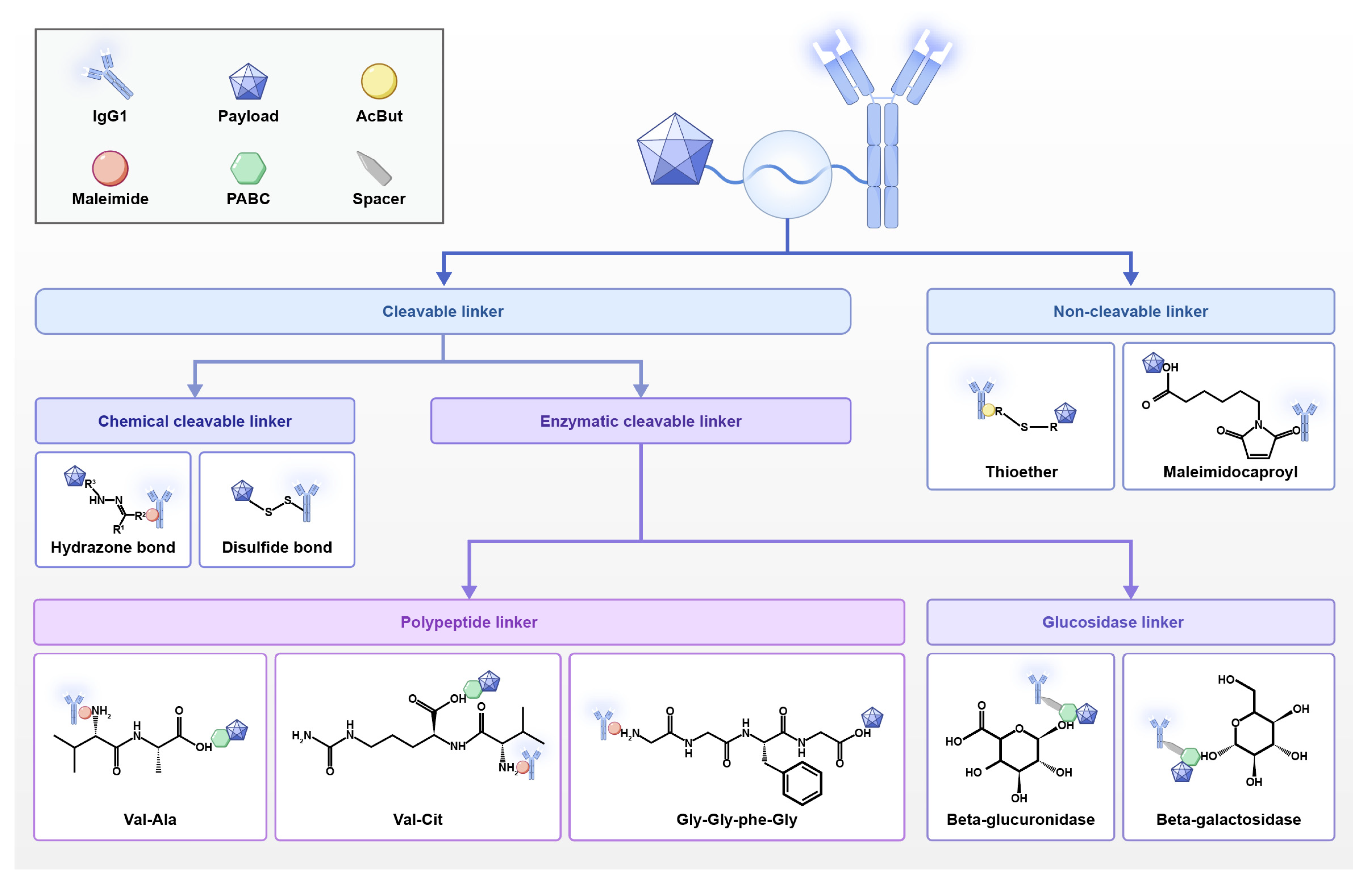
2.1.3. Linker
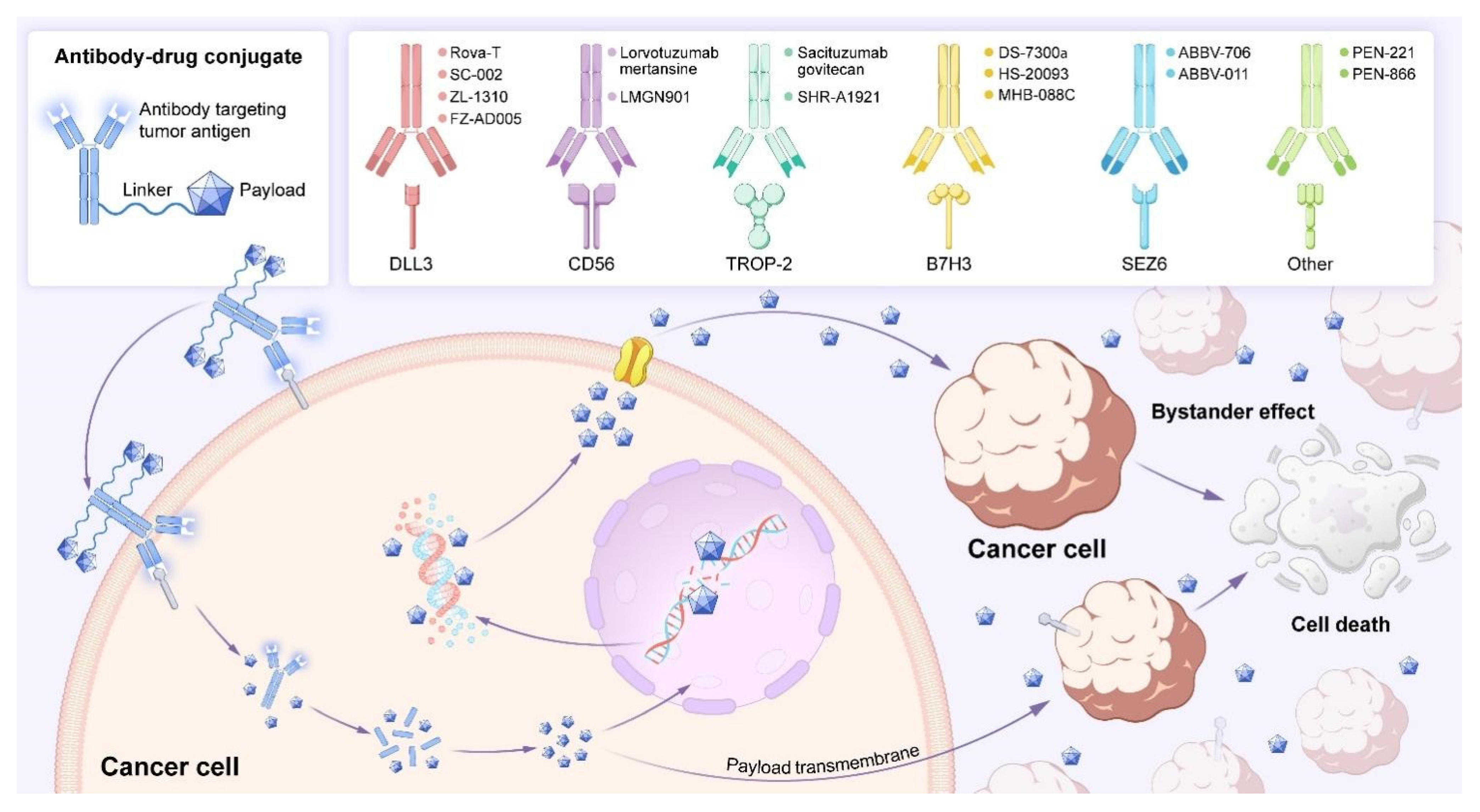
2.2. The Mechanism of Action of ADCs
3. Targeting SCLC: Potential Targets and Advanced ADCs
3.1. DLL3-Targeting ADCs
| Target | Agent Name | Linker/Payload Mechanism | Clinical Phase | Efficacy Outcomes (ORR/PFS) | Key Toxicities | Status/Outcome | References |
|---|---|---|---|---|---|---|---|
| DLL3 | Rovalpituzumab Tesirine (Rova-T) | Not specified/Tesirine (DNA-damaging agent) | I–III | Phase II: ORR 12.4% (all), mPFS ~4.6 mo (Phase I) | Fatigue, pleural effusion, thrombocytopenia, skin reactions, photosensitivity | Clinical development terminated. Failed to show survival benefit vs. standard care. | [36,37,38,39,40,41,42] |
| DLL3 | SC-002 | Different chemical linker vs. Rova-T/Not specified | Early-phase | ORR 14% (all), 11.8% (DLL3-positive) | Serous cavity effusion, dyspnea, thrombocytopenia | Study discontinued. Limited efficacy and significant toxicity. | [43] |
| DLL3 | ZL-1310 | Cleavable linker/Topoisomerase I inhibitor | I (Ongoing, NCT06179069) | ORR in brain metastases: 80%; DCR: 100% | Anaemia, neutropenia, thrombocytopenia | Active, promising. Manageable safety and notable activity, especially in brain metastases. | [44,45] |
| DLL3 | FZ-AD005 | Cathepsin-cleavable linker/DXd (Topo I inhibitor) | I (Ongoing, NCT06424665) | Primary endpoints include ORR (data pending) | DLTs, AEs under evaluation | Active, early-stage. First-in-human study assessing safety and preliminary activity. | [46,47] |
| DLL3 | SHR-4849 | Not specified/Not specified | I | ORR 73.2% (at ≥2.4 mg/kg); DCR 93.0%; DCR in brain metastases: 100% | Manageable safety profile, well-tolerated | Active, highly promising. High response rates support further clinical development. | [48] |
| B7-H3 | DS-7300a (I-DXd) | Cleavable tetrapeptide linker/I-DXd (Topo I inhibitor) | II | ORR 48.2%; DCR 87.6%; mPFS 4.9 mo; mOS 10.3 mo; intracranial ORR 46.2% | Safety profile deemed manageable | Breakthrough therapy designation. Robust and durable activity in pre-treated ES-SCLC. | [49,50,51,52] |
| B7-H3 | HS-20093 | Not specified/Not specified | I/II | ORR: 61.3% (8 mg/kg), 50.0% (10 mg/kg); mPFS: 5.9 mo, 7.3 mo | Neutropenia, leukopenia, thrombocytopenia, anaemia | Active. Promising antitumor activity with haematological toxicities. | [53] |
| B7-H3 | MHB088C (QLC5508) | Not specified/SuperTopoi (potent Topo I inhibitor) | I/II | ORR: 42.9–57.6%; mPFS: 5.5–5.9 mo | Neutropenia, thrombocytopenia, anaemia; mild ILD (1%) | Active, Phase III planned. Encouraging efficacy and acceptable safety. | [54] |
| SEZ6 | ABBV-011 | Non-cleavable linker/Calicheamicin | I | ORR 19% (all), 25% (1 mg/kg cohort); mPFS 3.5 mo | Fatigue, nausea, thrombocytopenia, hepatotoxicity | Development discontinued. Significant hepatotoxicity. | [55,56,57] |
| SEZ6 | ABBV-706 | Valine-alanine linker/Topoisomerase I inhibitor | I | ORR in 1.8 mg/kg group: 62.1% (Top1i-naïve); ORR in brain metastases: 62.5% | Anaemia, neutropenia | Active, Phase II initiated. Manageable safety and favourable antitumor activity. | [55,58] |
| CD56 | Lorvotuzumab Mertansine (LM) | Not specified/DM1 (microtubule inhibitor) | I/II | ORR 67.1% (combo); mPFS 6.2 mo | Significant TRAEs leading to death (18/94 patients) | Efficacy limited, toxicity high. Requires optimised therapeutic regimens. | [59,60,61,62,63] |
| TROP-2 | Sacituzumab Govitecan (SG) | Cleavable CL2A linker/SN-38 (Topo I inhibitor) | I/II | TROPiCS-03 (2L): ORR 41.9%; mPFS 4.4 mo; mOS 13.6 mo | Neutropenia, diarrhoea; one death from neutropenic sepsis | Clinically meaningful activity. Favourable tolerability, requires Phase III validation. | [64,65,66,67,68,69] |
| TROP-2 | SHR-A1921 | Tetrapeptide cleavable linker/Topoisomerase I inhibitor | I | ORR 33.3%; DCR 66.7%; mPFS 3.8 mo | Stomatitis, nausea, vomiting | Active, promising. Encouraging efficacy and manageable safety in heavily pre-treated patients. | [70,71] |
| SSTR2 | PEN-221 (SMDC) | Not specified/DM1 (maytansinoid) | I/II | Disease stabilisation up to 12 weeks | Well-tolerated in early studies | Early clinical activity. Showed preliminary efficacy in SSTR2-positive SCLC. | [72,73,74,75] |
| HSP90 | PEN-866 (SMDC) | Not specified/SN-38 (Topo I inhibitor) | I/II | Antitumor activity in advanced solid tumours | Expected AE profile, well-tolerated | Active. Evaluated in basket trials; entering clinical development in China. | [76,77] |
