Simple Summary
This metabolomics study compared muscle composition in three all-female rainbow trout strains: Chinese “All-Female No. 1,” and Spanish and Danish strains. Analysis of 2198 metabolites identified 228 differential compounds, primarily organic acids, benzene derivatives, and amino acids. Enrichment revealed the phenylalanine, tyrosine, and tryptophan biosynthesis pathway as most significantly affected. Targeted quantification identified 11 key differential amino acids, with L-tyrosine, tryptamine, and L-phenylalanine being crucial for this pathway. The research provides essential data for evaluating nutritional value and supports future breeding for muscle quality in rainbow trout.
Abstract
Rainbow trout (Oncorhynchus mykiss) is an economically important fish species, in which the muscle nutritional composition of market-size fish can vary significantly due to differences in genetic background and breeding environments. This study employed a metabolomics approach to conduct an in-depth comparative analysis of muscle samples from our independently bred new variety “All-Female No. 1”, as well as Spanish and Danish all-female strains. A total of 2198 metabolites were identified. Following screening with PCA and PLS-DA, 228 differential metabolites were obtained. These were most abundantly enriched in the categories of organic acids and derivatives, benzene and substituted derivatives, and amino acids and metabolites. KEGG pathway enrichment analysis revealed that the differential metabolites had the most significant impact on the phenylalanine, tyrosine, and tryptophan biosynthesis pathway. Further targeted quantification of amino acid metabolites identified 11 differentially expressed amino acids, which also exerted the strongest influence on this key pathway. Consequently, L-tyrosine, tryptamine, and L-phenylalanine were determined to be the key metabolites affecting the phenylalanine, tyrosine, and tryptophan biosynthesis pathway in the muscle of rainbow trout from different germplasms. This study provides a comprehensive evaluation of the nutritional value, particularly regarding amino acid profiles, of rainbow trout from different genetic sources, offering critical data and a scientific basis for subsequent related research.
1. Introduction
The rainbow trout (Oncorhynchus mykiss) is an economically important fish species belonging to the order Salmoniformes, family Salmonidae, and genus Oncorhynchus [1]. Native to the rivers, lakes, and mountain streams along the Pacific coast of North America, it has become one of the key species promoted by the FAO for aquaculture and is widely farmed globally [2]. It has emerged as a mainstream cultivated species in countries such as the United States, Chile, Denmark, and Iran [3]. As the largest freshwater-cultivated variety in the global salmon industry, rainbow trout possesses both nutritional value and commercial significance [4]. China has over 60 years of history in rainbow trout farming, achieving a series of technological innovations—from “independent breeding of genetic resources” to “controlled breeding of triploid varieties” and further to “applications in deep-sea aquaculture scenarios”—thereby restructuring the entire industrial chain development layout. However, no study has yet systematically analyzed the muscle composition of rainbow trout farming products derived from domestic genetic sources compared to those introduced from foreign sources [5,6].
A comparative analysis of the muscle composition between rainbow trout derived from domestic germplasms and those from foreign-sourced strains holds significant multifaceted importance, which can be articulated as follows: (a) Scientific evaluation of key economic traits [7]. Through comparative analysis, it is possible to objectively assess the differences in core economic traits—such as muscle yield and nutritional quality—between domestic germplasms (e.g., selectively bred local varieties) and introduced strains (e.g., lineages imported from countries such as Spain and Denmark) [8,9]. (b) Identification of unique advantages of domestic germplasms [10]. For instance, domestic rainbow trout may exhibit distinctive characteristics in specific unsaturated fatty acids (e.g., EPA and DHA), mineral content, or flavor-related amino acids, thereby fostering a competitive niche against imported products. (c) Elucidation of genetic influence on muscle composition [11,12]. By analyzing muscle composition differences among various genetic sources under identical farming conditions, it becomes possible to determine which nutritional variations are primarily attributable to genetic background. (d) Accelerated breeding through biomarker discovery [13,14]. The analysis can help identify key metabolites or nutritional components associated with desirable traits such as high quality and yield, which may serve as molecular markers to expedite the breeding process. In summary, conducting a systematic comparison of muscle composition between domestic and foreign-sourced rainbow trout is not merely a subject of basic scientific inquiry but also a critical step toward ensuring germplasm security in China, enhancing industrial competitiveness, optimizing aquaculture practices, and guiding consumer markets. The findings from such research will directly benefit all segments of the rainbow trout industry and contribute profoundly to the high-quality and sustainable development of Chinese rainbow trout industry.
Metabolomics involves the systematic analysis of all small molecule metabolites (<1000 Da) within an organism, directly reflecting its physiological status, nutritional condition, and responses to environmental changes [15,16]. In the analysis of muscle tissues in aquatic animals, it goes beyond traditional nutrient measurements (such as crude protein and crude fat) to delve into molecular mechanisms underlying flavor formation, quality variations, stress responses, dietary effects, and species identification. For instance, a GC-MS-based metabolomic study on the taste differences between lake-cultured and pond-cultured Chinese mitten crabs (Eriocheir sinensis) objectively explained, at the molecular level, why consumers perceive lake-cultured crabs as having superior flavor, thereby providing a scientific basis for quality traceability and pricing [17]. Another metabolomic investigation into the effects of replacing fishmeal with plant-based protein sources in juvenile turbot (Scophthalmus maximus) revealed that the plant protein substitution group exhibited decreased levels of lysophosphatidylcholine (LPC), reduced content of certain essential amino acids, and markers of bile acid metabolism disruption. These findings offer precise targets for optimizing feed formulations and incorporating specific additives (such as supplementing limiting amino acids or protecting bile acid metabolism) [18]. Furthermore, metabolomic research on the impact of transport stress on fish muscle metabolism demonstrated that stress activates the “fight-or-flight” response, leading to anaerobic glycolysis of glycogen and lactate accumulation (affecting pH and tenderness), along with rapid ATP degradation generating inosine monophosphate (IMP, which increases initially and then decreases). These metabolic shifts ultimately contribute to the deterioration of muscle quality.
