Research Advances in the Regulation of Fruit Size: An Integrated Perspective of Genetic, Hormonal, Epigenetic, and Environmental Control
Simple Summary
Abstract
1. Introduction
2. Genetic Basis of Fruit Size Regulation
2.1. Research Progress on Major Fruit Size/Weight QTLs
2.1.1. fw2.2
2.1.2. fw3.2
2.1.3. Fas and Lc
2.2. Other Related QTLs
| s.n. | Crop | Gene/QTL | Main Function | References |
|---|---|---|---|---|
| 1 | Tomato | fw2.2 | Negatively regulates cell division; encodes CNR protein; affects fruit size. | [26,27,28] |
| 2 | Tomato | fw3.2/SlKLUH | Positively regulates fruit size; encodes CYP78A subfamily P450 enzyme; promotes cell proliferation. | [35,36,37] |
| 3 | Tomato | FAS/SlCLV3 | Regulates carpel (locule) number; affects fruit size. | [38] |
| 4 | Tomato | LC/SlWUS | Regulates locule number; affects fruit size. | [40] |
| 5 | Tomato | fw11.3/CSR | Regulates cell volume; affects fruit size. | [42] |
| 6 | Tomato | OVATE | Negatively regulates fruit longitudinal elongation; affects fruit shape and size. | [43] |
| 7 | Tomato | SUN | Promotes fruit elongation; affects fruit shape and size. | [44] |
| 8 | Tomato | SlOFP20 | Cooperates with OVATE to regulate fruit shape and size. | [45] |
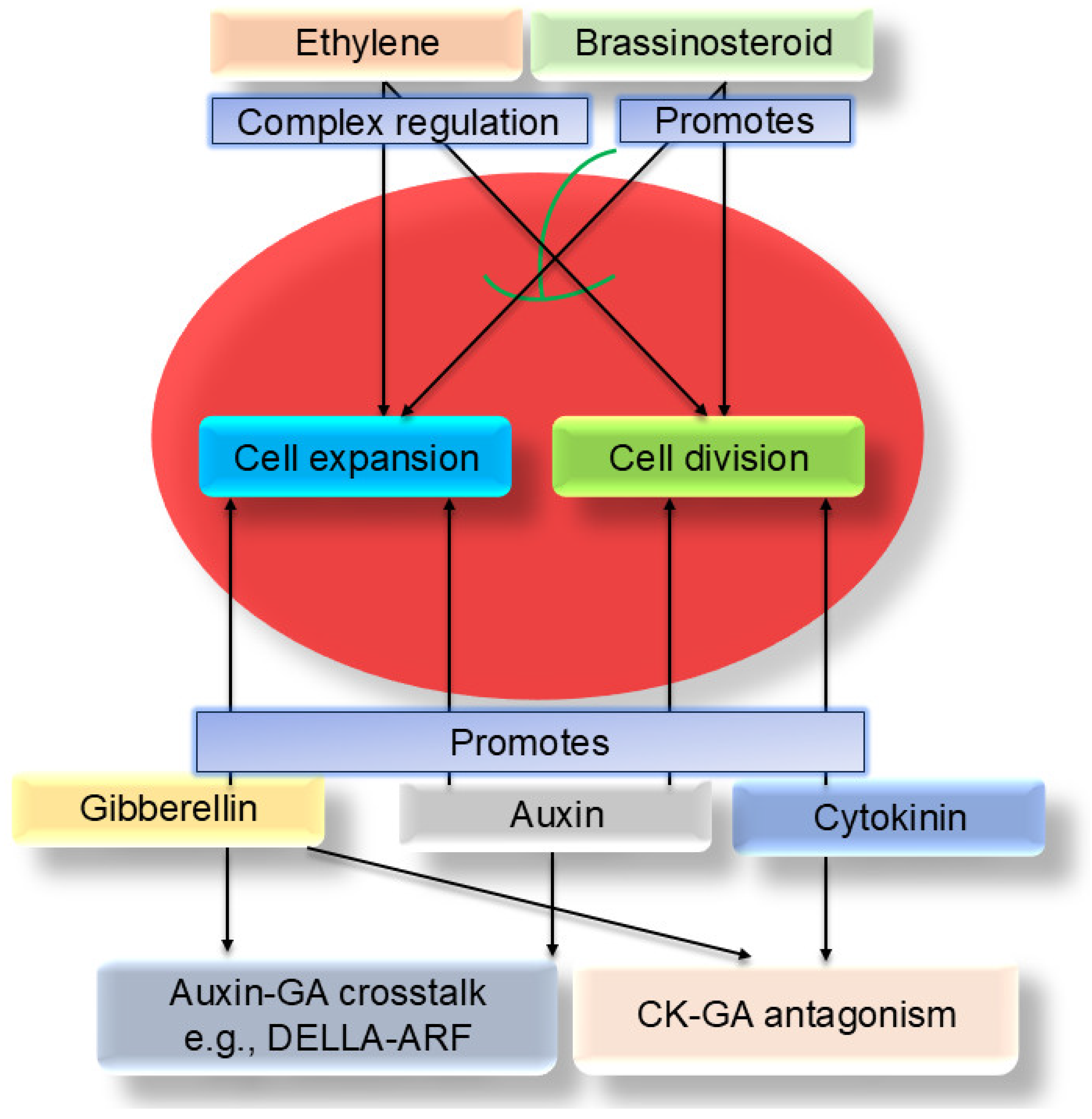
3. Core Role of Plant Hormones in Regulating Fruit Size
3.1. Auxin
3.2. Gibberellin (GA)
3.3. Cytokinin (CK)
3.4. Brassinosteroid (BR)
3.5. Ethylene and Other Hormones
4. Fine-Tuning of Fruit Size by Transcriptional Regulatory Networks
4.1. YABBY Transcription Factor Family
4.2. WOX Transcription Factor Family
4.3. Other Important Transcription Factors
| s.n. | Crop | Transcription Factor | Function | References |
|---|---|---|---|---|
| 1 | Tomato | SlYABBY2a | Positively regulates fruit septum development and ripening. | [22] |
| 2 | Tomato | SlWUS | Regulates meristem size and locule number; a key domestication gene. | [40,89] |
| 3 | Tomato | OVATE | Negatively regulates longitudinal fruit elongation; controls pear-shaped fruit. | [43] |
| 4 | Tomato | SlCRCa (YABBY) | Involved in feedback regulation of GA biosynthesis, affecting cell division. | [68] |
| 5 | Tomato | SlGAMYB2 | Positively regulates fruit size by activating SlGA3ox2 expression. | [69] |
| 6 | Tomato | SlPRE2 (bHLH) | Influences fruit size by regulating GA metabolism and cell proliferation-related genes. | [95] |
| 7 | Apple | MdARF106 | Associated with fruit cell division and expansion (Auxin Response Factor). | [49] |
| 8 | Apple | MdNAC1 | Overexpression results in smaller organs. | [92] |
| 9 | Watermelon | ClNAC100 | Directly upregulates ClEXPA1 and ClGA3oxs, promoting plant height and fruit development. | [93] |
| 10 | Strawberry | FvERF3 | Directly binds to the promoter of FvNAC073 to activate its expression, regulating fruit enlargement and ripening. | [94] |
| 11 | Apple | MdANT1/MdANT2 (AP2/ERF) | Affect early fruit development by regulating cell division. | [96] |
| 12 | Grape | VvYABBY4 | Ectopic expression leads to smaller fruits and seeds; may affect seed development. | [86] |
| 13 | Grape | VvNAC26 | Polymorphisms associate with berry size variation. | [91] |
| 14 | Kumquat | CsMYB77 | Overexpression delays fruit ripening and results in smaller fruits. | [98] |
| 15 | Melon | CmFYF | Overexpression promotes male flower formation but suppresses fruit size. | [106] |
| 16 | Tomato | AS2 and AS2L | Directly control pericarp development by modulating cell layer number and cell area. | [107] |
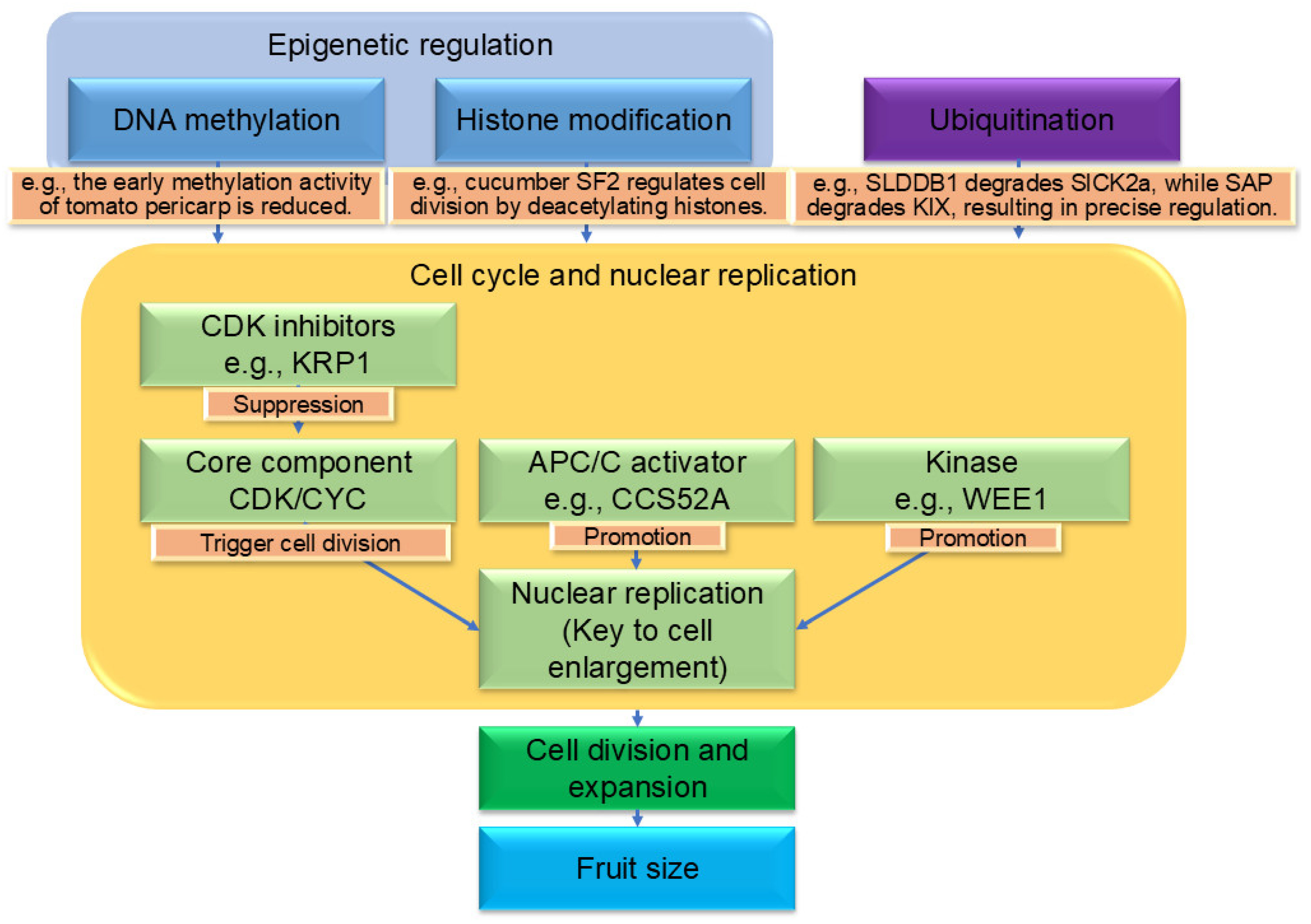
5. Epigenetic Regulation, Endoreduplication and Protein Ubiquitination’s Impact on Fruit Size Determination
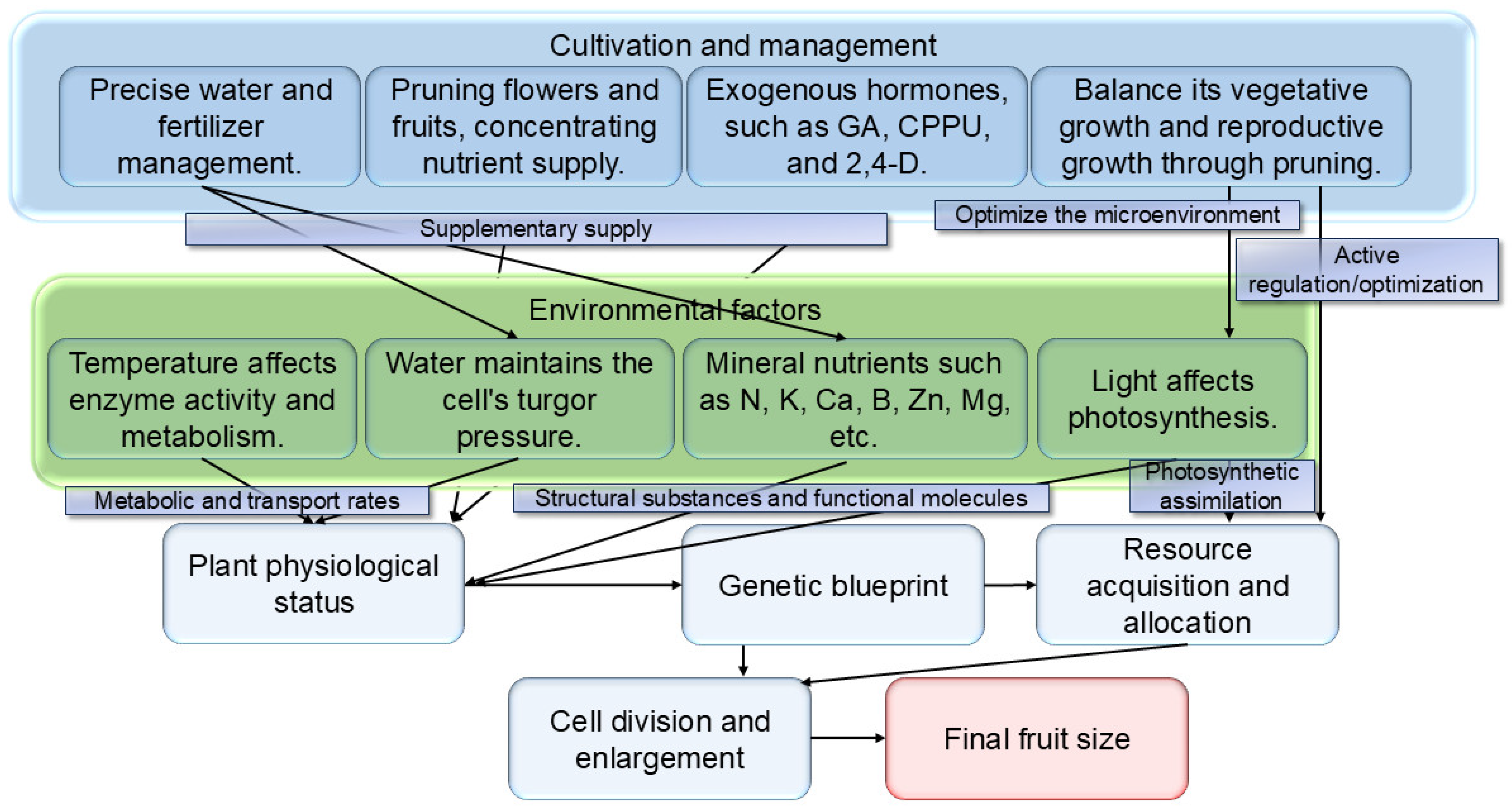
6. Regulation of Fruit Size by Environmental Factors and Cultivation Management
7. Future Research Directions and Prospects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gillaspy, G.; Ben-David, H.; Gruissem, W. Fruits: A developmental perspective. Plant Cell 1993, 5, 1439. [Google Scholar] [CrossRef]
- Chevalier, C.; Bourdon, M.; Pirrello, J.; Cheniclet, C.; Gévaudant, F.; Frangne, N. Endoreduplication and fruit growth in tomato: Evidence in favour of the karyoplasmic ratio theory. J. Exp. Bot. 2014, 65, 2731–2746. [Google Scholar] [CrossRef]
- Hu, D.L.; Richards, P.; Alexeev, A. The growth of giant pumpkins: How extreme weight influences shape. Int. J. Non-Linear Mech. 2011, 46, 637–647. [Google Scholar] [CrossRef]
- Harada, T.; Kurahashi, W.; Yanai, M.; Wakasa, Y.; Satoh, T. Involvement of cell proliferation and cell enlargement in increasing the fruit size of Malus species. Sci. Hortic. 2005, 105, 447–456. [Google Scholar] [CrossRef]