| Trial/NCT Number | Phase | Agent | Mechanism of Action | Eligibility | Intervention | Primary Endpoint(s) | Key Efficacy Outcomes | Toxicity (≥3 AEs) | Current Status |
|---|---|---|---|---|---|---|---|---|---|
| NCT03061812 | III | Rova-T | Anti-DLL3 ADC | DLL3-high ES-SCLC | Rova-T 0.3 mg/kg vs. Topotecan 1.5 mg/m2 D1–5 | Median PFS, OS | PFS: 3.0 mo (Rova-T) vs. 4.3 mo (Topo); OS: 6.3 mo vs. 8.6 mo | 64% vs. 54% | Completed |
| NCT01901653 | I | Rova-T | Anti-DLL3 ADC | Relapsed SCLC | Rova-T 0.2 or 0.3 mg/kg Q6W | ORR, AEs | ORR: 12% (0.3 mg/kg) | 39% (Gr 3+ AEs) | Completed |
| NCT03334487 | IIIb | Rova-T | Anti-DLL3 ADC | Relapsed/Refractory DLL3+ SCLC | Rova-T 0.3 mg/kg Q6W + Dexamethasone | PFS, OS | PFS: 3.5 mo; OS: 5.6 mo | 58% (Gr 3+) | Completed |
| NCT03086239 | I | Rova-T | Anti-DLL3 ADC | Advanced SCLC | Rova-T 0.2/0.3 mg/kg Q6W + Dexamethasone | PFS, OS | ORR: 14%; PFS: 2.7 mo | 52% (Gr 3+) | Completed |
| NCT03033511 | III | Rova-T | Anti-DLL3 ADC | ES-SCLC | Rova-T 0.3 mg/kg Q6W vs. Placebo | PFS, AEs | PFS: 3.0 mo vs. 2.8 mo (NS) | 61% vs. 45% | Terminated |
| NCT02819999 | -- | Rova-T | Anti-DLL3 ADC | SCLC | Rova-T + Cisplatin/Etoposide | AEs | ORR: 40% | 70% (Gr 3+) | Terminated |
| NCT03026166 | I/II | Rova-T | Anti-DLL3 ADC | ES-SCLC | Rova-T + Nivo vs. Rova-T + Nivo/Ipi | ORR, DOR, PFS, OS | ORR: 17% (combo) | 54% (Gr 3+) | Completed |
| NCT03000257 | I | Rova-T | Anti-DLL3 ADC | SCLC | Rova-T + Bogriamab (BMS-986012) | ORR | ORR: 15% | 48% (Gr 3+) | Completed |
| (Early-phase ref) | I/II | Rova-T | Anti-DLL3 ADC | Recurrent SCLC | Rova-T Monotherapy | ORR, OS | ORR: 10–15%; OS: ~6 mo | ~50% (Gr 3+) | Development Terminated |
| NCT02500914 | I | SC-002 | Anti-DLL3 ADC | Refractory SCLC/LCNEC | SC-002 Monotherapy | ORR, Safety | ORR: 0% (early stop) | 45% (Gr 3+) | Discontinued |
| NCT06179069 | I | ZL-1310 | Anti-DLL3 ADC | r/r ES-SCLC | ZL-1310 Monotherapy | Safety, Efficacy | Pending | Pending | Recruiting |
| NCT06424665 | I | FZ-AD005 | Anti-DLL3 ADC | Advanced Solid Tumours | FZ-AD005 (FIH) | DLTs, MTD, AEs | Pending | Pending | Recruiting |
| NCT06443489 | I | SHR-4849 | Anti-DLL3 ADC | Advanced Solid Tumours | SHR-4849 Monotherapy | Safety, Tolerability | Pending | Pending | Recruiting |
| NCT06613009 | I | IBJ3009 | Anti-DLL3 ADC | SCLC | IBJ3009 Monotherapy | Safety, Tolerability | Pending | Pending | Recruiting |
| B7-H3 | |||||||||
| NCT05280470 | II | Ifinatamab Deruxtecan (I-DXd) | Anti-B7-H3 ADC | Pre-treated ES-SCLC | I-DXd 12 mg/kg Q3W | ORR | ORR: 52% (interim) | 35% (Gr 3+) | Active, not recruiting |
| NCT06203210 | II/III | Ifinatamab Deruxtecan (I-DXd) | Anti-B7-H3 ADC | Relapsed SCLC | I-DXd vs. Topotecan/Amrubicin | OS, PFS | Pending | Pending | Recruiting |
| NCT05276609/NCT06498479 | I | HS-20093 | Anti-B7-H3 ADC | Advanced Solid Tumours | HS-20093 Monotherapy | Safety, Tolerability | Pending | Pending | Recruiting |
| NCT05914116 | I/IIa | HS-20093 | Anti-B7-H3 ADC | SCLC | HS-20093 + Topotecan | Safety, Efficacy | Pending | Pending | Recruiting |
| NCT05652855 | I/II | DB-1311 | Anti-B7-H3 ADC | Advanced Solid Tumours (incl. SCLC) | DB-1311 Monotherapy | Safety, ORR | Pending | Pending | Not yet recruiting |
| NCT06801834 | I | MHB088C | Anti-B7-H3 ADC | Advanced Solid Tumours | MHB088C Monotherapy | Safety, Tolerability | Pending | Pending | Recruiting |
| NCT06612151 | I | YL201 | Anti-B7-H3 ADC | SCLC | YL201 + Topotecan | Safety, Tolerability | Pending | Pending | Recruiting |
| TROP2 | |||||||||
| (Ongoing trial) | III | Sacituzumab Govitecan | Anti-TROP2 ADC | Relapsed SCLC | SG vs. Topotecan/Amrubicin | PFS, OS | Pending | Pending | Recruiting |
| NCT05154604 | I | SHR-A1921 | Anti-TROP2 ADC | Advanced Solid Tumours (incl. SCLC) | SHR-A1921 Monotherapy | Safety, Tolerability | Pending | Pending | Unknown |
| SEZ6 | |||||||||
| NCT05154604 | I | ABBV-706 | Anti-SEZ6 ADC | Advanced Solid Tumours (incl. SCLC) | ABBV-706 ± Chemo ± Budigalimab | Safety, Tolerability | Pending | Pending | Unknown |
3.1.1. Rovalpituzumab Tesirine (Rova-T)
3.1.2. SC-002
3.1.3. ZL-1310
3.1.4. FZ-AD005
3.1.5. SHR-4849
3.2. B7H3-Targeting ADCs
3.2.1. DS-7300a
3.2.2. HS-20093
3.2.3. MHB088C (QLC5508)
3.3. Seizure-Related Homologue 6 (SEZ6) -Targeting ADCs
3.3.1. ABBV-011
3.3.2. ABBV-706
3.4. CD56-Targeting ADCs
Lorvotuzumab Mertansine (LMGN901, LM)
3.5. TROP-2-Targeting ADCs
3.5.1. Sacituzumab Govitecan (SG)
