Characterising PMP22-Proximal Partners in a Schwann Cell Model of Charcot–Marie–Tooth Disease Type1A
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Immortalised Schwann Cell Culture and Geneticin “Kill Curve” Determination
2.2. Expression Vectors
2.3. Transfection of Schwann Cells and Establishment of Stable Cell Lines
2.4. Immunofluorescence Microscopy
2.5. SDS-Polyacrylamide Gel Electrophoresis and Western Blotting
2.6. Myelination Potential Analysis
2.7. RNA Isolation and RT-qPCR
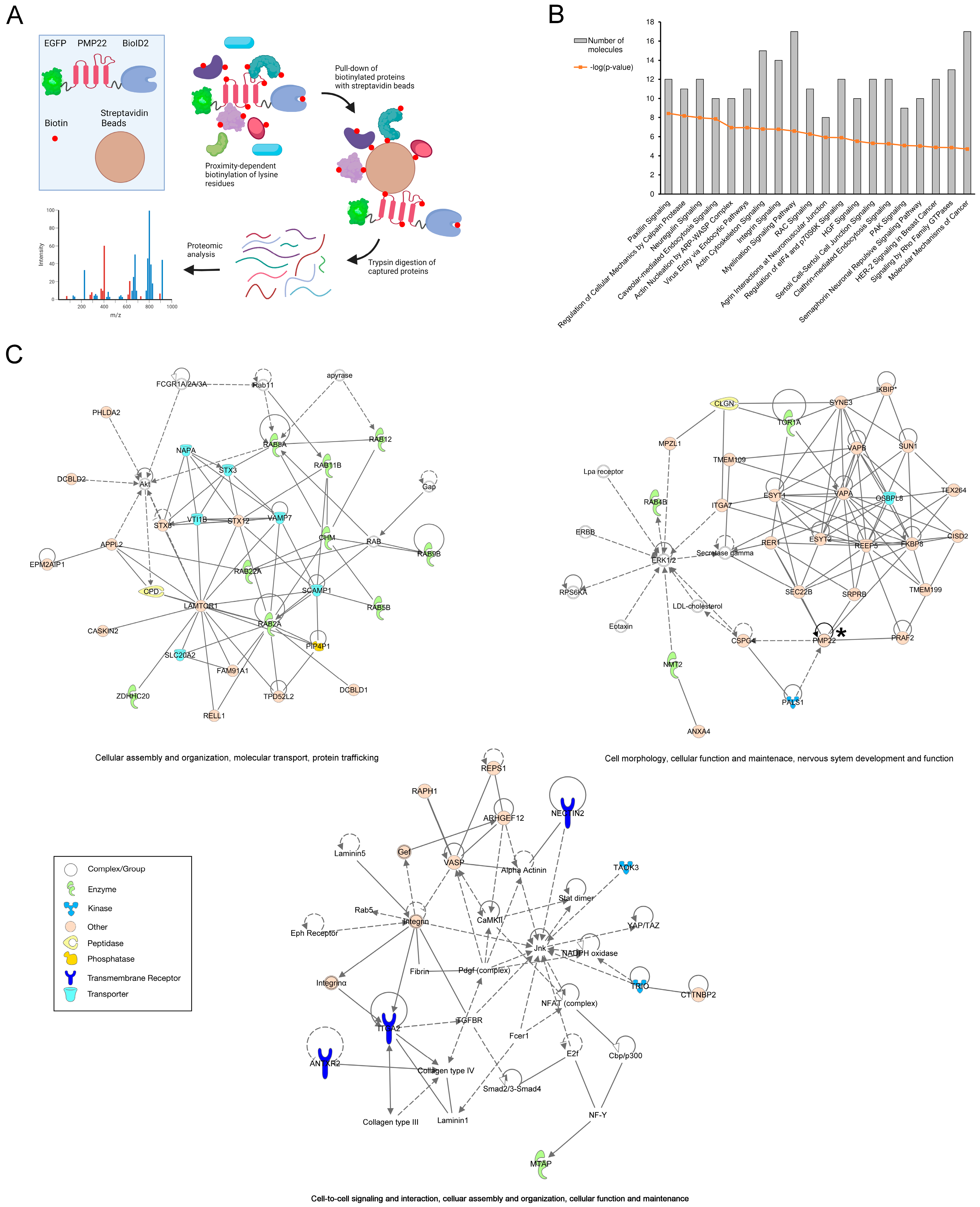
2.8. Cell Extraction and Pull-Down of Biotinylated Proteins
2.9. Mass Spectrometry Analysis of Pull-Downs
2.10. Ingenuity Pathway Analysis (IPA)
3. Results
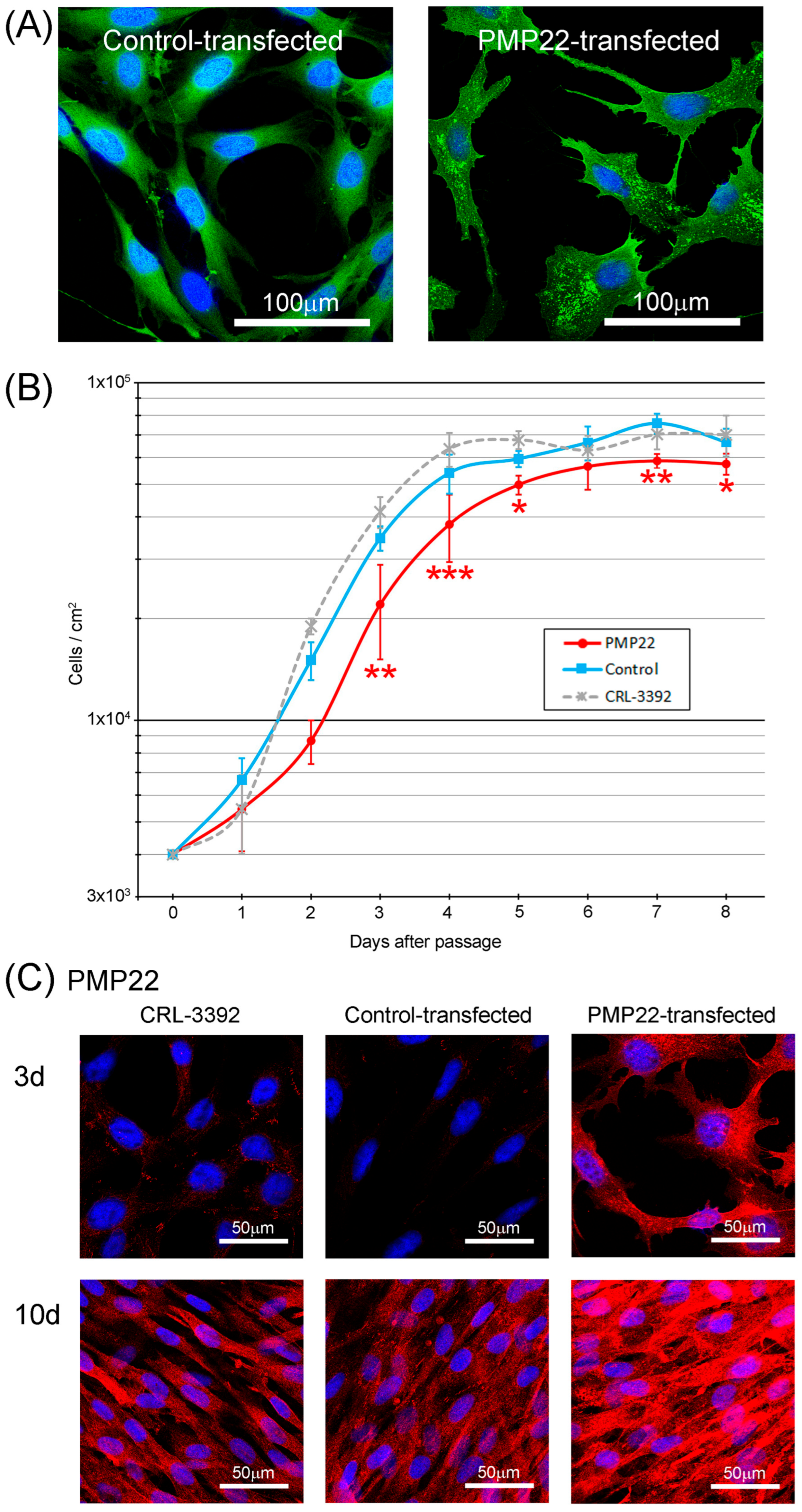
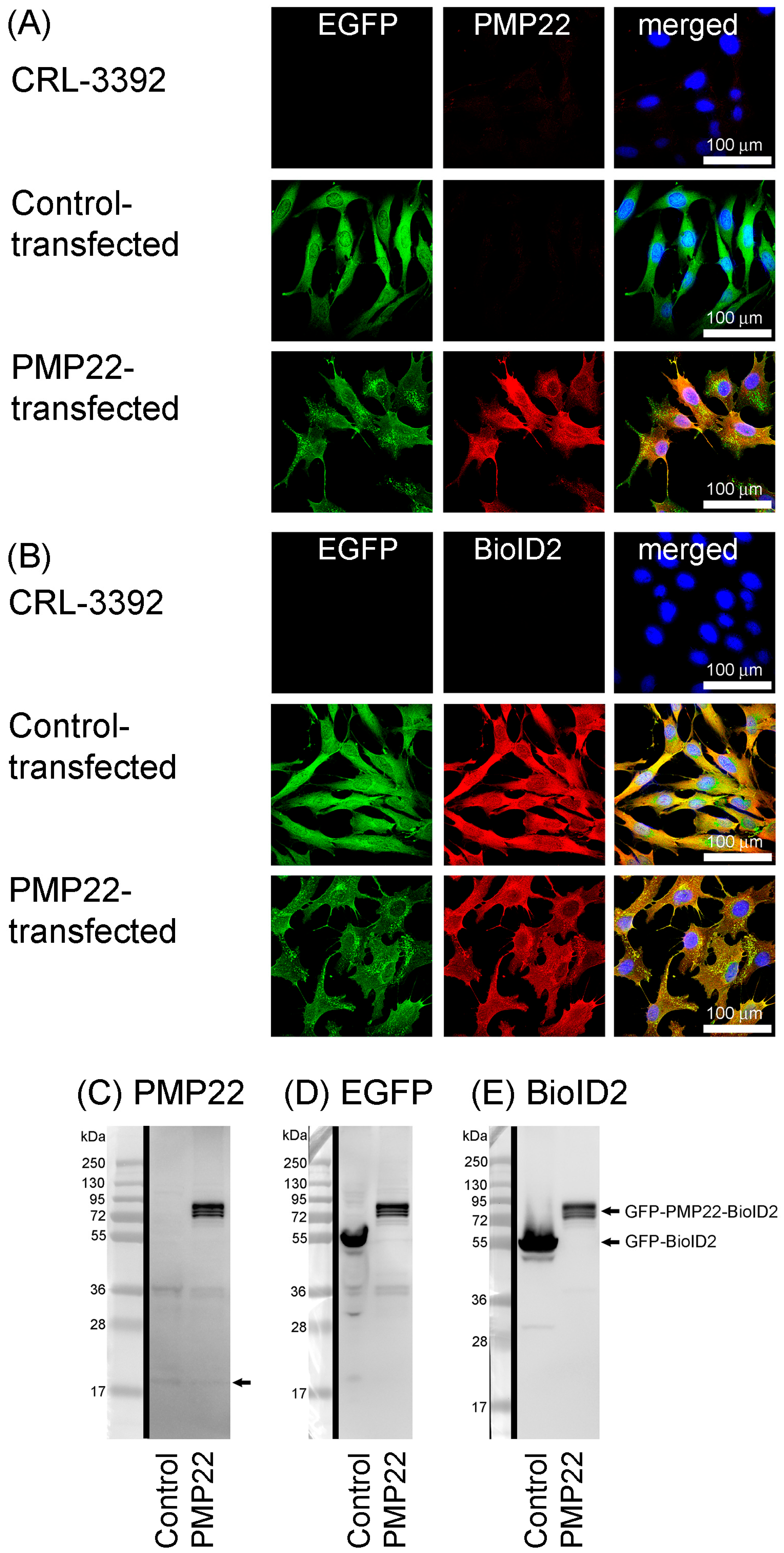
3.1. Generation of Stable Cell Lines
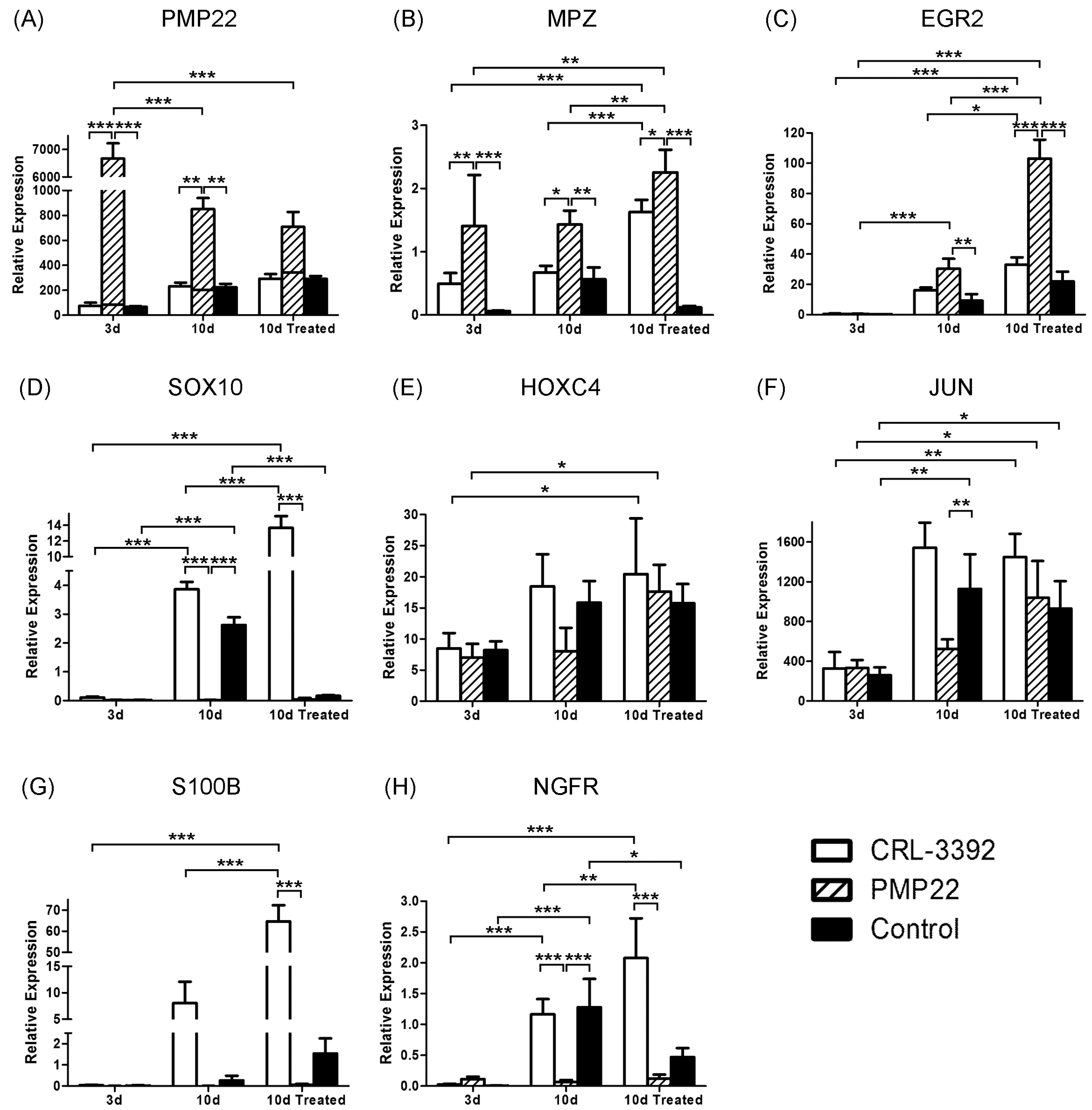
3.2. Cell Line Characteristics
3.3. Myelination Potential of the Cell Lines
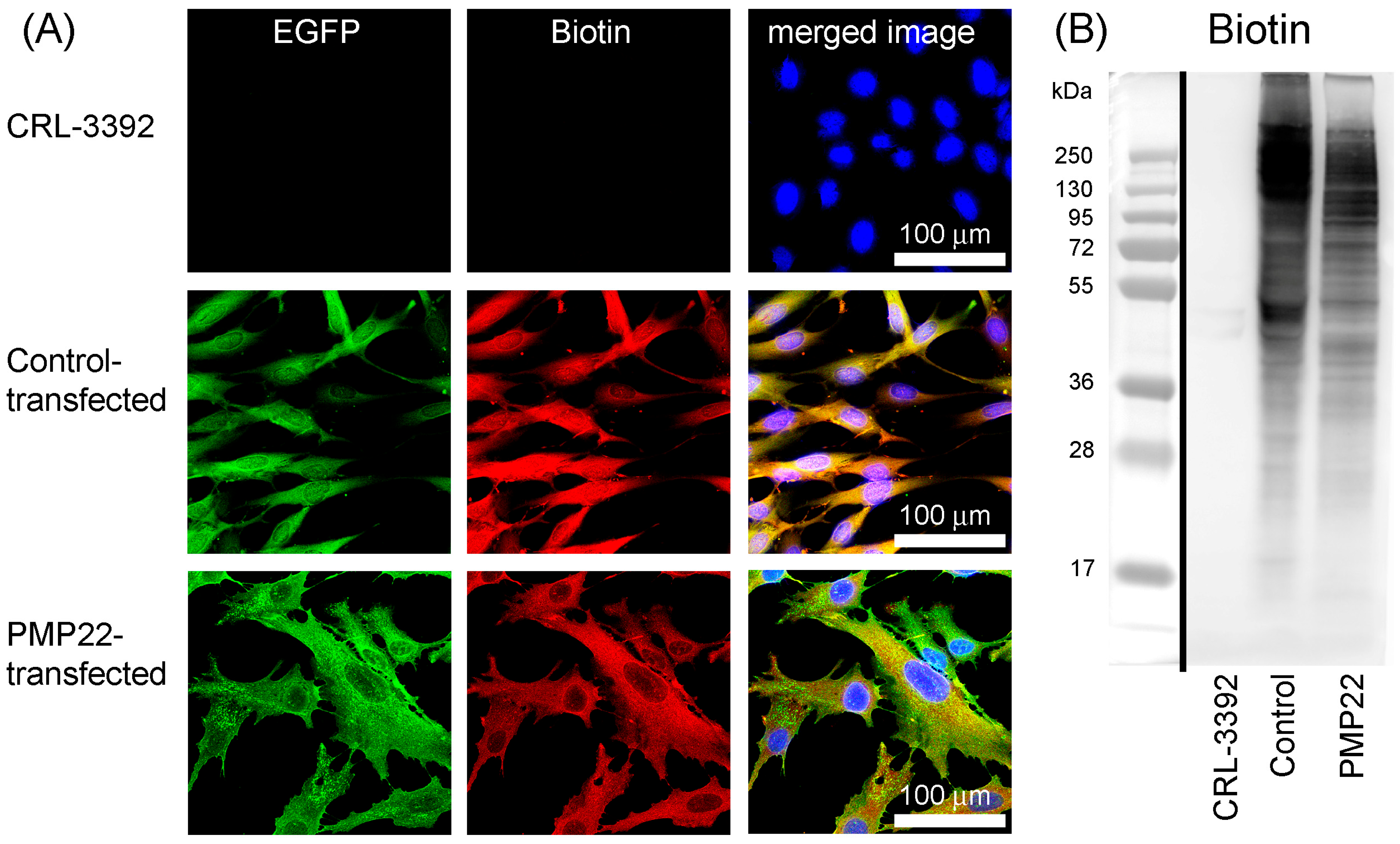
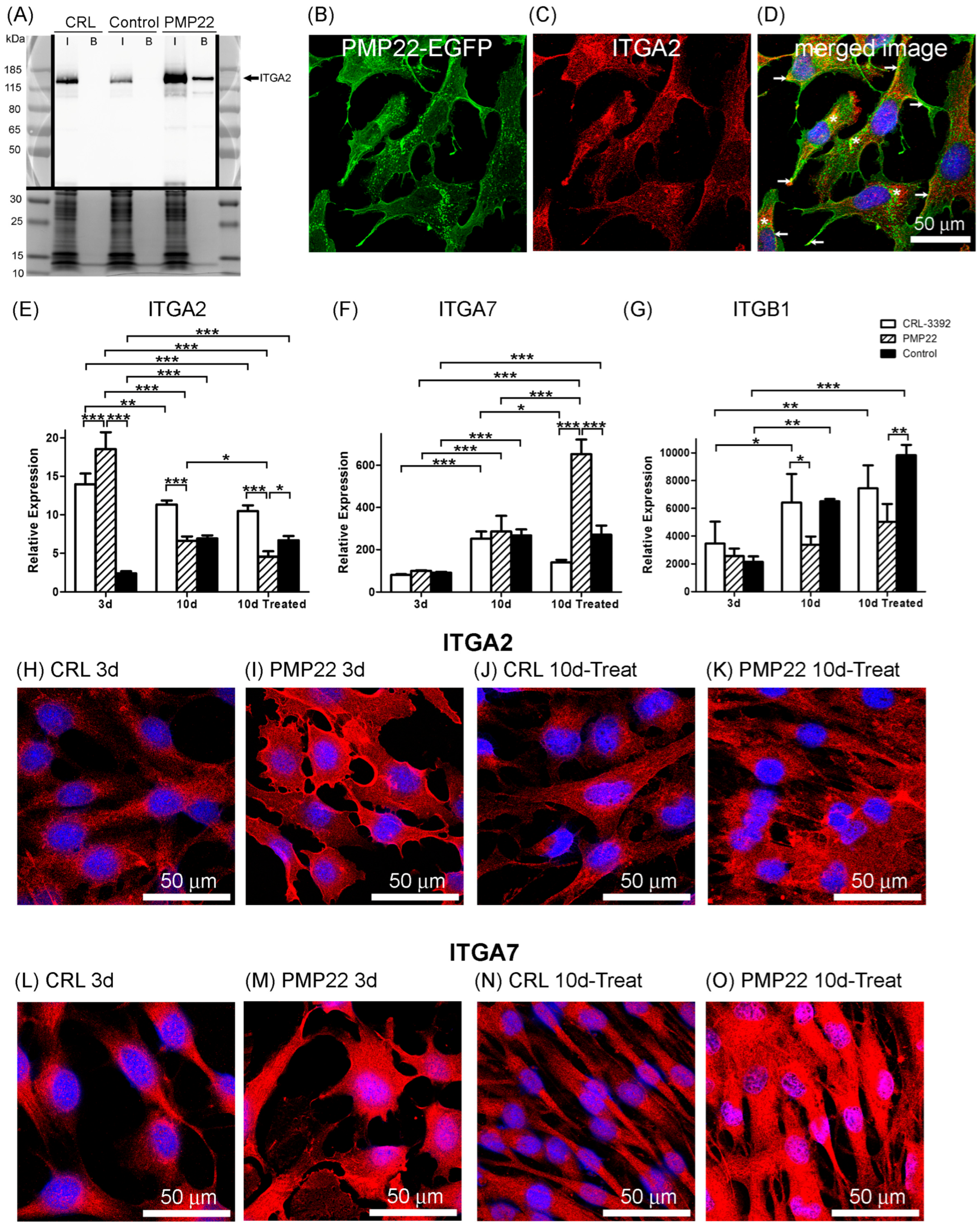
3.4. BioID2 Identification of PMP22-Associated Proteins in the CMT1A Schwann Cell Model
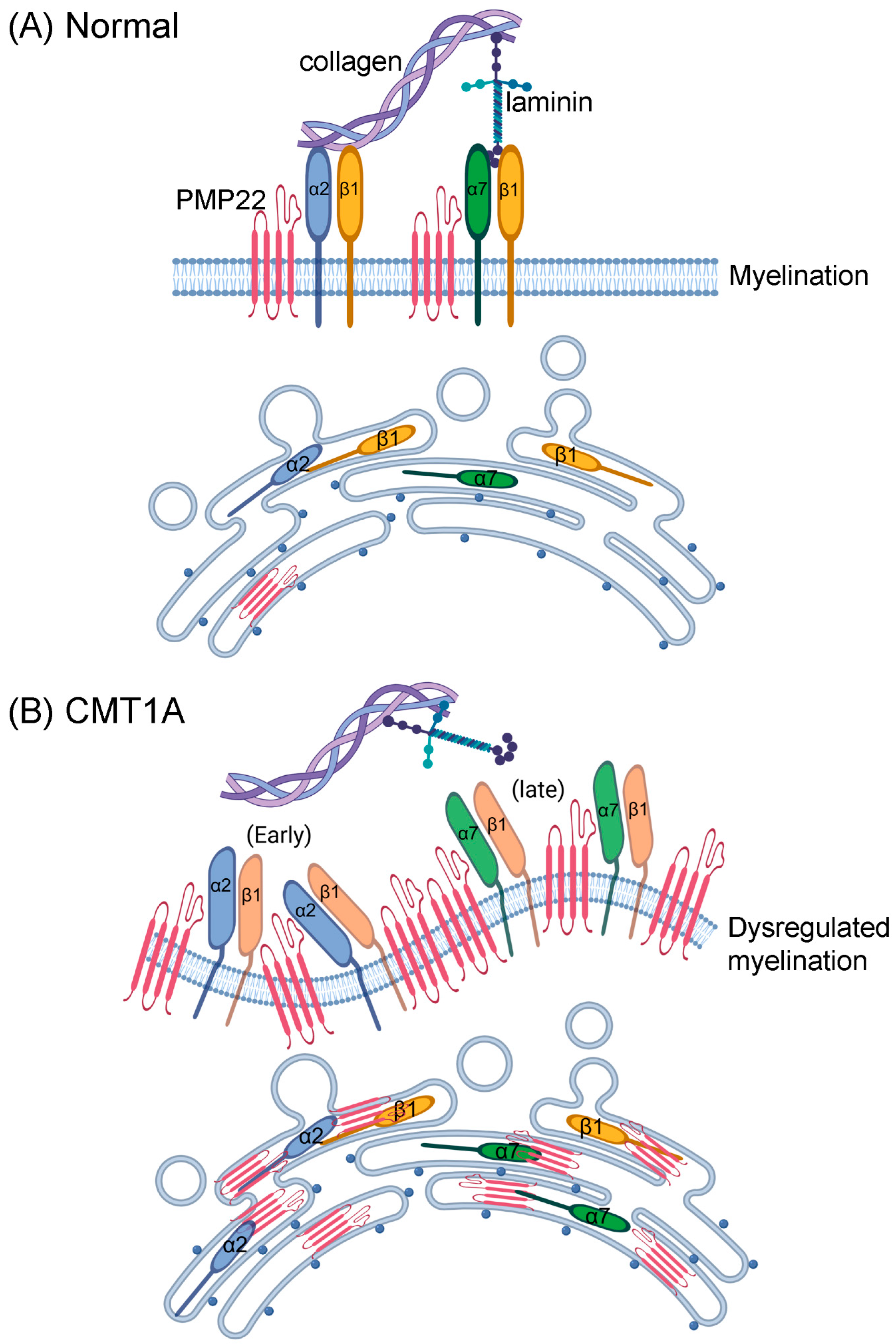
3.5. Integrins and Myelination
4. Discussion
4.1. Overexpression of PMP22
4.2. Myelination Potential of Schwann Cell Lines
4.3. Proximity Analysis of Overexpressed PMP22
4.4. Mitosis in Schwann Cell Lines
4.5. Integrins in CMT1A
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BioID | Proximity-dependent biotin identification |
| BioID2 | Proximity-dependent biotin identification 2 |
| CRL | CRL-3392. Parent cell line. Immortalised human Schwann cell |
| CMT1 | Charcot–Marie–Tooth disease type 1 |
| CMT1A | Charcot–Marie–Tooth disease type 1A |
| CMT2 | Charcot–Marie–Tooth disease type 2 |
| DPBS | Dulbecco’s phosphate-buffered saline |
| EGF | Epidermal growth factor |
| EGFP | Enhanced green fluorescent protein |
| EGR2 | Early growth response 2; KROX20 |
| ER | Endoplasmic reticulum |
| ERAD | Endoplasmic reticulum-associated degradation |
| ESYT1 | Extended synaptotagmin-1 |
| hiPSC | Human induced pluripotent stem cells |
| HNPP | Hereditary neuropathy with pressure palsies |
| HOXC4 | Homeobox C4 |
| IPA | Ingenuity Pathway Analysis |
| ITGA2 | Integrin alpha 2 |
| ITGA7 | Integrin alpha 7 |
| ITGB1 | Integrin beta 1 |
| JUN | Jun proto-oncogene, AP-1 transcription factor subunit; c-JUN |
| MPZ | Myelin protein zero; P0; MPP |
| NGFR | Nerve growth factor receptor; P75NTR |
| NRG1 | Neuregulin 1 |
| PAGE | Polyacrylamide gel electrophoresis |
| PALS1 | Protein associated with LIN7 1, MAGUK P55 family member |
| PBS | Phosphate-buffered saline |
| PMP22 | Peripheral myelin protein 22 |
| RIPA buffer | Radioimmunoprecipitation assay buffer |
| S100B | S100 calcium binding protein B |
| SDS | Sodium dodecyl sulfate |
| SOX10 | SRY-Box transcription factor 10 |
References
- Murphy, S.M.; Laura, M.; Fawcett, K.; Pandraud, A.; Liu, Y.-T.; Davidson, G.L.; Rossor, A.M.; Polke, J.M.; Castleman, V.; Manji, H.; et al. Charcot-Marie-Tooth disease: Frequency of genetic subtypes and guidelines for genetic testing. J. Neurol. Neurosurg. Psychiatry 2012, 83, 706–710. [Google Scholar] [CrossRef]