- Tanksley, S.D. The genetic, developmental, and molecular bases of fruit size and shape variation in tomato. Plant Cell 2004, 16, S181–S189. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Li, C.; Zhang, S.; Niyaz, A.; Du, R. Flowering biology and fruit development characteristics of apricot cultivar ‘Kezilang’ in Xinjiang. Agric. Sci. Technol. 2016, 17, 1838. [Google Scholar]
- Pei, M.; Cao, S.; Wu, L.; Wang, G.; Xie, Z.; Gu, C.; Zhang, S. Comparative transcriptome analyses of fruit development among pears, peaches, and strawberries provide new insights into single sigmoid patterns. BMC Plant Biol. 2020, 20, 108. [Google Scholar] [CrossRef]
- Christodoulou, M.D.; Culham, A. When do apples stop growing, and why does it matter? PLoS ONE 2021, 16, e0252288. [Google Scholar] [CrossRef] [PubMed]
- Bohner, J.; Bangerth, F. Effects of fruit set sequence and defoliation on cell number, cell size and hormone levels of tomato fruits (Lycopersicon esculentum Mill.) within a truss. Plant Growth Regul. 1988, 7, 141–155. [Google Scholar] [CrossRef]
- Yu, Z.; Huang, H.; Cao, S.; Wang, Q. Analysis of the causes of split pit in peaches. Int. J. Mol. Sci. 2025, 26, 5460. [Google Scholar] [CrossRef]
- Wan, S.; Li, Y.; Xie, Z. Exploring factors influencing the consumption of grape skins: A Review. Horticulturae 2025, 11, 962. [Google Scholar] [CrossRef]
- Wan, X.; Wu, Z.; Sun, D.; Long, L.; Song, Q.; Gao, C. Cytological characteristics of blueberry fruit development. BMC Plant Biol. 2024, 24, 184. [Google Scholar] [CrossRef]
- Miura, H.; Imada, S.; Yabuuchi, S. Double sigmoid growth curve of strawberry fruit. J. Jpn. Soc. Hortic. Sci. 1990, 59, 527–531. [Google Scholar] [CrossRef]
- Pratt, H.K.; Reid, M.S. Chinese gooseberry: Seasonal patterns in fruit growth and maturation, ripening, respiration and the role of ethylene. J. Sci. Food Agric. 1974, 25, 747–757. [Google Scholar] [CrossRef]
- Olmstead, J.W.; Iezzoni, A.F.; Whiting, M.D. Genotypic differences in sweet cherry fruit size are primarily a function of cell number. J. Am. Soc. Hortic. Sci. 2007, 132, 697–703. [Google Scholar] [CrossRef]
- Cowan, A.K.; Cripps, R.F.; Richings, E.W.; Taylor, N.J. Fruit size: Towards an understanding of the metabolic control of fruit growth using avocado as a model system. Physiol. Plant. 2001, 111, 127–136. [Google Scholar] [CrossRef]
- Malladi, A.; Hirst, P.M. Increase in fruit size of a spontaneous mutant of ‘Gala’ apple (Malus × domestica Borkh.) is facilitated by altered cell production and enhanced cell size. J. Exp. Bot. 2010, 61, 3003–3013. [Google Scholar] [CrossRef]
- Hamada, K.; Hasegawa, K.; Kitajima, A.; Ogata, T. The relationship between fruit size and cell division and enlargement in cultivated and wild persimmons. J. Hortic. Sci. Biotechnol. 2008, 83, 218–222. [Google Scholar] [CrossRef]
- Azzi, L.; Deluche, C.; Gévaudant, F.; Frangne, N.; Delmas, F.; Hernould, M.; Chevalier, C. Fruit growth-related genes in tomato. J. Exp. Bot. 2015, 66, 1075–1086. [Google Scholar] [CrossRef]
- Kumar, R.; Khurana, A.; Sharma, A.K. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 2013, 65, 4561–4575. [Google Scholar] [CrossRef]
- Peng, Z.; Zhao, C.; Li, S.; Guo, Y.; Xu, H.; Hu, G.; Liu, Z.; Chen, X.; Chen, J.; Lin, S. Integration of genomics, transcriptomics and metabolomics identifies candidate loci underlying fruit weight in loquat. Hortic. Res. 2022, 9, uhac037. [Google Scholar] [CrossRef]
- Shen, H.; Luo, B.; Ding, Y.; Xiao, H.; Chen, G.; Yang, Z.; Hu, Z.; Wu, T. The YABBY transcription factor, SLYABBY2A, positively regulates fruit septum development and ripening in tomatoes. Int. J. Mol. Sci. 2024, 25, 5206. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wang, X.; Dong, X.; Gao, M.; Dong, D.; Li, C.; Jing, S.; Guo, Y.D.; Zhang, N. CRISPR/Cas9 edited SlGT30 improved both drought resistance and fruit yield through endoreduplication. Plant Cell Environ. 2025, 48, 2581–2595. [Google Scholar] [CrossRef]
- Xia, W.; Chen, C.; Jin, S.; Chang, H.; Ding, X.; Fan, Q.; Zhang, Z.; Hua, B.; Miao, M.; Liu, J. Multi-Omics analysis reveals the distinct features of metabolism pathways supporting the fruit size and color variation of giant pumpkin. Int. J. Mol. Sci. 2024, 25, 3864. [Google Scholar] [CrossRef]
- Mizukami, Y.; Fischer, R.L. Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc. Natl. Acad. Sci. USA 2000, 97, 942–947. [Google Scholar] [CrossRef]
- Frary, A.; Nesbitt, T.C.; Frary, A.; Grandillo, S.; Knaap, E.v.d.; Cong, B.; Liu, J.; Meller, J.; Elber, R.; Alpert, K.B. fw2. 2: A quantitative trait locus key to the evolution of tomato fruit size. Science 2000, 289, 85–88. [Google Scholar] [CrossRef]
- Liu, J.; Cong, B.; Tanksley, S.D. Generation and analysis of an artificial gene dosage series in tomato to study the mechanisms by which the cloned quantitative trait locus fw2.2 controls fruit size. Plant Physiol. 2003, 132, 292–299. [Google Scholar] [CrossRef]