3.5.2. SHR-A1921
4. Small-Molecule Drug Conjugates (SMDCs): New Options for Targeted Therapy Based on ADCs
4.1. PEN-221
4.2. PEN-866
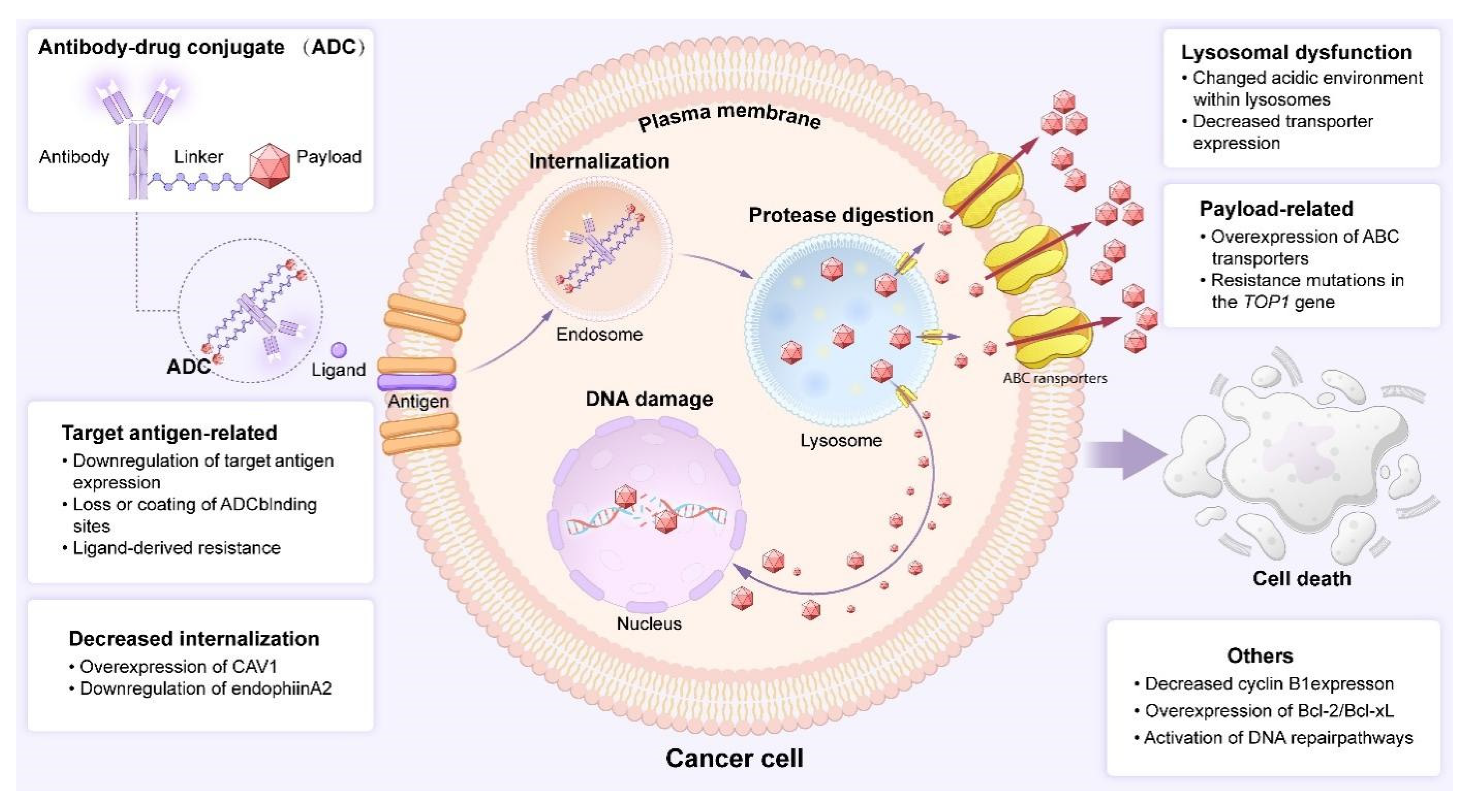
5. Challenges Facing ADC Drugs and Resolution Strategies
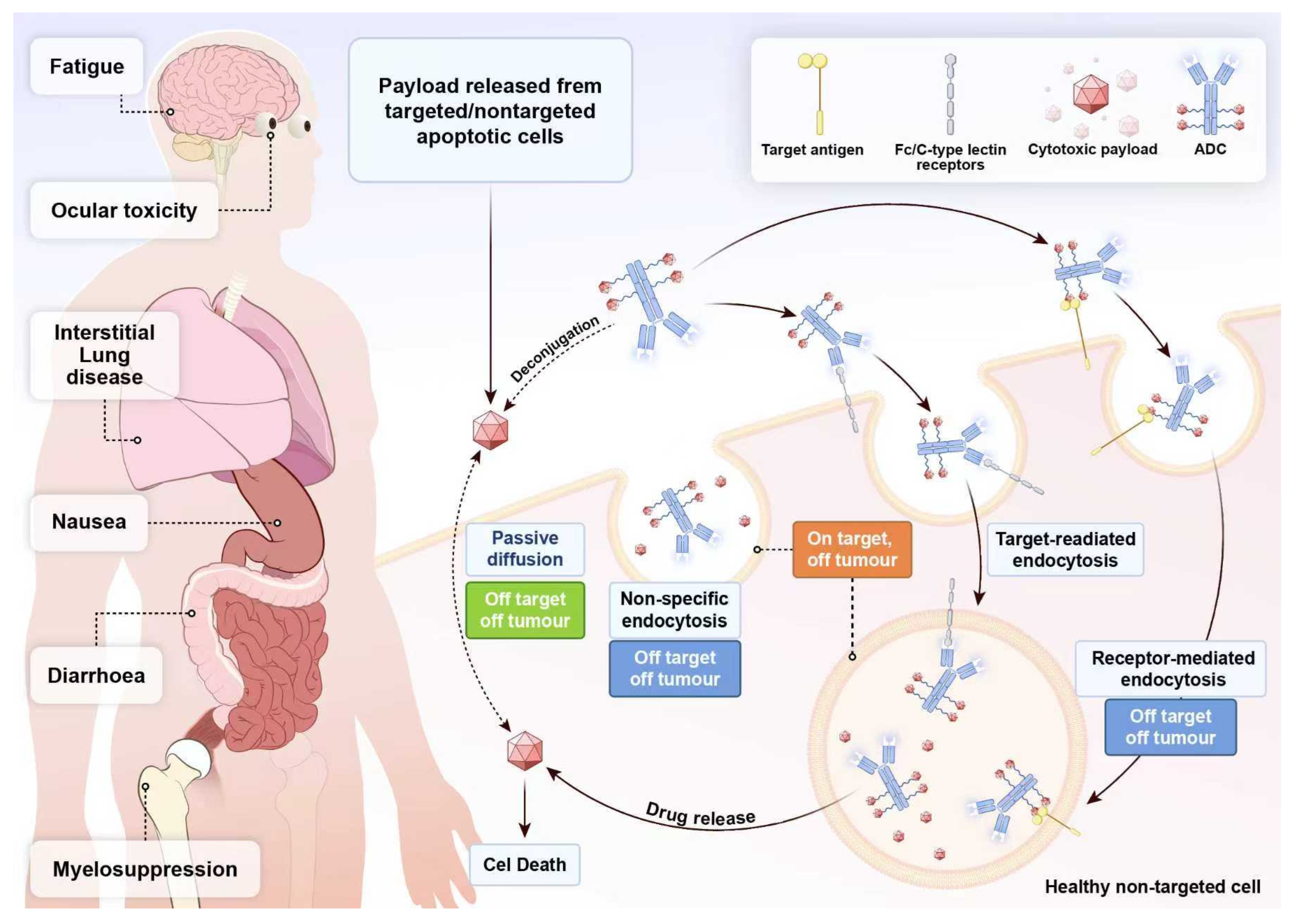
5.1. ADCs Drug Toxicity: A Critical Factor That Cannot Be Overlooked
5.2. ADCs Drug Resistance: The Looming Threat in Targeted Therapy
6. Future Development Direction of ADC
6.1. Synergy in Action: Reshaping the Therapeutic Landscape with ADC-Based Combinations
6.2. Optimising ADC Therapeutics: Rational Design and Enhanced Safety Management
7. Discussions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Antibody–drug conjugates |
| AEs | Adverse Events |
| BICR | Blinded Independent Central Review |
| Cmax | Maximum Plasma Concentration |
| CR | Complete Response |
| DCR | Disease Control Rate |
| DLTs | Dose-Limiting Toxicities |
| DOR | Duration of Response |
| ED | Extensive Disease |
| ESMO | European Society for Medical Oncology |
| FDA | U.S. Food and Drug Administration |
| ICI | Immune Checkpoint Inhibitors |
| ILD | Interstitial Lung Disease |
| LM | Lorvotuzumab Mertansine |
| mOS | Median overall survival |
| MTD | Maximum Tolerated Dose |
| NSCLC | Non-Small Cell Lung Cancer |
| ORR | Objective Response Rate |
| OS | Overall Survival |
| PDX | Patient-derived xenograft |
| PFS | Progression-Free Survival |
| RP2D | Recommended Phase II Dose |
| SCLC | Small Cell Lung Cancer |
| SEZ6 | Seizure-related homologue 6 |
| SG | Sacituzumab Govitecan |
| SMDC | Small-Molecule Drug Conjugates |
| STTR2 | Somatostatin Receptor 2 |
| TEAEs | Treatment-Emergent Adverse Events |
| TROP-2 | Trophoblast Cell Surface Antigen 2 |
References
- Kim, S.Y.; Park, H.S.; Chiang, A.C. Small Cell Lung Cancer: A Review. JAMA 2025, 333, 1906–1917. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D. Pathology of lung cancer. Clin. Chest Med. 2011, 32, 669–692. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Chansky, K.; Crowley, J.; Beyruti, R.; Kubota, K.; Turrisi, A.; Eberhardt, W.E.; van Meerbeeck, J.; Rami-Porta, R. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 300–311. [Google Scholar] [CrossRef]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers 2021, 7, 3. [Google Scholar] [CrossRef]
- Morabito, A.; Carillio, G.; Daniele, G.; Piccirillo, M.C.; Montanino, A.; Costanzo, R.; Sandomenico, C.; Giordano, P.; Normanno, N.; Perrone, F.; et al. Treatment of small cell lung cancer. Crit. Rev. Oncol. Hematol. 2014, 91, 257–270. [Google Scholar] [CrossRef]
- Waqar, S.N.; Morgensztern, D. Treatment advances in small cell lung cancer (SCLC). Pharmacol. Ther. 2017, 180, 16–23. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef]
- Hurwitz, J.; Haggstrom, L.R.; Lim, E. Antibody–drug conjugates: Ushering in a new era of cancer therapy. Pharmaceutics 2023, 15, 2017. [Google Scholar] [CrossRef]
- Strebhardt, K.; Ullrich, A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer 2008, 8, 473–480. [Google Scholar] [CrossRef]
- Zolot, R.S.; Basu, S.; Million, R.P. Antibody-drug conjugates. Nat. Rev. Drug Discov. 2013, 12, 259–260. [Google Scholar] [CrossRef]
- Tsuchikama, K.; An, Z. Antibody-drug conjugates: Recent advances in conjugation and linker chemistries. Protein Cell 2018, 9, 33–46. [Google Scholar] [CrossRef]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Abdollahpour-Alitappeh, M.; Lotfinia, M.; Gharibi, T.; Mardaneh, J.; Farhadihosseinabadi, B.; Larki, P.; Faghfourian, B.; Sepehr, K.S.; Abbaszadeh-Goudarzi, K.; Abbaszadeh-Goudarzi, G.; et al. Antibody-drug conjugates (ADCs) for cancer therapy: Strategies, challenges, and successes. J. Cell. Physiol. 2019, 234, 5628–5642. [Google Scholar] [CrossRef]
- Rezaei, M.A.; Pourasghari, H.; Karimi, F.; Rajaie, S.; Shahmohammady, A.; Behzadifar, M.; Dehqan, A.H.; Azari, S. Economic evaluation of antibody-drug conjugates (ADCs) in patients with breast cancer: Systematic review. Eur. J. Clin. Pharmacol. 2025; ahead of print. [Google Scholar] [CrossRef]
- Duan, R. Development of antibody-drug conjugates in gastric cancer: An overview of clinical trials from 2005 to 2025. Int. J. Surg. 2025; ahead of print. [Google Scholar] [CrossRef]