- Arnold, W.D.; Isfort, M.; Roggenbuck, J.; Hoyle, J.C. The genetics of Charcot-Marie-Tooth disease: Current trends and future implications for diagnosis and management. Appl. Clin. Genet. 2015, 8, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, K.F.; Marinko, J.T.; Hampton, C.M.; Ke, Z.; Hadziselimovic, A.; Schlebach, J.P.; Law, C.L.; Li, J.; Wright, E.R.; Sanders, C.R.; et al. Peripheral myelin protein 22 alters membrane architecture. Sci. Adv. 2017, 3, e1700220. [Google Scholar] [CrossRef] [PubMed]
- Marinko, J.T.; Wright, M.T.; Schlebach, J.P.; Clowes, K.R.; Heintzman, D.R.; Plate, L.; Sanders, C.R. Glycosylation limits forward trafficking of the tetraspan membrane protein PMP22. J. Biol. Chem. 2021, 296, 100719. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Parker, B.; Martyn, C.; Natarajan, C.; Guo, J. The PMP22 gene and its related diseases. Mol. Neurobiol. 2013, 47, 673–698. [Google Scholar] [CrossRef]
- Gabriel, J.-M.; Erne, B.; Pareyson, D.; Sghirlanzoni, A.; Taroni, F.; Steck, A.J. Gene dosage effects in hereditary peripheral neuropathy. Expression of peripheral myelin protein 22 in Charcot-Marie-Tooth disease type 1A and hereditary neuropathy with liability to pressure palsies nerve biopsies. Neurology 1997, 49, 1635–1640. [Google Scholar] [CrossRef]
- Hanemann, C.O.; D’Urso, D.; Gabreëls-Festen, A.A.W.M.; Müller, H.W. Mutation-dependent alteration in cellular distribution of peripheral myelin protein 22 in nerve biopsies from Charcot-Marie-Tooth type 1A. Brain 2000, 123 Pt 5, 1001–1006. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Nishimura, T.; Nakatsuji, Y.; Fujimura, H.; Himoro, M.; Hayasaka, K.; Sakoda, S.; Yanagihara, T. Elevated expression of messenger RNA for peripheral myelin protein 22 in biopsied peripheral nerves of patients with Charcot-Marie-Tooth disease type 1A. Ann. Neurol. 1994, 35, 445–450. [Google Scholar] [CrossRef]
- Huxley, C.; Passage, E.; Robertson, A.M.; Youl, B.; Huston, S.; Manson, A.; Sabéran-Djoniedi, D.; Figarella-Branger, D.; Pellissier, J.F.; Thomas, P.K.; et al. Correlation between varying levels of PMP22 expression and the degree of demyelination and reduction in nerve conduction velocity in transgenic mice. Hum. Mol. Genet. 1998, 7, 449–458. [Google Scholar] [CrossRef]
- Marinko, J.T.; Carter, B.D.; Sanders, C.R. Direct relationship between increased expression and mistrafficking of the Charcot-Marie-Tooth-associated protein PMP22. J. Biol. Chem. 2020, 295, 11963–11970. [Google Scholar] [CrossRef]
- Moss, K.R.; Bopp, T.S.; Johnson, A.E.; Höke, A. New evidence for secondary axonal degeneration in demyelinating neuropathies. Neurosci. Lett. 2021, 744, 135595. [Google Scholar] [CrossRef]
- Gautier, B.; Hajjar, H.; Soares, S.; Berthelot, J.; Deck, M.; Abbou, S.; Campbell, G.; Ceprian, M.; Gonzalez, S.; Fovet, C.-M.; et al. AAV2/9-mediated silencing of PMP22 prevents the development of pathological features in a rat model of Charcot-Marie-Tooth disease 1 A. Nat. Commun. 2021, 12, 2356. [Google Scholar] [CrossRef] [PubMed]