- Cong, B.; Liu, J.; Tanksley, S.D. Natural alleles at a tomato fruit size quantitative trait locus differ by heterochronic regulatory mutations. Proc. Natl. Acad. Sci. USA 2002, 99, 13606–13611. [Google Scholar] [CrossRef]
- Bai, Y.; Lindhout, P. Domestication and breeding of tomatoes: What have we gained and what can we gain in the future? Ann. Bot. 2007, 100, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zeng, B.; Luo, S.; Li, X.; Wu, B.; Li, J. Cloning, localization and expression analysis of two fw2.2-like genes in small-and large-fruited pear species. J. Integr. Agric. 2016, 15, 282–294. [Google Scholar] [CrossRef]
- Dahan, Y.; Rosenfeld, R.; Zadiranov, V.; Irihimovitch, V. A proposed conserved role for an avocado fw2.2-like gene as a negative regulator of fruit cell division. Planta 2010, 232, 663–676. [Google Scholar] [CrossRef] [PubMed]
- De Franceschi, P.; Stegmeir, T.; Cabrera, A.; Van Der Knaap, E.; Rosyara, U.; Sebolt, A.; Dondini, L.; Dirlewanger, E.; Quero-Garcia, J.; Campoy, J.A. Cell number regulator genes in Prunus provide candidate genes for the control of fruit size in sweet and sour cherry. Mol. Breed. 2013, 32, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xiong, W.; Cao, B.; Yan, T.; Luo, T.; Fan, T.; Luo, M. Molecular characterization and functional analysis of “fruit-weight2. 2-like” gene family in rice. Planta 2013, 238, 643–655. [Google Scholar] [CrossRef]
- Guo, M.; Rupe, M.A.; Dieter, J.A.; Zou, J.; Spielbauer, D.; Duncan, K.E.; Howard, R.J.; Hou, Z.; Simmons, C.R. Cell Number Regulator1 affects plant and organ size in maize: Implications for crop yield enhancement and heterosis. Plant Cell 2010, 22, 1057–1073. [Google Scholar] [CrossRef]
- Van der Knaap, E.; Tanksley, S. The making of a bell pepper-shaped tomato fruit: Identification of loci controlling fruit morphology in yellow stuffer tomato. Theor. Appl. Genet. 2003, 107, 139–147. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Zhang, N.; Sauvage, C.; Muños, S.; Blanca, J.; Cañizares, J.; Diez, M.J.; Schneider, R.; Mazourek, M.; McClead, J. A cytochrome P450 regulates a domestication trait in cultivated tomato. Proc. Natl. Acad. Sci. USA 2013, 110, 17125–17130. [Google Scholar] [CrossRef]
- Li, Q.; Chakrabarti, M.; Taitano, N.K.; Okazaki, Y.; Saito, K.; Al-Abdallat, A.M.; van der Knaap, E. Differential expression of SlKLUH controlling fruit and seed weight is associated with changes in lipid metabolism and photosynthesis-related genes. J. Exp. Bot. 2021, 72, 1225–1244. [Google Scholar] [CrossRef]
- Xu, C.; Liberatore, K.L.; MacAlister, C.A.; Huang, Z.; Chu, Y.-H.; Jiang, K.; Brooks, C.; Ogawa-Ohnishi, M.; Xiong, G.; Pauly, M. A cascade of arabinosyltransferases controls shoot meristem size in tomato. Nat. Genet. 2015, 47, 784–792. [Google Scholar] [CrossRef]
- Chu, Y.H.; Jang, J.C.; Huang, Z.; van der Knaap, E. Tomato locule number and fruit size controlled by natural alleles of lc and fas. Plant Direct 2019, 3, e00142. [Google Scholar] [CrossRef]
- Muños, S.; Ranc, N.; Botton, E.; Bérard, A.; Rolland, S.; Duffé, P.; Carretero, Y.; Le Paslier, M.-C.; Delalande, C.; Bouzayen, M. Increase in tomato locule number is controlled by two single-nucleotide polymorphisms located near WUSCHEL. Plant Physiol. 2011, 156, 2244–2254. [Google Scholar] [CrossRef] [PubMed]
- Carles, C.C.; Fletcher, J.C. Shoot apical meristem maintenance: The art of a dynamic balance. Trends Plant Sci. 2003, 8, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Huang, Z.; Chakrabarti, M.; Illa-Berenguer, E.; Liu, X.; Wang, Y.; Ramos, A.; van der Knaap, E. Fruit weight is controlled by cell size regulator encoding a novel protein that is expressed in maturing tomato fruits. PLoS Genet. 2017, 13, e1006930. [Google Scholar] [CrossRef]
- Liu, J.; Van Eck, J.; Cong, B.; Tanksley, S.D. A new class of regulatory genes underlying the cause of pear-shaped tomato fruit. Proc. Natl. Acad. Sci. USA 2002, 99, 13302–13306. [Google Scholar] [CrossRef]
- Xiao, H.; Jiang, N.; Schaffner, E.; Stockinger, E.J.; van der Knaap, E. A retrotransposon-mediated gene duplication underlies morphological variation of tomato fruit. Science 2008, 319, 1527–1530. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Q.; Keyhaninejad, N.; Taitano, N.; Sapkota, M.; Snouffer, A.; van der Knaap, E. A combinatorial TRM-OFP module bilaterally fine-tunes tomato fruit shape. New Phytol. 2023, 238, 2393–2409. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Yan, H.; Zhang, W.; Tian, D.; Che, G.; Hasi, A. OVATE family gene CmOFP6-19b negatively regulates fruit size in melon (Cucumis melo L.). Hortic. Res. 2025, 12, uhaf148. [Google Scholar] [CrossRef]
- Kenis, K.; Keulemans, J.; Davey, M.W. Identification and stability of QTLs for fruit quality traits in apple. Tree Genet. Genomes 2008, 4, 647–661. [Google Scholar] [CrossRef]
