Transcriptome Reveals Growth Responses of Populus qamdoensis Under Blue and Green Films
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Light Treatment
2.2. Determination of Phenotypic Indicators and Leaf Tissue Structure Parameters
2.3. Measurement of Photosynthetic Characteristics Under Different Treatments
2.4. Determination of Photosynthetic Pigments and Sugar Content
2.5. Transcriptome Data Analysis
2.6. Prediction of Light-Responsive Protein Interaction Networks
2.7. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.8. Statistical Analysis
3. Results
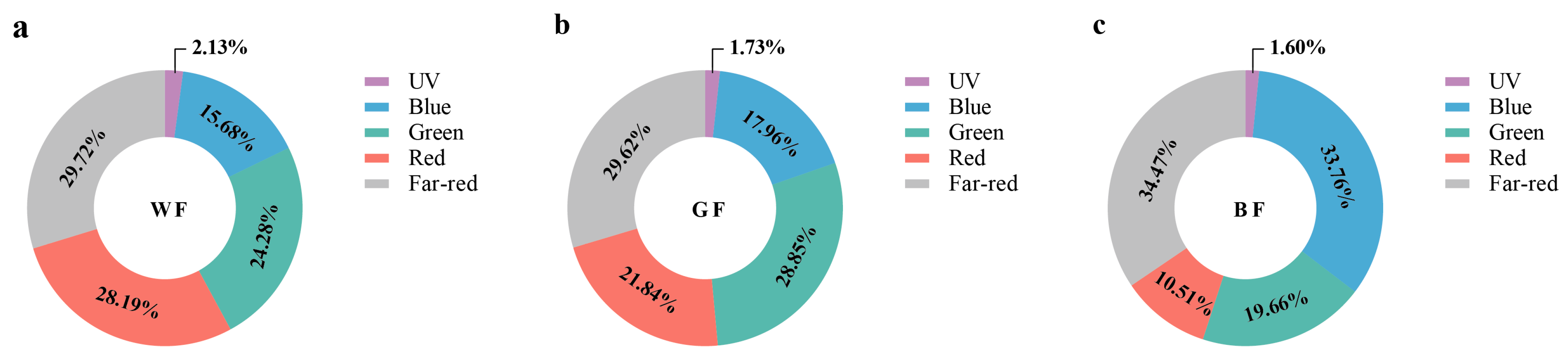
3.1. Ratio Analysis of Transmission Spectra in the Shed
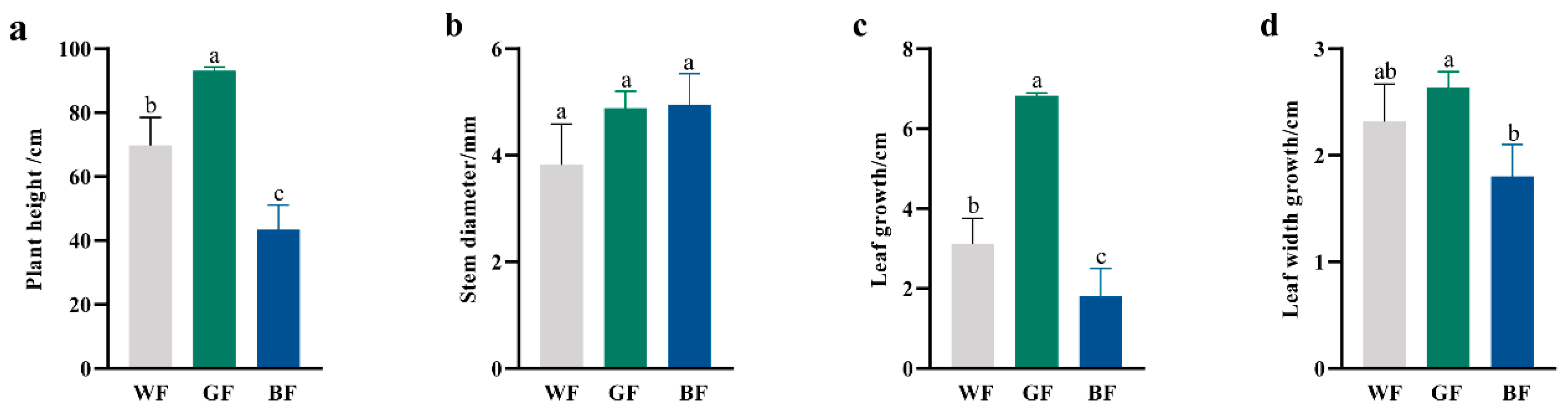
3.2. Effects of Different Film Treatment on P. qamdoensis Growth
3.3. Effects of Different Film Treatment on the Leaf Tissue Structure of P. qamdoensis
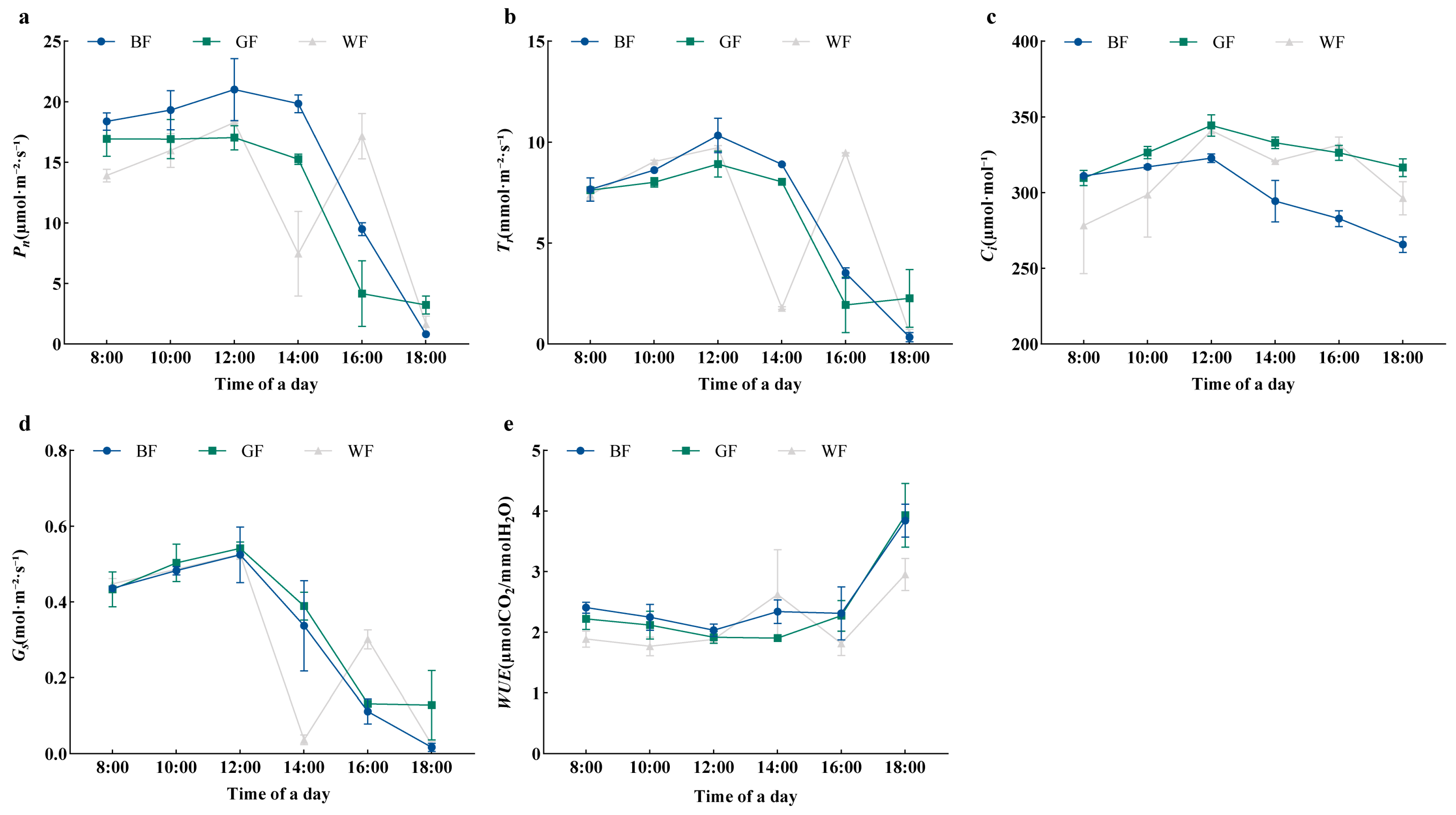
3.4. Effects of Different Colored Films on Photosynthetic Characteristics of P. qamdoensis
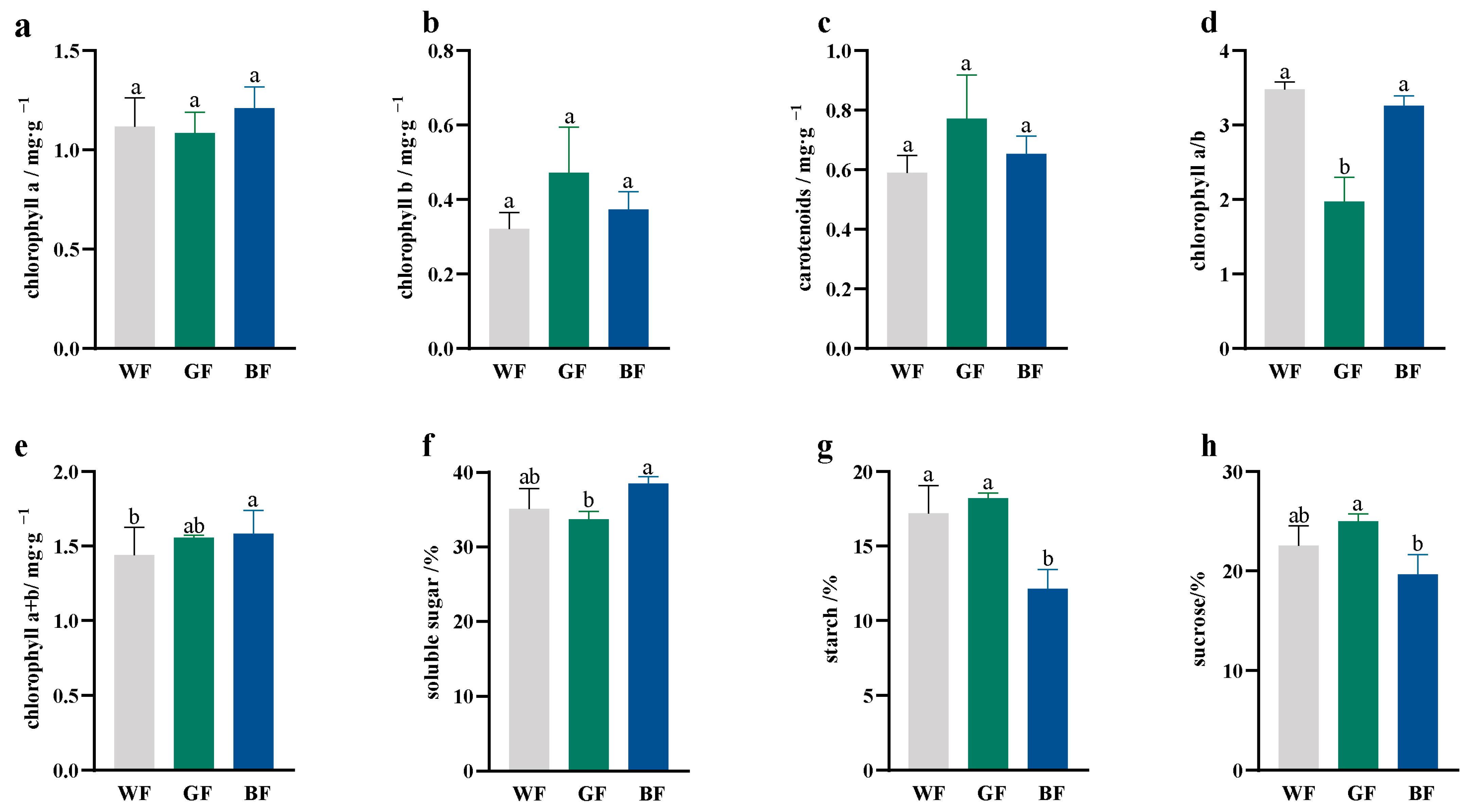
3.5. Effects of Different Colored Films on Photosynthetic Pigments and Sugar Content of P. qamdoensis
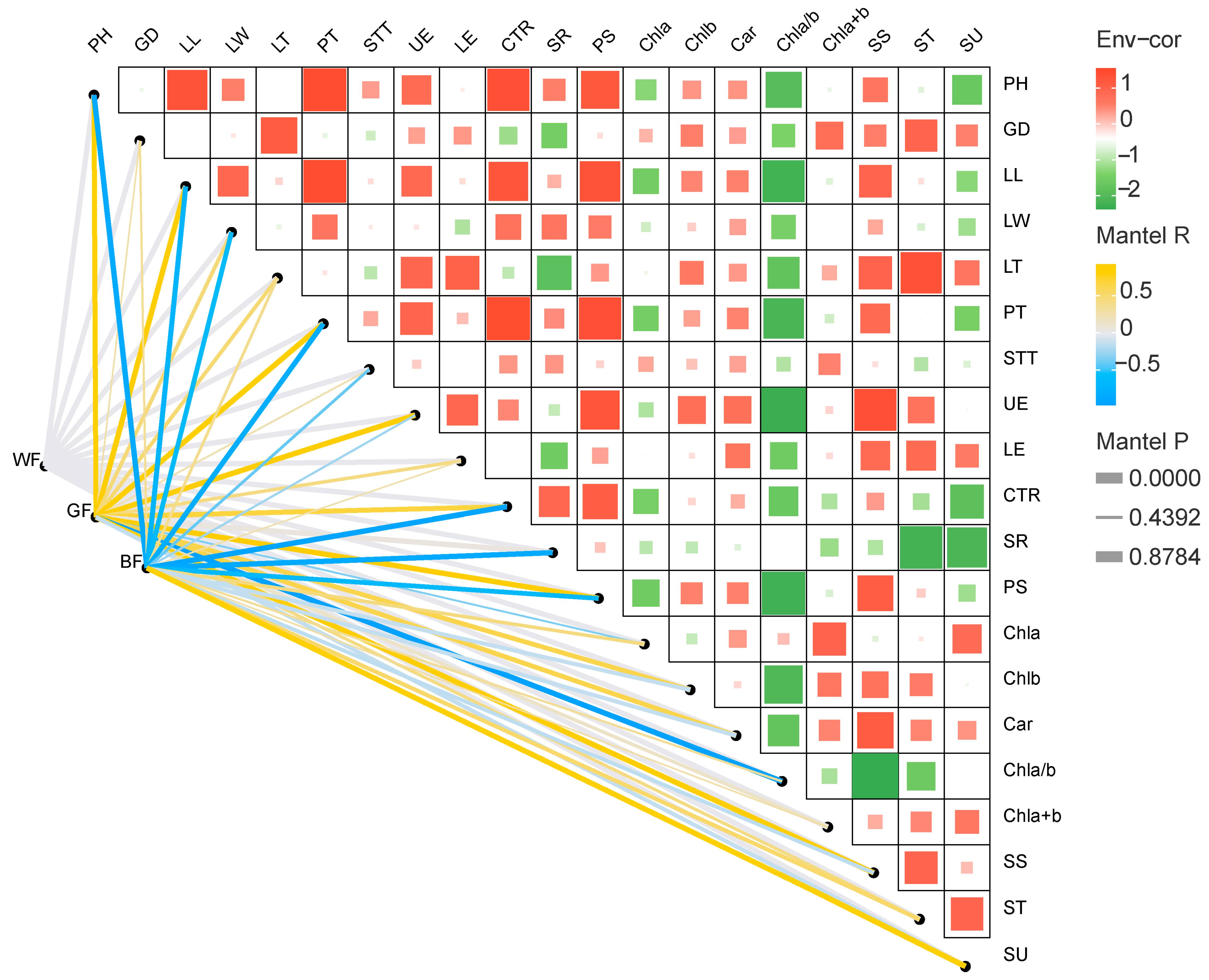
3.6. Correlation Analysis
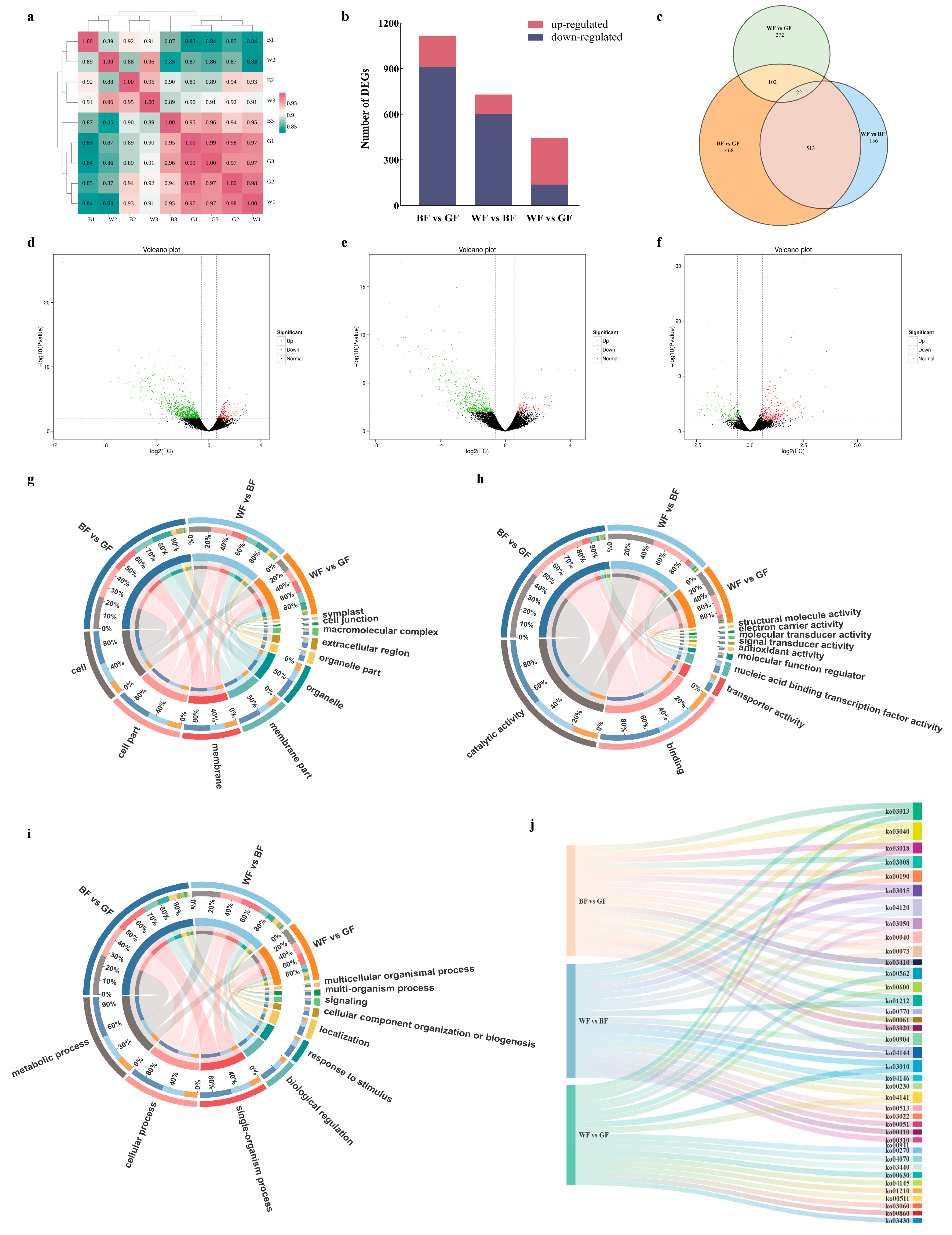
3.7. Analysis of DEGs Following the BF and GF Treatments by RNA-Seq
3.8. Pathway Analysis of P. qamdoensis in Response to Different Colored Film Treatments
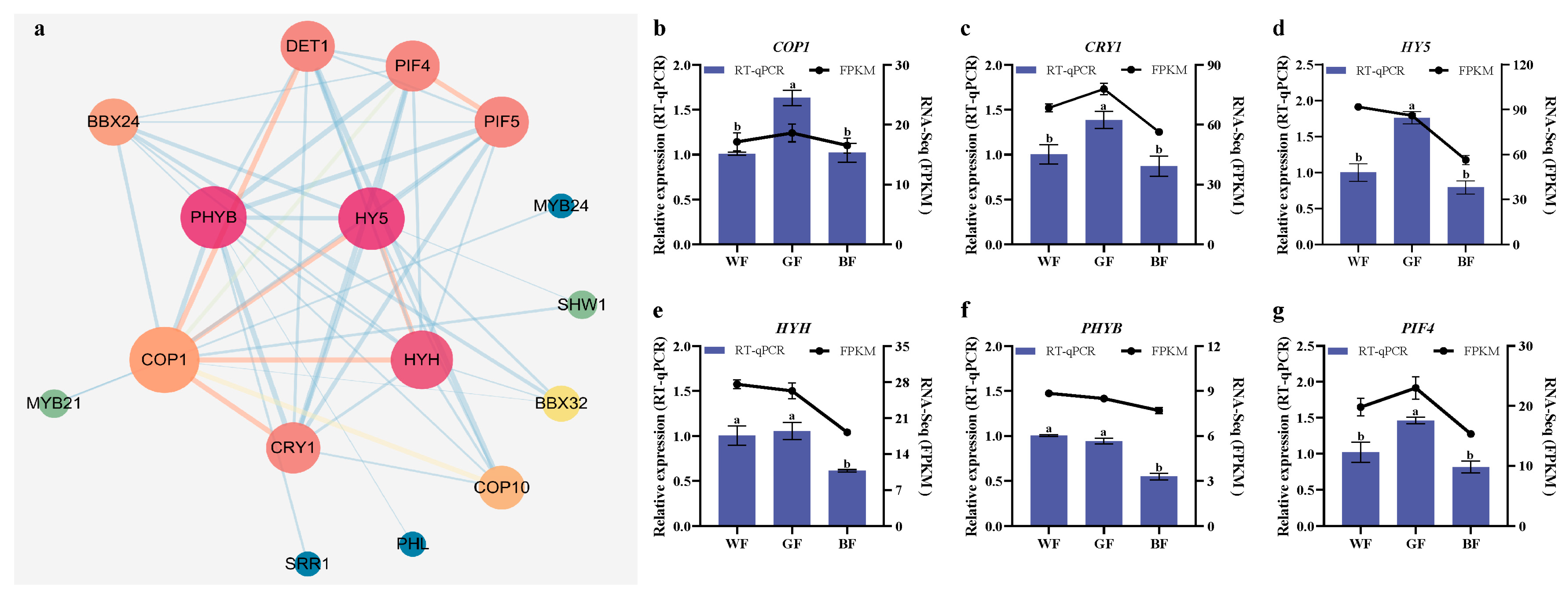
3.9. Expression Patterns of Light Signaling-Related Transcription Factors (TFs)
4. Discussion
4.1. Effects of Different Colored Film Treatments on the Growth and Development of P. qamdoensis
4.2. Specific Response Characteristics of Photosynthetic Pigments in P. qamdoensis to Different Light Qualities
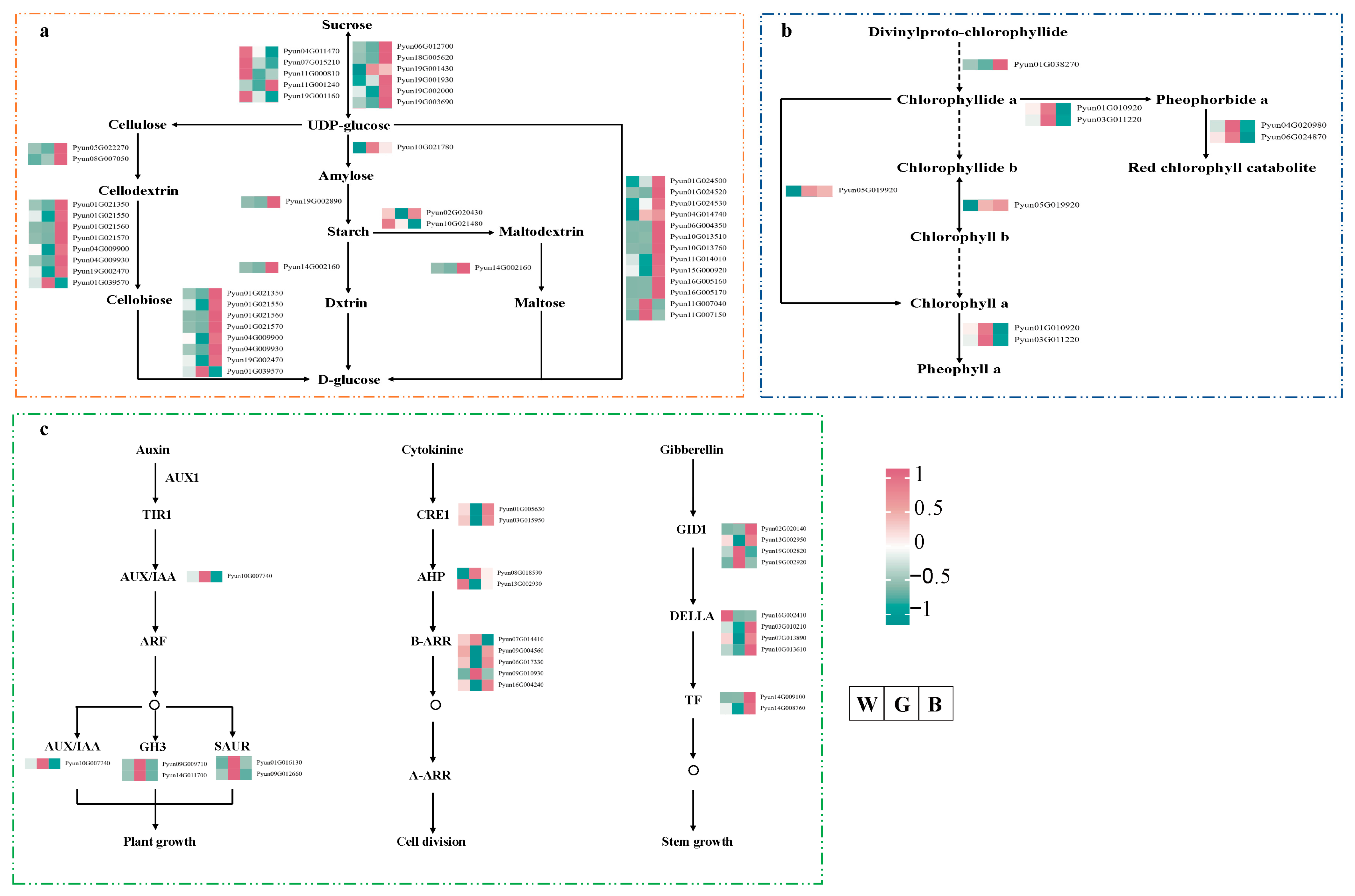
4.3. Association Between Metabolic Gene Expression and Photosynthate Allocation in P. qamdoensis Under BF and GF Treatments
4.4. Expression Characteristics of Hormone Signaling Pathway Genes in P. qamdoensis Under BF and GF Treatments