- Sereda, M.; Griffiths, I.; Pühlhofer, A.; Stewart, H.; Rossner, M.J.; Zimmermann, F.; Magyar, J.P.; Schneider, A.; Hund, E.; Meinck, H.-M.; et al. A transgenic rat model of Charcot-Marie-Tooth disease. Neuron 1996, 16, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Niemann, S.; Sereda, M.W.; Rossner, M.; Stewart, H.; Suter, U.; Meinck, H.; Griffiths, I.R.; Nave, K. The “CMT Rat”: Peripheral Neuropathy and Dysmyelination Caused by Transgenic Overexpression of PMP22. Ann. N. Y. Acad. Sci. 1999, 883, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Huxley, C.; Passage, E.; Manson, A.; Putzu, G.; Figarella-Branger, D.; Pellissier, J.F.; Fontés, M. Construction of a mouse model of Charcot-Marie-Tooth disease type 1A by pronuclear injection of human YAC DNA. Hum. Mol. Genet. 1996, 5, 563–569. [Google Scholar] [CrossRef]
- Magyar, J.P.; Martini, R.; Ruelicke, T.; Aguzzi, A.; Adlkofer, K.; Dembic, Z.; Zielasek, J.; Toyka, K.V.; Suter, U. Impaired differentiation of Schwann cells in transgenic mice with increased PMP22 gene dosage. J. Neurosci. 1996, 16, 5351–5360. [Google Scholar] [CrossRef]
- Robertson, A.M.; Perea, J.; McGuigan, A.; King, R.H.M.; Muddle, J.R.; Gabreëls-Festen, A.A.; Thomas, P.K.; Huxley, C. Comparison of a new pmp22 transgenic mouse line with other mouse models and human patients with CMT1A. J. Anat. 2002, 200, 377–390. [Google Scholar] [CrossRef]
- Suter, U.; Moskow, J.J.; A Welcher, A.; Snipes, G.J.; Kosaras, B.; Sidman, R.L.; Buchberg, A.M.; Shooter, E.M. A leucine-to-proline mutation in the putative first transmembrane domain of the 22-kDa peripheral myelin protein in the trembler-J mouse. Proc. Natl. Acad. Sci. USA 1992, 89, 4382–4386. [Google Scholar] [CrossRef]
- Juneja, M.; Burns, J.; A Saporta, M.; Timmerman, V. Challenges in modelling the Charcot-Marie-Tooth neuropathies for therapy development. J. Neurol. Neurosurg. Psychiatry 2019, 90, 58–67. [Google Scholar] [CrossRef]
- Shi, L.; Huang, L.; He, R.; Huang, W.; Wang, H.; Lai, X.; Zou, Z.; Sun, J.; Ke, Q.; Zheng, M.; et al. Modeling the Pathogenesis of Charcot-Marie-Tooth Disease Type 1A Using Patient-Specific iPSCs. Stem Cell Rep. 2018, 10, 120–133. [Google Scholar] [CrossRef]
- Van Lent, J.; Vendredy, L.; Adriaenssens, E.; Authier, T.D.S.; Asselbergh, B.; Kaji, M.; Weckhuysen, S.; Bosch, L.V.D.; Baets, J.; Timmerman, V. Downregulation of PMP22 ameliorates myelin defects in iPSC-derived human organoid cultures of CMT1A. Brain 2023, 146, 2885–2896. [Google Scholar] [CrossRef]
- Prior, R.; Silva, A.; Vangansewinkel, T.; Idkowiak, J.; Tharkeshwar, A.K.; Hellings, T.P.; Michailidou, I.; Vreijling, J.; Loos, M.; Koopmans, B.; et al. PMP22 duplication dysregulates lipid homeostasis and plasma membrane organization in developing human Schwann cells. Brain 2024, 147, 3113–3130. [Google Scholar] [CrossRef]
- Liu, X.; Ishikawa, K.-I.; Hattori, N.; Akamatsu, W. Generation of one induced pluripotent stem cell line JUCGRMi004-A from a Charcot-Marie-Tooth disease type 1A (CMT1A) patient with PMP22 duplication. Stem Cell Res. 2024, 77, 103401. [Google Scholar] [CrossRef]
- Callizot, N.; Combes, M.; Steinschneider, R.; Poindron, P. A new long term in vitro model of myelination. Exp. Cell Res. 2011, 317, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Chumakov, I.; Milet, A.; Cholet, N.; Primas, G.; Boucard, A.; Pereira, Y.; Graudens, E.; Mandel, J.; Laffaire, J.; Foucquier, J.; et al. Polytherapy with a combination of three repurposed drugs (PXT3003) down-regulates Pmp22 over-expression and improves myelination, axonal and functional parameters in models of CMT1A neuropathy. Orphanet J. Rare Dis. 2014, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Cosgaya, J.M.; Chan, J.R.; Shooter, E.M. The neurotrophin receptor p75NTR as a positive modulator of myelination. Science 2002, 298, 1245–1248. [Google Scholar] [CrossRef]