- Dujak, C.; Coleto-Alcudia, V.; Aranzana, M.J. Genomic analysis of fruit size and shape traits in apple: Unveiling candidate genes through GWAS analysis. Hortic. Res. 2024, 11, uhad270. [Google Scholar] [CrossRef] [PubMed]
- Devoghalaere, F.; Doucen, T.; Guitton, B.; Keeling, J.; Payne, W.; Ling, T.J.; Ross, J.J.; Hallett, I.C.; Gunaseelan, K.; Dayatilake, G. A genomics approach to understanding the role of auxin in apple (Malus x domestica) fruit size control. BMC Plant Biol. 2012, 12, 7. [Google Scholar] [CrossRef]
- Rosyara, U.R.; Bink, M.C.; van de Weg, E.; Zhang, G.; Wang, D.; Sebolt, A.; Dirlewanger, E.; Quero-Garcia, J.; Schuster, M.; Iezzoni, A.F. Fruit size QTL identification and the prediction of parental QTL genotypes and breeding values in multiple pedigreed populations of sweet cherry. Mol. Breed. 2013, 32, 875–887. [Google Scholar] [CrossRef]
- Qi, X.L.; Liu, C.L.; Song, L.L.; Dong, Y.X.; Chen, L.; Li, M. Functional characterization of PavCYP78As affecting sweet cherry fruit size. Acta Hortic. 2024, 1408, 151–156. [Google Scholar] [CrossRef]
- Cao, K.; Zhao, P.; Zhu, G.; Fang, W.; Chen, C.; Wang, X.; Wang, L. Expansin genes are candidate markers for the control of fruit weight in peach. Euphytica 2016, 210, 441–449. [Google Scholar] [CrossRef]
- Doligez, A.; Bertrand, Y.; Farnos, M.; Grolier, M.; Romieu, C.; Esnault, F.; Dias, S.; Berger, G.; François, P.; Pons, T. New stable QTLs for berry weight do not colocalize with QTLs for seed traits in cultivated grapevine (Vitis vinifera L.). BMC Plant Biol. 2013, 13, 217. [Google Scholar] [CrossRef]
- Klee, H.J.; Giovannoni, J.J. Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef]
- Pattison, R.J.; Catalá, C. Evaluating auxin distribution in tomato (Solanum lycopersicum) through an analysis of the PIN and AUX/LAX gene families. Plant J. 2012, 70, 585–598. [Google Scholar] [CrossRef]
- Lavy, M.; Estelle, M. Mechanisms of auxin signaling. Development 2016, 143, 3226–3229. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.; Wolters-Arts, M.; Schimmel, B.C.; Stultiens, C.L.; de Groot, P.F.; Powers, S.J.; Tikunov, Y.M.; Bovy, A.G.; Mariani, C.; Vriezen, W.H. Solanum lycopersicum AUXIN RESPONSE FACTOR 9 regulates cell division activity during early tomato fruit development. J. Exp. Bot. 2015, 66, 3405–3416. [Google Scholar] [CrossRef] [PubMed]
- Jong, M.D.; Wolters-Arts, M.; García-Martínez, J.L.; Mariani, C.; Vriezen, W.H. The Solanum lycopersicum AUXIN RESPONSE FACTOR 7 (SlARF7) mediates cross-talk between auxin and gibberellin signalling during tomato fruit set and development. J. Exp. Bot. 2011, 62, 617. [Google Scholar] [CrossRef]
- Kim, J.-S.; Ezura, K.; Lee, J.; Ariizumi, T.; Ezura, H. Genetic engineering of parthenocarpic tomato plants using transient SlIAA9 knockdown by novel tissue-specific promoters. Sci. Rep. 2019, 9, 18871. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.; Sun, X.; Yue, P.; Qiao, J.; Sun, J.; Wang, A.; Yuan, H.; Yu, W. The MdAux/IAA2 transcription repressor regulates cell and fruit size in apple fruit. Int. J. Mol. Sci. 2022, 23, 9454. [Google Scholar] [CrossRef]
- Davière, J.-M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef]
- Daviere, J.-M.; Achard, P. A pivotal role of DELLAs in regulating multiple hormone signals. Mol. Plant 2016, 9, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Israeli, A.; Ori, N.; Sun, T.-p. The interaction between DELLA and ARF/IAA mediates crosstalk between gibberellin and auxin signaling to control fruit initiation in tomato. Plant Cell 2018, 30, 1710–1728. [Google Scholar] [CrossRef]
- Carrera, E.; Ruiz-Rivero, O.; Peres, L.E.P.; Atares, A.; Garcia-Martinez, J.L. Characterization of the procera tomato mutant shows novel functions of the SlDELLA protein in the control of flower morphology, cell division and expansion, and the auxin-signaling pathway during fruit-set and development. Plant Physiol. 2012, 160, 1581–1596. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, X.; Zhang, L.; Lin, S.; Liu, D.; Wang, Q.; Cai, S.; El-Tanbouly, R.; Gan, L.; Wu, H. Identification and characterization of tomato gibberellin 2-oxidases (GA2oxs) and effects of fruit-specific SlGA2ox1 overexpression on fruit and seed growth and development. Hortic. Res. 2016, 3, 16059. [Google Scholar] [CrossRef]
- Jong, M.D.; Mariani, C.; Vriezen, W.H. The role of auxin and gibberellin in tomato fruit set. J. Exp. Bot. 2009, 60, 1523–1532. [Google Scholar] [CrossRef]
- Renau-Morata, B.; Carrillo, L.; Cebolla-Cornejo, J.; Molina, R.V.; Martí, R.; Domínguez-Figueroa, J.; Vicente-Carbajosa, J.; Medina, J.; Nebauer, S.G. The targeted overexpression of SlCDF4 in the fruit enhances tomato size and yield involving gibberellin signalling. Sci. Rep. 2020, 10, 10645. [Google Scholar] [CrossRef]