4.5. Characteristics and Adaptive Strategies of Light Signal Gene Expression in P. qamdoensis Regulated by Different Light Qualities
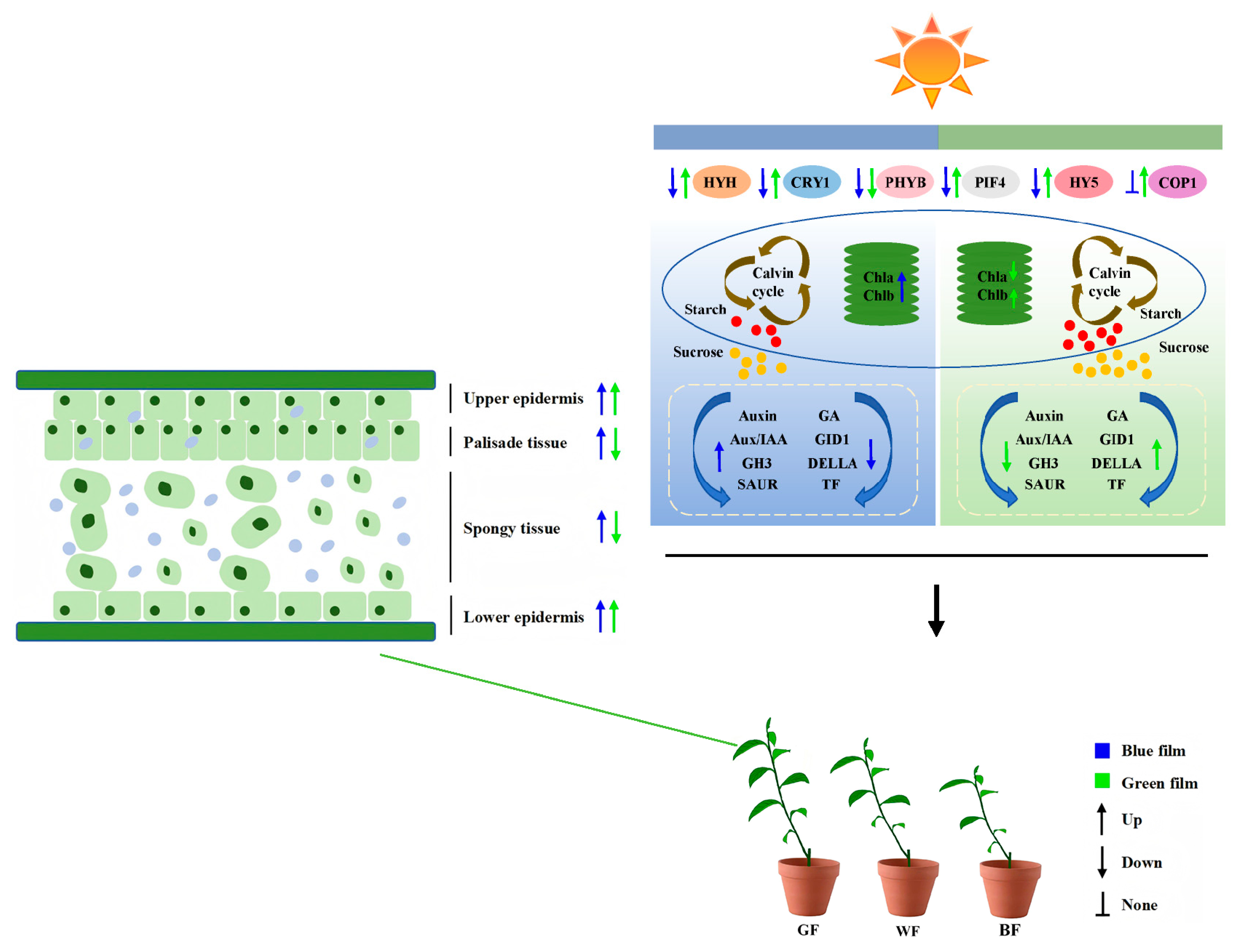
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| WF | Transparent colorless film |
| BF | Blue film |
| GF | Green film |
| Gs | Stomatal conductance |
| Ci | Intercellular CO2 concentration |
| WUE | Water use efficiency |
| Chla | Chlorophyll a |
| Chlb | Chlorophyll b |
| Car | Carotenoids |
| DEGs | Differentially expressed genes |
| FDR | False discovery rate |
References
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Marmiroli, M.; Imperiale, D.; Pagano, L.; Villani, M.; Zappettini, A.; Marmiroli, N. The proteomic response of Arabidopsis thaliana to cadmium sulfide quantum dots, and its correlation with the transcriptomic response. Front. Plant Sci. 2015, 6, 1104. [Google Scholar] [CrossRef]
- Franklin, K. Light and temperature signal crosstalk in plant development. Curr. Opin. Plant Biol. 2009, 12, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Townsend, A.; Retkute, R.; Chinnathambi, K.; Randall, J.; Foulkes, J.; Carmo, S.; Murchie, E. Suboptimal acclimation of photosynthesis to light in wheat canopies. Plant Physiol. 2018, 176, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Paik, I.; Zhu, L.; Huq, E. Illuminating progress in phytochrome-mediated light signaling pathways. Trends Plant Sci. 2015, 20, 641–650. [Google Scholar] [CrossRef]
- Jeong, H.W.; Lee, H.R.; Kim, H.M.; Kim, H.M.; Hwang, H.S.; Hwang, S.J. Using light quality for growth control of cucumber seedlings in closed-type plant production system. Plants 2020, 9, 639. [Google Scholar] [CrossRef]
- Mcausland, L.; Vialet-Chabrand, S.; Davey, P.; Baker, N.R.; Brendel, O.; Lawson, T. Effects of kinetics of light-induced stomatal responses on photosynthesis and water-use efficiency. New Phytol. 2016, 211, 1209–1220. [Google Scholar] [CrossRef]