- Rangaraju, S.; Madorsky, I.; Pileggi, J.G.; Kamal, A.; Notterpek, L. Pharmacological induction of the heat shock response improves myelination in a neuropathic model. Neurobiol. Dis. 2008, 32, 105–115. [Google Scholar] [CrossRef]
- Park, N.-Y.; Kwak, G.; Doo, H.-M.; Kim, H.-J.; Jang, S.-Y.; Lee, Y.-I.; Choi, B.-O.; Hong, Y.-B. Farnesol Ameliorates Demyelinating Phenotype in a Cellular and Animal Model of Charcot-Marie-Tooth Disease Type 1A. Curr. Issues Mol. Biol. 2021, 43, 2011–2021. [Google Scholar] [CrossRef]
- Stefanski, K.M.; Li, G.C.; Marinko, J.T.; Carter, B.D.; Samuels, D.C.; Sanders, C.R. How T118M peripheral myelin protein 22 predisposes humans to Charcot-Marie-Tooth disease. J. Biol. Chem. 2023, 299, 102839. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Noto, Y.-I.; Shiga, K.; Tsuji, Y.; Mizuta, I.; Higuchi, Y.; Hashiguchi, A.; Takashima, H.; Nakagawa, M.; Mizuno, T. Nerve ultrasound depicts peripheral nerve enlargement in patients with genetically distinct Charcot-Marie-Tooth disease. J. Neurol. Neurosurg. Psychiatry 2015, 86, 378–384. [Google Scholar] [CrossRef]
- Syed, N.; Reddy, K.; Yang, D.P.; Taveggia, C.; Salzer, J.L.; Maurel, P.; Kim, H.A. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J. Neurosci. 2010, 30, 6122–6131. [Google Scholar] [CrossRef] [PubMed]
- Lundgaard, I.; Luzhynskaya, A.; Stockley, J.H.; Wang, Z.; Evans, K.A.; Swire, M.; Volbracht, K.; Gautier, H.O.B.; Franklin, R.J.M.; Ffrench-Constant, C.; et al. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 2013, 11, e1001743. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Duong, N.T.; Morris, G.E.; Lam, L.T.; Zhang, Q.; Sewry, C.A.; Shanahan, C.M.; Holt, I. Nesprins: Tissue-specific expression of epsilon and other short isoforms. PLoS ONE 2014, 9, e94380. [Google Scholar] [CrossRef]
- PrimerBank—PCR Primers for Gene Expression Detection and Quantification. Available online: https://pga.mgh.harvard.edu/primerbank/ (accessed on 12 June 2024).
- Perkins, D.N.; Pappin, D.J.; Creasy, D.M.; Cottrell, J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 1999, 20, 3551–3567. [Google Scholar] [CrossRef]
- Ishihama, Y.; Oda, Y.; Tabata, T.; Sato, T.; Nagasu, T.; Rappsilber, J.; Mann, M. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteom. 2005, 4, 1265–1272. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Askar, P.; Xu, J.; Hu, J.; Shangguan, J.; Sun, H.; Zhou, S.; Yang, X.; Chen, G.; Su, W.; Gu, Y. The Etv1/Er81 transcription factor coordinates myelination-related genes to regulate Schwann cell differentiation and myelination. Ann. Transl. Med. 2022, 10, 875. [Google Scholar] [CrossRef]
- Fridman, V.; Saporta, M.A. Mechanisms and Treatments in Demyelinating CMT. Neurotherapeutics 2021, 18, 2236–2268. [Google Scholar] [CrossRef]
- Syed, N.; Kim, H.A. Soluble Neuregulin and Schwann Cell Myelination: A Therapeutic Potential for Improving Remyelination of Adult Axons. Mol. Cell. Pharmacol. 2010, 2, 161–167. [Google Scholar] [CrossRef]
