Allies in the Skin Defense System: The Role of Thread Cells in the Evolution of Hagfish (Myxiniformes)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
2.2. Tissue Preparation and Histological Analysis
2.3. Rationale for Antibody Selection for Thread Cells
2.4. Immunoperoxidase
2.5. Immunofluorescence and Confocal Microscopy Analysis
2.6. Quantitative and Statistical Analysis
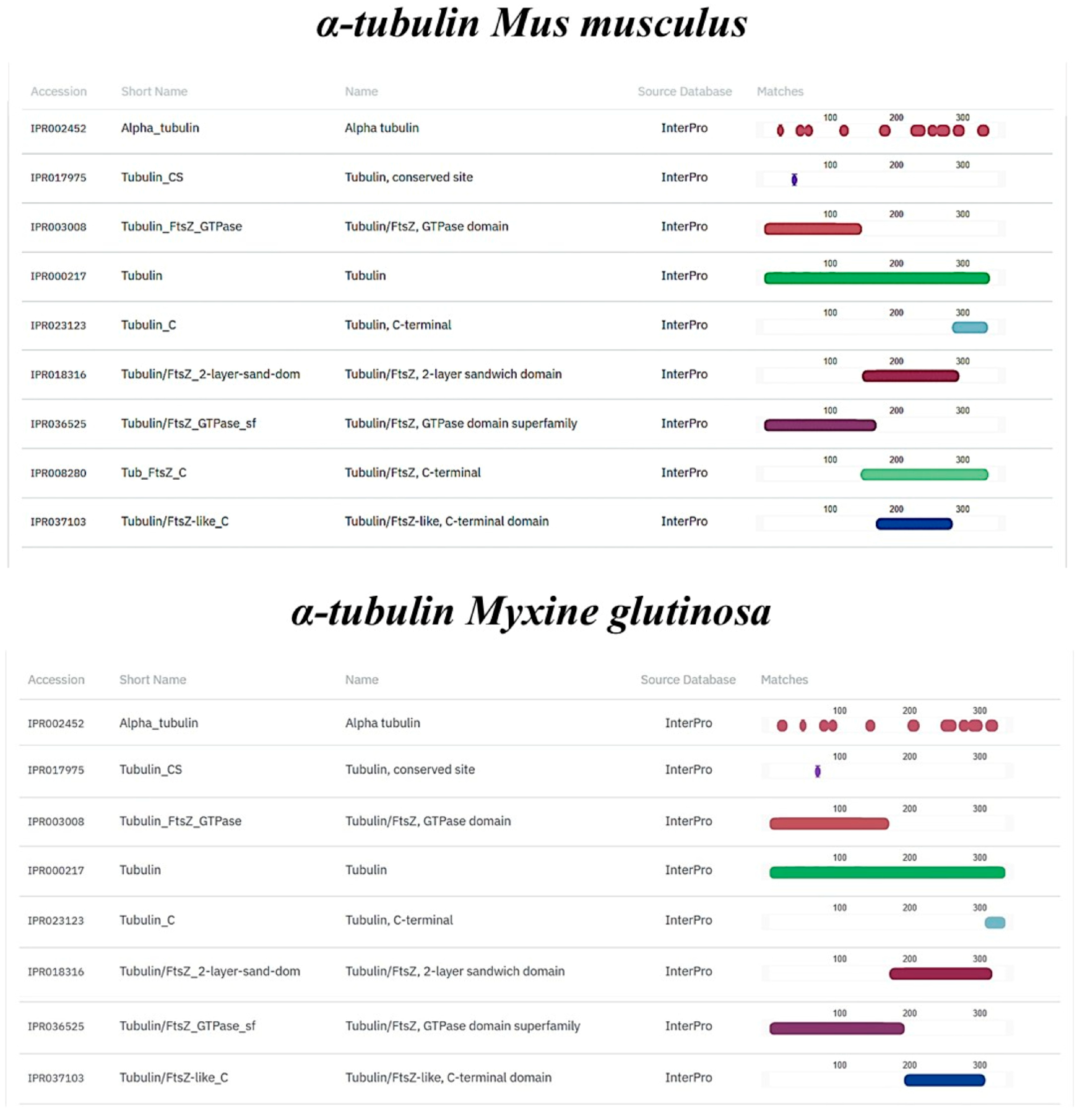
2.7. In Silico Comparison of Muc2, TLR2, Vimentin and α-Tubulin Protein Domains
3. Results
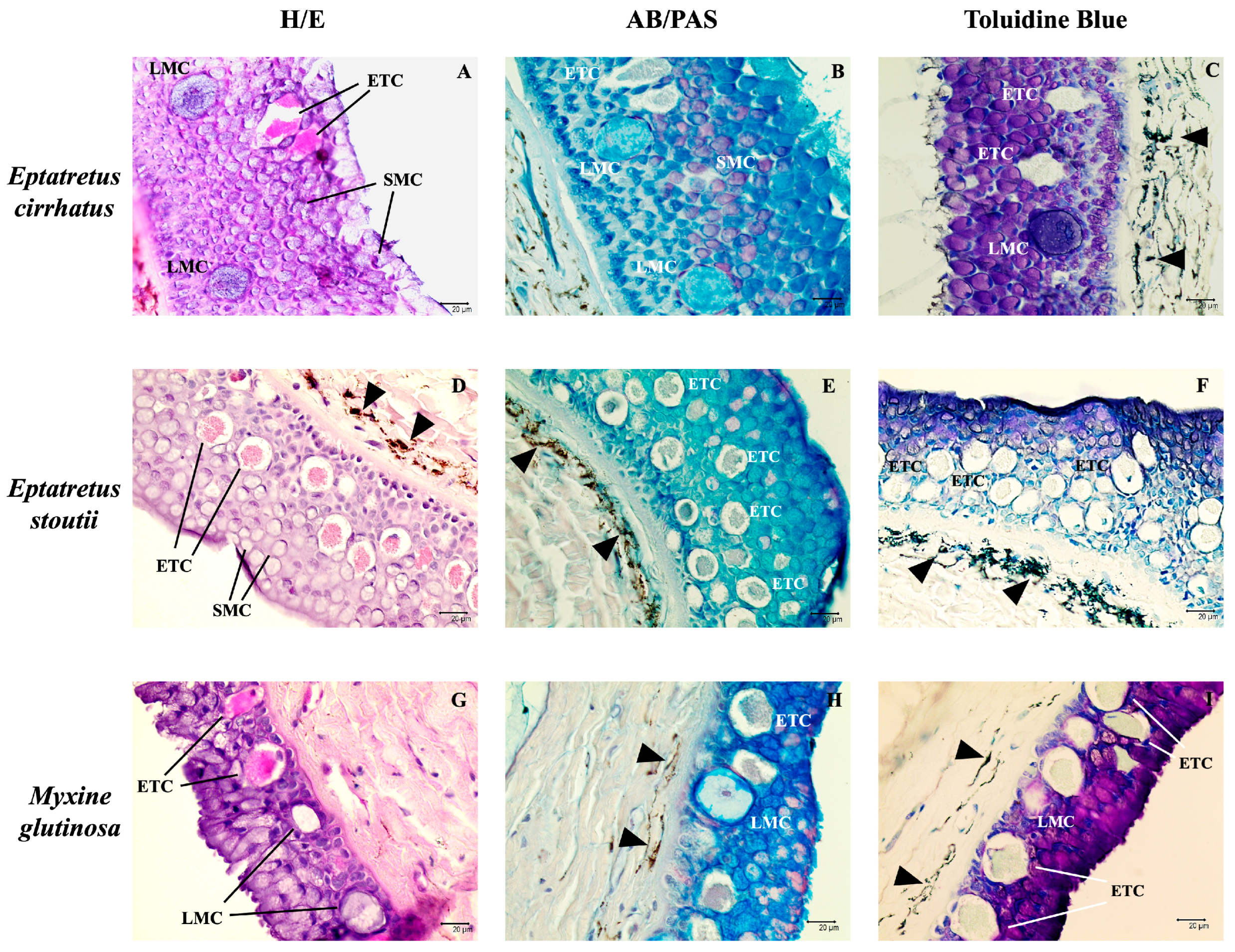
3.1. Histological Analysis
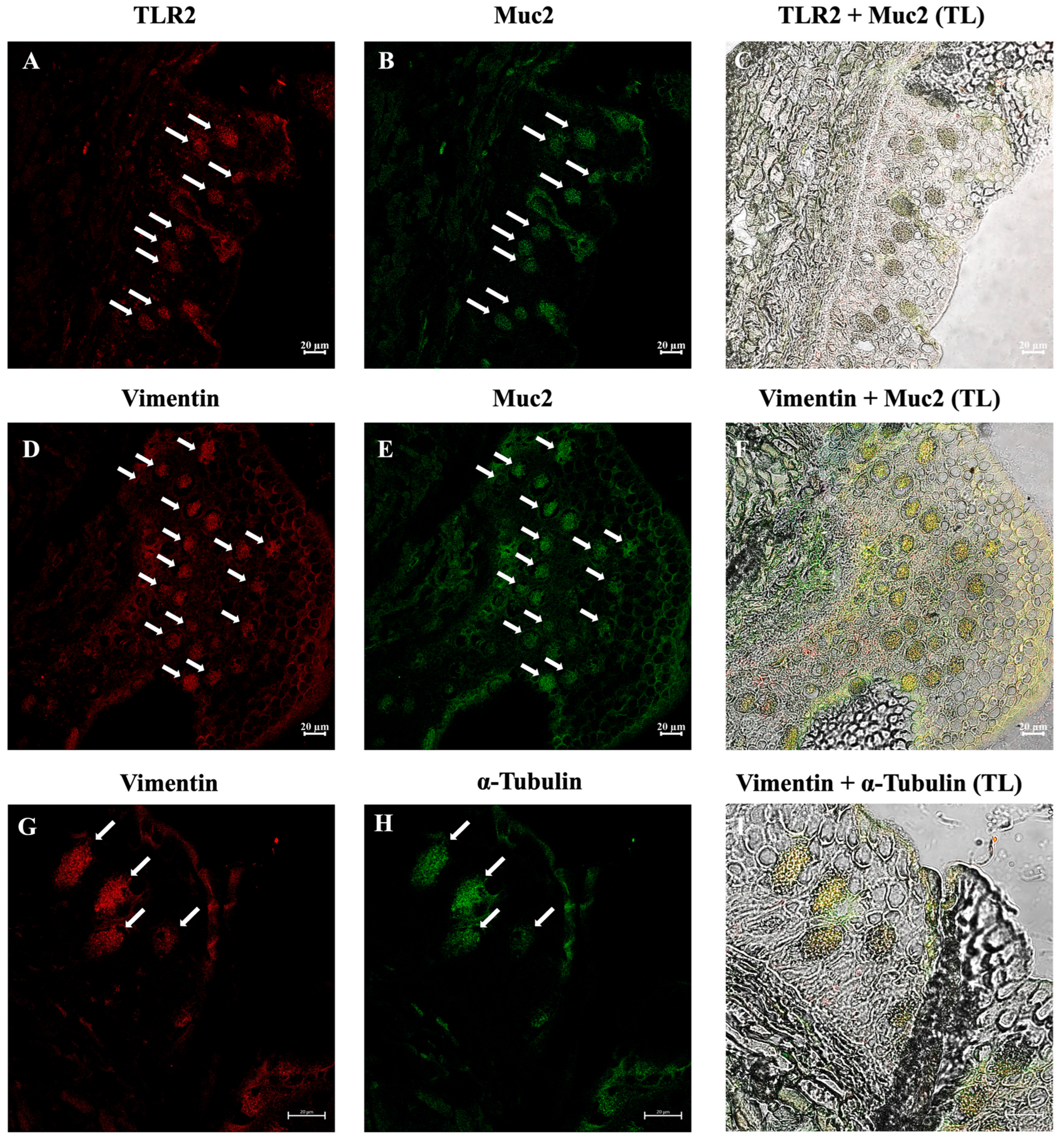
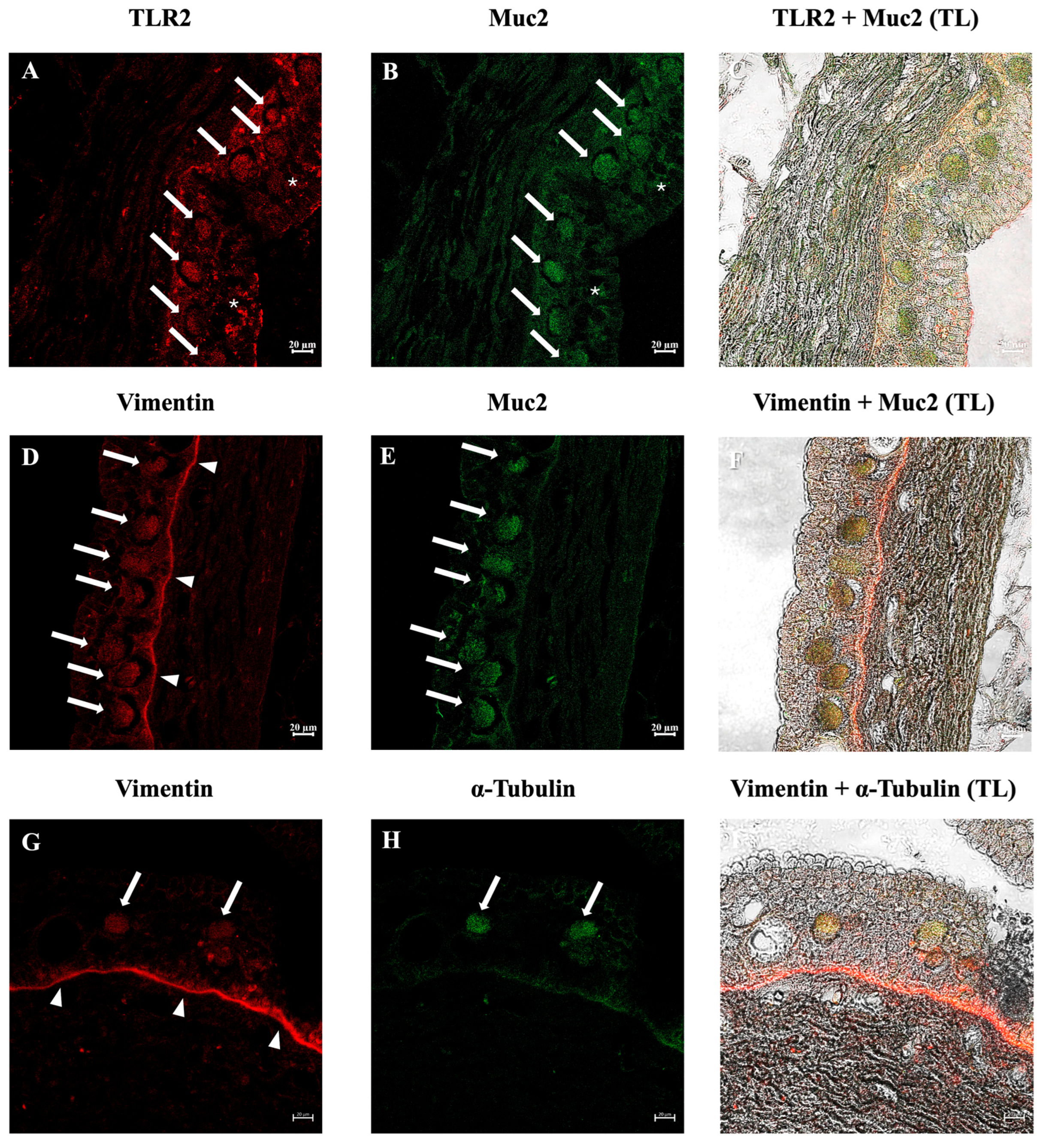
3.2. Immunohistochemical Analysis
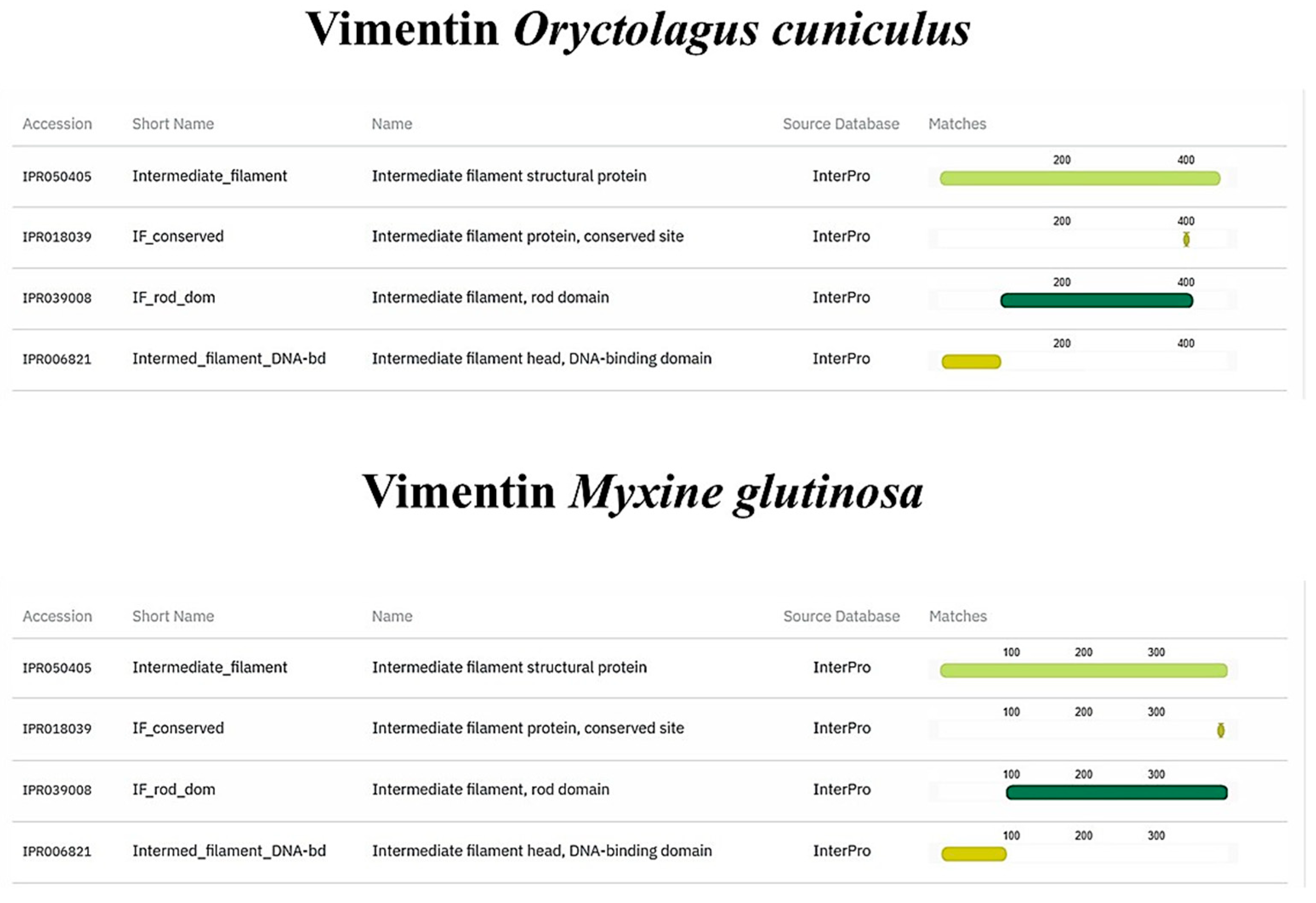
3.3. In Silico Analysis of TLR2, Vimentin, Muc2, and α-Tubulin Orthologues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, Y.S.; Kim, J.-K. A New Species of Hagfish, Eptatretus albiderma Sp. Nov. (Agnatha: Myxinidae), from Vietnam, with the Keys to Species of Eptatretus in East Asia. Mar. Biodivers. 2020, 50, 71. [Google Scholar] [CrossRef]
- Miyashita, T.; Coates, M.I.; Farrar, R.; Larson, P.; Manning, P.L.; Wogelius, R.A.; Edwards, N.P.; Anné, J.; Bergmann, U.; Palmer, A.R.; et al. Hagfish from the Cretaceous Tethys Sea and a Reconciliation of the Morphological–Molecular Conflict in Early Vertebrate Phylogeny. Proc. Natl. Acad. Sci. USA 2019, 116, 2146–2151. [Google Scholar] [CrossRef]
- Akat, E.; Yenmiş, M.; Pombal, M.A.; Molist, P.; Megías, M.; Arman, S.; Veselỳ, M.; Anderson, R.; Ayaz, D. Comparison of Vertebrate Skin Structure at Class Level: A Review. Anat. Rec. 2022, 305, 3543–3608. [Google Scholar] [CrossRef]
- Orlov, A. Jawless Fishes of the World: Volume 1, 1st ed.; Cambridge Scholars Publishing: Newcastle-upon-Tyne, UK, 2016; ISBN 978-1-4438-8582-9. [Google Scholar]
- Zintzen, V.; Roberts, C.D.; Anderson, M.J.; Stewart, A.L.; Struthers, C.D.; Harvey, E.S. Hagfish Predatory Behaviour and Slime Defence Mechanism. Sci. Rep. 2011, 1, 131. [Google Scholar] [CrossRef] [PubMed]
- Herr, J.E.; Clifford, A.; Goss, G.G.; Fudge, D.S. Defensive Slime Formation in Pacific Hagfish Requires Ca2+ and Aquaporin Mediated Swelling of Released Mucin Vesicles. J. Exp. Biol. 2014, 217, jeb.101584. [Google Scholar] [CrossRef]
- Randle, E.; Sansom, R.S. Bite Marks and Predation of Fossil Jawless Fish during the Rise of Jawed Vertebrates. Proc. R. Soc. B 2019, 286, 20191596. [Google Scholar] [CrossRef]