This study employed liquid chromatography–tandem mass spectrometry (LC-MS/MS) to conduct non-targeted metabolomic and targeted amino acid quantitative analyses on the variety “All-Female No. 1” rainbow trout, alongside all-female rainbow trout strains from Spain and Denmark, all cultivated under identical flowing pond conditions. Using chemometric techniques such as principal component analysis (PCA) and orthogonal partial least squares–discriminant analysis (OPLS-DA) for in-depth data processing and interpretation, the research aims to systematically summarize and compare the metabolic profile characteristics among sample groups of different genetic origins. KEGG enrichment analysis was subsequently performed on the filtered differentially expressed metabolites. The metabolomic analytical methodology established and the results obtained in this study will provide important data references and a scientific basis for further evaluation of the nutritional value and subsequent related research on rainbow trout from different germplasm sources.
2. Materials and Methods
2.1. Experimental Animals
2.1.1. Animal Ethics
All procedures involving fish in this study were conducted in accordance with the experimental animal ethical guidelines and protocols of the Heilongjiang River Fisheries Research Institute, Chinese Academy of Fishery Sciences. The experimental protocols were reviewed and approved by the Institutional Animal Welfare and Ethics Committee of the Heilongjiang River Fisheries Research Institute (Approval No. 20250408-001).
2.1.2. Animal Preparation and Sample Collection
The germplasm resources of the three species were obtained from different sources: the independently selected and bred new variety of rainbow trout “All-Female No. 1” from the Heilongjiang River Fisheries Research Institute, the all-female strain from Spain, and the all-female strain from Denmark. All fish were reared in flowing water ponds at the Bohai Cold-water Fish Experiment Station of the Heilongjiang River Fisheries Research Institute (cistern: 13 × 5 × 1.2 m). Three individuals (female, 2.3 ± 0.2 kg) were collected from each group, and muscle tissue was sampled from below the dorsal fin and adjacent areas. For each individual, 10 g of muscle tissue were collected for subsequent untargeted and targeted metabolomic analyses.
2.2. Non-Targeted Metabolomics Analysis
A 20 mg sample was mixed with 400 μL of an internal standard solution containing 70% methanol, homogenized, and incubated on ice for 15 min. After centrifugation at 12,000 rpm and 4 °C for 10 min, 300 μL of supernatant was collected and allowed to stand at −20 °C for 30 min. The supernatant was then centrifuged again under the same conditions, yielding a final volume of 200 μL for LC-MS analysis.
Chromatographic separation was performed using a Thermo Scientific Q Exactive HF-X mass spectrometer coupled with a vanquish liquid chromatography system (LC: Q Exactive HF-X; MS: Vanquish, Thermo Scientific, Waltham, MA, USA). Separation was achieved on a Waters ACQUITY Premier HSS T3 column (1.8 µm, 2.1 mm × 100 mm; Waters Corporation, Milford, MA, USA) maintained at 40 °C, with a flow rate of 0.4 mL/min and an injection volume of 4 μL. The mobile phase (A: 0.1% formic acid in water, B: 0.1% formic acid in acetonitrile) was eluted using the following gradient program: an increase in phase B from 5% to 20% within 2 min, followed by a rise to 60% over 3 min, then to 99% within 1 min, holding at 99% for 1.5 min, and finally returning to 5% in 0.1 min with re-equilibration for 2.4 min [19].
Mass spectrometric detection was conducted using an electrospray ionization source in both positive and negative ion modes (Vanquish, Waltham, MA, USA), with a full scan range of m/z 75–1000 and a resolution of 35,000. The ion source parameters were set as follows: ion spray voltage at 3.5 kV for positive mode and 3200 V for negative mode, sheath gas at 30 arb, auxiliary gas at 5 arb, ion transfer tube temperature at 320 °C, and vaporizer temperature at 300 °C. Data acquisition was performed using a full MS scan combined with data-dependent MSn switching mode. The collision energies were set at 30, 40, and 50 V, with a signal intensity threshold of 1 × 106 cps. The “Top N vs. Top Speed” was set to 10, and the dynamic exclusion time was 3 s [20].
2.3. Amino Acid Composition Analysis
A 500 μL aliquot of pre-cooled (−20 °C) 70% methanol solution was added to 50 mg of the sample. After thorough mixing, the mixture was centrifuged at 4 °C and 12,000 r/min for 10 min, and 300 μL of supernatant was collected. The supernatant was allowed to stand at −20 °C for 30 min, followed by another 10-min centrifugation under the same conditions. Then, 200 μL of the supernatant was passed through a protein precipitation plate for purification and subsequently analyzed using an LC-ESI-MS/MS system (LC: AB Sciex, Framingham, MA, USA; ESI: AB Sciex, Framingham, MA, USA; MS: QTRAP® 6500+ System, SCIEX, Framingham, MA, USA). The system consisted of an ExionLC AD ultra-high-performance liquid chromatograph coupled with a QTRAP® 6500+ mass spectrometer (SCIEX, Framingham, MA, USA) [21].