- Yang, T.; He, Y.; Niu, S.; Zhang, Y. A YABBY gene CRABS CLAW a (CRCa) negatively regulates flower and fruit sizes in tomato. Plant Sci. 2022, 320, 111285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, F.; Deng, Y.; Zhong, F.; Tian, P.; Lin, D.; Deng, J.; Zhang, Y.; Huang, T. Sly-miR159 regulates fruit morphology by modulating GA biosynthesis in tomato. Plant Biotechnol. J. 2022, 20, 833–845. [Google Scholar] [CrossRef]
- Gan, L.; Song, M.; Wang, X.; Yang, N.; Li, H.; Liu, X.; Li, Y. Cytokinins are involved in regulation of tomato pericarp thickness and fruit size. Hortic. Res. 2022, 9, uhab041. [Google Scholar] [CrossRef]
- Matsuo, S.; Kikuchi, K.; Fukuda, M.; Honda, I.; Imanishi, S. Roles and regulation of cytokinins in tomato fruit development. J. Exp. Bot. 2012, 63, 5569–5579. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rojas, M.; Díaz-Ramírez, D.; Ortíz-Ramírez, C.I.; Galaz-Ávalos, R.M.; Loyola-Vargas, V.M.; Ferrándiz, C.; Abraham-Juárez, M.d.R.; Marsch-Martínez, N. The role of cytokinins during the development of strawberry flowers and receptacles. Plants 2023, 12, 3672. [Google Scholar] [CrossRef]
- Su, W.; Shao, Z.; Wang, M.; Gan, X.; Yang, X.; Lin, S. EjBZR1 represses fruit enlargement by binding to the EjCYP90 promoter in loquat. Hortic. Res. 2021, 8, 152. [Google Scholar] [CrossRef]
- Bhat, Z.; Reddy, Y.; Srihari, D.; Bhat, J.; Rashid, R.; Rather, J. New generation growth regulators—Brassinosteroids and CPPU improve bunch and berry characteristics in ‘Tas-A-Ganesh’ grape. Int. J. Fruit Sci. 2011, 11, 309–315. [Google Scholar] [CrossRef]
- Kshirsagar, D.; Warusavitharana, A.; Tambe, T. Effect of cytokinins and brassinosteroid with gibberellic acid on yield and quality of Thompson Seedless grapes. In Proceedings of the International Symposium on Grape Production and Processing, Maharashtra, India, 6–11 February 2006; pp. 217–224. [Google Scholar]
- Mori, K.; Lemaire-Chamley, M.; Jorly, J.; Carrari, F.; Conte, M.; Asamizu, E.; Mizoguchi, T.; Ezura, H.; Rothan, C. The conserved brassinosteroid-related transcription factor BIM1a negatively regulates fruit growth in tomato. J. Exp. Bot. 2021, 72, 1181–1197. [Google Scholar] [CrossRef]
- Wang, F.; Cui, X.; Sun, Y.; Dong, C. Ethylene signaling and regulation in plant growth and stress responses. Plant Cell Rep. 2013, 32, 1099–1109. [Google Scholar] [CrossRef]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef]
- Lin, Z.; Arciga-Reyes, L.; Zhong, S.; Alexander, L.; Hackett, R.; Wilson, I.; Grierson, D. SlTPR1, a tomato tetratricopeptide repeat protein, interacts with the ethylene receptors NR and LeETR1, modulating ethylene and auxin responses and development. J. Exp. Bot. 2008, 59, 4271–4287. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Q.; Li, Y.; Yang, L.; Zhang, Y.; Cai, Y. Effect of exogenous gibberellin, paclobutrazol, abscisic acid, and ethrel application on bulblet development in Lycoris radiata. Front. Plant Sci. 2021, 11, 615547. [Google Scholar] [CrossRef]
- Brookbank, B.P.; Patel, J.; Gazzarrini, S.; Nambara, E. Role of basal ABA in plant growth and development. Genes 2021, 12, 1936. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, Y.; Hao, S.; Kojima, M.; Sakakibara, H.; Ozeki-Iida, Y.; Zheng, Y.; Fei, Z.; Zhong, S.; Giovannoni, J.J.; Rose, J.K. Ethylene suppresses tomato (Solanum lycopersicum) fruit set through modification of gibberellin metabolism. Plant J. 2015, 83, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Su, L.; Chen, X.; Qi, X. Hormonal interactions underlying parthenocarpic fruit formation in horticultural crops. Hortic. Res. 2022, 9, uhab024. [Google Scholar] [CrossRef]
- Cong, B.; Barrero, L.S.; Tanksley, S.D. Regulatory change in YABBY-like transcription factor led to evolution of extreme fruit size during tomato domestication. Nat. Genet. 2008, 40, 800–804. [Google Scholar] [CrossRef]
- Huang, Z.; van der Knaap, E. Tomato fruit weight 11.3 maps close to fasciated on the bottom of chromosome 11. Theor. Appl. Genet. 2011, 123, 465–474. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Sun, X.; Li, Y.; Yao, J.; Nocker, S.v.; Wang, X. Genome-wide analysis of the YABBY gene family in grapevine and functional characterization of VvYABBY4. Front. Plant Sci. 2019, 10, 1207. [Google Scholar] [CrossRef]
- Zhang, T.; Li, C.; Li, D.; Liu, Y.; Yang, X. Roles of YABBY transcription factors in the modulation of morphogenesis, development, and phytohormone and stress responses in plants. J. Plant Res. 2020, 133, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Zhao, J.; He, C. Evolutionary developmental genetics of fruit morphological variation within the Solanaceae. Front. Plant Sci. 2015, 6, 248. [Google Scholar] [CrossRef]