- Fudge, D.S.; Schorno, S.; Ferraro, S. Physiology, Biomechanics, and Biomimetics of Hagfish Slime. Annu. Rev. Biochem. 2015, 84, 947–967. [Google Scholar] [CrossRef] [PubMed]
- Fudge, D.; Schorno, S. The Hagfish Gland Thread Cell: A Fiber-Producing Cell Involved in Predator Defense. Cells 2016, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Schaffeld, M.; Schultess, J. Genes Coding for Intermediate Filament Proteins Closely Related to the Hagfish “Thread Keratins (TK)” α and γ Also Exist in Lamprey, Teleosts and Amphibians. Exp. Cell Res. 2006, 312, 1447–1462. [Google Scholar] [CrossRef]
- Winegard, T.; Herr, J.; Mena, C.; Lee, B.; Dinov, I.; Bird, D.; Bernards, M.; Hobel, S.; Van Valkenburgh, B.; Toga, A.; et al. Coiling and Maturation of a High-Performance Fibre in Hagfish Slime Gland Thread Cells. Nat. Commun. 2014, 5, 3534. [Google Scholar] [CrossRef]
- Spitzer, R.H.; Koch, E.A. Hagfish Skin and Slime Glands. In The Biology of Hagfishes; Springer: Dordrecht, The Netherlands, 1998; pp. 109–132. ISBN 978-94-010-6465-1. [Google Scholar]
- Winegard, T.M.; Fudge, D.S. Deployment of Hagfish Slime Thread Skeins Requires the Transmission of Mixing Forces via Mucin Strands. J. Exp. Biol. 2010, 213, 1235–1240. [Google Scholar] [CrossRef]
- Zeng, Y.; Petrichko, S.; Nieders, K.; Plachetzki, D.; Fudge, D. Evolution of a remarkable intracellular polymer and extreme cell allometry in hagfishes. Curr. Biol. 2021, 31, 5062–5068.e4. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Plachetzki, D.C.; Nieders, K.; Campbell, H.; Cartee, M.; Pankey, M.S.; Guillen, K.; Fudge, D. Epidermal Threads Reveal the Origin of Hagfish Slime. eLife 2023, 12, e81405. [Google Scholar] [CrossRef] [PubMed]
- Mincarone, M.M. Eptatretus cirrhatus. The IUCN Red List of Threatened Species. e.T154825A4644581. 2011. Available online: https://www.iucnredlist.org/species/154825/4644581 (accessed on 19 November 2025).
- IUCN. Hagfish IUCN Red List of Threatened Species: Eptatretus Stoutii; IUCN: Gland, Switzerland, 2009. [Google Scholar]
- Lesser, M.P.; Martini, F.H.; Heiser, J.B. Ecology of the Hagfish, Myxine glutinosa L. in the Gulf of Maine I. Metabolic rates and energetics. J. Exp. Mar. Biol. Ecol. 1997, 208, 215–225. [Google Scholar] [CrossRef]
- Cox, G.K.; Sandblom, E.; Richards, J.G.; Farrell, A.P. Anoxic Survival of the Pacific Hagfish (Eptatretus stoutii). J. Comp. Physiol. B 2011, 181, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Glover, C.N.; Bucking, C.; Wood, C.M. Adaptations to in Situ Feeding: Novel Nutrient Acquisition Pathways in an Ancient Vertebrate. Proc. R. Soc. B 2011, 278, 3096–3101. [Google Scholar] [CrossRef]