Chromatographic separation was performed on an ACQUITY BEH Amide column (1.7 μm, 100 mm × 2.1 mm internal diameter; Waters Corporation, Milford, MA, USA) maintained at 40 °C. The mobile phase consisted of an aqueous solution containing 2 mM ammonium acetate and 0.04% formic acid (phase A) and an acetonitrile solution containing 2 mM ammonium acetate and 0.04% formic acid (phase B), with a flow rate set at 0.4 mL/min and an injection volume of 2 μL. The gradient elution program was as follows: 90% phase B was maintained from 0 to 1.2 min, decreased to 60% phase B by 9 min, further decreased to 40% phase B between 10 and 11 min, rapidly returned to 90% phase B at 11.01 min, and maintained until the end of the 15-min run [22].
Mass spectrometric analysis was conducted using an electrospray ionization source with the ion source temperature set at 550 °C. The ionization voltage was 5500 V in positive ion mode and −4500 V in negative ion mode, with a curtain gas pressure of 35 psi. Detection was performed in multiple reaction monitoring mode, with declustering voltage and collision energy parameters optimized for the target compounds to ensure sensitivity and specificity [23,24].
2.4. Statistical Analysis
Data are expressed as mean ± standard deviation. All statistical analyses were performed using DPS statistical software (version 18.10). After initial preprocessing by unit variance scaling, unsupervised principal component analysis was conducted using the prcomp function in R language 3.5.1 (https://www.r-project.org; Accessed: 15 May 2025). Differential metabolites were screened based on a variable importance in projection (VIP) value greater than 1 and a p-value less than 0.05 (based on analysis of variance). VIP values were extracted from the OPLS-DA model and calculated using the R package MetaboAnalystR 1.0.1. Prior to OPLS-DA modeling, the data underwent log2 transformation and mean centering, and the model’s validity was verified through 200 permutation tests to avoid overfitting. Additionally, hierarchical clustering analysis was performed and Pearson correlation coefficients were calculated using the R package pheatmap. For KEGG pathway enrichment analysis, Fisher’s exact test was employed to evaluate the significance of metabolite distribution in each pathway, with all metabolites used as the background. KEGG bubble diagrams were generated using the online software MetaboAnalyst 6.0 (https://www.metaboanalyst.ca/home.xhtml; Accessed: 10 July 2025) [25].
3. Results
3.1. Classification of Metabolite Categories
In this study, muscle samples from three different sources of rainbow trout were analyzed using liquid chromatography–mass spectrometry (LC-MS). Following mass spectral peak extraction and calibration, a total of 2198 metabolites were detected. A circular diagram (Figure 1) visualizing these metabolites revealed that the top seven metabolite categories by proportion were as follows: Benzene and substituted derivatives (354 species, accounting for 16.11%); Organic acids and their derivatives (336 species, accounting for 15.29%); Amino acids and their metabolites (245 species, accounting for 11.15%); Heterocyclic compounds (224 species, accounting for 10.19%); Aldehydes, ketones, and esters (189 species, accounting for 8.60%); Fatty acids (158 species, accounting for 7.19%); and Glycerophospholipids (143 species, accounting for 6.51%).
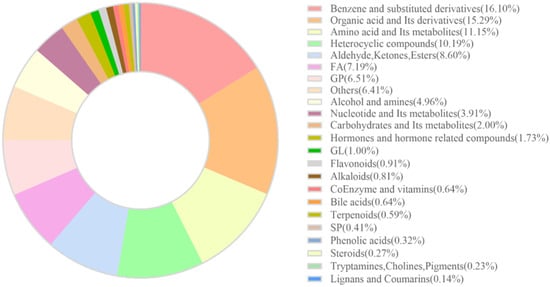
Figure 1.
Doughnut chart illustrating the composition of metabolite categories.
3.2. Screening of Differential Metabolites
Principal Component Analysis (PCA) reduces the dimensionality of complex, high-dimensional data while retaining maximal original information, thereby establishing reliable mathematical models to describe and summarize the metabolic characteristics of the studied subjects. Figure 2A displays the PCA score plot of the three experimental muscle groups and quality control (QC) samples. The clustering of QC samples indicates excellent data stability. PC1 (x-axis) accounts for 15.07% of the total variance, while PC2 (y-axis) accounts for 7.85%. Meanwhile, Figure 2B illustrates the variance associated with PC1 and subsequent principal components. The variance values plateau after PC3, suggesting that PC1 and PC2 represent genuine biological signals, consistent with the expectations of dimensionality reduction via PCA. Overall, the results demonstrate effective separation among Groups A, B, and C, though there remains room for improvement in intra-group compactness and inter-group separability.
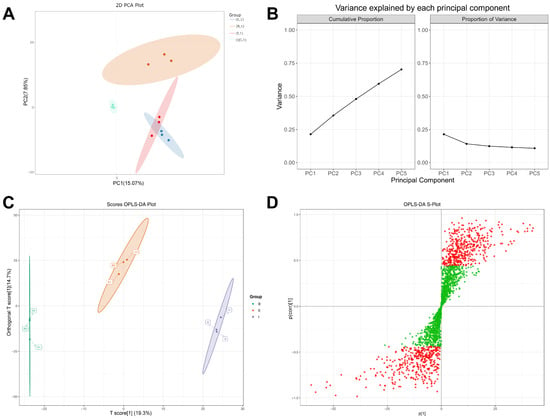
Figure 2.
Screening of differential metabolites in all-female strain from Denmark (Group B), the “All-Female No. 1” variety (Group E), and the all-female Spanish (Group I) rainbow trout strain. (A) PCA Score Plot; (B) Component Loading Plots; (C) OPLS-DA Scores Plot; (D) OPLS-DA S-Plot.
