1. Introduction
Poultry exhibit unique glucose homeostasis. Firstly, blood glucose concentrations are twofold higher than those observed in mammals [
1,
2]. Secondly, the chronically elevated concentrations of blood glucose (chronic hyperglycemia) have not caused any adverse effects even in a fasted state, including diabetes mellitus and its associated complications [
3,
4]. It has been preliminarily proven that glucose homeostasis of poultry was correlated with the markedly higher standard metabolic rate and body temperature of birds compared to mammals [
5,
6]. Studies have shown that hummingbirds and bats can use hyperglycemia to provide fuel for flight [
7,
8]. However, these conventions in mammals dictate that these parameters are expected to result in an increased level of tissue damage, such as retinopathy, nephropathy, neuropathy, microvascular damage, and macrovascular damage [
9,
10]. Previous studies have focused only on physiological-level correlates (e.g., metabolic rate and body temperature) and failed to explore the underlying causes of poultry’s resistance to hyperglycemic complications. Chickens, as a type of poultry, have lost the ability to fly and migrate, but still maintain high glucose concentration. To the best of our knowledge, the underlying mediators that regulate blood glucose homeostasis to prevent diabetic complications under chronic hyperglycemia in chickens have yet to be clarified.
Maillard reaction (MR) is one of the main factors contributing to diabetic complications [
11] and is a reaction involving the condensation between a carbonyl group of reducing sugars, aldehydes, or ketones, and an amino group of amino acids, proteins or any nitrogenous compound [
12]. MR is a chemical procedure that involves three steps through which the initial products are identified as the slow formation of Schiff bases that via rearrangement reaction could form Amadori products, which undergo further reactions to produce irreversible advanced glycation end products (AGEs) [
13,
14]. Fructosamine (FA) is a product of the Schiff base reaction in the early stage of the Maillard reaction, referring to all ketoamine linkages that result from the glycation of serum proteins [
15,
16]. While methylglyoxal (MG) is an intermediate product of the Maillard reaction, it has two adjacent carbonyl groups that can directly form AGEs [
17,
18]. AGEs are deleterious molecules and they transduce inflammatory and fibrotic signals in immune-related cells to induce somatic cell damage, and lastly, tissue fibrosis in various tissue types and organs [
19,
20]. Accordingly, understanding the generation and development of AGEs is of crucial importance in the research of the underlying mechanisms of diabetic complications.
Streptozotocin (STZ) is an antibiotic that raises blood glucose by destructing pancreatic islet β-cells and is widely used experimentally to produce a model of diabetes mellitus (DM) [
21]. Also, previous research suggests that blood glucose concentrations in chickens and rats could be increased by intraperitoneal injection of STZ [
22,
23]. However, the physiological differences in blood glucose regulation between them have been rarely reported. Additionally, studies have indicated that the breakthrough of using poultry as a non-pathological model for T2DM lies in their low concentration of AGEs, which may be attributed to the anti-glycosylation properties of poultry, but there is currently no data to support this claim [
24,
25]. To clarify the potential link between poultry physiology and AGE inhibition, prior studies have provided critical evidence for the anti-glycation role of free amino acids. Specifically, it has been proven that many free amino acids inhibit the formation of AGEs, including histidine, alanine, taurine, and arginine [
26,
27]. In addition to any potential hypoglycemic effects, their more important function is to bind to the carbonyl groups of glucose to competitively inhibit the carbonyl–amine reactions [
28,
29]. Thus, free amino acids are also known as “carbonyl scavengers” for their ability to intercept glucose-derived carbonyl groups and block AGE formation, thereby inhibiting the MR.
Therefore, in this study, chickens and rats were chosen as representatives of poultry and mammals, respectively, to investigate the differences in substances such as insulin, AGEs, and free amino acids in the blood under normal conditions and STZ-induced hyperglycemia. The objective of this study was to explore the internal causes of why mammals develop hyperglycemic complications while poultry show no clinical symptoms, and specifically to investigate whether the reduction in free amino acid concentration inhibits the occurrence of MRs by comparing blood glucose regulation between chickens and rats under hyperglycemia. We hypothesize that higher concentrations of free amino acids in chicken plasma reduce the formation of AGEs under hyperglycemic conditions. In contrast, the insufficiency of free amino acids in rat plasma fails to prevent the occurrence of MR and the accumulation of AGEs, ultimately contributing to the development of hyperglycemic complications. This difference is expected to be the key internal reason for the absence of clinical symptoms in hyperglycemic poultry. The results not only shed light on the anti-inflammatory effect of chronic hyperglycemia in chickens but also offer new targets for developing therapeutic agents to regulate hyperglycemia.
2. Materials and Methods
2.1. Ethics Statement
Animal care and handling protocols were approved by the Yangzhou University Ethics Committee for Animal Experiments (Permit Number: 2023004742). All procedures adhered to the “Regulations on Laboratory Animal Management” (Yangzhou University, 2012) and the “Standards for Experimental Practices” (Jiangsu, China, 2008).
2.2. Experimental Animals
Thirty 20-day-old male Ross 308 chickens (body weight: 0.7–1 kg) were sourced from the Hongwei Chicken Farm (Suqian, China), and thirty male Sprague Dawley (SD) rats at 4 weeks of age (body weights: 70–100 g) were obtained from the Animal Center of Yangzhou University (Yangzhou, China). Thirty animals per species were used to account for potential losses (e.g., health issues and accidental injury) and align with the animal facility’s capacity, with only healthy individuals included per preset screening criteria. The healthy chickens were housed in an 18.5-square-foot area with 6 wire cages, each containing 4–6 birds, and were kept under a 16 h light and 8 h dark cycle at a temperature range of 22–26 °C for 7 days. The rats were housed in a 20-square-foot room with 9 cages, each containing 3–4 rats and were maintained under a 12 h light and 12 h dark cycle, with room temperature set between 23 and 26 °C. The composition of the basal diets and nutrient levels for chickens and rats is presented in
Table 1 and
Table 2. There was no additional glucose added to the daily diets consumed by the experimental animals.