- Li, H.; Qi, M.; Sun, M.; Liu, Y.; Liu, Y.; Xu, T.; Li, Y.; Li, T. Tomato transcription factor SlWUS plays an important role in tomato flower and locule development. Front. Plant Sci. 2017, 8, 457. [Google Scholar] [CrossRef] [PubMed]
- van der Graaff, E.; Laux, T.; Rensing, S.A. The WUS homeobox-containing (WOX) protein family. Genome Biol. 2009, 10, 248. [Google Scholar] [CrossRef]
- Tello, J.; Torres-Pérez, R.; Grimplet, J.; Carbonell-Bejerano, P.; Martínez-Zapater, J.M.; Ibáñez, J. Polymorphisms and minihaplotypes in the VvNAC26 gene associate with berry size variation in grapevine. BMC Plant Biol. 2015, 15, 253. [Google Scholar] [CrossRef]
- Jia, D.; Gong, X.; Li, M.; Li, C.; Sun, T.; Ma, F. Overexpression of a novel apple NAC transcription factor gene, MdNAC1, confers the dwarf phenotype in transgenic apple (Malus domestica). Genes 2018, 9, 229. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; Li, M.; Zhang, H.; Zhang, X.; Tian, S.; Ren, Y.; Yu, Y.; Liao, S.; Gong, G. The NAC transcription factor ClNAC100 positively regulates plant height and fruit size in watermelon. Plant J. 2025, 123, e70292. [Google Scholar] [CrossRef]
- Fan, J.; Cao, M.; Bi, X.; Zhu, Y.; Gao, Q.; Zhang, L.; Liu, Z.; Lian, H.; Xu, P. A FvERF3-FvNAC073 module regulates strawberry fruit size and ripening. Plant J. 2025, 122, e70262. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liang, H.; Chen, G.; Li, F.; Wang, Y.; Liao, C.; Hu, Z. The bHLH transcription factor SlPRE2 regulates tomato fruit development and modulates plant response to gibberellin. Plant Cell Rep. 2019, 38, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Malladi, A. The AINTEGUMENTA genes, MdANT1 and MdANT2, are associated with the regulation of cell production during fruit growth in apple (Malus× domestica Borkh.). BMC Plant Biol. 2012, 12, 98. [Google Scholar] [CrossRef]
- Long, T.; Wang, Y.; Liu, Z.; Wang, Y.; Mao, C.; Wang, D.; Qin, A.; Liao, Q.; Yang, J.; Fan, X. ZmMYB127 modulates maize dernel texture and size by integrating the synthesis of starch, Zein proteins and auxin. Plant Biotechnol. J. 2025; in press. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Y.; Li, Y.; Zheng, S.; Zhao, Z.; Chen, M.; Yang, H.; Yi, H.; Wu, J. Transcription factor CsMYB77 negatively regulates fruit ripening and fruit size in citrus. Plant Physiol. 2024, 194, 867–883. [Google Scholar] [CrossRef]
- Cheng, K.; Lin, D.; Ma, L.; Lu, Y.; Li, J.; Zhu, G.; Lin, T.; Qu, G.; Zhu, B.; Fu, D. A SlRBP1-SlFBA7/SlGPIMT module regulates fruit size in tomato. Hortic. Res. 2025, 12, uhaf089. [Google Scholar] [CrossRef]
- Li, W.; Liu, M.; Wang, R.; He, L.; Zhou, S.; Zhao, B.; Mao, Y.; Wu, Q.; Wang, D.; Ji, X. The F-box protein SlSAP 1 and SlSAP 2 redundantly control leaf and fruit size by modulating the stability of SlKIX 8 and SlKIX 9 in tomato. New Phytol. 2025, 246, 2617–2633. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lu, S.; Li, W.; Wang, Z.; Hu, D.; Su, X.; Wu, F.; Dong, S.; Cui, X.; Zhang, Y. A major latex protein, GsMLP328, modulates seed traits in soybean. J. Integr. Plant Biol. 2025; in press. [Google Scholar] [CrossRef]
- Li, Y.; Huang, K.; Zhang, L.; Zhang, B.; Duan, P.; Zhang, G.; Huang, X.; Zhou, C.; Han, N.; Zheng, L. A molecular framework for the GS2-SUG1 module-mediated control of grain size and weight in rice. Nat. Commun. 2025, 16, 3944. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Huang, Q.; Zhang, B.; Guo, W.; Lv, G.; Wang, D.; Yang, Y.; Wang, C.; Wu, X.; Zhang, Z. Histological and transcriptomic analysis reveals the cell number and plant hormone related to fruit size in Litchi chinensis Sonn. Sci. Hortic. 2025, 341, 114007. [Google Scholar] [CrossRef]
- Fernández-Lozano, A.; Yuste-Lisbona, F.J.; Pérez-Martín, F.; Pineda, B.; Moreno, V.; Lozano, R.; Angosto, T. Mutation at the tomato EXCESSIVE NUMBER OF FLORAL ORGANS (ENO) locus impairs floral meristem development, thus promoting an increased number of floral organs and fruit size. Plant Sci. 2015, 232, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Yuste-Lisbona, F.J.; Fernández-Lozano, A.; Pineda, B.; Bretones, S.; Ortíz-Atienza, A.; García-Sogo, B.; Müller, N.A.; Angosto, T.; Capel, J.; Moreno, V. ENO regulates tomato fruit size through the floral meristem development network. Proc. Natl. Acad. Sci. USA 2020, 117, 8187–8195. [Google Scholar] [CrossRef]
- Ren, T.; Ma, J.; Zhu, K.; Zhao, J.; Yang, H.; Feng, L.; Nie, L.; Zhao, W. The MADS-box transcription factor CmFYF promotes the production of male flowers and inhibits the fruit development in melon (Cucumis melo L.). Plant Physiol. Biochem. 2025, 221, 109634. [Google Scholar] [CrossRef]
- Dong, R.; Yuan, Y.; Liu, Z.; Sun, S.; Wang, H.; Ren, H.; Cui, X.; Li, R. ASYMMETRIC LEAVES 2 and ASYMMETRIC LEAVES 2-LIKE are partially redundant genes and essential for fruit development in tomato. Plant J. 2023, 114, 1285–1300. [Google Scholar] [CrossRef]