- Gambarotta, G.; Ronchi, G.; Geuna, S.; Perroteau, I. Neuregulin 1 isoforms could be an effective therapeutic candidate to promote peripheral nerve regeneration. Neural Regen. Res. 2014, 9, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Monje, P.V.; Sant, D.; Wang, G. Phenotypic and Functional Characteristics of Human Schwann Cells as Revealed by Cell-Based Assays and RNA-SEQ. Mol. Neurobiol. 2018, 55, 6637–6660. [Google Scholar] [CrossRef] [PubMed]
- Arthur-Farraj, P.J.; Latouche, M.; Wilton, D.K.; Quintes, S.; Chabrol, E.; Banerjee, A.; Woodhoo, A.; Jenkins, B.; Rahman, M.; Turmaine, M.; et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 2012, 75, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Hantke, J.; Carty, L.; Wagstaff, L.J.; Turmaine, M.; Wilton, D.K.; Quintes, S.; Koltzenburg, M.; Baas, F.; Mirsky, R.; Jessen, K.R. c-Jun activation in Schwann cells protects against loss of sensory axons in inherited neuropathy. Brain 2014, 137 Pt 11, 2922–2937. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The Role of c-Jun and Autocrine Signaling Loops in the Control of Repair Schwann Cells and Regeneration. Front. Cell. Neurosci. 2022, 15, 820216. [Google Scholar] [CrossRef]
- Fujiwara, S.; Hoshikawa, S.; Ueno, T.; Hirata, M.; Saito, T.; Ikeda, T.; Kawaguchi, H.; Nakamura, K.; Tanaka, S.; Ogata, T. SOX10 transactivates S100B to suppress Schwann cell proliferation and to promote myelination. PLoS ONE 2014, 9, e115400. [Google Scholar] [CrossRef]
- Kim, D.I.; Kc, B.; Zhu, W.; Motamedchaboki, K.; Doye, V.; Roux, K.J. Probing nuclear pore complex architecture with proximity-dependent biotinylation. Proc. Natl. Acad. Sci. USA 2014, 111, E2453–E2461. [Google Scholar] [CrossRef]
- Kim, D.I.; Jensen, S.C.; Noble, K.A.; Kc, B.; Roux, K.H.; Motamedchaboki, K.; Roux, K.J. An improved smaller biotin ligase for BioID proximity labeling. Mol. Biol. Cell 2016, 27, 1188–1196. [Google Scholar] [CrossRef]
- Özçelik, M.; Cotter, L.; Jacob, C.; Pereira, J.A.; Relvas, J.B.; Suter, U.; Tricaud, N. Pals1 is a major regulator of the epithelial-like polarization and the extension of the myelin sheath in peripheral nerves. J. Neurosci. 2010, 30, 4120–4131. [Google Scholar] [CrossRef] [PubMed]
- Vanoye, C.G.; Sakakura, M.; Follis, R.M.; Trevisan, A.J.; Narayan, M.; Li, J.; Sanders, C.R.; Carter, B.D. Peripheral myelin protein 22 modulates store-operated calcium channel activity, providing insights into Charcot-Marie-Tooth disease etiology. J. Biol. Chem. 2019, 294, 12054–12065. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Yoshikawa, H.; Fujimura, H.; Sakoda, S.; Yanagihara, T. Accumulation of peripheral myelin protein 22 in onion bulbs and Schwann cells of biopsied nerves from patients with Charcot-Marie-Tooth disease type 1A. Acta Neuropathol. 1996, 92, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Bazick, H.; Chittoor-Vinod, V.; Al Salihi, M.O.; Xia, G.; Notterpek, L. Elevated Peripheral Myelin Protein 22, Reduced Mitotic Potential, and Proteasome Impairment in Dermal Fibroblasts from Charcot-Marie-Tooth Disease Type 1A Patients. Am. J. Pathol. 2018, 188, 728–738. [Google Scholar] [CrossRef]