- Lindback, L.N.; Hu, Y.; Ackermann, A.; Artz, O.; Pedmale, U.V. UBP12 and UBP13 deubiquitinases destabilize the CRY2 blue light receptor to regulate Arabidopsis growth. Curr. Biol. 2022, 32, 3221–3231.e6. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Kaiser, E.; Kromdijk, J.; Harbinson, J.; Heuvelink, E.; Marcelis, L.F.M. Photosynthetic induction and its diffusional, carboxylation and electron transport processes as affected by CO2 partial pressure, temperature, air humidity and blue irradiance. Ann. Bot. 2017, 119, 191–205. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Hao, Y.; Zeng, Z.; Zhang, X.; Xie, D.; Li, X.; Ma, L.; Liu, M.; Liu, H. Green means go: Green light promotes hypocotyl elongation via brassinosteroid signaling. Plant Cell 2023, 35, 1304–1317. [Google Scholar] [CrossRef]
- Zhang, T.; Maruhnich, S.A.; Folta, K.M. Green light induces shade avoidance symptoms. Plant Physiol. 2011, 157, 1528–1536. [Google Scholar] [CrossRef]
- Schenkels, L.; Saeys, W.; Lauwers, A.; De Proft, M.P. Green light induces shade avoidance to alter plant morphology and increases biomass production in Ocimum basilicum L. Sci. Hortic. 2020, 261, 109002. [Google Scholar] [CrossRef]
- Jiao, Y.; Lau, O.S.; Deng, X.W. Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 2007, 8, 217–230. [Google Scholar] [CrossRef]
- Song, Z.; Bian, Y.; Liu, J.; Sun, Y.; Xu, D. B-box proteins: Pivotal players in light-mediated development in plants. J. Integr. Plant Biol. 2020, 62, 1293–1309. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, X.; Li, L.; Lian, H.; Mao, Z.; Xu, P.; Guo, T.; Xu, F.; Du, S.; Cao, X.; et al. Photoexcited CRYPTOCHROME1 interacts with dephosphorylated BES1 to regulate brassinosteroid signaling and photomorphogenesis in Arabidopsis. Plant Cell 2018, 30, 1989–2005. [Google Scholar] [CrossRef]
- He, G.; Liu, J.; Dong, H.; Sun, J. The blue-light receptor CRY1 interacts with BZR1 and BIN2 to modulate the phosphorylation and nuclear function of BZR1 in repressing BR signaling in Arabidopsis. Mol. Plant 2019, 12, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Xu, D. COP1 and BBXs-HY5-mediated light signal transduction in plants. New Phytol. 2020, 228, 1748–1753. [Google Scholar] [CrossRef]