- Glover, C.N.; Blewett, T.A.; Wood, C.M. Determining the Functional Role of Waterborne Amino Acid Uptake in Hagfish Nutrition: A Constitutive Pathway When Fasting or a Supplementary Pathway When Feeding? J. Comp. Physiol. B 2016, 186, 843–853. [Google Scholar] [CrossRef]
- Knapp, L.; Mincarone, M.M.; Harwell, H.; Polidoro, B.; Sanciangco, J.; Carpenter, K. Conservation Status of the World’s Hagfish Species and the Loss of Phylogenetic Diversity and Ecosystem Function. Aquat. Conserv. 2011, 21, 401–411. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Savoca, S.; Fumia, A.; Mangano, A.; Albano, M.; Messina, E.; Aragona, M.; Lo Cascio, P.; Capillo, G.; et al. Detecting Intestinal Goblet Cells of the Broadgilled Hagfish Eptatretus cirrhatus (Forster, 1801): A Confocal Microscopy Evaluation. Biology 2022, 11, 1366. [Google Scholar] [CrossRef]
- Pergolizzi, S.; Fumia, A.; D’Angelo, R.; Mangano, A.; Lombardo, G.P.; Giliberti, A.; Messina, E.; Alesci, A.; Lauriano, E.R. Expression and Function of Toll-like Receptor 2 in Vertebrate. Acta Histochem. 2023, 125, 152028. [Google Scholar] [CrossRef]
- Ilan-Ber, T.; Ilan, Y. The Role of Microtubules in the Immune System and as Potential Targets for Gut-Based Immunotherapy. Mol. Immunol. 2019, 111, 73–82. [Google Scholar] [CrossRef]
- Dash, S.; Das, S.K.; Samal, J.; Thatoi, H.N. Epidermal Mucus, a Major Determinant in Fish Health: A Review. Iran. J. Vet. Res. 2018, 19, 72–81. [Google Scholar] [CrossRef]
- Arrindell, J.; Desnues, B. Vimentin: From a Cytoskeletal Protein to a Critical Modulator of Immune Response and a Target for Infection. Front. Immunol. 2023, 14, 1224352. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Deng, C.; Peng, L.; Shang, L.; Jiang, J.; Yu, W.; Yang, H.; Liu, J.; Jiang, L.; Zuo, T.; et al. Self-Assembling TLR2 Agonists Promote Mucosal Immune Responses without Pulmonary Immunopathologic Injuries in Mice. Npj Vaccines 2025, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Glassy, M.C. Cell Surface Vimentin: A Natural Human Immune Response Target for Immunotherapy. Front. Mol. Med. 2025, 5, 1552323. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Janmey, P.A.; McCulloch, C.A. Structure and Function of Vimentin in the Generation and Secretion of Extracellular Vimentin in Response to Inflammation. Cell Commun. Signal. 2025, 23, 187. [Google Scholar] [CrossRef]