- Niemann, S.; Sereda, M.W.; Suter, U.; Griffiths, I.R.; Nave, K.-A. Uncoupling of myelin assembly and schwann cell differentiation by transgenic overexpression of peripheral myelin protein 22. J. Neurosci. 2000, 20, 4120–4128. [Google Scholar] [CrossRef]
- Fortun, J.; Go, J.C.; Li, J.; Amici, S.A.; Dunn, W.A.; Notterpek, L. Alterations in degradative pathways and protein aggregation in a neuropathy model based on PMP22 overexpression. Neurobiol. Dis. 2006, 22, 153–164. [Google Scholar] [CrossRef]
- Ha, N.; Choi, Y.I.; Jung, N.; Song, J.Y.; Bae, D.K.; Kim, M.C.; Lee, Y.J.; Song, H.; Kwak, G.; Jeong, S.; et al. A novel histone deacetylase 6 inhibitor improves myelination of Schwann cells in a model of Charcot-Marie-Tooth disease type 1A. Br. J. Pharmacol. 2020, 177, 5096–5113. [Google Scholar] [CrossRef]
- Ryan, M.C.; Shooter, E.M.; Notterpekb, L. Aggresome formation in neuropathy models based on peripheral myelin protein 22 mutations. Neurobiol. Dis. 2002, 10, 109–118. [Google Scholar] [CrossRef]
- Fortun, J.; Dunn, W.A.; Joy, S.; Li, J.; Notterpek, L. Emerging role for autophagy in the removal of aggresomes in Schwann cells. J. Neurosci. 2003, 23, 10672–10680. [Google Scholar] [CrossRef]
- Fortun, J.; Verrier, J.D.; Go, J.C.; Madorsky, I.; Dunn, W.A.; Notterpek, L. The formation of peripheral myelin protein 22 aggregates is hindered by the enhancement of autophagy and expression of cytoplasmic chaperones. Neurobiol. Dis. 2007, 25, 252–265. [Google Scholar] [CrossRef]
- Svaren, J.; Moran, J.J.; Wu, X.; Zuccarino, R.; Bacon, C.; Bai, Y.; Ramesh, R.; Gutmann, L.; Anderson, D.M.; Pavelec, D.; et al. Schwann cell transcript biomarkers for hereditary neuropathy skin biopsies. Ann. Neurol. 2019, 85, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Florio, F.; Ferri, C.; Scapin, C.; Feltri, M.L.; Wrabetz, L.; D’ANtonio, M. Sustained Expression of Negative Regulators of Myelination Protects Schwann Cells from Dysmyelination in a Charcot-Marie-Tooth 1B Mouse Model. J. Neurosci. 2018, 38, 4275–4287. [Google Scholar] [CrossRef]
- Raasakka, A.; Ruskamo, S.; Kowal, J.; Han, H.; Baumann, A.; Myllykoski, M.; Fasano, A.; Rossano, R.; Riccio, P.; Bürck, J.; et al. Molecular structure and function of myelin protein P0 in membrane stacking. Sci. Rep. 2019, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Tammia, M.; Mi, R.; Sluch, V.M.; Zhu, A.; Chung, T.; Shinn, D.; Zack, D.J.; Höke, A.; Mao, H.-Q. Egr2 overexpression in Schwann cells increases myelination frequency in vitro. Heliyon 2018, 4, e00982. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.E.G.; Roberts, K.; Tuck, E.; Li, T.; Mamanova, L.; Balogh, P.; Usher, I.; Piapi, A.; Mazin, P.; Anderson, N.D.; et al. HOX gene expression in the developing human spine. Nat. Commun. 2024, 15, 10023. [Google Scholar] [CrossRef]
- Parkinson, D.B.; Bhaskaran, A.; Arthur-Farraj, P.; Noon, L.A.; Woodhoo, A.; Lloyd, A.C.; Feltri, M.L.; Wrabetz, L.; Behrens, A.; Mirsky, R.; et al. c-Jun is a negative regulator of myelination. J. Cell Biol. 2008, 181, 625–637. [Google Scholar] [CrossRef]
- Roux, K.J.; Kim, D.I.; Raida, M.; Burke, B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 2012, 196, 801–810. [Google Scholar] [CrossRef]
- Chen, X.; Zaro, J.L.; Shen, W.-C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef]