- Xu, J.; Ma, Y.; Hu, P.; Yao, J.; Chen, H.; Ma, Q. Recent advances in antibody-drug conjugates for metastatic castration-resistant prostate cancer. Zhejiang Da Xue Xue Bao Yi Xue Ban 2025, 54, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Teicher, B.A.; Hassan, R. Antibody-drug conjugates for cancer therapy. Lancet Oncol. 2016, 17, e254–e262. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, B.; Pan, Z.; Mo, C.; Zhao, X.; Liu, G.; Hou, P.; Cui, Q.; Xu, Z.; Wang, W.; et al. Antibody-Drug Conjugates (ADCs): Current and future biopharmaceuticals. J. Hematol. Oncol. 2025, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Goldmacher, V.S. Antibody-drug conjugates in cancer and beyond: Progress, promise, and perspectives. Antib. Ther. 2025, 8, 239–241. [Google Scholar] [CrossRef]
- Goulet, D.R.; Atkins, W.M. Considerations for the Design of Antibody-Based Therapeutics. J. Pharm. Sci. 2020, 109, 74–103. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Li, Y.-Y.; Liu, X.-Y.; Lu, X.-L.; Cao, X.; Jiao, B.-H. Marine antibody–drug conjugates: Design strategies and research progress. Mar. Drugs 2017, 15, 18. [Google Scholar] [CrossRef]
- Schumacher, D.; Helma, J.; Schneider, A.F.L.; Leonhardt, H.; Hackenberger, C.P.R. Nanobodies: Chemical Functionalization Strategies and Intracellular Applications. Angew. Chem. Int. Ed. Engl. 2018, 57, 2314–2333. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Adjei, A.A. Antibody-Drug Conjugates for the Therapy of Thoracic Malignancies. J. Thorac. Oncol. 2019, 14, 358–376. [Google Scholar] [CrossRef]
- Leung, D.; Wurst, J.M.; Liu, T.; Martinez, R.M.; Datta-Mannan, A.; Feng, Y. Antibody Conjugates-Recent Advances and Future Innovations. Antibodies 2020, 9, 2. [Google Scholar] [CrossRef]
- Mehrling, T.; Soltis, D. Challenges in optimising the successful construction of antibody drug conjugates in cancer therapy. Antibodies 2018, 7, 11. [Google Scholar] [CrossRef]
- He, J.; Zeng, X.; Wang, C.; Wang, E.; Li, Y. Antibody-drug conjugates in cancer therapy: Mechanisms and clinical studies. MedComm 2024, 5, e671. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.L.; Schwettmann, B.; McArthur, H.L.; Chan, I.S. Antibody-drug conjugates in breast cancer: Overcoming resistance and boosting immune response. J. Clin. Investig. 2023, 133, e172156. [Google Scholar] [CrossRef]
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 327–344. [Google Scholar] [CrossRef]
- Gu, F.; Zhao, K.; Yan, H.; Sui, D. Research Progress on the Role of Notch Signaling Pathway in Small Cell Lung Cancer. Zhongguo Fei Ai Za Zhi 2025, 28, 513–520. [Google Scholar] [PubMed]
- Saunders, L.R.; Bankovich, A.J.; Anderson, W.C.; Aujay, M.A.; Bheddah, S.; Black, K.; Desai, R.; Escarpe, P.A.; Hampl, J.; Laysang, A. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci. Transl. Med. 2015, 7, ra136–ra302. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Li, X.; Yuan, X.; Chu, Q. Targeting the Notch signaling pathway and the Notch ligand, DLL3, in small cell lung cancer. Biomed. Pharmacother. 2023, 159, 114248. [Google Scholar] [CrossRef]
- Li, B.; Wang, L.; Qi, X.; Liu, Y.; Li, J.; Lv, J.; Zhou, X.; Cai, X.; Shan, J.; Ma, X. NOTCH signaling inhibition after DAPT treatment exacerbates alveolar echinococcosis hepatic fibrosis by blocking M1 and enhancing M2 polarization. FASEB J. 2023, 37, e22901. [Google Scholar] [CrossRef]
- Lashari, B.H.; Vallatharasu, Y.; Kolandra, L.; Hamid, M.; Uprety, D. Rovalpituzumab Tesirine: A Novel DLL3-Targeting Antibody-Drug Conjugate. Drugs R D 2018, 18, 255–258. [Google Scholar] [CrossRef]
- Rudin, C.M.; Pietanza, M.C.; Bauer, T.M.; Ready, N.; Morgensztern, D.; Glisson, B.S.; Byers, L.A.; Johnson, M.L.; Burris, H.A., 3rd; Robert, F.; et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: A first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017, 18, 42–51. [Google Scholar] [CrossRef]
- Morgensztern, D.; Besse, B.; Greillier, L.; Santana-Davila, R.; Ready, N.; Hann, C.L.; Glisson, B.S.; Farago, A.F.; Dowlati, A.; Rudin, C.M. Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: Results from the phase II TRINITY study. Clin. Cancer Res. 2019, 25, 6958–6966. [Google Scholar] [CrossRef]
- Blackhall, F.; Jao, K.; Greillier, L.; Cho, B.C.; Penkov, K.; Reguart, N.; Majem, M.; Nackaerts, K.; Syrigos, K.; Hansen, K.; et al. Efficacy and Safety of Rovalpituzumab Tesirine Compared with Topotecan as Second-Line Therapy in DLL3-High SCLC: Results from the Phase 3 TAHOE Study. J. Thorac. Oncol. 2021, 16, 1547–1558. [Google Scholar] [CrossRef]