- Mandal, S.; Kumari, S. Tubulin with a Twist: Acetylated Highways at the Immune Synapse. J. Cell Biol. 2025, 224, e202506017. [Google Scholar] [CrossRef]
- Fiyouzi, T.; Subiza, J.L.; Lafuente, E.M.; Reche, P.A. Peptides Derived from α-Tubulin Induce Functional T Regulatory Cells. Int. J. Mol. Sci. 2025, 26, 8356. [Google Scholar] [CrossRef]
- Fan, F.; Guo, R.; Pan, K.; Xu, H.; Chu, X. Mucus and Mucin: Changes in the Mucus Barrier in Disease States. Tissue Barriers 2025, 2499752. [Google Scholar] [CrossRef]
- Marín-Parra, C.; Serna-Duque, J.A.; Espinosa-Ruiz, C.; Albadalejo-Riad, N.; Bardera, G.; Thompson, K.; Esteban, M.Á. Piscidin 1 and 2 of Gilthead Seabream (Sparus Aurata): New Insights into Their Role as Host Defense Peptides. Fish Shellfish Immunol. 2025, 165, 110501. [Google Scholar] [CrossRef]
- Lauriano, E.R.; Icardo, J.M.; Zaccone, D.; Kuciel, M.; Satora, L.; Alesci, A.; Alfa, M.; Zaccone, G. Expression patterns and quantitative assessment of neurochemical markers in the lung of the gray bichir, Polypterus senegalus (Cuvier, 1829). Acta Histochem. 2015, 117, 738–746. [Google Scholar] [CrossRef]
- Mcdonald, J.H.; Dunn, K.W. Statistical Tests for Measures of Colocalization in Biological Microscopy. J. Microsc. 2013, 252, 295–302. [Google Scholar] [CrossRef]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef]
- Boggett, S.; Stiles, J.-L.; Summers, A.P.; Fudge, D.S. Flaccid Skin Protects Hagfishes from Shark Bites. J. R. Soc. Interface 2017, 14, 20170765. [Google Scholar] [CrossRef]
- Chiu, F.-C.; Kuo, H.-M.; Yu, C.-L.; Selvam, P.; Su, I.-L.; Tseng, C.-C.; Yuan, C.-H.; Wen, Z.-H. Marine-Derived Antimicrobial Peptide Piscidin-1 Triggers Extrinsic and Intrinsic Apoptosis in Oral Squamous Cell Carcinoma through Reactive Oxygen Species Production and Inhibits Angiogenesis. Free Radic. Biol. Med. 2024, 220, 28–42. [Google Scholar] [CrossRef]
- Alesci, A.; Capillo, G.; Mokhtar, D.M.; Fumia, A.; D’Angelo, R.; Lo Cascio, P.; Albano, M.; Guerrera, M.C.; Sayed, R.K.A.; Spanò, N.; et al. Expression of Antimicrobic Peptide Piscidin1 in Gills Mast Cells of Giant Mudskipper Periophthalmodon schlosseri (Pallas, 1770). Int. J. Mol. Sci. 2022, 23, 13707. [Google Scholar] [CrossRef]