- Zhong, S.; Fei, Z.; Chen, Y.-R.; Zheng, Y.; Huang, M.; Vrebalov, J.; McQuinn, R.; Gapper, N.; Liu, B.; Xiang, J. Single-base resolution methylomes of tomato fruit development reveal epigenome modifications associated with ripening. Nat. Biotechnol. 2013, 31, 154–159. [Google Scholar] [CrossRef]
- Liu, R.; How-Kit, A.; Stammitti, L.; Teyssier, E.; Rolin, D.; Mortain-Bertrand, A.; Halle, S.; Liu, M.; Kong, J.; Wu, C. A DEMETER-like DNA demethylase governs tomato fruit ripening. Proc. Natl. Acad. Sci. USA 2015, 112, 10804–10809. [Google Scholar] [CrossRef]
- Schmitz, R.J.; Schultz, M.D.; Lewsey, M.G.; O’Malley, R.C.; Urich, M.A.; Libiger, O.; Schork, N.J.; Ecker, J.R. Transgenerational epigenetic instability is a source of novel methylation variants. Science 2011, 334, 369–373. [Google Scholar] [CrossRef]
- Daccord, N.; Celton, J.-M.; Linsmith, G.; Becker, C.; Choisne, N.; Schijlen, E.; Van de Geest, H.; Bianco, L.; Micheletti, D.; Velasco, R. High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat. Genet. 2017, 49, 1099–1106. [Google Scholar] [CrossRef]
- Peng, M.; Ying, P.; Liu, X.; Li, C.; Xia, R.; Li, J.; Zhao, M. Genome-wide identification of histone modifiers and their expression patterns during fruit abscission in litchi. Front. Plant Sci. 2017, 8, 639. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Wang, S.; Lin, T.; Yang, L.; Zhao, Z.; Zhang, Z.; Huang, S.; Yang, X. Genome-wide target mapping shows histone deacetylase complex1 regulates cell proliferation in cucumber fruit. Plant Physiol. 2020, 182, 167–184. [Google Scholar] [CrossRef]
- Czerednik, A.; Busscher, M.; Bielen, B.A.; Wolters-Arts, M.; de Maagd, R.A.; Angenent, G.C. Regulation of tomato fruit pericarp development by an interplay between CDKB and CDKA1 cell cycle genes. J. Exp. Bot. 2012, 63, 2605–2617. [Google Scholar] [CrossRef]
- Czerednik, A.; Busscher, M.; Angenent, G.C.; de Maagd, R.A. The cell size distribution of tomato fruit can be changed by overexpression of CDKA 1. Plant Biotechnol. J. 2015, 13, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, N.; Gévaudant, F.; Hernould, M.; Chevalier, C.; Mouras, A. The cell cycle-associated protein kinase WEE1 regulates cell size in relation to endoreduplication in developing tomato fruit. Plant J. 2007, 51, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Mathieu-Rivet, E.; Gévaudant, F.; Sicard, A.; Salar, S.; Do, P.T.; Mouras, A.; Fernie, A.R.; Gibon, Y.; Rothan, C.; Chevalier, C. Functional analysis of the anaphase promoting complex activator CCS52A highlights the crucial role of endo-reduplication for fruit growth in tomato. Plant J. 2010, 62, 727–741. [Google Scholar] [CrossRef]
- Fernandez, A.I.; Viron, N.; Alhagdow, M.; Karimi, M.; Jones, M.; Amsellem, Z.; Sicard, A.; Czerednik, A.; Angenent, G.; Grierson, D. Flexible tools for gene expression and silencing in tomato. Plant Physiol. 2009, 151, 1729–1740. [Google Scholar] [CrossRef]
- Wang, H.; Tang, X.; Liu, Y. SlCK2α as a novel substrate for CRL4 E3 ligase regulates fruit size through maintenance of cell division homeostasis in tomato. Planta 2023, 257, 38. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.; Sharples, R. The influence of shade and within-tree position on apple fruit size, colour and storage quality. J. Hortic. Sci. 1971, 46, 277–287. [Google Scholar] [CrossRef]
- Barritt, B.H.; Rom, C.R.; Konishi, B.J.; Dilley, M.A. Light level influences spur quality and canopy development and light interception influence fruit production in apple. HortScience 1991, 26, 993–999. [Google Scholar] [CrossRef]
- Lopez, G.; Johnson, R.S.; DeJong, T.M. High spring temperatures decrease peach fruit size. Calif. Agric. 2007, 61, 31–34. [Google Scholar] [CrossRef]
- Woznicki, T.L.; Heide, O.M.; Sønsteby, A.; Måge, F.; Remberg, S.F. Climate warming enhances flower formation, earliness of blooming and fruit size in plum (Prunus domestica L.) in the cool Nordic environment. Sci. Hortic. 2019, 257, 108750. [Google Scholar] [CrossRef]
- Naor, A. Irrigation and crop load influence fruit size and water relations in field-grown Spadona’ Pear. J. Am. Soc. Hortic. Sci. 2001, 126, 252–255. [Google Scholar] [CrossRef]
- Naor, A.; Klein, I.; Doron, I.; Gal, Y.; Ben-David, Z.; Bravdo, B. The effect of irrigation and crop load on stem water potential and apple fruit size. J. Hortic. Sci. 1997, 72, 765–771. [Google Scholar] [CrossRef]
- Peng, Y.; Rabe, E. Effect of differing irrigation regimes on fruit quality, yield, fruit size and net CO2 assimilation of ‘Mihowase’ satsuma. J. Hortic. Sci. Biotechnol. 1998, 73, 229–234. [Google Scholar] [CrossRef]
- Wargo, J.M.; Merwin, I.A.; Watkins, C.B. Fruit size, yield, and market value of gold rush’ Apple are affected by amount, timing and method of nitrogen Fertilization. HortTechnology 2003, 13, 153–161. [Google Scholar] [CrossRef]