- Zhou, Y.; Miles, J.R.; Tavori, H.; Lin, M.; Khoshbouei, H.; Borchelt, D.R.; Bazick, H.; Landreth, G.E.; Lee, S.; Fazio, S.; et al. PMP22 Regulates Cholesterol Trafficking and ABCA1-Mediated Cholesterol Efflux. J. Neurosci. 2019, 39, 5404–5418. [Google Scholar] [CrossRef]
- Bian, X.; De Camilli, P. In Vitro Assays to Measure the Membrane Tethering and Lipid Transport Activities of the Extended Synaptotagmins. Methods Mol. Biol. 2019, 1949, 201–212. [Google Scholar] [CrossRef]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- van Schaik, T.; Manzo, S.G.; E Vouzas, A.; Liu, N.Q.; Teunissen, H.; de Wit, E.; Gilbert, D.M.; van Steensel, B. Dynamic chromosomal interactions and control of heterochromatin positioning by Ki-67. EMBO Rep. 2022, 23, e55782. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Min, M.; Yang, C.; Tian, C.; Gookin, S.; Carter, D.; Spencer, S.L. Ki67 is a Graded Rather than a Binary Marker of Proliferation versus Quiescence. Cell Rep. 2018, 24, 1105–1112.e5. [Google Scholar] [CrossRef] [PubMed]
- Adorno-Cruz, V.; Liu, H. Regulation and functions of integrin α2 in cell adhesion and disease. Genes. Dis. 2018, 6, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119 Pt 19, 3901–3903. [Google Scholar] [CrossRef]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a004994. [Google Scholar] [CrossRef]
- Berti, C.; Nodari, A.; Wrabetz, L.; Feltri, M.L. Role of integrins in peripheral nerves and hereditary neuropathies. Neuromol. Med. 2006, 8, 191–204. [Google Scholar] [CrossRef]
- Feltri, M.L.; Porta, D.G.; Previtali, S.C.; Nodari, A.; Migliavacca, B.; Cassetti, A.; Littlewood-Evans, A.; Reichardt, L.F.; Messing, A.; Quattrini, A.; et al. Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J. Cell Biol. 2002, 156, 199–209. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, X.; Yue, C.; Yang, W.; Zhang, S.; Ou, Z.; Chen, Y. Ninj2 regulates Schwann cells development by interfering laminin-integrin signaling. Theranostics 2022, 12, 7307–7318. [Google Scholar] [CrossRef]
- Amici, S.A.; Dunn, W.A.; Notterpek, L. Developmental abnormalities in the nerves of peripheral myelin protein 22-deficient mice. J. Neurosci. Res. 2007, 85, 238–249. [Google Scholar] [CrossRef]
- Wadehra, M.; Iyer, R.; Goodglick, L.; Braun, J. The tetraspan protein epithelial membrane protein-2 interacts with beta1 integrins and regulates adhesion. J. Biol. Chem. 2002, 277, 41094–41100. [Google Scholar] [CrossRef]
- Amici, S.A.; Dunn, W.A.; Murphy, A.J.; Adams, N.C.; Gale, N.W.; Valenzuela, D.M.; Yancopoulos, G.D.; Notterpek, L. Peripheral myelin protein 22 is in complex with alpha6beta4 integrin, and its absence alters the Schwann cell basal lamina. J. Neurosci. 2006, 26, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Pellegatta, M.; De Arcangelis, A.; D’Urso, A.; Nodari, A.; Zambroni, D.; Ghidinelli, M.; Matafora, V.; Williamson, C.; Georges-Labouesse, E.; Kreidberg, J.; et al. α6β1 and α7β1 integrins are required in Schwann cells to sort axons. J. Neurosci. 2013, 33, 17995–18007. [Google Scholar] [CrossRef] [PubMed]
- Salicioni, A.M.; Gaultier, A.; Brownlee, C.; Cheezum, M.K.; Gonias, S.L. Low density lipoprotein receptor-related protein-1 promotes beta1 integrin maturation and transport to the cell surface. J. Biol. Chem. 2004, 279, 10005–10012. [Google Scholar] [CrossRef] [PubMed]