- Alesci, A.; Albano, M.; Savoca, S.; Mokhtar, D.M.; Fumia, A.; Aragona, M.; Lo Cascio, P.; Hussein, M.M.; Capillo, G.; Pergolizzi, S.; et al. Confocal Identification of Immune Molecules in Skin Club Cells of Zebrafish (Danio Rerio, Hamilton 1882) and Their Possible Role in Immunity. Biology 2022, 11, 1653. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.M.O.; Ruangsri, J.; Kiron, V. Atlantic Cod Piscidin and Its Diversification through Positive Selection. PLoS ONE 2010, 5, e9501. [Google Scholar] [CrossRef] [PubMed]
- Ruangsri, J.; Fernandes, J.M.O.; Rombout, J.H.W.M.; Brinchmann, M.F.; Kiron, V. Ubiquitous Presence of Piscidin-1 in Atlantic Cod as Evidenced by Immunolocalisation. BMC Vet. Res. 2012, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Ruangsri, J.; Salger, S.A.; Caipang, C.M.A.; Kiron, V.; Fernandes, J.M.O. Differential Expression and Biological Activity of Two Piscidin Paralogues and a Novel Splice Variant in Atlantic Cod (Gadus morhua L.). Fish Shellfish. Immunol. 2012, 32, 396–406. [Google Scholar] [CrossRef]
- Zaccone, G.; Capillo, G.; Fernandes, J.M.O.; Kiron, V.; Lauriano, E.R.; Alesci, A.; Lo Cascio, P.; Guerrera, M.C.; Kuciel, M.; Zuwala, K.; et al. Expression of the Antimicrobial Peptide Piscidin 1 and Neuropeptides in Fish Gill and Skin: A Potential Participation in Neuro-Immune Interaction. Mar. Drugs 2022, 20, 145. [Google Scholar] [CrossRef]
- Alesci, A.; Marino, S.; D’Iglio, C.; Morgante, S.; Miller, A.; Rigano, G.; Ferri, J.; Fernandes, J.M.O.; Capillo, G. Investigating Development and Defense Systems in Early Reproductive Stages of Male and Female Gonads in Black Scorpionfish Scorpaena porcus (Linnaeus, 1758). Biology 2024, 13, 587. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Lauriano, E.R.; Aragona, M.; Capillo, G.; Pergolizzi, S. Marking Vertebrates Langerhans Cells, from Fish to Mammals. Acta Histochem. 2020, 122, 151622. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Lo Cascio, P.; Capillo, G.; Lauriano, E.R. Localization of vasoactive intestinal peptide and toll-like receptor 2 immunoreactive cells in endostyle of urochordate Styela plicata (Lesueur, 1823). Microsc. Res. Tech. 2022, 85, 2651–2658. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Capillo, G.; Fumia, A.; Albano, M.; Messina, E.; Spanò, N.; Pergolizzi, S.; Lauriano, E.R. Coelomocytes of the Oligochaeta Earthworm Lumbricus Terrestris (Linnaeus, 1758) as Evolutionary Key of Defense: A Morphological Study. Zool. Lett. 2023, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Di Paola, D.; Fumia, A.; Marino, S.; D’Iglio, C.; Famulari, S.; Albano, M.; Spanò, N.; Lauriano, E.R. Internal Defense System of Mytilus Galloprovincialis (Lamarck, 1819): Ecological Role of Hemocytes as Biomarkers for Thiacloprid and Benzo[a]Pyrene Pollution. Toxics 2023, 11, 731. [Google Scholar] [CrossRef]