2.3. Hyperglycemic Animal Model
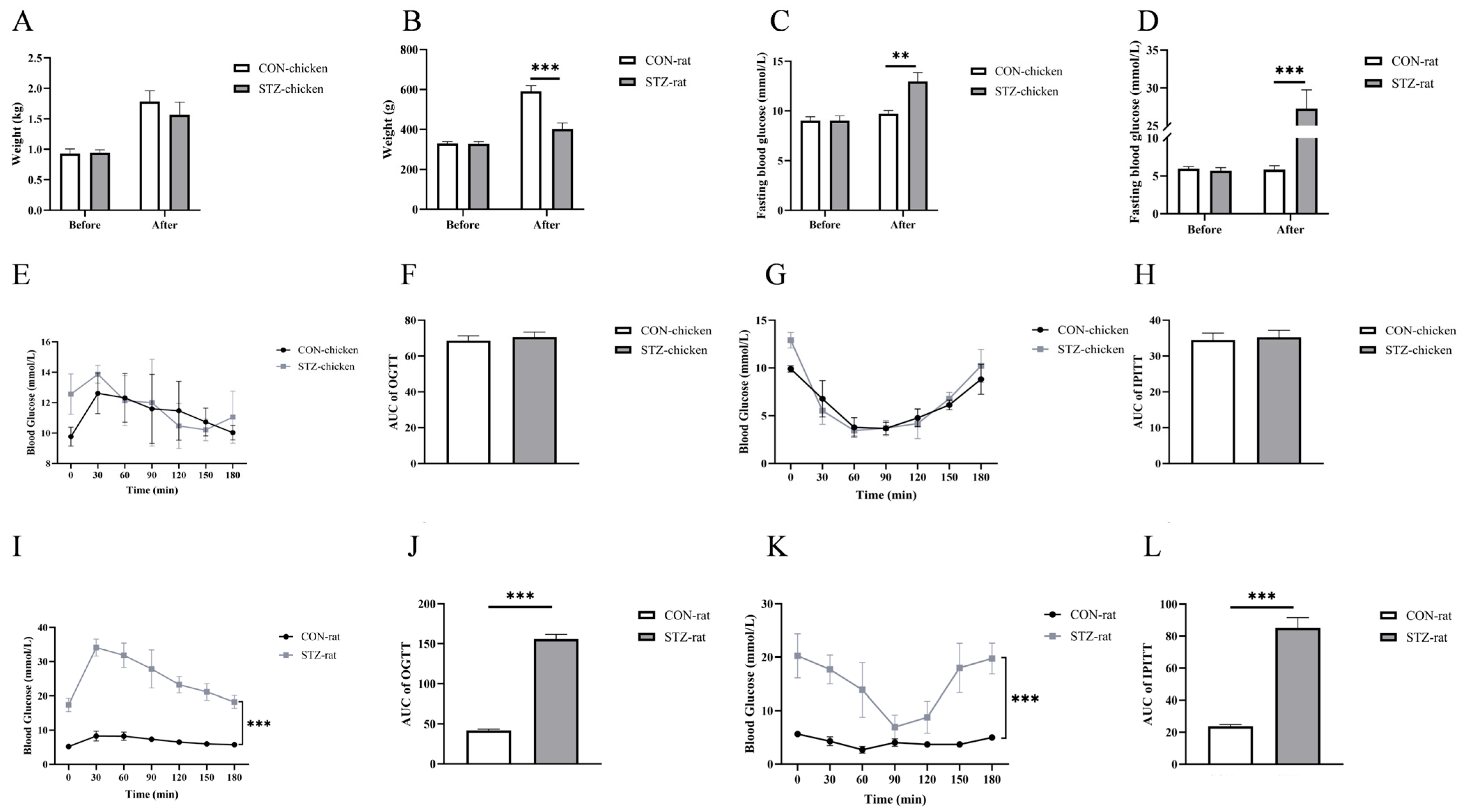
After 7 days of acclimation, thirty chickens and thirty rats were fasted for 8 h with ad libitum access to water before the experiment. These animals were randomized into two groups, respectively. Fifteen chickens and fifteen rats received intraperitoneal injections of streptozotocin (STZ; 70 mg/kg in 0.1 M citrate-buffered saline, pH 4.5) to induce diabetes (572201, Sigma-Aldrich, St. Louis, MO, USA), while the remaining animals were injected with PBS (1 mL) as a control. The injection was provided once every three days, twice at the beginning of the trial, totaling six days. Subsequently, the STZ-treated chickens and rats fasted for 12 h prior to blood glucose measurements. The fast blood glucose (FBG) concentrations were measured with an ACCU-CHEKA Performa glucometer (Roche Diagnostics GmbH, Mannheim, Germany). Chickens with fasting glucose concentrations > 14 mmol/L were classified as hyperglycemic [
22]. Rats with fasting glucose concentrations > 12 mmol/L were considered hyperglycemic [
30].
2.4. Oral Glucose Tolerance Tests and Intraperitoneal Insulin Tolerance Tests
For oral glucose tolerance tests (OGTTs), chickens and rats were fasted overnight without water restriction on day 14, after which glucose (2 g/kg body weight, BW) was administered orally. Blood glucose levels were measured at 0, 30, 60, 90, and 120 min post-administration using an ACCU-CHEK Performa glucometer.