Although PCA efficiently extracts primary information, it is less sensitive to variables with low correlations. In contrast, Partial Least Squares–Discriminant Analysis (PLS-DA) can screen marker metabolites from extensive metabolomic datasets and establish accurate discriminant models. Orthogonal Partial Least Squares–Discriminant Analysis (OPLS-DA) was further applied to classify the three tested muscle samples, yielding cumulative values of R2X (cum) = 0.34, R2Y (cum) = 0.999, and Q2 (cum) = 0.575 (Supplementary Figure S1). Similar to the PCA model, clear separation was observed among Groups A, B, and C. However, the OPLS-DA score plot demonstrated higher clustering density and classification clarity (Figure 2C), indicating that OPLS-DA outperforms PCA in distinguishing different samples, with high reliability and predictive capability.
Additionally, the S-plot from OPLS-DA (Figure 2D) displays the covariance between principal components and metabolites on the x-axis and the correlation coefficients between principal components and metabolites on the y-axis. Metabolites with VIP values greater than 1.0 are highlighted in red. Metabolites meeting the criteria of VIP > 1.0 and p-value < 0.05 (based on ANOVA) were selected, and hierarchical clustering analysis was performed on the resulting differential metabolites. Ultimately, 228 differential metabolites were identified as potential biomarkers for subsequent analysis (Supplementary Table S1).
3.3. Analysis of Differential Metabolites
The 228 identified biomarker metabolites were subjected to Unit Variance Scaling (UV) pretreatment, and a heatmap was generated to visualize their abundance variations (Figure 3A). Groups B (Denmark), E (All-Female No. 1), and I (Spanish) exhibited distinct clustering patterns in the heatmap. The categorical results revealed that the top differential metabolites primarily belonged to organic acids and their derivatives (37 species), benzene and substituted derivatives (24 species), and amino acids and their metabolites (21 species). Additionally, a considerable number of metabolites were detected in the following categories: glycerophospholipids (20 species), nucleotides and their metabolites (20 species), heterocyclic compounds (18 species), fatty acids (16 species), and alcohols and amines (14 species).
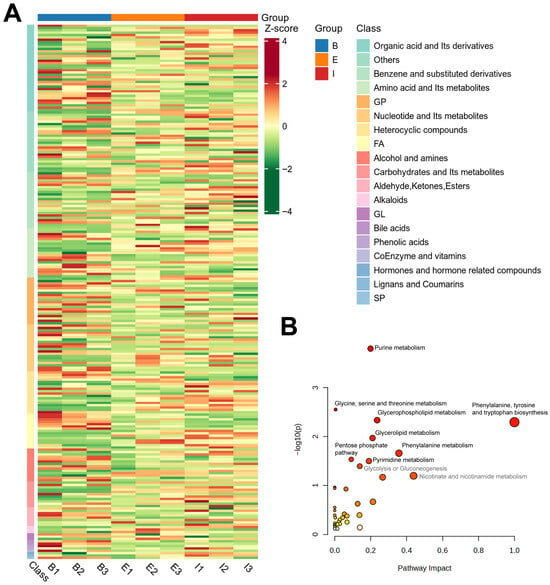
Figure 3.
Hierarchical clustering analysis of differential metabolites (A) and KEGG enrichment bubble plot (B) of metabolites in all-female Danish (Group B), the new variety “All-Female No. 1” (Group E), and the all-female Spanish (Group I) rainbow trout strain.
The KEGG database provides functional annotations and interaction networks for genes, proteins, and metabolites, enabling the exploration of metabolism-related pathways. As shown in Figure 3B, all 228 detected metabolites were enriched in 39 metabolic pathways, including purine metabolism, glycine, serine and threonine metabolism, glycerophospholipid metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, glycerolipid metabolism, and phenylalanine metabolism. In the KEGG enrichment bubble plot, the Y-axis (−Log10(p)) represents the significance of pathway enrichment, with higher values indicating greater significance. The X-axis represents the impact of metabolites on the pathway, where larger values denote stronger influences. Accordingly, differential metabolites were most enriched in Purine metabolism and exhibited the greatest impact on Phenylalanine, tyrosine and tryptophan biosynthesis.
3.4. LC-MS/MS-Based Targeted Quantitative Detection of Amino Acids
Targeted quantitative analysis of amino acids enabled precise quantification of the differences in muscle amino acid composition among all-female Danish rainbow trout (B), the new variety “All-Female No. 1” rainbow trout (E), and the all-female Spanish rainbow trout strain (I). The PCA plot indicated good intra-group clustering of muscle samples from the three sources, but inter-group overlap was observed, suggesting room for improvement in separability (Figure 4A, Supplementary Figure S2). The constructed OPLS-DA model demonstrated better discrimination among the different samples (Figure 4B), with optimal model performance (Supplementary Figure S3). Consequently, by combining the OPLS-DA model with univariate analysis (screening based on p-value/FDR), 11 differentially expressed amino acids were identified (Supplementary Figure S4, Table 1).
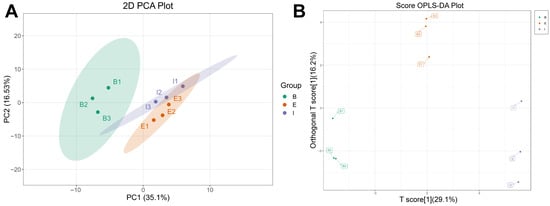
Figure 4.
PCA score plot (A) and OPLS-DA scores plot (B) of all-female Danish (Group B), the new variety “All-Female No. 1” (Group E), and the all-female Spanish (Group I) rainbow trout strain.