- Knoop, K.A.; McDonald, K.G.; McCrate, S.; McDole, J.R.; Newberry, R.D. Microbial Sensing by Goblet Cells Controls Immune Surveillance of Luminal Antigens in the Colon. Mucosal Immunol. 2015, 8, 198–210. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, C. The Relationship between Intestinal Goblet Cells and the Immune Response. Biosci. Rep. 2020, 40, BSR20201471. [Google Scholar] [CrossRef]
- Ridler, C. Sentinel Goblet Cells Flush out Bacteria from Crypts. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 438. [Google Scholar] [CrossRef]
- Alesci, A.; Marino, S.; Miller, A.; Giliberti, A.; Rigano, G.; Giuffrè, L.; Fernandes, J.M.O.; Savoca, S.; Capillo, G. Role of Mucous Cells in the Defence System of the Cnidarian Actinia Equina (Linnaeus, 1758): The Keystone of Evolution. Acta Zool. 2024, 106, azo.12523. [Google Scholar] [CrossRef]
- Ridge, K.M.; Eriksson, J.E.; Pekny, M.; Goldman, R.D. Roles of Vimentin in Health and Disease. Genes Dev. 2022, 36, 391–407. [Google Scholar] [CrossRef]
- Chen, Z.; Fang, Z.; Ma, J. Regulatory Mechanisms and Clinical Significance of Vimentin in Breast Cancer. Biomed. Pharmacother. 2021, 133, 111068. [Google Scholar] [CrossRef]
- Cheng, F.; Eriksson, J.E. Intermediate Filaments and the Regulation of Cell Motility during Regeneration and Wound Healing. Cold Spring Harb. Perspect. Biol. 2017, 9, a022046. [Google Scholar] [CrossRef]
- Kidd, M.E.; Shumaker, D.K.; Ridge, K.M. The Role of Vimentin Intermediate Filaments in the Progression of Lung Cancer. Am. J. Respir. Cell Mol. Biol. 2014, 50, 1–6. [Google Scholar] [CrossRef]
- Ivaska, J.; Pallari, H.-M.; Nevo, J.; Eriksson, J.E. Novel Functions of Vimentin in Cell Adhesion, Migration, and Signaling. Exp. Cell Res. 2007, 313, 2050–2062. [Google Scholar] [CrossRef]
- Alesci, A.; Fumia, A.; Mastrantonio, L.; Marino, S.; Miller, A.; Albano, M. Functional Adaptations of Hemocytes of Aplysia Depilans (Gmelin, 1791) and Their Putative Role in Neuronal Regeneration. Fishes 2024, 9, 32. [Google Scholar] [CrossRef]
- Patteson, A.E.; Vahabikashi, A.; Goldman, R.D.; Janmey, P.A. Mechanical and Non-Mechanical Functions of Filamentous and Non-Filamentous Vimentin. BioEssays 2020, 42, 2000078. [Google Scholar] [CrossRef] [PubMed]