For intraperitoneal insulin tolerance tests (IPITTs), chickens and rats were fasted overnight without water restriction on day 17. Subcutaneous insulin injection (2 IU/kg BW) was administered, and blood glucose concentrations were determined at 0, 30, 60, 90, and 120 min post-injection using the same glucometer. The homeostatic model assessment for insulin resistance (HOMA-IR) index was calculated using the following formula: HOMA-IR = (FBG [mmol/L] × FINS [units/L])/22.5 [
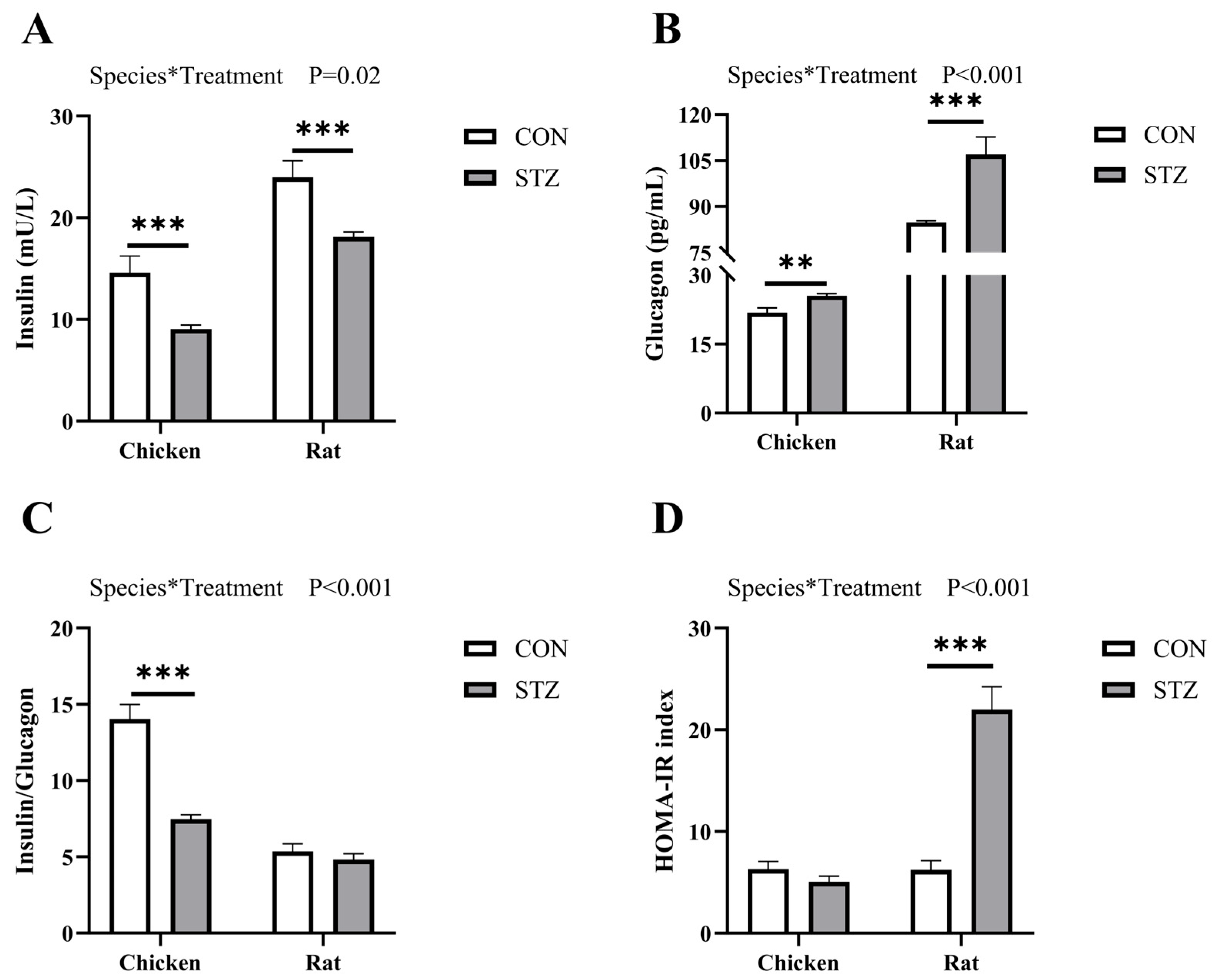
31], where FBG denotes fasting blood glucose and FINS represents fasting insulin.
