The Effect of a Dominant Inhibitory p53 Protein on Stress Responses Induced by Toxic and Non-Toxic Concentrations of Anisomycin in PC12 Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Western Blotting
2.3. Extraction of Exosomes from Cell Culture Media
3. Results
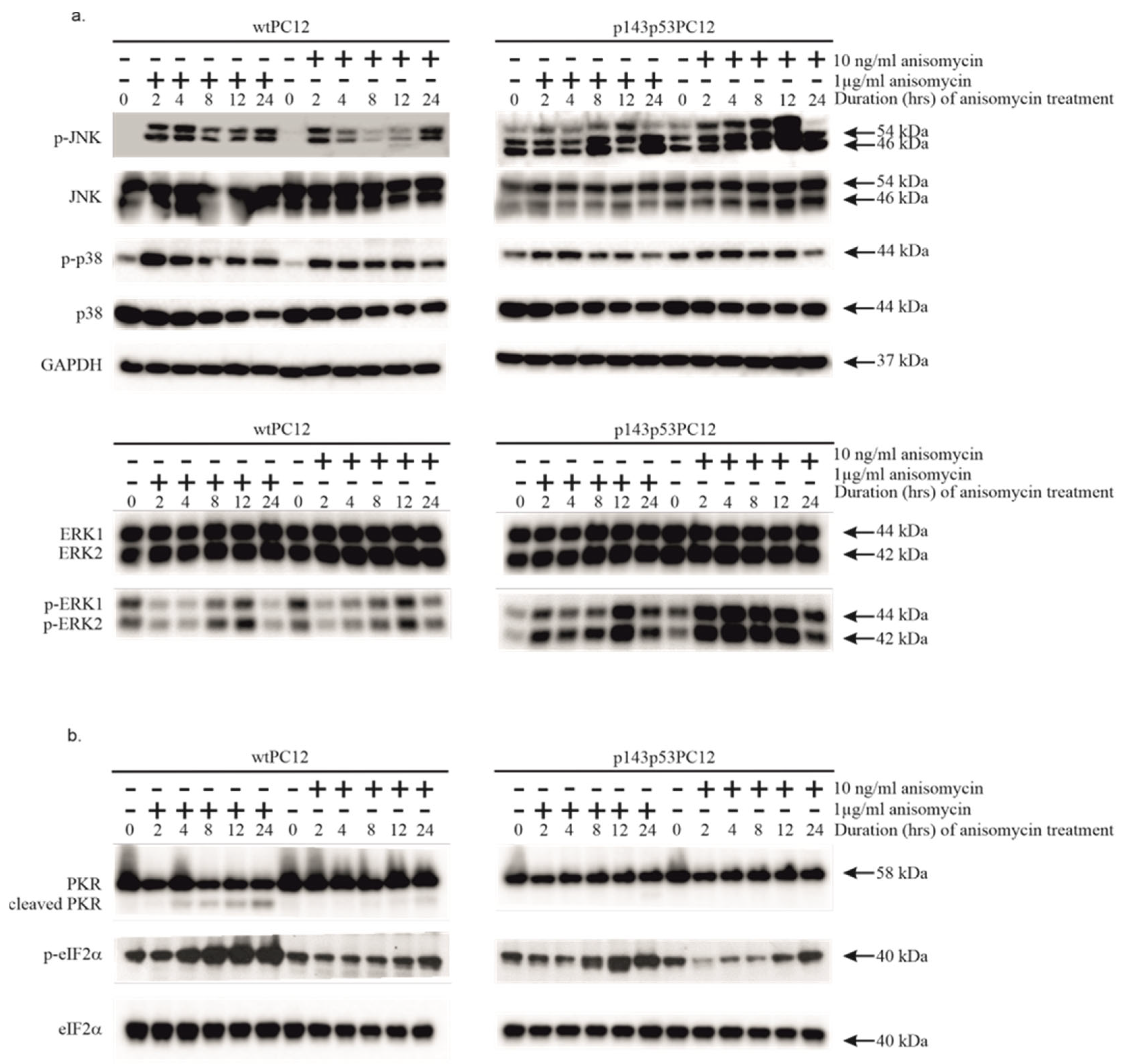
3.1. Both Low and High Concentrations of Anisomycin Cause Activation of Stress Kinases in wtPC12 Cells and PC12 Cells Expressing a Dominant Negative p53 Protein
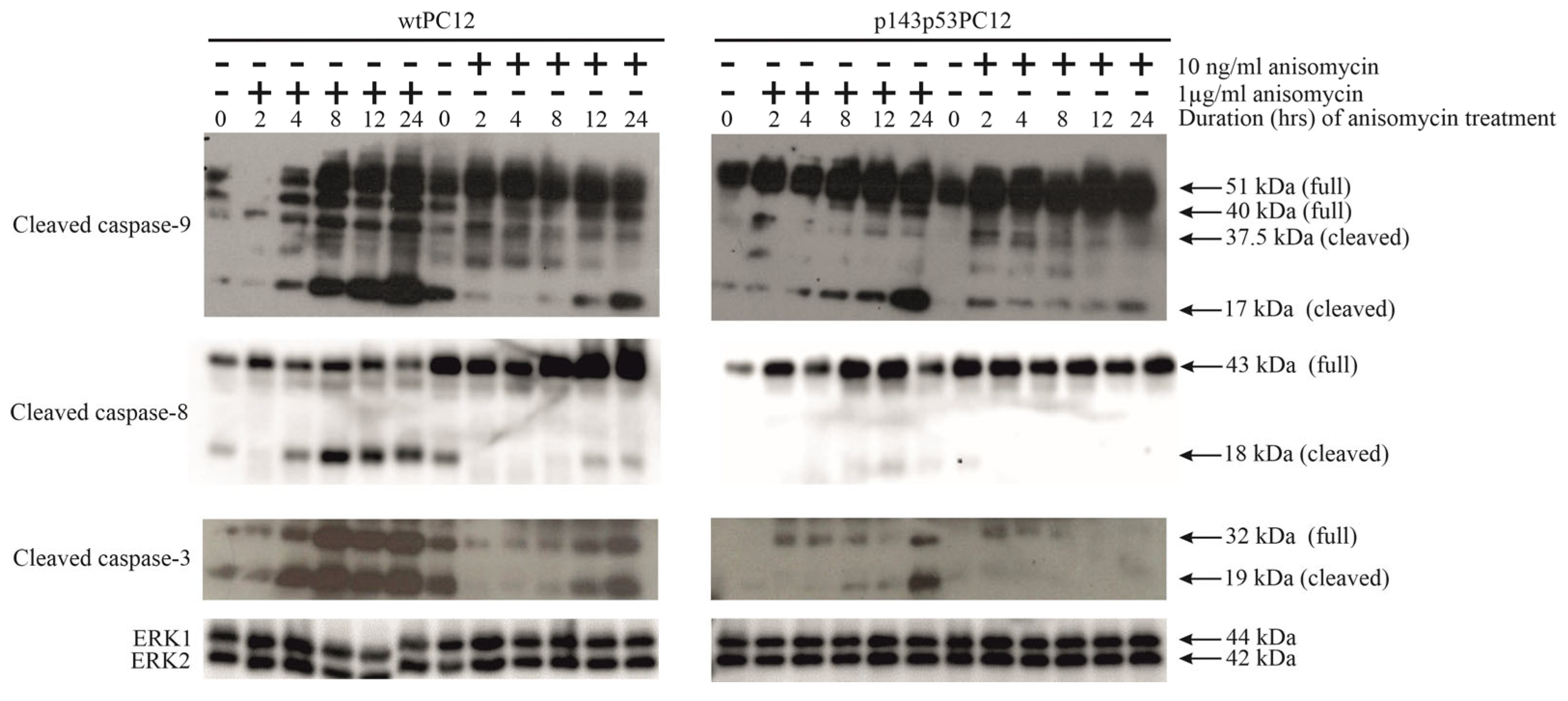
3.2. p53-Dependence of Anisomycin-Induced Caspase Activation
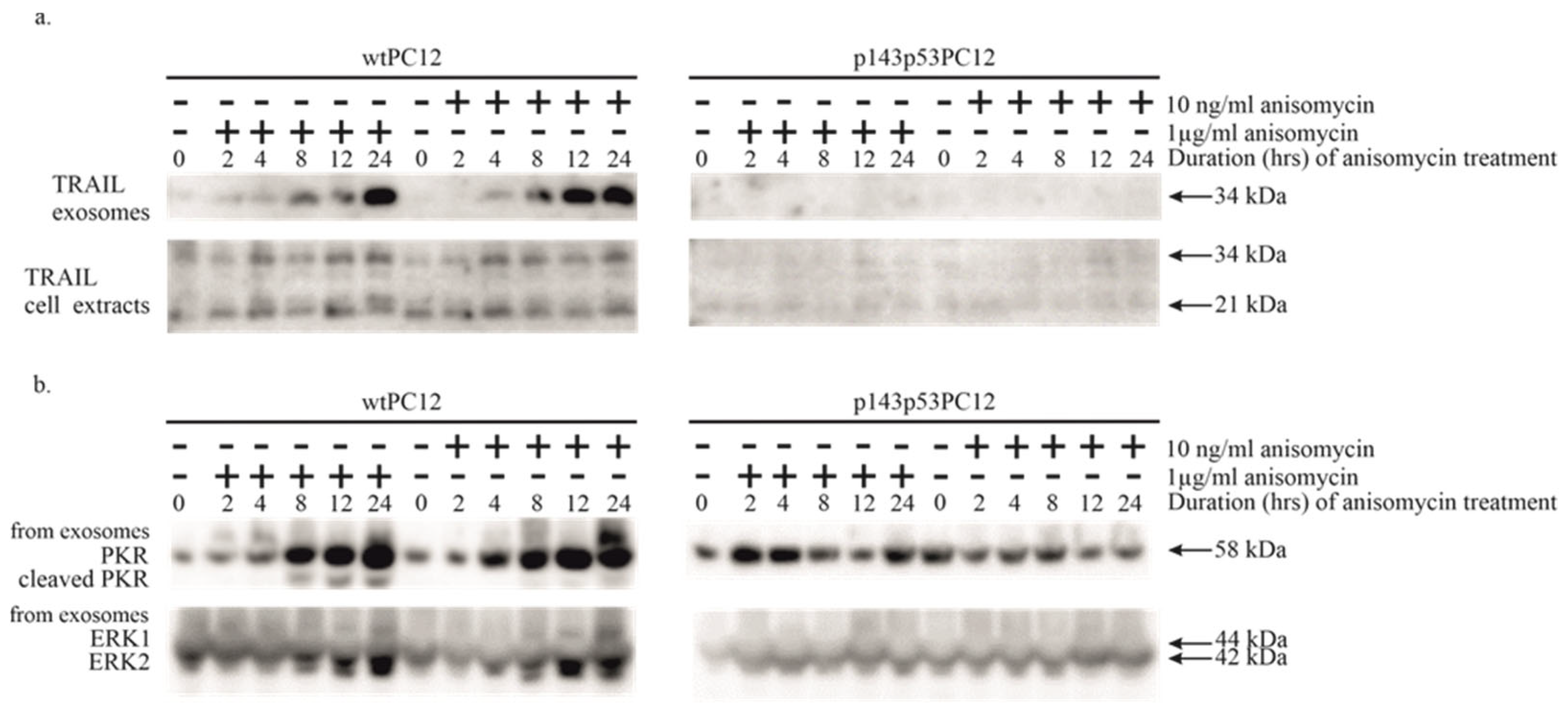
3.3. Potential Involvement of Exosomal Signaling in the Apoptosis of Anisomycin-Treated PC12 Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DR4, DR5 | death receptors 4/5 |
| eIF2α | eukaryotic translation initiation factor 2 α |
| ERK1,2 | extracellular signal-regulated kinase 1/2 |
| JNK | c-Jun N-terminal kinase |
| p38 MAPK | p38 mitogen-activated protein kinase |
| p143p53PC12 | PC12 subclone expressing a dominant inhibitory p53 protein |
| PKR | dsRNA-activated protein kinase |
| RSR | ribotoxic stress response |
| TRAIL | tumor necrosis factor-related apoptosis-inducing ligand |
| wtPC12 | wild-type PC12, rat pheochromocytoma cell line |
References
- Iordanov, M.S.; David, P.; Jennifer, L.M.; Thanh-Hoai, D.; Jean, A.P.; Steven Li-Ye, C.; Magun, B.E. Ribotoxic Stress Response: Activation of the Stress-Activated Protein Kinase JNK1 by Inhibitors of the Peptidyl Transferase Reaction and by Sequence-Specific RNA Damage to the α-Sarcin/Ricin Loop in the 28S RRNA. Mol. Cell Biol. 1997, 17, 3373–3381. [Google Scholar] [CrossRef]
- Vázquez, D. Protein Synthesis and Translation Inhibitors. In Inhibitors of Protein Biosynthesis; Springer: Berlin/Heidelberg, Germany, 1979; pp. 1–14. ISBN 978-3-642-81309-2. [Google Scholar]
- Barbacid, M.; Vazquez, D. [3H]Anisomycin Binding to Eukaryotic Ribosomes. J. Mol. Biol. 1974, 84, 603–623. [Google Scholar] [CrossRef]