- Corfield, A.P. Mucins: A Biologically Relevant Glycan Barrier in Mucosal Protection. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2015, 1850, 236–252. [Google Scholar] [CrossRef]
- Lang, T.; Hansson, G.C.; Samuelsson, T. Gel-Forming Mucins Appeared Early in Metazoan Evolution. Proc. Natl. Acad. Sci. USA 2007, 104, 16209–16214. [Google Scholar] [CrossRef] [PubMed]
- Downing, S.W.; Salo, W.L.; Spitzer, R.H.; Koch, E.A. The Hagfish Slime Gland: A Model System for Studying the Biology of Mucus. Science 1981, 214, 1143–1145. [Google Scholar] [CrossRef]
- Salo, W.L.; Downing, S.W.; Lidinsky, W.A.; Gallagher, W.H.; Spitzer, R.H.; Koch, E.A. Fractionation of Hagfish Slime Gland Secretions: Partial Characterization of the Mucous Vesicle Fraction. Prep. Biochem. 1983, 13, 103–135. [Google Scholar] [CrossRef] [PubMed]
- Breviario, D.; Gianì, S.; Morello, L. Multiple Tubulins: Evolutionary Aspects and Biological Implications. Plant J. 2013, 75, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Breuss, M.W.; Leca, I.; Gstrein, T.; Hansen, A.H.; Keays, D.A. Tubulins and Brain Development—The Origins of Functional Specification. Mol. Cell. Neurosci. 2017, 84, 58–67. [Google Scholar] [CrossRef]
- Hookway, C.; Ding, L.; Davidson, M.W.; Rappoport, J.Z.; Danuser, G.; Gelfand, V.I. Microtubule-dependent transport and dynamics of vimentin intermediate filaments. Mol. Biol. Cell 2015, 26, 1675–1686. [Google Scholar] [CrossRef]
- Robert, A.; Herrmann, H.; Davidson, M.W.; Gelfand, V.I. Microtubule-dependent transport of vimentin filament precursors is regulated by actin and by the concerted action of Rho- and p21-activated kinases. FASEB J. 2014, 28, 2879–2890. [Google Scholar] [CrossRef]
- Robert, A.; Hookway, C.; Gelfand, V.I. Intermediate filament dynamics: What we can see now and why it matters. BioEssays 2016, 38, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Puertas, R.; Adamek, M.; Mallavia, R.; Falco, A. Fish Skin Mucus Extracts: An Underexplored Source of Antimicrobial Agents. Mar. Drugs 2023, 21, 350. [Google Scholar] [CrossRef]
- Pandey, S.; Stockwell, C.A.; Snider, M.R.; Wisenden, B.D. Epidermal Club Cells in Fishes: A Case for Ecoimmunological Analysis. Int. J. Mol. Sci. 2021, 22, 1440. [Google Scholar] [CrossRef]




| Antibody | Supplier | Dilution µL/µL | Animal Source |
|---|---|---|---|
| Piscidin-1 | GenScript, Piscataway, NJ, USA | 1:200 | Rabbit |
| TLR2 | Active Motif, La Hulpe, Belgium (AB_2750977) | 1:200 | Rabbit |