2.5. Sample Collection
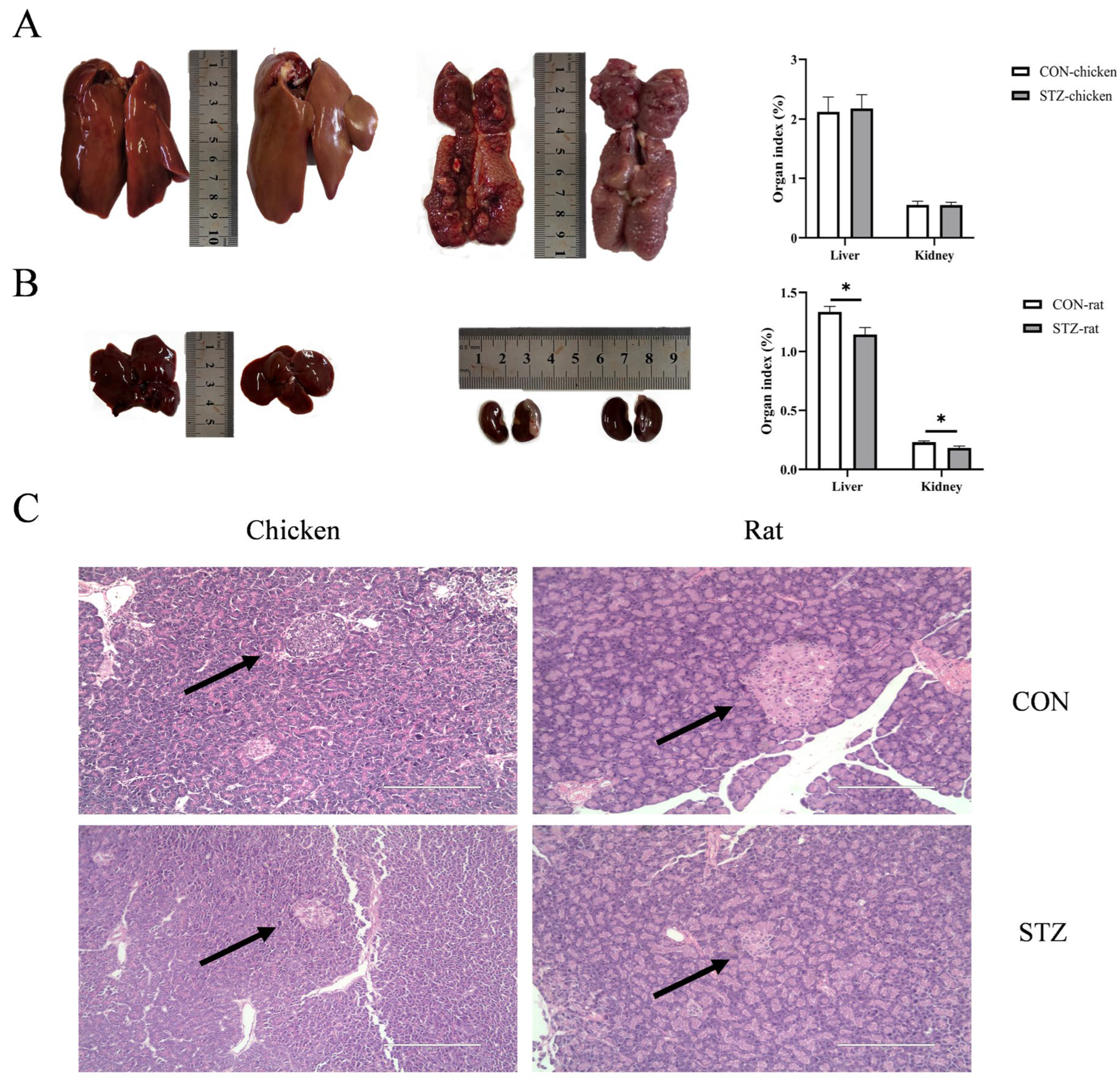
All animals were monitored daily for any health-related problems. The experimental study lasted for 21 days after successful induction with STZ. At the end of the experiment, six chickens and six rats per group were selected as replicates using a random sequence generated via Microsoft Excel (Microsoft Corp., Redmond, WA, USA), with priority given to healthy individuals free of specific symptoms; blood collection was then performed at the Animal Center of Yangzhou University. The serum was separated from the blood samples by centrifuging at 3000× g for 10 min at 4 °C. Following labeling, the serum samples were rapidly frozen in liquid nitrogen for 10 min and then cryostored at −80 °C for subsequent experimental use. After the treatment ended, all chickens and rats were anesthetized using 10% chloral hydrate and then humanely sacrificed. The kidney, liver, heart, muscle (pectoral muscle), and pancreas were collected sequentially. The kidney and liver were weighed after removing related contents and adherent substances. Organ indices were determined using the following formula: [organ weight (g)/live body weight (g)] × 100%. Then, different organs were promptly collected into cryotubes, frozen in liquid nitrogen, and stored at −80 °C for real-time RT-qPCR analysis. Additionally, the pancreas was also harvested for Hematoxylin–Eosin (H&E) staining.
2.6. Determination of Serum Hormone Concentrations by ELISA
Serum concentrations of insulin, glucagon, fructosamine (FA), and advanced glycation end products (AGEs) in chickens and rats were detected by ELISA, respectively, following the protocols from commercial kits (for chickens, MM-60632O2, MM-60623O2, MM-60621O2, and MM-60615O2; for rats, MM-1049R2, MM-8272R2, MM-21260R2, and MM-0270R2, Jiangsu Kete Biotechnology Co., Ltd., Yancheng, China). The concentrations of nuclear factor-κB phosphorylated p65 (NF-κB p-p65), interleukin-1β (IL-1β), and interleukin-10 (IL-10) were also measured by ELISA using commercially available kits (for chickens: ml002789, ml059835, and ml059830; for rats: ml259671, ml037361, and ml037371; Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China). Briefly, 50 µL of the prepared sample, standards, and biotin antigen were added to each well and incubated for 30 min at 37 °C. After washing the plate 5 times, 50 µL of horseradish peroxidase conjugate reagent was added, followed by another 30 min incubation at 37 °C. The plate was washed again 5 times, and 50 µL of chromogen solution A and B was added, with further incubation for 30 min at 37 °C. Lastly, 50 µL of stop solution was added, and optical density (OD) values were recorded for calculation within 10 min.