- Macías-Silva, M.; Vázquez-Victorio, G.; Hernández-Damián, J. Anisomycin Is a Multifunctional Drug: More than Just a Tool to Inhibit Protein Synthesis. Curr. Chem. Biol. 2010, 4, 124–132. [Google Scholar] [PubMed]
- Ouyang, D.-Y.; Wang, Y.; Zheng, Y.T. Activation of C-Jun N-Terminal Kinases by Ribotoxic Stresses. Cell Mol. Immunol. 2006, 2, 419–425. [Google Scholar]
- Vind, A.C.; Genzor, A.V.; Bekker-Jensen, S. Ribosomal Stress-Surveillance: Three Pathways Is a Magic Number. Nucleic Acids Res. 2020, 48, 10648–10661. [Google Scholar] [CrossRef]
- Törocsik, B.; Szeberényi, J. Anisomycin Uses Multiple Mechanisms to Stimulate Mitogen-activated Protein Kinases and Gene Expression and to Inhibit Neuronal Differentiation in PC12 Phaeochromocytoma Cells. Eur. J. Neurosci. 2000, 12, 527–532. [Google Scholar] [CrossRef]
- Gal-Ben-Ari, S.; Barrera, I.; Ehrlich, M.; Rosenblum, K. PKR: A Kinase to Remember. Front. Mol. Neurosci. 2019, 11, 480. [Google Scholar] [CrossRef]
- Saelens, X.; Kalai, M.; Vandenabeele, P. Translation Inhibition in Apoptosis: Caspase-Dependent PKR Activation and EIF2-α Phosphorylation. J. Biol. Chem. 2001, 276, 41620–41628. [Google Scholar] [CrossRef] [PubMed]
- Kalai, M.; Suin, V.; Festjens, N.; Meeus, A.; Bernis, A.; Wang, X.M.; Saelens, X.; Vandenabeele, P. The Caspase-Generated Fragments of PKR Cooperate to Activate Full-Length PKR and Inhibit Translation. Cell Death Differ. 2007, 14, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Törőcsik, B.; Szeberényi, J. Anisomycin Affects Both Pro- and Antiapoptotic Mechanisms in PC12 Cells. Biochem. Biophys. Res. Commun. 2000, 278, 550–556. [Google Scholar] [CrossRef]
- Hernández Borrero, L.J.; El-Deiry, W.S. Tumor Suppressor P53: Biology, Signaling Pathways, and Therapeutic Targeting. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef] [PubMed]
- Lavin, M.F.; Gueven, N. The Complexity of P53 Stabilization and Activation. Cell Death Differ. 2006, 13, 941–950. [Google Scholar] [CrossRef]