- Johnson, M.L.; Zvirbule, Z.; Laktionov, K.; Helland, A.; Cho, B.C.; Gutierrez, V.; Colinet, B.; Lena, H.; Wolf, M.; Gottfried, M.; et al. Rovalpituzumab Tesirine as a Maintenance Therapy After First-Line Platinum-Based Chemotherapy in Patients with Extensive-Stage-SCLC: Results from the Phase 3 MERU Study. J. Thorac. Oncol. 2021, 16, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.; Nikolinakos, P.; Leal, T.; Lehman, J.; Morgensztern, D.; Patel, J.D.; Wrangle, J.M.; Curigliano, G.; Greillier, L.; Johnson, M.L.; et al. A Phase 1-2 Study of Rovalpituzumab Tesirine in Combination with Nivolumab Plus or Minus Ipilimumab in Patients with Previously Treated Extensive-Stage SCLC. J. Thorac. Oncol. 2021, 16, 1559–1569. [Google Scholar] [CrossRef]
- Hann, C.L.; Burns, T.F.; Dowlati, A.; Morgensztern, D.; Ward, P.J.; Koch, M.M.; Chen, C.; Ludwig, C.; Patel, M.; Nimeiri, H.; et al. A Phase 1 Study Evaluating Rovalpituzumab Tesirine in Frontline Treatment of Patients with Extensive-Stage SCLC. J. Thorac. Oncol. 2021, 16, 1582–1588. [Google Scholar] [CrossRef]
- Morgensztern, D.; Johnson, M.; Rudin, C.M.; Rossi, M.; Lazarov, M.; Brickman, D.; Fong, A. SC-002 in patients with relapsed or refractory small cell lung cancer and large cell neuroendocrine carcinoma: Phase 1 study. Lung Cancer 2020, 145, 126–131. [Google Scholar] [CrossRef]
- Lin, L.; Wan, B.; Ye, Q.; Wang, L.; Wang, C.; Peng, W.; Wang, J.; Dai, X.; Chen, M.; Lv, C. 241P Development and characterization of a novel DLL3-targeting antibody drug conjugate (ADC) for the treatment of solid tumors. ESMO Open 2024, 9, 102891. [Google Scholar] [CrossRef]
- Patel, M.R.; Wu, Y.-L.L.C.; Wang, Z.; Rocha, P.; Wang, Q.; Du, Y.; Dy, G.K.; Dowlati, A.; Spira, A.; Dong, X. ZL-1310, a DLL3 ADC, in patients with extensive stage small cell lung cancer: Ph1 trial update. J. Clin. Oncol. 2025, 43, 3041. [Google Scholar] [CrossRef]
- Guo, Q.; Gao, B.; Song, R.; Li, W.; Zhu, S.; Xie, Q.; Lou, S.; Wang, L.; Shen, J.; Zhao, T. FZ-AD005, a Novel DLL3-Targeted Antibody–Drug Conjugate with Topoisomerase I Inhibitor, Shows Potent Antitumor Activity in Preclinical Models. Mol. Cancer Ther. 2024, 23, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Bragasin, E.I.; Cheng, J.; Ford, L.; Poei, D.; Ali, S.; Hsu, R. Advances in adoptive cell therapies in small cell lung cancer. Explor. Target. Anti-Tumor Ther. 2025, 6, 1002302. [Google Scholar] [CrossRef] [PubMed]
- Zinberg, Y. ADC for SCLC Yields High Response Rates in Early Trial; American Association for Cancer Research: Philadelphia, PA, USA, 2025. [Google Scholar]
- Fabrizio, F.P.; Muscarella, L.A.; Rossi, A. B7-H3/CD276 and small-cell lung cancer: What’s new? Transl. Oncol. 2024, 39, 101801. [Google Scholar] [CrossRef]
- Yamato, M.; Hasegawa, J.; Maejima, T.; Hattori, C.; Kumagai, K.; Watanabe, A.; Nishiya, Y.; Shibutani, T.; Aida, T.; Hayakawa, I. DS-7300a, a DNA topoisomerase I inhibitor, DXd-based antibody–drug conjugate targeting B7-H3, exerts potent antitumor activities in preclinical models. Mol. Cancer Ther. 2022, 21, 635–646. [Google Scholar] [CrossRef]
- Johnson, M.; da Rocha, P.S.; Kim, Y.; Chen, Y.; Paz-Ares, L.; Girard, N.; Hann, C.; Nishio, M.; Ahn, M.; Qian, M. 1787P Intracranial response in patients (pts) with baseline (BL) brain metastases (BM) and extensive-stage (ES) small cell lung cancer (SCLC) treated with ifinatamab deruxtecan (I-DXd) in the IDeate-Lung01 study. Ann. Oncol. 2024, 35, S1062–S1063. [Google Scholar] [CrossRef]
- Lézard, L. Ifinatamab Deruxtecan Granted Breakthrough Therapy Designation by US FDA for Patients with Pretreated Extensive-Stage Small Cell Lung Cancer; Merck & Co., Inc.: Rahway, NJ, USA, 2025. [Google Scholar]
- Wang, J.; Duan, J.; Sun, Y.; Xing, L.; Han, L.; Wang, Q.; Wu, L.; Chen, J.; Lu, P.; Guo, W. ARTEMIS-001: Data from a phase 1a/b study of HS-20093 in patients with relapsed small cell lung cancer (SCLC). J. Clin. Oncol. 2024, 42, 8093. [Google Scholar] [CrossRef]
- Shen, L.; Zhou, C.; Yu, Y.; Yu, X.; Meng, X.; Sun, Y.; Ji, Y.; Ji, Y.; Ning, F.; Jia, Y. Efficacy and safety of MHB088C, a novel B7-H3-targeted ADC, in patients with relapsed extensive-stage small cell lung cancer (ES-SCLC): Subgroup analysis from a phase 1/2 multicenter study. J. Clin. Oncol. 2025, 43, 8510. [Google Scholar] [CrossRef]
- Ajay, A.; Wang, H.; Rezvani, A.; Savari, O.; Grubb, B.J.; McColl, K.S.; Yoon, S.; Joseph, P.L.; Kopp, S.R.; Kresak, A.M.; et al. Assessment of targets of antibody drug conjugates in SCLC. NPJ Precis. Oncol. 2025, 9, 1. [Google Scholar] [CrossRef]