- Zhong, M.; Zeng, B.; Tang, D.; Yang, J.; Qu, L.; Yan, J.; Wang, X.; Li, X.; Liu, X.; Zhao, X. The blue light receptor CRY1 interacts with GID1 and DELLA proteins to repress GA signaling during photomorphogenesis in Arabidopsis. Mol. Plant 2021, 14, 1328–1342. [Google Scholar] [CrossRef]
- Lian, H.; Xu, P.; He, S.; Wu, J.; Pan, J.; Wang, W.; Xu, F.; Wang, S.; Pan, J.; Huang, J.; et al. Photoexcited CRYPTOCHROME 1 interacts directly with G-Protein β subunit AGB1 to regulate the DNA-binding activity of HY5 and photomorphogenesis in Arabidopsis. Mol. Plant 2018, 11, 1248–1263. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Gao, S.; Martinez, C.; He, G.; Zhou, Z.; Huang, X.; Lee, J.-H.; Zhang, H.; Shen, Y.; et al. Arabidopsis transcription factor ELONGATED HYPOCOTYL5 plays a role in the feedback regulation of phytochrome A signaling. Plant Cell 2010, 22, 3634–3649. [Google Scholar] [CrossRef]
- Huang, X.; Ouyang, X.; Yang, P.; Lau, O.S.; Li, G.; Li, J.; Chen, H.; Deng, X.W. Arabidopsis FHY3 and HY5 positively mediate induction of COP1 transcription in response to photomorphogenic UV-B light. Plant Cell 2012, 24, 4590–4606. [Google Scholar] [CrossRef]
- Abbas, N.; Maurya, J.P.; Senapati, D.; Gangappa, S.N.; Chattopadhyay, S. Arabidopsis CAM7 and HY5 physically interact and directly bind to the HY5 promoter to regulate its expression and thereby promote photomorphogenesis. Plant Cell 2014, 26, 1036–1052. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh, M.; Dayandan, S. Phylogeny of Populus (Salicaceae) based on nucleotide sequences of chloroplast trnT-trnF region and nuclear Rdna. Am. J. Bot. 2004, 91, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Korpelainen, H.; Li, C. Populus yunnanensis males adopt more efficient protective strategies than females to cope with excess zinc and acid rain. Chemosphere 2013, 91, 1213–1220. [Google Scholar] [CrossRef]