| α-tubulin | Santa Cruz Biotechnology, Dallas, TX, USA (sc-23950) | 1:300 | Mouse, clone 6-11B-1 |
| Muc2 | Santa Cruz Biotechnology, Dallas, TX, USA (sc-515032) | 1:200 | Mouse, clone F-2 |
| Vimentin | Sigma-Aldrich, St. Louis, MO, USA. (SAB1305445) | 1:200 | Rabbit |
| Alexa Fluor 488 donkey anti-mouse IgG FITC conjugated | Molecular Probes, Invitrogen, Waltham, MA, USA | 1:300 | Donkey |
| Alexa Fluor 594 donkey anti-rabbit IgG TRITC conjugated | Molecular Probes, Invitrogen, Waltham, MA, USA | 1:300 | Donkey |
| Antibody | Immunogen Sequence/Region from the Supplier Company | Accession (NCBI) | Closest Species | Homology (%) |
|---|---|---|---|---|
| TLR2 | a.a. 180–196, 353–370, and 473–489 | XP_067975384.1 | NP_036035.3 | 29.56 |
| α-tubulin | 15 S dynein fraction from sea urchin sperm axoneme | XP_067992527.1 | NP_001019507.1 | 92.84 |
| Muc2 | a.a. C-terminus 4880–5179 | XP_067977617.1 | GAB1292918.1 | 33.06 |
| Vimentin | a.a. 63–90 (includes Ser82) | XP_067959164.1 | BAE29426.1 | 44.01 |
| E. cirrhatus | E. stoutii | M. glutinosa | Negative Control E. cirrhatus | Negative Control E. stoutii | Negative Control M. glutinosa | |
|---|---|---|---|---|---|---|
| Vim + Muc2 | 78.358 ± 30.184 | 85.620 ± 32.338 | 77.936 ± 31.973 | 201.467 ± 27.482 | 190.938 ± 26.030 | 182.364 ± 25.876 |
| TLR2 + Muc2 | 73.047 ± 33.332 | 83.576 ± 32.957 | 75.462 ± 42.112 | 179.475 ± 23.786 | 184.365 ± 25. 983 | 192.614 ± 26.572 |
| Vim + α-tub | 79.379 ± 29.981 | 93.355 ± 38.377 | 81.743 ± 37.957 | 187.849 ± 26.001 | 177.832 ± 21.374 | 198.583 ± 27.130 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, S.; Alesci, A. Allies in the Skin Defense System: The Role of Thread Cells in the Evolution of Hagfish (Myxiniformes). Biology 2025, 14, 1662. https://doi.org/10.3390/biology14121662
Marino S, Alesci A. Allies in the Skin Defense System: The Role of Thread Cells in the Evolution of Hagfish (Myxiniformes). Biology. 2025; 14(12):1662. https://doi.org/10.3390/biology14121662
Chicago/Turabian StyleMarino, Sebastian, and Alessio Alesci. 2025. "Allies in the Skin Defense System: The Role of Thread Cells in the Evolution of Hagfish (Myxiniformes)" Biology 14, no. 12: 1662. https://doi.org/10.3390/biology14121662
APA StyleMarino, S., & Alesci, A. (2025). Allies in the Skin Defense System: The Role of Thread Cells in the Evolution of Hagfish (Myxiniformes). Biology, 14(12), 1662. https://doi.org/10.3390/biology14121662