- Wiedemeyer, W.R.; Gavrilyuk, J.; Schammel, A.; Zhao, X.; Sarvaiya, H.; Pysz, M.; Gu, C.; You, M.; Isse, K.; Sullivan, T.; et al. ABBV-011, A Novel, Calicheamicin-Based Antibody-Drug Conjugate, Targets SEZ6 to Eradicate Small Cell Lung Cancer Tumors. Mol. Cancer Ther. 2022, 21, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Morgensztern, D.; Ready, N.; Johnson, M.L.; Dowlati, A.; Choudhury, N.; Carbone, D.P.; Schaefer, E.; Arnold, S.M.; Puri, S.; Piotrowska, Z.; et al. A Phase I First-in-Human Study of ABBV-011, a Seizure-Related Homolog Protein 6-Targeting Antibody-Drug Conjugate, in Patients with Small Cell Lung Cancer. Clin. Cancer Res. 2024, 30, 5042–5052. [Google Scholar] [CrossRef] [PubMed]
- Chandana, S.R.; Choudhury, N.J.; Dowlati, A.; Chiang, A.C.; Garmezy, B.; Kim, J.-H.; Byers, L.A.; Ahn, M.-J.; Kim, T.M.; Kim, Y.-C. First-in-human study of ABBV-706, a seizure-related homolog protein 6 (SEZ6)–targeting antibody-drug conjugate (ADC), in patients (pts) with advanced solid tumors. J. Clin. Oncol. 2024, 42, 3001. [Google Scholar] [CrossRef]
- Baine, M.K.; Hsieh, M.S.; Lai, W.V.; Egger, J.V.; Jungbluth, A.A.; Daneshbod, Y.; Beras, A.; Spencer, R.; Lopardo, J.; Bodd, F.; et al. SCLC Subtypes Defined by ASCL1, NEUROD1, POU2F3, and YAP1: A Comprehensive Immunohistochemical and Histopathologic Characterization. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, 1823–1835. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Abdayem, P.; Adjei, A.A.; Planchard, D. Antibody-drug conjugates: A promising novel therapeutic approach in lung cancer. Lung Cancer 2022, 163, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Berdeja, J.G. Lorvotuzumab mertansine: Antibody-drug-conjugate for CD56+ multiple myeloma. Front. Biosci. 2014, 19, 163–170. [Google Scholar] [CrossRef]
- Whiteman, K.R.; Johnson, H.A.; Mayo, M.F.; Audette, C.A.; Carrigan, C.N.; LaBelle, A.; Zukerberg, L.; Lambert, J.M.; Lutz, R.J. Lorvotuzumab mertansine, a CD56-targeting antibody-drug conjugate with potent antitumor activity against small cell lung cancer in human xenograft models. mAbs 2014, 6, 556–566. [Google Scholar] [CrossRef]
- Socinski, M.A.; Kaye, F.J.; Spigel, D.R.; Kudrik, F.J.; Ponce, S.; Ellis, P.M.; Majem, M.; Lorigan, P.; Gandhi, L.; Gutierrez, M.E.; et al. Phase 1/2 Study of the CD56-Targeting Antibody-Drug Conjugate Lorvotuzumab Mertansine (IMGN901) in Combination with Carboplatin/Etoposide in Small-Cell Lung Cancer Patients with Extensive-Stage Disease. Clin. Lung Cancer 2017, 18, 68–76.e2. [Google Scholar] [CrossRef]
- Trerotola, M.; Cantanelli, P.; Guerra, E.; Tripaldi, R.; Aloisi, A.L.; Bonasera, V.; Lattanzio, R.; de Lange, R.; Weidle, U.H.; Piantelli, M.; et al. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene 2013, 32, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Sacituzumab Govitecan: First Approval. Drugs 2020, 80, 1019–1025. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Sharkey, R.M. Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy. Expert Opin. Biol. Ther. 2020, 20, 871–885. [Google Scholar] [CrossRef]
- Gray, J.E.; Heist, R.S.; Starodub, A.N.; Camidge, D.R.; Kio, E.A.; Masters, G.A.; Purcell, W.T.; Guarino, M.J.; Misleh, J.; Schneider, C.J.; et al. Therapy of Small Cell Lung Cancer (SCLC) with a Topoisomerase-I-inhibiting Antibody-Drug Conjugate (ADC) Targeting Trop-2, Sacituzumab Govitecan. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 5711–5719. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Messersmith, W.A.; Kio, E.A.; Berlin, J.D.; Vahdat, L.; Masters, G.A.; Moroose, R.; Santin, A.D.; Kalinsky, K.; Picozzi, V.; et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: Final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 746–756. [Google Scholar] [CrossRef]
- Dowlati, A.; Chiang, A.C.; Cervantes, A.; Babu, S.; Hamilton, E.; Wong, S.F.; Tazbirkova, A.; Sullivan, I.G.; van Marcke, C.; Italiano, A.; et al. Phase 2 Open-Label Study of Sacituzumab Govitecan as Second-Line Therapy in Patients with Extensive-Stage SCLC: Results from TROPiCS-03. J. Thorac. Oncol. 2025, 20, 799–808. [Google Scholar] [CrossRef]
- He, N.; Yang, C.; Yang, Y.; Xue, Z.; Xu, J.; Zhao, L.; Feng, J.; Ye, X.; Zhang, Z.; He, F. Abstract LB030: SHR-A1921, a novel TROP-2 ADC with an optimized design and well-balanced profile between efficacy and safety. Cancer Res. 2023, 83 (Suppl. S8), LB030. [Google Scholar] [CrossRef]