- Batista-Silva, W.; da Fonseca-Pereira, P.; Martins, A.; Zsögön, A.; Nunes-Nesi, A.; Araújo, W. Engineering improved photosynthesis in the era of synthetic biology. Plant Commun. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Li, J.; Qiao, Z.; Zong, D.; He, C. Effects of different light qualities on the growth characteristics of Populus trinervis. Phyton Int. J. Exp. Bot. 2024, 93, 1043–1056. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Chen, Z.; Ma, S.; Zong, D.; He, C. Effects of different light quality on growth and photosynthetic characteristics of Populus szechuanica var. tibetica seedlings. Southwest. China J. Agric. Sci. 2024, 37, 1019–1027. [Google Scholar]
- Gong, G. The geographic distribution and origin of Populus L. J. Sichuan For. Sci. Technol. 2004, 25, 25–30. [Google Scholar]
- Jia, C.; Gu, Y.; He, C.; Zhou, A.; Luo, J. Growth characteristics of five native poplars in western Sichuan plateau. J. Northwest A&F Univ. 2017, 45, 79–87. [Google Scholar]
- Yu, S.; Liu, J.; Fu, D.; Liu, D.; Liu, Y. Characteristics of tacamachaca genes in the western Sichuan plateau. J. Zhejiang A&F Univ. 2003, 20, 29–33. [Google Scholar]
- Zhou, N.; Zhang, J.; Liu, H.; Zha, W.; Pei, D. New Protocols for paraffin sections of heterogeneous tissues of woody plants. Chin. Bull. Bot. 2018, 53, 653–660. [Google Scholar]
- Cai, Y. Experimental Supervision of Plant Physiology; China Agricultural University Press: Beijing, China, 2014; pp. 42–45. [Google Scholar]
- Gao, J. Experimental Supervision of Plant Physiology; Higher Education Press: Beijing, China, 2006; pp. 144–148. [Google Scholar]
- Yun, T.; Li, J.; Zu, Y.; Zhou, A.; Zong, D.; Wang, S.; Li, D.; He, C. Selection of reference genes for RT-qPCR analysis in the bark of Populus yunnanensis cuttings. J. Environ. Biol. 2019, 40, 584–591. [Google Scholar] [CrossRef]