- Klein, A.M.; De Queiroz, R.M.; Venkatesh, D.; Prives, C. The Roles and Regulation of MDM2 and MDMX: It Is Not Just about P53. Genes Dev. 2021, 35, 575–601. [Google Scholar] [CrossRef] [PubMed]
- Haupt, S.; Berger, M.; Goldberg, Z.; Haupt, Y. Apoptosis—The P53 Network. J. Cell Sci. 2003, 116, 4077–4085. [Google Scholar] [CrossRef]
- Fridman, J.S.; Lowe, S.W. Control of Apoptosis by P53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef]
- Feroz, W.; Sheikh, A.M.A. Exploring the Multiple Roles of Guardian of the Genome: P53. Egypt. J. Med. Hum. Genet. 2020, 21, 49. [Google Scholar] [CrossRef]
- Pavlakis, E.; Neumann, M.; Stiewe, T. Extracellular Vesicles: Messengers of P53 in Tumor–Stroma Communication and Cancer Metastasis. Int. J. Mol. Sci. 2020, 21, 9648. [Google Scholar] [CrossRef]
- Yu, X.; Harris, S.L.; Levine, A.J. The Regulation of Exosome Secretion: A Novel Function of the P53 Protein. Cancer Res. 2006, 66, 4795–4801. [Google Scholar] [CrossRef]
- Théry, C. Exosomes: Secreted Vesicles and Intercellular Communications. F1000 Biol. Rep. 2011, 3, 15. [Google Scholar] [CrossRef]
- Dilsiz, N. Hallmarks of Exosomes. Future Sci. OA 2022, 8, FSO764. [Google Scholar] [CrossRef]
- Stenqvist, A.-C.; Nagaeva, O.; Baranov, V.; Mincheva-Nilsson, L. Exosomes Secreted by Human Placenta Carry Functional Fas Ligand and TRAIL Molecules and Convey Apoptosis in Activated Immune Cells, Suggesting Exosome-Mediated Immune Privilege of the Fetus. J. Immunol. 2013, 191, 5515–5523. [Google Scholar] [CrossRef]
- Pimentel, J.M.; Zhou, J.-Y.; Wu, G.S. The Role of TRAIL in Apoptosis and Immunosurveillance in Cancer. Cancers 2023, 15, 2752. [Google Scholar] [CrossRef]
- Gonzalvez, F.; Ashkenazi, A. New Insights into Apoptosis Signaling by Apo2L/TRAIL. Oncogene 2010, 29, 4752–4765. [Google Scholar] [CrossRef]
- Naval, J.; de Miguel, D.; Gallego-Lleyda, A.; Anel, A.; Martinez-Lostao, L. Importance of TRAIL Molecular Anatomy in Receptor Oligomerization and Signaling. Implic. Cancer Therapy Cancers 2019, 11, 444. [Google Scholar] [CrossRef]