- Darom, A.; Bening-Abu-Shach, U.; Broday, L. RNF-121 is an endoplasmic reticulum-membrane E3 ubiquitin ligase involved in the regulation of beta-integrin. Mol. Biol. Cell 2010, 21, 1788–1798. [Google Scholar] [CrossRef]
- Chittoor-Vinod, V.G.; Lee, S.; Judge, S.M.; Notterpek, L. Inducible HSP70 is critical in preventing the aggregation and enhancing the processing of PMP22. ASN Neuro 2015, 7, 1759091415569909. [Google Scholar] [CrossRef]
- Pisciotta, C.; Saveri, P.; Pareyson, D. Challenges in Treating Charcot-Marie-Tooth Disease and Related Neuropathies: Current Management and Future Perspectives. Brain Sci. 2021, 11, 1447. [Google Scholar] [CrossRef]
| Population-Doubling Time (Hours) a | Cells/cm2 (3 Days) b | Ki67 Quantitation (3 Days): Mean Grey Value per Nucleus e Mean ± SD (n = 50) | ||
|---|---|---|---|---|
| Adherent Mean ± SD (n = 4) | Non-Adherent Mean ± SD (n = 4) | |||
| CRL-3392 | 22.3 | 41,400 ± 4400 | 77 ± 14 | 33.4 ± 13.0 |
| Control-transfected | 23.0 | 34,600± 2875 | 16 ± 6 | 20.8 ± 6.0 |
| PMP22-transfected | 30.7 | 22,000± 6866 (*) c | 696 ± 108 (***) d | 13.6 ± 10.7 (***) f |
| 3 d | 10 d | |
|---|---|---|
| CRL-3392 | 0.28 ± 0.11 | 1.28 ± 0.60 ** |
| Control-trans | 0.05 ± 0.02 | 1.61 ± 0.74 *** |
| PMP22-trans | 4.19 ± 2.79 | 4.85 ± 1.95 |
| Protein | Accession | Number of Significant Peptides |
|---|---|---|
| Isoform 2 of Extended synaptotagmin-1 (ESYT1) | Q9BSJ8-2 | 28 |
| Integrin alpha-2 (ITGA2) | P17301 | 20 |
| Treacle protein (TCOF1) | Q13428 | 19 |
| Structural maintenance of chromosomes protein (SMC1A) | G8JLG1 | 18 |
| Plasma membrane calcium-transporting ATPase 1 (ATP2B1) | P20020 | 17 |
| DNA replication licencing factor MCM5 (MCM5) | P33992 | 16 |
| Glutamine--tRNA ligase (QARS1) | P47897 | 16 |
| Isoform 13 of Sodium bicarbonate cotransporter 3 (SLC4A7) | Q9Y6M7-13 | 16 |
| Phospholipid-transporting ATPase IC (ATP8B1) | O43520 | 15 |
| Polyadenylate-binding protein 1 (PABPC1) | A0A7I2V649 | 15 |
| Integrin alpha-7 (ITGA7) | J3KNV4 | 14 |
| Vigilin (Fragment) (HDLBP) | H0Y394 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holt, I.; Emery, N.; Gates, M.A.; Brown, S.J.; Shirran, S.L.; Fuller, H.R. Characterising PMP22-Proximal Partners in a Schwann Cell Model of Charcot–Marie–Tooth Disease Type1A. Biology 2025, 14, 1552. https://doi.org/10.3390/biology14111552
Holt I, Emery N, Gates MA, Brown SJ, Shirran SL, Fuller HR. Characterising PMP22-Proximal Partners in a Schwann Cell Model of Charcot–Marie–Tooth Disease Type1A. Biology. 2025; 14(11):1552. https://doi.org/10.3390/biology14111552
Chicago/Turabian StyleHolt, Ian, Nicholas Emery, Monte A. Gates, Sharon J. Brown, Sally L. Shirran, and Heidi R. Fuller. 2025. "Characterising PMP22-Proximal Partners in a Schwann Cell Model of Charcot–Marie–Tooth Disease Type1A" Biology 14, no. 11: 1552. https://doi.org/10.3390/biology14111552
APA StyleHolt, I., Emery, N., Gates, M. A., Brown, S. J., Shirran, S. L., & Fuller, H. R. (2025). Characterising PMP22-Proximal Partners in a Schwann Cell Model of Charcot–Marie–Tooth Disease Type1A. Biology, 14(11), 1552. https://doi.org/10.3390/biology14111552