2.7. Blood Biochemical Analysis
After blood collection, centrifugation, and storage, the serum biochemical indicators were determined using a fully automatic biochemical analyzer (AU480, Beckman Coulter K.K, Tokyo, Japan). Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), the AST/ALT, alkaline phosphatase (ALP), total protein (TP), albumin (ALB), globulin (GLOB), and ALB/GLOB were determined. The blood lipid indicators measured were triglyceride (TG), total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C).
2.8. RT-qPCR
Total RNA was isolated from the kidney, liver, heart, muscle, and pancreas. Primers were designed via Primer 5.0 software (Premier Biosoft International, Palo Alto, CA, USA) and custom-synthesized by Tsingke Biotechnology Co., Ltd. (Nanjing, China) to detect the expression levels of the following target genes: nuclear factor kappa B (NF-κB), interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNFα), monocyte chemoattractant protein-1 (MCP1), and interleukin-10 (IL-10) (
Table 3). The reaction was performed using 2× Q3 SYBR qPCR Master Mix (Universal) (22204, Tolo Biotech Co., Ltd., Shanghai, China). For each experimental group, six samples per tissue were selected, with three technical replicates per sample. Data were normalized against the housekeeping gene β-actin, and relative abundance was calculated by applying the comparative CT method 2−∆∆CT.
2.9. Western Blot Analysis
Tissues were lysed with RIPA laced with proteinase inhibitors for 30 min, and the protein concentration was measured using the BCA assay (P0010S, Beyotime Biotechnology, Shanghai, China) following the manufacturer’s instructions. The protein samples were denatured at 100 °C for 7 min containing 5× loading buffer (P0285, Beyotime Biotechnology, Shanghai, China). The 12% sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel was prepared using an SDS-PAGE Gel Kit (P1200, Solarbio Science & Technology Co., Ltd., Beijing, China). Then, 20 μg of total protein was subjected to 12% SDS-PAGE, which was performed on ice using an electrophoresis system (PowerPac HC, Bio-Rad, Hercules, CA, USA). The electrophoresis parameters were set as follows: 90 V for 20 min for the stacking gel and 120 V for 40 min for the resolving gel. After electrophoresis, proteins were transferred onto 0.45 μm polyvinylidene fluoride (PVDF) membranes, which were incubated in 5% bovine serum albumin-blocking solution for 1 h at room temperature.
To detect protein markers of inflammatory factors, the membranes were incubated with primary antibodies including rabbit anti-NF-κB phosphorylated p65 (1:1000, AP1294, ABclonal Technology Co., Ltd., Wuhan, China), NF-κB total p65 (1:1000, A2547, ABclonal Technology Co., Ltd., Wuhan, China), IL1β (1:1000, A16288, ABclonal Technology Co., Ltd., Wuhan, China), and β-actin (1:2000, CW0097, CWBIO Technology Co., Ltd., Taizhou, China) at 4 °C overnight. After the excess primary antibodies on the PVDF membranes were cleaned, the membranes were incubated with secondary goat anti-rabbit antibodies which conjugated with horseradish peroxidase (HRP) (1:7500, CW0103S, CWBIO Technology Co., Ltd., Taizhou, China). After the excess secondary antibodies on the PVDF membranes were cleaned, the membranes were incubated with a chemiluminescence detection kit (P10100, NCM Biotech Co., Ltd., Soochow, China) following the manufacturer’s instructions and were detected with a chemiluminescence detector (Tanon5200, Shanghai, China).
2.10. H&E Staining and Masson Staining
After fixation, the pancreas samples were embedded in paraffin for 24 h first and then sectioned into 5 µm-thick sections using a microtome (RM2016, Leica Biosystems, Wetzlar, Germany). The sections were subjected to H&E staining (GP1031, Wuhan Servicebio Biotechnology Co., Ltd., Wuhan, China) for histological inspection. Histological images were captured using a micro-image analysis system (CaseViewer 2.4, 3DHISTECH Ltd., Budapest, Hungary).
2.11. Liquid Chromatography–Mass Spectrometry (LCMS)
AGILENT 1290–6470 liquid chromatography–mass spectrometry (Agilent Technologies Inc., Santa Clara, CA, USA) was used to obtain the detailed mass spectra of methylglyoxal (MG), taurine and free amino acids in the serum. The serum was filtered with a 0.22 µm nylon filter, and the sample was injected into the analytical column. The LCMS conditions for MG were as follows. Chromatographic column: Agilent C18 column (2.1 mm × 100 mm, 1.8 μm); column temperature: 35 °C; flow rate: 0.3 mL/min; acquisition mode: ESI+; parent ion: 195; daughter ions: 127, 115; injection volume: 2 μL; mobile phase: mobile phase A: 0.1% formic acid aqueous solution; mobile phase B: acetonitrile. The mobile phase gradient is shown in
Table 4.
The LCMS conditions for 17 free amino acids were as follows: chromatographic column: C18 SHISEIDO column (4.6 mm × 250 mm × 5 μm, Shiseido Co., Ltd., Tokyo, Japan); detector: FLD detector; column temperature: 40 °C; injection volume: 10 μL; wavelength: 254 nm. Mobile phases: Mobile phase A: a mixture of 0.1 mol/L anhydrous sodium acetate and acetonitrile at a ratio of 97:3. After mixing, the pH was adjusted to 6.5 (prepared by dissolving 31.815 g of sodium acetate in 3880 mL of water and then adding 120 mL of acetonitrile). Mobile phase B: a mixture of acetonitrile and water at a ratio of 80:20. The mobile phase gradient is shown in
Table 5. The LCMS unit was directly connected with Agilent Technologies version acquisition method info for the detailed analysis and mass fragmentation was identified by using a spectrum database for organic compounds.