- Rivoltini, L.; Chiodoni, C.; Squarcina, P.; Tortoreto, M.; Villa, A.; Vergani, B.; Bürdek, M.; Botti, L.; Arioli, I.; Cova, A.; et al. TNF-Related Apoptosis-Inducing Ligand (TRAIL)–Armed Exosomes Deliver Proapoptotic Signals to Tumor Site. Clin. Cancer Res. 2016, 22, 3499–3512. [Google Scholar] [CrossRef]
- Ke, C.; Hou, H.; Li, J.; Su, K.; Huang, C.; Lin, Y.; Lu, Z.; Du, Z.; Tan, W.; Yuan, Z. Extracellular Vesicle Delivery of TRAIL Eradicates Resistant Tumor Growth in Combination with CDK Inhibition by Dinaciclib. Cancers 2020, 12, 1157. [Google Scholar] [CrossRef]
- Jella, K.K.; Nasti, T.H.; Li, Z.; Malla, S.R.; Buchwald, Z.S.; Khan, M.K. Exosomes, Their Biogenesis and Role in Inter-Cellular Communication, Tumor Microenvironment and Cancer Immunotherapy. Vaccines 2018, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Pap, M.; Szeberényi, J. Differential Ras-Dependence of Gene Induction by Nerve Growth Factor and Second Messenger Analogs in PC12 Cells. Neurochem. Res. 1998, 23, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Balogh, A.; Bátor, J.; Markó, L.; Németh, M.; Pap, M.; Sétáló, G.; Müller, D.N.; Csatary, L.K.; Szeberényi, J. Gene Expression Profiling in PC12 Cells Infected with an Oncolytic Newcastle Disease Virus Strain. Virus Res. 2014, 185, 10–22. [Google Scholar] [CrossRef]
- Teng, K.K.; Angelastro, J.M.; Cunningham, M.E.; Greene, L.A. Chapter 21—Cultured PC12 Cells: A Model for Neuronal Function, Differentiation, and Survival. In Cell Biology, 3rd ed.; Celis, J.E., Ed.; Academic Press: Burlington, NJ, USA, 2006; pp. 171–176. ISBN 978-0-12-164730-8. [Google Scholar]
- Varga, J.; Bátor, J.; Péter, M.; Árvai, Z.; Pap, M.; Sétáló, G.; Szeberényi, J. The Role of the P53 Protein in Nitrosative Stress-Induced Apoptosis of PC12 Rat Pheochromocytoma Cells. Cell Tissue Res. 2014, 358, 65–74. [Google Scholar] [CrossRef]