- Shuai, P.; Liang, D.; Tang, S.; Zhang, Z.; Ye, C.-Y.; Su, Y.; Xia, X.; Yin, W. Genome-wide identification and functional prediction of novel and drought-responsive lincRNAs in Populus trichocarpa. J. Exp. Bot. 2014, 65, 4975–4983. [Google Scholar] [CrossRef]
- Trouwborst, G.; Oosterkamp, J.; Hogewoning, S.W.; Harbinson, J.; van Ieperen, W. The responses of light interception, photosynthesis and fruit yield of cucumber to LED-lighting within the canopy. Physiol. Plant 2010, 138, 289–300. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern led systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Hormonal impact on photosynthesis and photoprotection in plants. Plant Physiol. 2021, 185, 1500–1522. [Google Scholar] [CrossRef]
- Roni, Z.K.; Islam, S.; Shimasaki, K. Response of Eustoma leaf phenotype and photosynthetic performance to LED light quality. Horticulturae 2017, 3, 50. [Google Scholar] [CrossRef]
- Lim, S.; Kim, J. Light quality affects water use of sweet basil by changing its stomatal development. Agronomy 2021, 11, 303. [Google Scholar] [CrossRef]
- Dörken, V.; Lepetit, B. Morpho-anatomical and physiological differences between sun and shade leaves in Abies alba MILL. (Pinaceae, Coniferales): A combined approach. Plant Cell Environ. 2018, 41, 1683–1697. [Google Scholar] [CrossRef] [PubMed]
- Carriquí, M.; Nadal, M.; Flexas, J. Acclimation of mesophyll conductance and anatomy to light during leaf aging in Arabidopsis thaliana. Physiol. Plant. 2021, 172, 1894–1907. [Google Scholar] [CrossRef] [PubMed]
- Allorent, G.; Petroutsos, D. Photoreceptor-dependent regulation of photoprotection. Curr. Opin. Plant Biol. 2017, 37, 102–108. [Google Scholar] [CrossRef]
- Griffin, J.H.C.; Toledo-Ortiz, G. Plant photoreceptors and their signalling components in chloroplastic anterograde and retrograde communication. J. Exp. Bot. 2022, 73, 7126–7138. [Google Scholar] [CrossRef]