2.12. Statistical Analysis
SPSS software (version 25.0, IBM Corp, Armonk, NY, USA) was used for statistical analysis. Each experiment was performed six times, and the data were expressed as mean ± SE. Prior to data analysis, the normality of the data was verified by the Shapiro–Wilk test, and the homogeneity of variances was confirmed by the Levene test. For comparisons involving two independent groups, an unpaired Student’s t-test was used. To investigate the effects of species (chicken/rat) and treatment (CON/STZ) and their interaction, a two-way ANOVA was conducted. Where a significant interaction was found, simple effects analysis was performed. Statistical significance was set at p < 0.05. GraphPad Prism 10.1 (GraphPad Software, San Diego, CA, USA) was used for processing figures.
4. Discussion
Poultry are unique among vertebrates in maintaining relatively high blood glucose concentrations. They live healthily without adverse effects despite high blood glucose concentrations compared with mammals [
2,
32]. Glycosylation of proteins and subsequent production of advanced glycation end products (AGEs) are the most important factors leading to hyperglycemia-induced pathological changes [
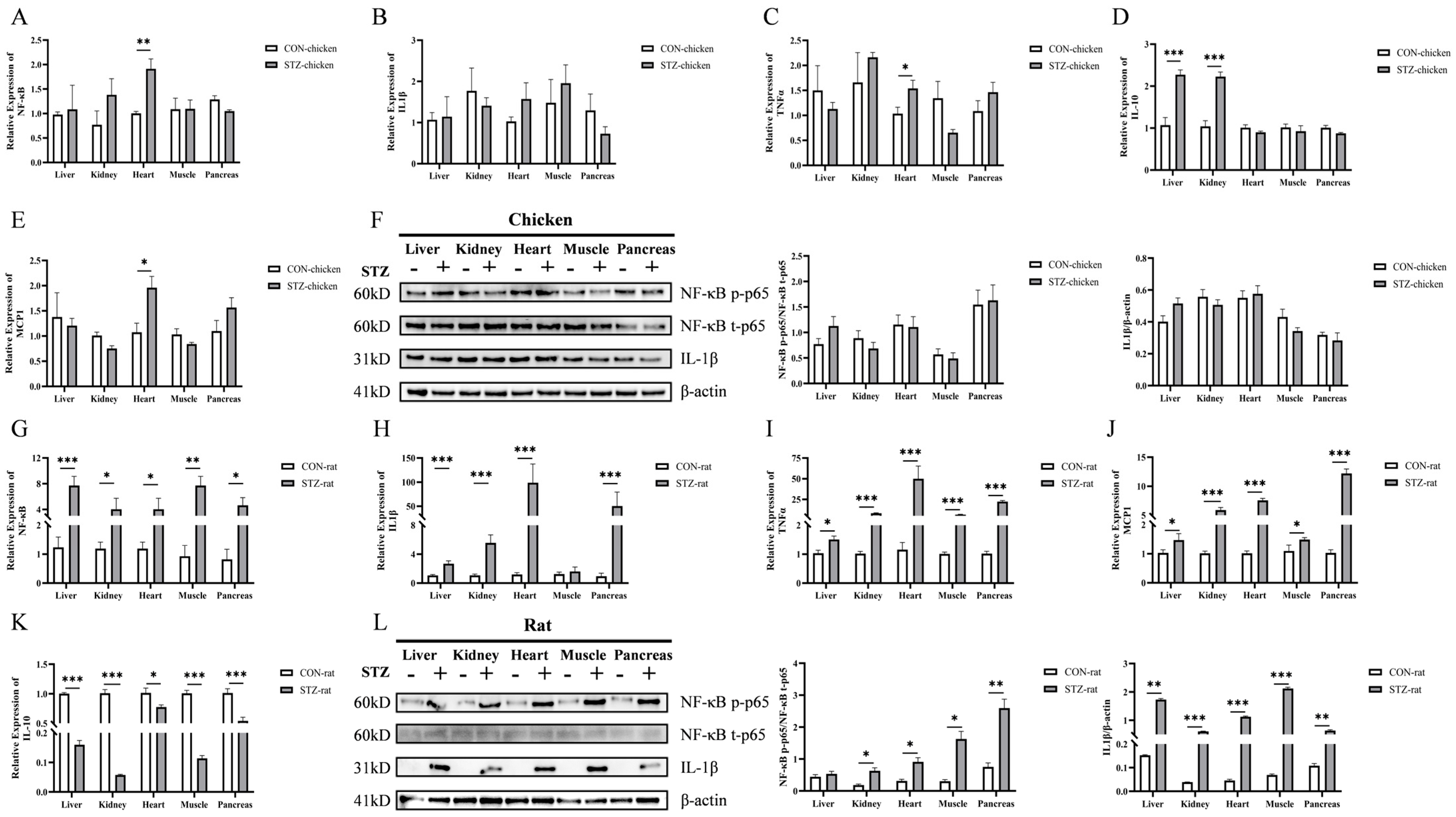
33]. However, there are no studies investigating the relationship between the development of AGEs and potential side effects on chronic hyperglycemia in poultry. In this study, we compared the blood physiological differences between chickens and rats and assessed whether there were biomolecules in chicken blood that could prevent inflammation induced by streptozotocin (STZ)-induced hyperglycemia. We found that after STZ treatment, fasting blood glucose (FBG) levels in both chickens and rats increased significantly, but those in rats were higher than those in chickens. Moreover, STZ induction had no obvious effects on body weight, organ development, tissue damage, and inflammatory factor expression in chickens. In contrast, STZ-treated rats showed delayed body weight and organ development, and the expression levels of inflammatory factors were significantly increased in the target organs of hyperglycemia. For the expression of AGEs, chickens showed an upward trend but no significant change after STZ induction, while rats showed a significant increase. However, for the precursor substances of AGEs, fructosamine (FA) and methylglyoxal (MG), both chickens and rats showed increases after STZ treatment. The concentration of some free amino acids (including taurine), which inhibit the formation of AGEs, was significantly decreased in chickens, whereas disorder was observed in rats. Taken together, the main reason for the no deleterious effects exhibited with chronic hyperglycemia by chickens is that taurine and some free amino acids act as carbonyl scavengers to inhibit the formation of AGEs from FA and MG. In contrast, in hyperglycemic rats, free amino acids were not consumed, leading to the continuous accumulation of FA, MG, and even AGEs. Ultimately, inflammation occurred. This study revealed that taurine and other free amino acids in chickens act as carbonyl scavengers to inhibit the formation of AGEs, thereby providing a novel explanation for their resistance to hyperglycemic damage, which will provide new targets for developing therapeutic agents.