Table 1.
Differential expression of free amino acid and related metabolites in the muscle of all-female Danish (Group B), the new variety “All-Female No. 1” (Group E), and the all-female Spanish (Group I) rainbow trout strain.
L-Threonine was the predominant component in Group B, accounting for 61.33%, followed by L-Proline (23.21%). In contrast, the main component in samples E and I was L-Proline, with proportions of 46.84% and 41.14%, respectively, while L-Threonine accounted for 25.09% and 17.70%, respectively. Notably, L-Theanine was not detected in Group B.
3.5. Amino Acid Enrichment Analysis
The visualized heatmap of differentially expressed amino acid metabolites revealed distinct expression patterns among the three groups (Figure 5A). These differentially expressed amino acid metabolites were most enriched in the phenylalanine, tyrosine and tryptophan biosynthesis pathway and also exhibited the greatest impact on this pathway (Figure 5B). L-Tyrosine, 5-hydroxy-tryptamine, and L-phenylalanine were identified as the key amino acid metabolites influencing the phenylalanine, tyrosine and tryptophan biosynthesis pathway (Supplementary Figure S5).
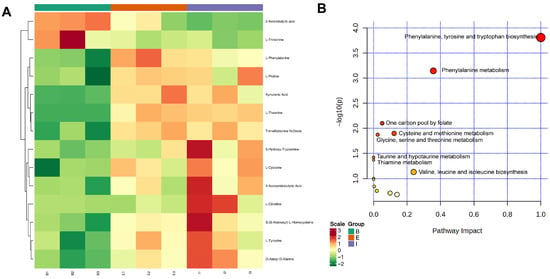
Figure 5.
Hierarchical clustering analysis of differential metabolites (A) and KEGG enrichment bubble plot of amino acid metabolites (B) in all-female Danish (Group B), the new variety “All-Female No. 1” (Group E), and the all-female Spanish (Group I) rainbow trout strain.
Compared to the total differential amino acid content, the proportions of L-Tyrosine in groups B, E, and I were 4.31%, 8.47%, and 8.39%, respectively; 5-Hydroxy-Tryptamine accounted for 0.54%, 0.86%, and 1.26%, respectively; and L-Phenylalanine constituted 2.13%, 4.67%, and 3.40%, respectively (Table 1). The impact of differentially expressed amino acid metabolites on Phenylalanine metabolism was second only to that on Phenylalanine, tyrosine and tryptophan biosynthesis, with L-Tyrosine and L-Phenylalanine being the key metabolites in this pathway (Supplementary Figure S6). In summary, L-tyrosine, 5-hydroxy-tryptamine, and L-phenylalanine were the most influential key amino acid metabolites identified in this analysis.
4. Discussion
Rainbow trout with different genetic backgrounds exhibit distinct advantages in core economic traits, which also impart varying nutritional qualities to their muscle tissue. A total of 2198 metabolites were detected in the rainbow trout muscle samples. Benzene and substituted derivatives were the most abundant category, accounting for 16.11% of all metabolites. Certain benzene derivatives, such as benzaldehyde and phenylethanal, can enhance the flavor of fish meat [26]. Organic acids and their derivatives (336 species, 15.29%) are central to muscle movement and energy metabolism [27]. Fish muscle protein contains all essential amino acids required by the human body, offering high nutritional value [28]. Amino acids are crucial organic compounds that play vital roles in many biological processes, including protein synthesis, cell growth and development, and energy production [29]. The eating quality and nutritional value of muscle tissue largely depend on its amino acid profile and the composition of related metabolites [30,31]. The 245 detected amino acids and their metabolites accounted for 11.15% of all metabolites. The subsequent PCA, while showing some separation between groups, revealed limitations of the model due to partial overlap, prompting the introduction of the OPLS-DA model to screen for biomarker metabolites [25,32].
Purine metabolism plays a crucial role in energy supply, participating in the synthesis, degradation, and recycling of nucleic acids. Furthermore, the purine metabolism process is responsible for producing inosinate and inosine monophosphate (IMP), which are key contributors to the umami taste of fish meat. Therefore, the significant enrichment of differential metabolites in purine metabolism is directly related to the flavor experienced when consuming fish.
In the phenylalanine, tyrosine and tryptophan biosynthesis pathway, phenylalanine, tyrosine, and tryptamine—all aromatic amino acids—can be converted into volatile metabolites, enhancing flavor [33,34]. In animals, aromatic amino acids are considered “essential [35]. Phenylalanine is a precursor of tyrosine and has been shown to be associated with sugar and lipid metabolism [36,37,38]. Additionally, phenylalanine serves as the “source” of the aromatic flavor spectrum and is an essential nutrient; its thermal degradation produces aromatic compounds that enrich the flavor profile of fish meat [39]. L-Tyrosine is commonly used as a dietary supplement, primarily due to reports of its ability to stimulate brain activity for improved memory and mental alertness, act as an appetite suppressant (aiding in controlling depression and anxiety), and enhance physical performance [35]. The new variety “All-Female No. 1” rainbow trout, containing higher levels of phenylalanine and L-tyrosine, thus offers greater nutritional value.
Certain amino acids, known as umami or flavor amino acids, contribute to the sensory properties of meat by enhancing its taste profile. For example, proline, threonine, serine, glycine, and alanine can directly increase the sweetness of fish meat [40,41]. Among the 11 differential amino acids detected in this study, the combined proportions of the umami amino acids L-threonine and L-proline in the three groups reached 84.54%, 71.93%, and 58.84%, respectively. The all-female Danish rainbow trout had the highest proportion, followed by the “All-Female No. 1” variety. This indicates that the all-female Danish rainbow trout and the “All-Female No. 1” rainbow trout possess more desirable flavor characteristics.