- Ye, S.; Shao, Q.; Xu, M.; Li, S.; Wu, M.; Tan, X.; Su, L. Effects of light quality on morphology, enzyme activities, and bioactive compound contents in Anoectochilus roxburghii. Front. Plant Sci. 2017, 8, 857. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, S.; Ren, Y.; Chen, S.; Liesche, J. Regulation of sucrose transporters and phloem loading in response to environmental cues. Plant Physiol. 2018, 176, 930–945. [Google Scholar] [CrossRef]
- Braun, D.M. Phloem loading and unloading of sucrose: What a long, strange trip from source to sink. Annu. Rev. Plant Biol. 2022, 73, 553–584. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Li, T.; Yang, Q.; Guo, W. Sugar accumulation and growth of lettuce exposed to different lighting modes of red and blue LED light. Sci. Rep. 2019, 9, 6926. [Google Scholar] [CrossRef]
- Li, C.; Xu, Z.; Dong, R.; Chang, S.; Wang, L.; Khalil-Ur-Rehman, M.; Tao, J. An RNA-Seq analysis of grape plantlets grown in vitro reveals different responses to blue, green, red LED light, and white fluorescent light. Front. Plant Sci. 2017, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Sassi, M.; Wang, J.; Ruberti, I.; Vernoux, T.; Xu, J. Shedding light on auxin movement: Light-regulation of polar auxin transport in the photocontrol of plant development. Plant Signal. Behav. 2013, 8, e23355. [Google Scholar] [CrossRef]
- Zhou, Y.; Ahammed, G.J.; Wang, Q.; Wu, C.; Wan, C.; Yang, Y. Transcriptomic insights into the blue light-induced female floral sex expression in cucumber (Cucumis sativus L.). Sci. Rep. 2018, 8, 14261. [Google Scholar] [CrossRef] [PubMed]
- OuYang, F.; Mao, J.-F.; Wang, J.; Zhang, S.; Li, Y. Transcriptome analysis reveals that red and blue light regulate growth and phytohormone metabolism in Norway Spruce [Picea abies (L.) Karst.]. PLoS ONE 2015, 10, e0127896. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.N.; Kathare, P.K.; Huq, E. Phytochromes and phytochrome interacting factors. Plant Physiol. 2018, 176, 1025–1038. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, C. Mechanisms of cryptochrome-mediated photoresponses in plants. Annu. Rev. Plant Biol. 2020, 71, 103–129. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, H.; Zhao, Y.; Cheng, X.; Wang, L.; Guo, X. Effect of light quality on polyphenol biosynthesis in three varieties of mung bean sprouts with different color seed coats. Plant Cell Rep. 2023, 42, 253–268. [Google Scholar]