At present, chickens have been studied as potentially useful animal models in the case of diabetes mellitus [
24,
25]. For mammals, these chronically elevated concentrations of blood glucose lead over time to pathophysiological changes collectively known as diabetic complications, including retinopathy, nephropathy neuropathy, microvascular damage, and macrovascular damage [
9,
10]. However, there are no adverse effects on chickens, and the process in which substances in their blood are involved in blood glucose regulation remains unclear. Here, we first compared the factors that affect blood glucose concentrations of chickens and rats. Notably, consistent with previous results [
34,
35], the fasting blood glucose level of chickens was 1.5–2 times that of rats when both were in a normal physiological state and had no additional glucose intake. Chickens are an insulin-resistant species characterized by a low concentration of insulin and high level of blood glucose [
2,
20]. This is consistent with our results showing that in the oral glucose tolerance test (OGTT) and intraperitoneal insulin tolerance test (IPITT), as well as in the insulin and glucagon detection assays, chickens exhibit distinct insulin-resistant physiological characteristics that differ from those of rats. As a glucosamine–nitrosourea antibiotic, streptozotocin (STZ) induces specific damage to pancreatic β-cells [
36,
37]. Due to its chemical resemblance to glucose, STZ penetrates the cell membrane via glucose transporter 2 (GLUT2) after injection. The subsequent intracellular toxicity leads to irreversible necrosis and a severe reduction in insulin production, thus elevating the blood glucose levels [
38,
39]. Therefore, we induced hyperglycemia in chickens and rats by intraperitoneal injection of STZ and found that the blood glucose concentrations of both chickens and rats increased significantly, but those in rats were higher than those in chickens. The OGTT and IPITT values of STZ-induced rats were significantly higher than those of the STZ-induced chickens. However, there was no significant difference between STZ-induced chickens and the control group. Moreover, the concentrations of both insulin and glucagon in rats were significantly higher than those in chickens. From the results of STZ induction, we found that chickens exhibited blood glucose metabolic stability that differs from that of rats.
Advanced glycation end products (AGEs) are a series of irreversible products formed by carbonyl and amino groups through the Schiff base and the Amadori rearrangement, and their formation is accelerated in hyperglycemia [
40]. This process is known as the Maillard reaction due to the associated yellow–brown color change [
41]. Since both types of compounds are indispensable for life and have to coexist in cells and organisms, Maillard reactions are unavoidable and lead to the formation of AGEs that are invariably deleterious to cell and organism function [
42,
43,
44]. One way by which AGEs exert their damaging effects is through interaction with specific receptors known as the receptor for advanced glycation end products (RAGEs), via which AGEs largely activate signaling mechanisms that cause cell stress, contribute to cellular dysfunction, and damage target organs, leading to complications [
45,
46,
47]. Therefore, RAGEs are considered to be one of the important factors contributing to diabetic complications [
24]. The engagement of AGEs with RAGEs activates a plethora of downstream signaling pathways, and the nuclear factor-kappa B (NF-κB) pathway is one of the central and pivotal inflammatory pathways [
48,
49]. Upon the activation of the RAGE, the phosphorylation and degradation of the inhibitory protein IκBα are induced, and the NF-κB dimer (primarily the p65/p50 heterodimer) is thereby released, enabling it to translocate into the nucleus [
50]. Within the nucleus, NF-κB, particularly the phosphorylated active form of p65 (p-p65), serves as a master transcriptional switch, thereby triggering the expression of a wide array of pro-inflammatory cytokines and chemokines, including interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNFα), and monocyte chemoattractant protein 1 (MCP1) [
51,
52]. This cascade creates a sustained pro-inflammatory microenvironment, which is a fundamental driver of the tissue damage and dysfunction observed in diabetic complications [
53,
54]. In this study, we assessed the activation status of this pathway by measuring the mRNA and protein levels of NF-κB total p65 (t-p65), NF-κB phosphorylated p65 (p-p65), and one of its key downstream targets, IL1β. We found that STZ-induced hyperglycemic rats exhibited a significant upregulation of AGEs, alongside elevated p-p65 and IL1β, strongly supporting the notion that the AGEs/RAGE-NF-κB axis is robustly activated, culminating in inflammatory damage. Conversely, the absence of significant AGE accumulation in chickens precludes the initiation of this deleterious signaling cascade, as evidenced by the stable levels of p-p65 and IL1β. Our findings provide a more upstream and critical explanation: AGEs themselves failed to accumulate in hyperglycemic chickens, which is the direct reason for the absence of inflammation. This contrasts sharply with the significant AGEs accumulation and subsequent inflammatory response in STZ-induced rats. Accordingly, it is more crucial to explore the reasons for maintaining the low concentration of AGEs in chicken blood.