5. Conclusions
This study evaluated the nutritional components in the muscle tissue of rainbow trout with three distinct genetic backgrounds, identifying a total of 2198 metabolites and 228 differential metabolites. The results revealed that the differences in nutritional composition among the rainbow trout muscle tissues from different sources were closely associated with amino acid metabolism. The all-female Danish rainbow trout and the “All-Female No. 1” variety exhibited more desirable flavor characteristics due to their high content of the umami amino acids L-threonine and L-proline. Meanwhile, the “All-Female No. 1” rainbow trout demonstrated higher nutritional value owing to its elevated levels of phenylalanine and L-tyrosine. In conclusion, genetic background directly contributes to differences in nutritional value among rainbow trout from various germplasm sources. Future studies should build on this work and explore these findings further with a larger sample size. This study provides a foundation for the future selection and breeding of superior rainbow trout strains and the development of muscle quality evaluation criteria.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology14111613/s1. Table S1: Screen of 228 differentially expressed metabolites; Figure S1: PLS-DA simulation verification diagram; Figure S2: Component Loading Plots for Amino Acid Metabolites; Figure S3: PLS-DA simulation verification diagram for Amino Acid Metabolites; Figure S4: OPLS-DA S-Plot for Differentially Metabolised Amino Acids; Figure S5: KEGG pathway of phenylalanine, tyrosine and tryptophan biosynthesis; Figure S6: KEGG pathway of Phenylalanine metabolism.
Author Contributions
T.H. designed and performed the experiments; Y.S., E.L., W.G. and K.G. analysed the data and checked the manuscript; B.C., G.W. and J.T. cultured and sampled the fish; B.C., T.H. and D.L. drafted the article; J.T., G.P. and F.B. provided resources. G.P., F.B., P.F. and G.X. reviewed the manuscript. All authors contributed to the manuscript at various stages. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the STI2030—Major Projects (2023ZD0405505), the China Agriculture Research System of MOF and MARA (CARS-46), and the Central Public-interest Scientific Institution Basal Research Fund, CAFS (2025XT0101).
Institutional Review Board Statement
The experimental protocols were reviewed and approved by the Institutional Animal Welfare and Ethics Committee of the Heilongjiang River Fisheries Research Institute (Approval No. 20250408-001, date: 8 April 2025).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the reported results of this study can be provided from the corresponding author upon reasonable request.
Conflicts of Interest
Author Guoqing Pan was employed by the company Burqin Ertix River Endemic Cold-water Fish Breeding and Development Co., Ltd. Author Fuyang Bi was employed by the company Xinjiang Sailake Fisheries Science and Technology Development Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Liu, L.-P.; Fang, Y.-D.; Kang, P.-T.; Gao, X.-Y.; Zhang, G.-W.; Pan, J.; Lu, J.; Liu, J.-X.; Zhang, W.-D. Isolation, Identification and Characteristics of Aeromonas Sobria from Diseased Rainbow Trout (Oncorhynchus mykiss). Front. Microbiol. 2025, 15, 1499126. [Google Scholar] [CrossRef]
- Osmond, A.T.Y.; Arts, M.T.; Hall, J.R.; Rise, M.L.; Bazinet, R.P.; Armenta, R.E.; Colombo, S.M. Schizochytrium sp. (T18) Oil as a Fish Oil Replacement in Diets for Juvenile Rainbow Trout (Oncorhynchus mykiss): Effects on Growth Performance, Tissue Fatty Acid Content, and Lipid-Related Transcript Expression. Animals 2021, 11, 1185. [Google Scholar] [CrossRef]
- Farahnak Roudsari, S.; Rajabi Islami, H.; Mousavi, S.A.; Shamsaie Mehrgan, M. Folic Acid-Coated Nanochitosan Ameliorated the Growth Performance, Hematological Parameters, Antioxidant Status, and Immune Responses of Rainbow Trout (Oncorhynchus mykiss). Front. Vet. Sci. 2021, 8, 647722. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Aviles, P.; Torrealba, D.; Figueroa, C.; Mercado, L.; Dixon, B.; Conejeros, P.; Gallardo-Matus, J. Why Vaccines Fail against Piscirickettsiosis in Farmed Salmon and Trout and How to Avoid It: A Review. Front. Immunol. 2022, 13, 1019404. [Google Scholar] [CrossRef]
- Łuczyńska, J.; Paszczyk, B.; Nowosad, J.; Łuczyński, M.J. Mercury, Fatty Acids Content and Lipid Quality Indexes in Muscles of Freshwater and Marine Fish on the Polish Market. Risk Assessment of Fish Consumption. Int. J. Environ. Res. Public Health 2017, 14, 1120. [Google Scholar] [CrossRef] [PubMed]