- Fábián, Z.; Vecsernyés, M.; Pap, M.; Szeberényi, J. The Effects of a Mutant P53 Protein on the Proliferation and Differentiation of PC12 Rat Phaeochromocytoma Cells. J. Cell Biochem. 2006, 99, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Abayasiriwardana, K.S.; Barbone, D.; Kim, K.-U.; Vivo, C.; Lee, K.K.; Dansen, T.B.; Hunt, A.E.; Evan, G.I.; Broaddus, V.C. Malignant Mesothelioma Cells Are Rapidly Sensitized to TRAIL-Induced Apoptosis by Low-Dose Anisomycin via Bim. Mol. Cancer Ther. 2007, 6, 2766–2776. [Google Scholar] [CrossRef]
- Xia, S.; Li, Y.; Rosen, E.M.; Laterra, J. Ribotoxic Stress Sensitizes Glioblastoma Cells to Death Receptor–Induced Apoptosis: Requirements for c-Jun NH2-Terminal Kinase and Bim. Mol. Cancer Res. 2007, 5, 783–792. [Google Scholar] [CrossRef]
- Schipp, R.; Varga, J.; Bátor, J.; Vecsernyés, M.; Árvai, Z.; Pap, M.; Szeberényi, J. Partial P53-Dependence of Anisomycin-Induced Apoptosis in PC12 Cells. Mol. Cell Biochem. 2017, 434, 41–50. [Google Scholar] [CrossRef]
- Varga, J.; Bátor, J.; Nádasdi, G.; Árvai, Z.; Schipp, R.; Szeberényi, J. Partial Protection of PC12 Cells from Cellular Stress by Low-Dose Sodium Nitroprusside Pre-Treatment. Cell Mol. Neurobiol. 2016, 36, 1161–1168. [Google Scholar] [CrossRef]
- Pap, M.; Szeberényi, J. Involvement of Proteolytic Activation of Protein Kinase R in the Apoptosis of PC12 Pheochromocytoma Cells. Cell Mol. Neurobiol. 2008, 28, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Zhou, Y.; Long, R.; Chen, C.; Zhao, J.; Cui, P.; Guo, M.; Liang, G.; Xu, L. DUSP8 Phosphatase: Structure, Functions, Expression Regulation and the Role in Human Diseases. Cell Biosci. 2019, 9, 70. [Google Scholar] [CrossRef]
- Ha, J.; Kang, E.; Seo, J.; Cho, S. Phosphorylation Dynamics of JNK Signaling: Effects of Dual-Specificity Phosphatases (DUSPs) on the JNK Pathway. Int. J. Mol. Sci. 2019, 20, 6157. [Google Scholar] [CrossRef]