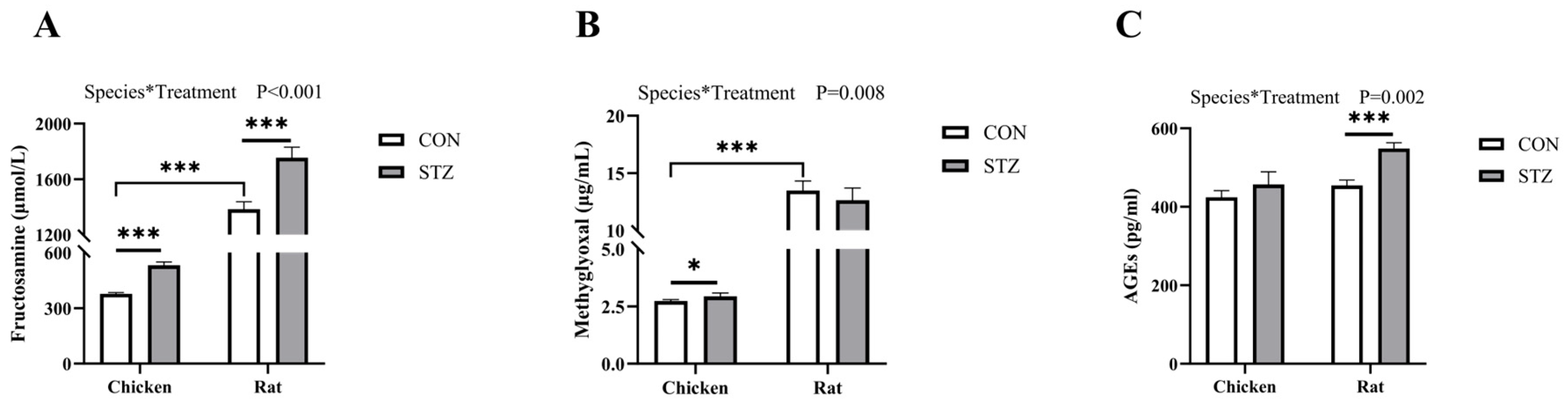
Consequently, this study further detected the concentrations of fructosamine (FA) and methylglyoxal (MG), which are important precursor substances of AGEs [
55]. FA is a product of the Schiff base reaction in the early stage of the Maillard reaction, referring to all ketoamine linkages that result from the glycation of serum proteins [
17,
18]. MG is an intermediate product of the Maillard reaction, having two adjacent carbonyl groups that can directly form AGEs [
46,
56]. We found that the concentration of FA in both chickens and rats with hyperglycemia was significantly higher than that in the control group. However, regarding the concentration of MG, there was no significant increase in hyperglycemic rats, while there was a significant increase in hyperglycemic chickens. For AGE concentrations, there was no significant difference in chickens between the two groups, while there was an extremely significant difference in rats. While both FA and MG increased significantly in hyperglycemic chickens, the final production of AGEs was effectively blocked. This critical disconnect between elevated precursors and stable AGE levels strongly suggests the presence of active scavenging substances in chicken blood that intercept the Maillard reaction pathway.
Our results demonstrated significantly higher basal levels of several free amino acids (e.g., taurine, arginine, histidine, and alanine) in chickens compared to rats. This finding is particularly intriguing in the context of their established role as carbonyl scavengers, which competitively inhibit the carbonyl-amine reactions that lead to AGE formation [
12,
28]. For example, it was found that taurine, as well as other free amino acids including arginine, leucine, and glycine, could inhibit the production of glycation products by reacting with aldehyde groups [
28,
29]. Moreover, free amino acids could directly further form dipeptides or polypeptides to enhance the effect of scavenging AGEs, such as homocarnosine [
57]. Crucially, upon STZ induction, we observed a significant decrease in the concentrations of these specific amino acids specifically in chickens, concomitant with a successful blockade of AGEs accumulation. We interpret this dynamic consumption as indirect evidence of their active engagement in neutralizing reactive carbonyl species derived from glucose and MG, thereby explaining the absence of hyperglycemic complications. This stands in stark contrast to rats, which lacked both high basal levels and this consumption response, resulting in significant AGEs accumulation. While our study was not designed to establish direct causality—a limitation we acknowledge—this correlative evidence is bolstered by preliminary functional data. Our preliminary research results showed that intraperitoneal injection of arginine and taurine in STZ-induced hyperglycemic rats significantly reduced AGE concentrations [
2]. This prior finding lends indirect support to the hypothesis that the higher availability and dynamic consumption of these amino acids in chickens constitute a key mechanism for their resistance to AGEs formation.
However, we recognize that our discussion attributes a primary protective role to free amino acids, while other factors—such as differences in mitochondrial metabolism or antioxidant systems—were not investigated and could also contribute to the observed phenotype [
58,
59,
60]. Furthermore, our study operated on the premise that STZ induces β-cell damage in chickens via a mechanism analogous to that in mammals, yet direct evidence for GLUT2 transporter expression in chicken β-cells and quantitative histological confirmation of islet damage are lacking. Future studies employing taurine synthesis inhibition in chickens or amino acid supplementation in hyperglycemic models, coupled with measurements of key enzyme activities (e.g., glyoxalase I), as well as direct quantification of pancreatic islet morphology and GLUT2 expression, are essential to definitively confirm the causal role of these candidate amino acids and elucidate their relative importance among other potential protective mechanisms.