- Skałecki, P.; Florek, M.; Kędzierska-Matysek, M.; Poleszak, E.; Domaradzki, P.; Kaliniak-Dziura, A. Mineral and Trace Element Composition of the Roe and Muscle Tissue of Farmed Rainbow Trout (Oncorhynchus mykiss) with Respect to Nutrient Requirements. J. Trace Elem. Med. Biol. 2020, 62, 126619. [Google Scholar] [CrossRef]
- Lan, Q.; Lian, Y.; Peng, P.; Yang, L.; Zhao, H.; Huang, P.; Ma, H.; Wei, H.; Yin, Y.; Liu, M. Association of Gut Microbiota and SCFAs with Finishing Weight of Diannan Small Ear Pigs. Front. Microbiol. 2023, 14, 1117965. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Q.; Song, Y.; Lei, Z.; Fu, L.; Cheng, S. Exploring Effects of Different Male Parent Crossings on Sheep Muscles and Related Regulatory Genes Using mRNA-Seq. Anim. Biosci. 2022, 35, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Hoa, V.-B.; Song, D.-H.; Seol, K.-H.; Kang, S.-M.; Kim, H.-W.; Bae, I.-S.; Kim, E.-S.; Park, Y.-S.; Cho, S.-H. A Comparative Study on the Carcass and Meat Chemical Composition, and Lipid-Metabolism-Related Gene Expression in Korean Hanwoo and Brindle Chikso Cattle. Curr. Issues Mol. Biol. 2023, 45, 3279–3290. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, W.; Guo, Y.; Wang, D.; Zhang, Y.; Zhi, Y.; Li, D.; Li, W.; Li, Z.; Jiang, R.; et al. Dynamic Alternations of Three-Dimensional Chromatin Architecture Contribute to Phenotypic Characteristics of Breast Muscle in Chicken. Commun. Biol. 2024, 7, 910. [Google Scholar] [CrossRef]
- Weimer, S.L.; Zuelly, S.; Davis, M.; Karcher, D.M.; Erasmus, M.A. Differences in Carcass Composition and Meat Quality of Conventional and Slow-Growing Broiler Chickens Raised at 2 Stocking Densities. Poult. Sci. 2022, 101, 101833. [Google Scholar] [CrossRef]
- Ding, R.; Zhuang, Z.; Qiu, Y.; Wang, X.; Wu, J.; Zhou, S.; Ruan, D.; Xu, C.; Hong, L.; Gu, T.; et al. A Composite Strategy of Genome-Wide Association Study and Copy Number Variation Analysis for Carcass Traits in a Duroc Pig Population. BMC Genom. 2022, 23, 590. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Masamba, P.; Kappo, A.P. Metabolomics and Chemoinformatics in Agricultural Biotechnology Research: Complementary Probes in Unravelling New Metabolites for Crop Improvement. Biology 2022, 11, 1156. [Google Scholar] [CrossRef]
- Neagu, M.; Longo, C.; Ribero, S. Omics Landscape in Disease Biomarkers Discovery. Dis. Markers 2016, 2016, 4068252. [Google Scholar] [CrossRef]
- Singh, R.; Fatima, E.; Thakur, L.; Singh, S.; Ratan, C.; Kumar, N. Advancements in CHO Metabolomics: Techniques, Current State and Evolving Methodologies. Front. Bioeng. Biotechnol. 2024, 12, 1347138. [Google Scholar] [CrossRef]
- Kim, H.M.; Kang, J.S. Metabolomic Studies for the Evaluation of Toxicity Induced by Environmental Toxicants on Model Organisms. Metabolites 2021, 11, 485. [Google Scholar] [CrossRef]
- Zhuang, K.; Wu, N.; Wang, X.; Wu, X.; Wang, S.; Long, X.; Wei, X. Effects of 3 Feeding Modes on the Volatile and Nonvolatile Compounds in the Edible Tissues of Female Chinese Mitten Crab (Eriocheir sinensis). J. Food Sci. 2016, 81, S968–S981. [Google Scholar] [CrossRef]
- Hoerterer, C.; Petereit, J.; Lannig, G.; Bock, C.; Buck, B.H. 1H-NMR-Based Metabolic Profiling in Muscle and Liver Tissue of Juvenile Turbot (Scophthalmus maximus) Fed with Plant and Animal Protein Sources. Metabolites 2023, 13, 612. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Rasmussen, M.-L.H.; Piening, B.; Shen, X.; Chen, S.; Röst, H.; Snyder, J.K.; Tibshirani, R.; Skotte, L.; Lee, N.C.; et al. Metabolic Dynamics and Prediction of Gestational Age and Time to Delivery in Pregnant Women. Cell 2020, 181, 1680–1692.e15. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.; Plumb, R.S.; Wilson, I.D.; Nicholson, J.K. A validated UPLC-MS/MS assay for the quantification of amino acids and biogenic amines in rat urine. J. Chromatogr. B 2019, 1106–1107, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Le, A.; Ng, A.; Kwan, T.; Cusmano-Ozog, K.; Cowan, T.M. A rapid, sensitive method for quantitative analysis of underivatized amino acids by liquid chromatography–tandem mass spectrometry (LC–MS/MS). J. Chromatogr. B 2014, 944, 166–174. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Hu, T.; Lv, Y.; Li, P.; Liu, L. Targeted Amino Acid and Related Amines Analysis Based on iTRAQ®-LC-MS/MS for Discovering Potential Hepatotoxicity Biomarkers. J. Pharm. Biomed. Anal. 2020, 178, 112812. [Google Scholar] [CrossRef]