- Wang, J.; Wu, L.; Li, X.; Xing, N.; Zhang, S.; Song, Z.; Chen, L.; Dang, Q.; Liu, C.; Li, Y. OA04. 05 SHR-A1921, A TROP-2 targeted antibody-drug conjugate (ADC), in patients (pts) with advanced small-cell lung cancer (SCLC). J. Thorac. Oncol. 2024, 19, S16–S17. [Google Scholar] [CrossRef]
- Wang, T.; Li, M.; Wei, R.; Wang, X.; Lin, Z.; Chen, J.; Wu, X. Small Molecule–Drug Conjugates Emerge as a New Promising Approach for Cancer Treatment. Mol. Pharm. 2024, 21, 1038–1055. [Google Scholar] [CrossRef]
- White, B.H.; Whalen, K.; Kriksciukaite, K.; Alargova, R.; Au Yeung, T.; Bazinet, P.; Brockman, A.; DuPont, M.; Oller, H.; Lemelin, C.A.; et al. Discovery of an SSTR2-Targeting Maytansinoid Conjugate (PEN-221) with Potent Activity in Vitro and in Vivo. J. Med. Chem. 2019, 62, 2708–2719. [Google Scholar] [CrossRef]
- Whalen, K.A.; White, B.H.; Quinn, J.M.; Kriksciukaite, K.; Alargova, R.; Au Yeung, T.P.; Bazinet, P.; Brockman, A.; DuPont, M.M.; Oller, H.; et al. Targeting the Somatostatin Receptor 2 with the Miniaturized Drug Conjugate, PEN-221: A Potent and Novel Therapeutic for the Treatment of Small Cell Lung Cancer. Mol. Cancer Ther. 2019, 18 (Suppl. S15), 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Meyer, T.; Halperin, D.M.; Fojo, A.T.; Cook, N.; Blaszkowsky, L.S.; Schlechter, B.L.; Yao, J.C.; Jemiai, Y.; Kriksciukaite, K.; et al. First in human phase 1/2a study of PEN-221 somatostatin analog (SSA)-DM1 conjugate for patients (PTS) with advanced neuroendocrine tumor (NET) or small cell lung cancer (SCLC): Phase 1 results. J. Clin. Oncol. 2018, 36, 4097. [Google Scholar] [CrossRef]
- Li, L.; Chen, N.N.; You, Q.D.; Xu, X.L. An updated patent review of anticancer Hsp90 inhibitors (2013-present). Expert Opin. Ther. Pat. 2021, 31, 67–80. [Google Scholar] [CrossRef]
- Bendell, J.; Falchook, G.; Sen, S.; Johnson, M.; Jerkovic, G.; Sarapa, N.; Vilimas, R.; Kriksciukaite, K.; Mei, L.; Wooster, R. First in human phase I/IIa study of PEN-866, a heat shock protein 90 (HSP90) ligand–SN38 conjugate for patients with advanced solid tumours: Phase I results. Ann. Oncol. 2019, 30, v172–v173. [Google Scholar] [CrossRef]
- Li, J.; Shen, G.; Liu, Z.; Liu, Y.; Wang, M.; Zhao, F.; Ren, D.; Xie, Q.; Li, Z.; Liu, Z. Treatment-related adverse events of antibody-drug conjugates in clinical trials: A systematic review and meta-analysis. Cancer Innov. 2023, 2, 346–375. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, K.; Wang, K.; Zhu, H. Treatment-related adverse events of antibody–drug conjugates in clinical trials: A systematic review and meta-analysis. Cancer 2023, 129, 283–295. [Google Scholar] [CrossRef]
- Hafeez, U.; Parakh, S.; Gan, H.K.; Scott, A.M. Antibody-Drug Conjugates for Cancer Therapy. Molecules 2020, 25, 4764. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Mohan, N.; Dokmanovic, M.; Wu, W.J. Mechanisms contributing to ado-trastuzumab emtansine-induced toxicities: A gateway to better understanding of ADC-associated toxicities. Antib. Ther. 2021, 4, 55–59. [Google Scholar] [CrossRef]
- Eaton, J.S.; Miller, P.E.; Mannis, M.J.; Murphy, C.J. Ocular adverse events associated with antibody–drug conjugates in human clinical trials. J. Ocul. Pharmacol. Ther. 2015, 31, 589–604. [Google Scholar] [CrossRef]
- Zippelius, A.; Tolaney, S.M.; Tarantino, P.; Balthasar, J.P.; Thurber, G.M. Unveiling the molecular and immunological drivers of antibody–drug conjugates in cancer treatment. Nat. Rev. Cancer 2025, 25, 925–944. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Abelman, R.O.; Wu, B.; Spring, L.M.; Ellisen, L.W.; Bardia, A. Mechanisms of Resistance to Antibody-Drug Conjugates. Cancers 2023, 15, 1278. [Google Scholar] [CrossRef]
- Li, S.; Zhao, X.; Fu, K.; Zhu, S.; Pan, C.; Yang, C.; Wang, F.; To, K.K.W.; Fu, L. Resistance to antibody-drug conjugates: A review. Acta Pharm. Sin. B 2025, 15, 737–756. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Tarantino, P.; Rich, J.R.; LoRusso, P.M.; de Vries, E.G.E. The Journey of Antibody-Drug Conjugates: Lessons Learned from 40 Years of Development. Cancer Discov. 2024, 14, 2089–2108. [Google Scholar] [CrossRef]
- Díaz-Tejeiro, C.; López de Sá, A.; Poyatos-Racionero, E.; Ballestín, P.; Bartolomé, J.; Calvo, E.; Moreno, V.; Moris, F.; Pérez-Segura, P.; Gyorffy, B. Understanding the preclinical efficacy of antibody–drug conjugates. Int. J. Mol. Sci. 2024, 25, 12875. [Google Scholar] [CrossRef] [PubMed]