- Gill, P.; Ganaie, M.; Dhillon, W.; Singh, N.P. Effect of foliar sprays of potassium on fruit size and quality of ‘Patharnakh’ pear. Indian J. Hortic. 2012, 69, 512–516. [Google Scholar]
- Alcaraz-Lopez, C.; Botia, M.; Alcaraz, C.F.; Riquelme, F. Effects of foliar sprays containing calcium, magnesium and titanium on plum (Prunus domestica L.) fruit quality. J. Plant Physiol. 2003, 160, 1441–1446. [Google Scholar] [CrossRef]
- Buccheri, M.; Di Vaio, C. Relationship among seed number, quality, and calcium content in apple fruits. J. Plant Nutr. 2005, 27, 1735–1746. [Google Scholar] [CrossRef]
- Bibi, F.; Ahmad, I.; Bakhsh, A.; Kiran, S.; Danish, S.; Ullah, H.; Rehman, A.-u. Effect of foliar application of boron with calcium and potassium on quality and yield of mango cv. summer bahisht (SB) Chaunsa. Open Agric. 2019, 4, 98–106. [Google Scholar] [CrossRef]
- Wojcik, P.; Wojcik, M.; Klamkowski, K. Response of apple trees to boron fertilization under conditions of low soil boron availability. Sci. Hortic. 2008, 116, 58–64. [Google Scholar] [CrossRef]
- Saraswat, N.; Pandey, U.; Tripathi, V. Influence of NAA and zinc sulphate on fruit set, fruit drop, cracking, fruit size, yield and quality of litchi cv. Calcuttia. J. Asian Hortic. 2006, 2, 255–269. [Google Scholar]
- Buler, Z.; Mika, A. The influence of canopy architecture on light interception and distribution in ‘Sampion’ apple trees. J. Fruit Ornam. Plant Res. 2009, 2, 17. [Google Scholar]
- Asrey, R.; Patel, V.B.; Barman, K.; Pal, R.K. Pruning affects fruit yield and postharvestquality in mango (Mangifera indica L.) cv. Amrapali. Fruits 2013, 68, 367–380. [Google Scholar] [CrossRef]
- Kumar, M.; Rawat, V.; Rawat, J.; Tomar, Y. Effect of pruning intensity on peach yield and fruit quality. Sci. Hortic. 2010, 125, 218–221. [Google Scholar] [CrossRef]
- Al-Saif, A.M.; Abdel-Aziz, H.F.; Khalifa, S.M.; Elnaggar, I.A.; Abd El-wahed, A.E.-w.N.; Farouk, M.H.; Hamdy, A.E. Pruning boosts growth, yield, and fruit quality of old Valencia orange trees: A field study. Agriculture 2023, 13, 1720. [Google Scholar] [CrossRef]
- Burge, G.; Spence, C.; Marshall, R. Kiwifruit: Effects of thinning on fruit size, vegetative growth, and return bloom. New Zealand J. Exp. Agric. 1987, 15, 317–324. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, H.; Zhang, Y.; Guo, S.; Wang, X.; Sun, M.; Yu, M.; Ma, R. Effects of blooming and fruit thinning on the yield, fruit quality, and leaf photosynthesis of peach cultivar ‘Xiahui 5’in China. Food Qual. Saf. 2024, 8, fyae019. [Google Scholar] [CrossRef]
- Hehnen, D.; Hanrahan, I.; Lewis, K.; McFerson, J.; Blanke, M. Mechanical flower thinning improves fruit quality of apples and promotes consistent bearing. Sci. Hortic. 2012, 134, 241–244. [Google Scholar] [CrossRef]
- Link, H. Significance of flower and fruit thinning on fruit quality. Plant Growth Regul. 2000, 31, 17–26. [Google Scholar] [CrossRef]
- Stern, R.A.; Moshe, F.; Ruth, B.-A. The effect of synthetic auxins on fruit development, quality and final fruit size in ‘Canino’ apricot (Prunus armeniaca L.). J. Hortic. Sci. Biotechnol. 2007, 82, 335–340. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Z.; Feng, L.; Zhi, Z.; Liu, Y.; Zhang, M.; Yue, H.; Zhu, G.; Gao, F. The transcriptional regulatory mechanisms exploration of jujube biological traits through multi-omics analysis. Forests 2024, 15, 395. [Google Scholar] [CrossRef]
- Guo, H.; Mao, M.; Deng, Y.; Sun, L.; Chen, R.; Cao, P.; Lai, J.; Zhang, Y.; Wang, C.; Li, C. Multi-omics analysis reveals that SlERF. D6 synergistically regulates SGAs and fruit development. Front. Plant Sci. 2022, 13, 860577. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, H.; Sun, X.; Hu, Y.; Gu, G.; Yang, Y.; Yu, W. Research Advances in the Regulation of Fruit Size: An Integrated Perspective of Genetic, Hormonal, Epigenetic, and Environmental Control. Biology 2025, 14, 1643. https://doi.org/10.3390/biology14121643
Bu H, Sun X, Hu Y, Gu G, Yang Y, Yu W. Research Advances in the Regulation of Fruit Size: An Integrated Perspective of Genetic, Hormonal, Epigenetic, and Environmental Control. Biology. 2025; 14(12):1643. https://doi.org/10.3390/biology14121643
Chicago/Turabian StyleBu, Haidong, Xiaohuan Sun, Yinghui Hu, Guangjun Gu, Yue Yang, and Wenquan Yu. 2025. "Research Advances in the Regulation of Fruit Size: An Integrated Perspective of Genetic, Hormonal, Epigenetic, and Environmental Control" Biology 14, no. 12: 1643. https://doi.org/10.3390/biology14121643
APA StyleBu, H., Sun, X., Hu, Y., Gu, G., Yang, Y., & Yu, W. (2025). Research Advances in the Regulation of Fruit Size: An Integrated Perspective of Genetic, Hormonal, Epigenetic, and Environmental Control. Biology, 14(12), 1643. https://doi.org/10.3390/biology14121643