- Toledo-Ortiz, G.; Johansson, H.; Lee, K.P.; Bou-Torrent, J.; Stewart, K.; Steel, G.; Rodríguez-Concepción, M.; Halliday, K.J. The HY5-PIF regulatory module coordinates light and temperature control of photosynthetic gene transcription. PLoS Genet. 2014, 10, e1004416. [Google Scholar] [CrossRef] [PubMed]
- Gangappa, S.N.; Botto, J.F. The multifaceted roles of HY5 in plant growth and development. Mol. Plant 2016, 9, 1353–1365. [Google Scholar] [CrossRef]
- Huai, J.; Jing, Y.; Lin, R. Functional analysis of ZmCOP1 and ZmHY5 reveals conserved light signaling mechanism in maize and Arabidopsis. Physiol. Plant. 2020, 169, 369–379. [Google Scholar] [CrossRef]
- Leivar, P.; Monte, E. PIFs: Systems integrators in plant development. Plant Cell 2014, 26, 56–78. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xu, R.; Wang, C.; Zhang, S.; Zhao, L.; Zhao, N.; Xu, Y.; Zong, D. Transcriptome Reveals Growth Responses of Populus qamdoensis Under Blue and Green Films. Biology 2025, 14, 1658. https://doi.org/10.3390/biology14121658
Zhang X, Xu R, Wang C, Zhang S, Zhao L, Zhao N, Xu Y, Zong D. Transcriptome Reveals Growth Responses of Populus qamdoensis Under Blue and Green Films. Biology. 2025; 14(12):1658. https://doi.org/10.3390/biology14121658
Chicago/Turabian StyleZhang, Xiaolin, Rong Xu, Cai Wang, Shihai Zhang, Lihong Zhao, Ning Zhao, Yulan Xu, and Dan Zong. 2025. "Transcriptome Reveals Growth Responses of Populus qamdoensis Under Blue and Green Films" Biology 14, no. 12: 1658. https://doi.org/10.3390/biology14121658
APA StyleZhang, X., Xu, R., Wang, C., Zhang, S., Zhao, L., Zhao, N., Xu, Y., & Zong, D. (2025). Transcriptome Reveals Growth Responses of Populus qamdoensis Under Blue and Green Films. Biology, 14(12), 1658. https://doi.org/10.3390/biology14121658