- Liu, Z.; Tu, M.-J.; Zhang, C.; Jilek, J.L.; Zhang, Q.-Y.; Yu, A.-M. A Reliable LC-MS/MS Method for the Quantification of Natural Amino Acids in Mouse Plasma: Method Validation and Application to a Study on Amino Acid Dynamics during Hepatocellular Carcinoma Progression. J. Chromatogr. B 2019, 1124, 72–81. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Li, L.; Yang, X.; Chen, S.; Wu, Y.; Zhao, Y.; Wang, J.; Wei, Y.; Yang, D. Application of UHPLC-Q/TOF-MS-Based Metabolomics in the Evaluation of Metabolites and Taste Quality of Chinese Fish Sauce (Yu-Lu) during Fermentation. Food Chem. 2019, 296, 132–141. [Google Scholar] [CrossRef]
- Guo, Y.; Shao, J.; Sun, J.; Wang, Z.; Jiang, B. Optimization of Extraction and Refining Parameters of Oil from Dotted Gizzard Shad (Konosirus punctatus). Foods 2024, 13, 1278. [Google Scholar] [CrossRef]
- Liu, X.; Yao, Z.; Zhang, L.; Shyh-Chang, N. Muscle-derived Bioactive Factors: MyoEVs and Myokines. Cell Prolif. 2024, 58, e13801. [Google Scholar] [CrossRef]
- Goulart, J.F.F.; Pereira, A.C.; Marques, A.M.B.; Martins, I.D.C.A. Nutritional value of seven demersal fish species from the North Atlantic Azores archipelago. Food Chem. X 2024, 24, 102046. [Google Scholar] [CrossRef]
- Yang, H.; Tian, L.; Qiu, H.; Qin, C.; Ling, S.; Xu, J. Metabolomics Analysis of Sporulation-Associated Metabolites of Metarhizium Anisopliae Based on Gas Chromatography–Mass Spectrometry. J. Fungi 2023, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Oladipupo, A.A.; Stuart, K.R.; Bruce, T.J.; Drawbridge, M.A.; Davis, D.A. Practical Diets for California Yellowtail, Seriola Dorsalis: Use of Advanced Soybean Meal Products on Growth Performance, Body Composition, Intestinal Morphology, and Immune Gene Expression. PLoS ONE 2024, 19, e0304679. [Google Scholar] [CrossRef]
- Liu, S.; Du, M.; Tu, Y.; You, W.; Chen, W.; Liu, G.; Li, J.; Wang, Y.; Lu, Z.; Wang, T.; et al. Fermented Mixed Feed Alters Growth Performance, Carcass Traits, Meat Quality and Muscle Fatty Acid and Amino Acid Profiles in Finishing Pigs. Anim. Nutr. 2023, 12, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhu, Y.; Zhang, H.; Zhang, X.; Li, Y.; Yao, Q.; Cai, Q.; Hu, Y. Differentiation of Three Commercial Tuna Species through GC-Q-TOF and UPLC-Q/Orbitrap Mass Spectrometry-Based Metabolomics and Chemometrics. Food Chem. 2024, 452, 139603. [Google Scholar] [CrossRef]
- Fu, A.; Zheng, Y.; Guo, J.; Grierson, D.; Zhao, X.; Wen, C.; Liu, Y.; Li, J.; Zhang, X.; Yu, Y.; et al. Telomere-to-Telomere Genome Assembly of Bitter Melon (Momordica charantia L. var. abbreviata Ser.) Reveals Fruit Development, Composition and Ripening Genetic Characteristics. Hortic. Res. 2023, 10, uhac228. [Google Scholar]
- Sanoppa, K.; Huang, T.-C.; Wu, M.-C. Effects of Saccharomyces cerevisiae in Association with Torulaspora delbrueckii on the Aroma and Amino Acids in Longan Wines. Food Sci. Nutr. 2019, 7, 2817–2826. [Google Scholar] [CrossRef]
- Lütke-Eversloh, T.; Santos, C.N.S.; Stephanopoulos, G. Perspectives of Biotechnological Production of L-Tyrosine and Its Applications. Appl. Microbiol. Biotechnol. 2007, 77, 751–762. [Google Scholar] [CrossRef]
- Zhuo, G.; Wang, L.; Ali, M.; Jing, Z.; Hassan, M.F. Effect of Hexavalent Chromium on Growth Performance and Metabolism in Broiler Chicken. Front. Vet. Sci. 2023, 10, 1273944. [Google Scholar] [CrossRef]
- Hua, Y.; Guo, S.; Xie, H.; Zhu, Y.; Yan, H.; Tao, W.; Shang, E.; Qian, D.; Duan, J. Ziziphus jujuba Mill. Var. spinosa (Bunge) Hu Ex, H.F. Chou Seed Ameliorates Insomnia in Rats by Regulating Metabolomics and Intestinal Flora Composition. Front. Pharmacol. 2021, 12, 653767. [Google Scholar] [CrossRef]
- Jing, Y.; Xu, X.; Wang, Y.; Qu, X.; Guo, Y.; Guo, A.; Dai, Y.; Liu, Y.; Yue, H. Preliminary Study on Metabolite Differences between Two Obesity Syndromes Based on Q Exactive Liquid Chromatography–Tandem Mass Spectrometry Metabolomics. PLoS ONE 2025, 20, e0331901. [Google Scholar] [CrossRef]
- Latoch, A.; Głuchowski, A.; Czarniecka-Skubina, E. Sous-Vide as an Alternative Method of Cooking to Improve the Quality of Meat: A Review. Foods 2023, 12, 3110. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, S.; Zhang, M.; Jiang, H.; Qian, Y.; Wang, R.; Li, M. Comprehensive Analysis of Metabolomics on Flesh Quality of Yellow Catfish (Pelteobagrus fulvidraco) Fed Plant-Based Protein Diet. Front. Nutr. 2023, 10, 1166393. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Song, X.; Wu, W.; Zhang, L.; Han, Z.; Wang, X.; Wang, R.; Yang, M.; Zhang, Z. Nutritional Profiling of Breast Muscle: A Comparative Study between Yuzhong Pigeons and European Meat Pigeons. Food Chem. X 2025, 25, 102157. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).