- Li, M.; Zhou, J.-Y.; Ge, Y.; Matherly, L.H.; Wu, G.S. The Phosphatase MKP1 Is a Transcriptional Target of P53 Involved in Cell Cycle Regulation. J. Biol. Chem. 2003, 278, 41059–41068. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, Y.-X.; Jin, Y.J.; Hall, E.J.; Barrett, J.C. PAC1 Phosphatase Is a Transcription Target of P53 in Signalling Apoptosis and Growth Suppression. Nature 2003, 422, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.H.; Wang, J.; Wu, J.; Zhurkin, V.B.; Yin, Y. Mitogen-Activated Protein Kinase Phosphatase 2: A Novel Transcription Target of P53 in Apoptosis. Cancer Res. 2006, 66, 6033–6039. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhang, Q.; Jiang, L. Current Knowledge on Exosome Biogenesis, Cargo-Sorting Mechanism and Therapeutic Implications. Membranes 2022, 12, 498. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schipp, R.; Varga, J.; Bátor, J.; Vecsernyés, M.; Árvai, Z.; Kele-Morvai, P.; Szeberényi, J.; Pap, M. The Effect of a Dominant Inhibitory p53 Protein on Stress Responses Induced by Toxic and Non-Toxic Concentrations of Anisomycin in PC12 Cells. Biology 2025, 14, 1634. https://doi.org/10.3390/biology14121634
Schipp R, Varga J, Bátor J, Vecsernyés M, Árvai Z, Kele-Morvai P, Szeberényi J, Pap M. The Effect of a Dominant Inhibitory p53 Protein on Stress Responses Induced by Toxic and Non-Toxic Concentrations of Anisomycin in PC12 Cells. Biology. 2025; 14(12):1634. https://doi.org/10.3390/biology14121634
Chicago/Turabian StyleSchipp, Renáta, Judit Varga, Judit Bátor, Mónika Vecsernyés, Zita Árvai, Petra Kele-Morvai, József Szeberényi, and Marianna Pap. 2025. "The Effect of a Dominant Inhibitory p53 Protein on Stress Responses Induced by Toxic and Non-Toxic Concentrations of Anisomycin in PC12 Cells" Biology 14, no. 12: 1634. https://doi.org/10.3390/biology14121634
APA StyleSchipp, R., Varga, J., Bátor, J., Vecsernyés, M., Árvai, Z., Kele-Morvai, P., Szeberényi, J., & Pap, M. (2025). The Effect of a Dominant Inhibitory p53 Protein on Stress Responses Induced by Toxic and Non-Toxic Concentrations of Anisomycin in PC12 Cells. Biology, 14(12), 1634. https://doi.org/10.3390/biology14121634