- Abel, M.L.; Takahashi, N.; Peer, C.; Redon, C.E.; Nichols, S.; Vilimas, R.; Lee, M.-J.; Lee, S.; Shelat, M.; Kattappuram, R. Targeting replication stress and chemotherapy resistance with a combination of sacituzumab govitecan and berzosertib: A phase I clinical trial. Clin. Cancer Res. 2023, 29, 3603–3611. [Google Scholar] [CrossRef] [PubMed]
- Nicolo, E.; Giugliano, F.; Ascione, L.; Tarantino, P.; Corti, C.; Tolaney, S.M.; Cristofanilli, M.; Curigliano, G. Combining antibody-drug conjugates with immunotherapy in solid tumors: Current landscape and future perspectives. Cancer Treat. Rev. 2022, 106, 102395. [Google Scholar] [CrossRef]
- Wei, Q.; Li, P.; Yang, T.; Zhu, J.; Sun, L.; Zhang, Z.; Wang, L.; Tian, X.; Chen, J.; Hu, C. The promise and challenges of combination therapies with antibody-drug conjugates in solid tumors. J. Hematol. Oncol. 2024, 17, 1. [Google Scholar] [CrossRef]
- Faivre, E.J.; Pysz, M.A.; Hainmhire, E.O.; Kreckler, L.; Doyle, K.; D’Souza, A.; Ding, Z.; Sondkar, T.; Maji, D.; Zhao, F. ABBV-706 is a novel SEZ6-targeted topoisomerase 1 inhibitor ADC for SCLC and other neuroendocrine cancers. Cancer Res. 2024, 84 (Suppl. S6), 3148. [Google Scholar] [CrossRef]
- Wang, D.; Wang, K.; An, R.; Yu, G.; Zhang, K.; Wang, D.; Jiang, K.; Gao, Y.; Cheng, Y.; Liu, Y. 715MO Safety and efficacy of sacituzumab tirumotecan (sac-TMT) in patients (pts) with previously treated advanced endometrial carcinoma (EC) and ovarian cancer (OC) from a phase II study. Ann. Oncol. 2024, 35, 1. [Google Scholar] [CrossRef]
- Le, X.; Hendriks, L.; Morabito, A.; Bonanno, L.; Okamoto, I.; Goldman, J.W.; Yu, H.A.; Brustugun, O.T.; Halvorsen, T.O.; Kim, Y.J. 1O: Osimertinib (osi) + datopotamab deruxtecan (Dato-DXd) in patients (pts) with EGFR-mutated (EGFRm) advanced NSCLC (aNSCLC) whose disease progressed on first-line (1L) osi: ORCHARD. J. Thorac. Oncol. 2025, 20, S2–S4. [Google Scholar] [CrossRef]
- Sharma, M.; Strickler, J.H.; Sommerhalder, D.; Kuboki, Y.; Perets, R.; Cohen, J.; Raimbourg, J.; Nakajima, T.E.; Yamamoto, N.; Cruz-Correa, M. First-in-human study of ABBV-400, a novel c-Met–targeting antibody-drug conjugate, in advanced solid tumors: Results in colorectal cancer. J. Clin. Oncol. 2024, 42, 3515. [Google Scholar] [CrossRef]
- Fu, C. Where does ISAC (immune-stimulating antibody conjugates) go from here? J. Immunother. Cancer 2025, 13, e012500. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Ma, Y.; Zhao, Y.; Fang, W.; Zhao, H.; Yang, Y.; Chen, L.; Hou, X.; Wang, Q. Phase I study of iza-bren (BL-B01D1), an EGFR x HER3 bispecific antibody-drug conjugate (ADC), in patients with locally advanced or metastatic small cell lung cancer (SCLC). J. Clin. Oncol. 2025, 43, 3002. [Google Scholar] [CrossRef]
- Marei, H.E.; Cenciarelli, C.; Hasan, A. Potential of antibody-drug conjugates (ADCs) for cancer therapy. Cancer Cell Int. 2022, 22, 255. [Google Scholar] [CrossRef]
- Smit, E.F.; Nakagawa, K.; Nagasaka, M.; Felip, E.; Goto, Y.; Li, B.T.; Pacheco, J.M.; Murakami, H.; Barlesi, F.; Saltos, A.N.; et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-mutated metastatic non-small cell lung cancer (NSCLC): Interim results of DESTINY-Lung01. J. Clin. Oncol. 2020, 38 (Suppl. S15), 9504. [Google Scholar] [CrossRef]
- Coleman, N.; Yap, T.A.; Heymach, J.V.; Meric-Bernstam, F.; Le, X. Antibody-drug conjugates in lung cancer: Dawn of a new era? NPJ Precis. Oncol. 2023, 7, 5. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Cui, Y.; Liao, D.; Sima, M.; Han, M.; Li, W.; Bi, Y.; Yue, D. Unlocking New Horizons: Antibody–Drug Conjugates in Small Cell Lung Cancer. Biology 2025, 14, 1677. https://doi.org/10.3390/biology14121677
Liu J, Cui Y, Liao D, Sima M, Han M, Li W, Bi Y, Yue D. Unlocking New Horizons: Antibody–Drug Conjugates in Small Cell Lung Cancer. Biology. 2025; 14(12):1677. https://doi.org/10.3390/biology14121677
Chicago/Turabian StyleLiu, Jiayu, Yan Cui, Dongying Liao, Mingwei Sima, Moxuan Han, Wanhao Li, Yan Bi, and Donghui Yue. 2025. "Unlocking New Horizons: Antibody–Drug Conjugates in Small Cell Lung Cancer" Biology 14, no. 12: 1677. https://doi.org/10.3390/biology14121677
APA StyleLiu, J., Cui, Y., Liao, D., Sima, M., Han, M., Li, W., Bi, Y., & Yue, D. (2025). Unlocking New Horizons: Antibody–Drug Conjugates in Small Cell Lung Cancer. Biology, 14(12), 1677. https://doi.org/10.3390/biology14121677






