Glycogen Synthase Kinase 3 Is Essential for Intestinal Cell Niche and Digestive Function
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Antibodies
2.3. Tissue Preparation and Histology
2.4. Quantitative RT-PCR
2.5. Flow Cytometry
2.6. Whole-Gut Transit Time
2.7. Nutritional Intervention with Liquid Diet
2.8. Western Blot
2.9. Antibiotic Treatment (Abx)
2.10. Image Acquisition
2.11. Statistical Analysis
3. Results
3.1. GSK3α or GSK3β Deletion Does Not Disrupt Intestinal Homeostasis and Tumorigenesis
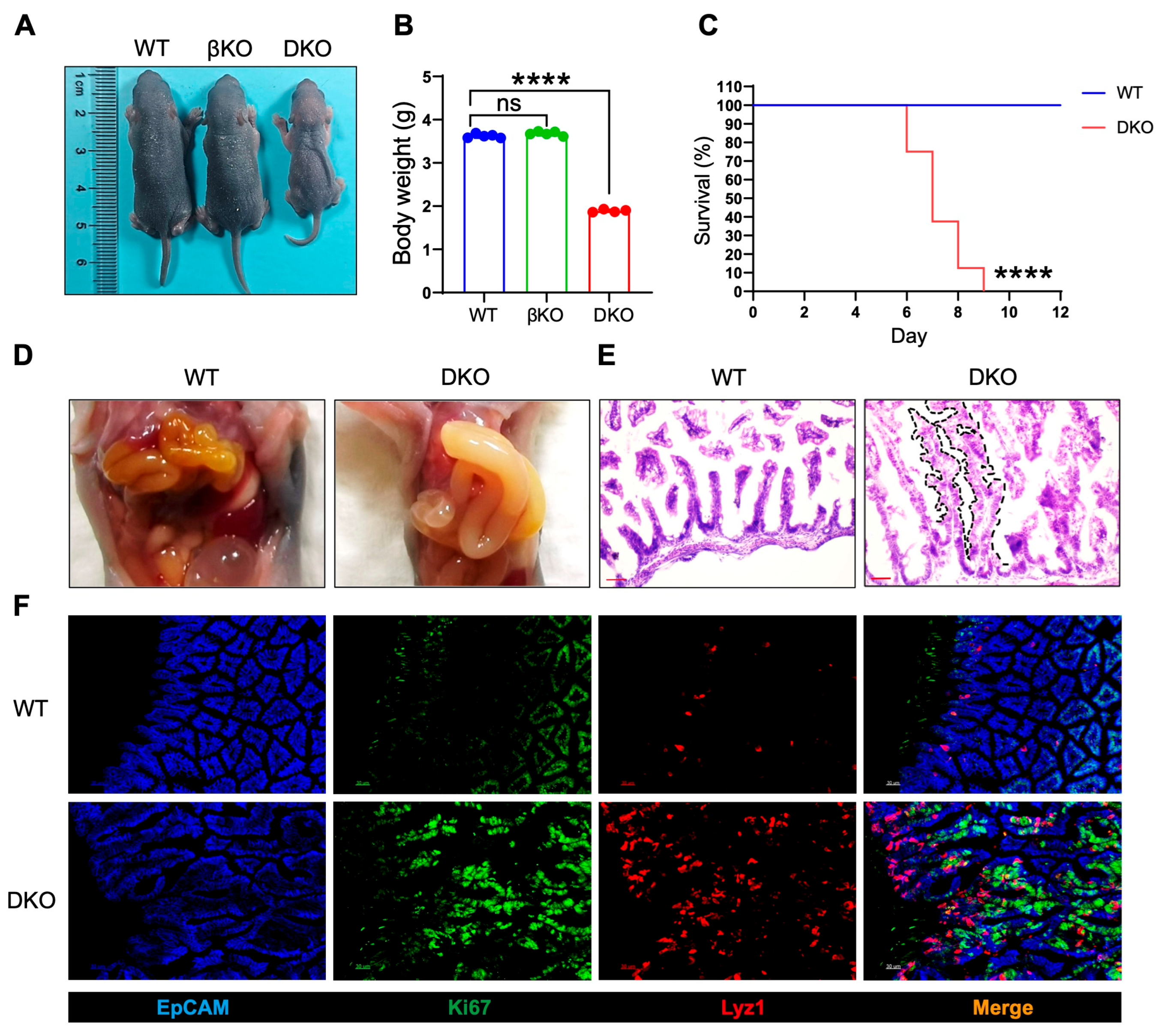
3.2. Intestinal GSK3α and GSK3β Deficiency (DKO) Leads to Abnormal Intestinal Development and Perinatal Death
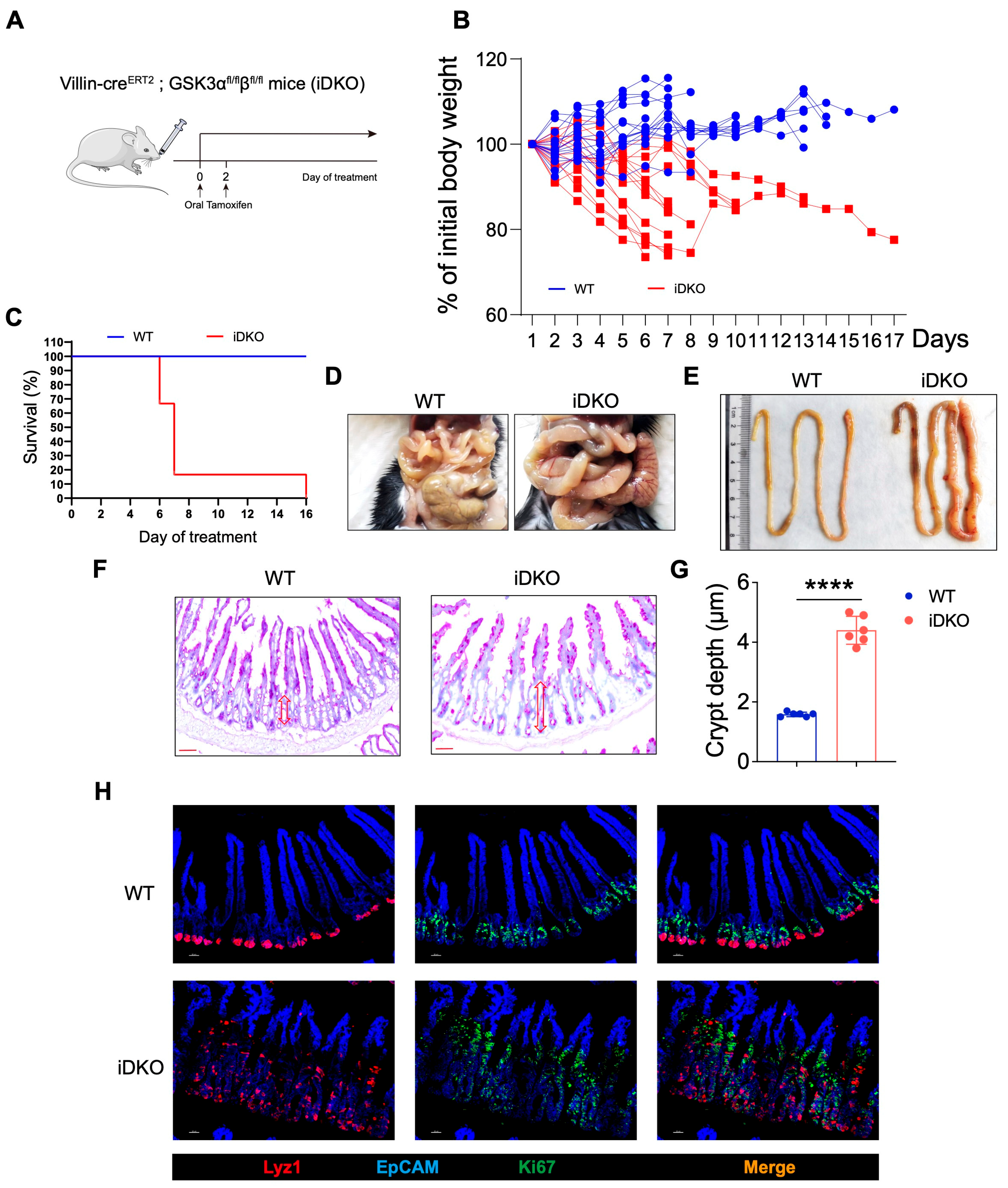
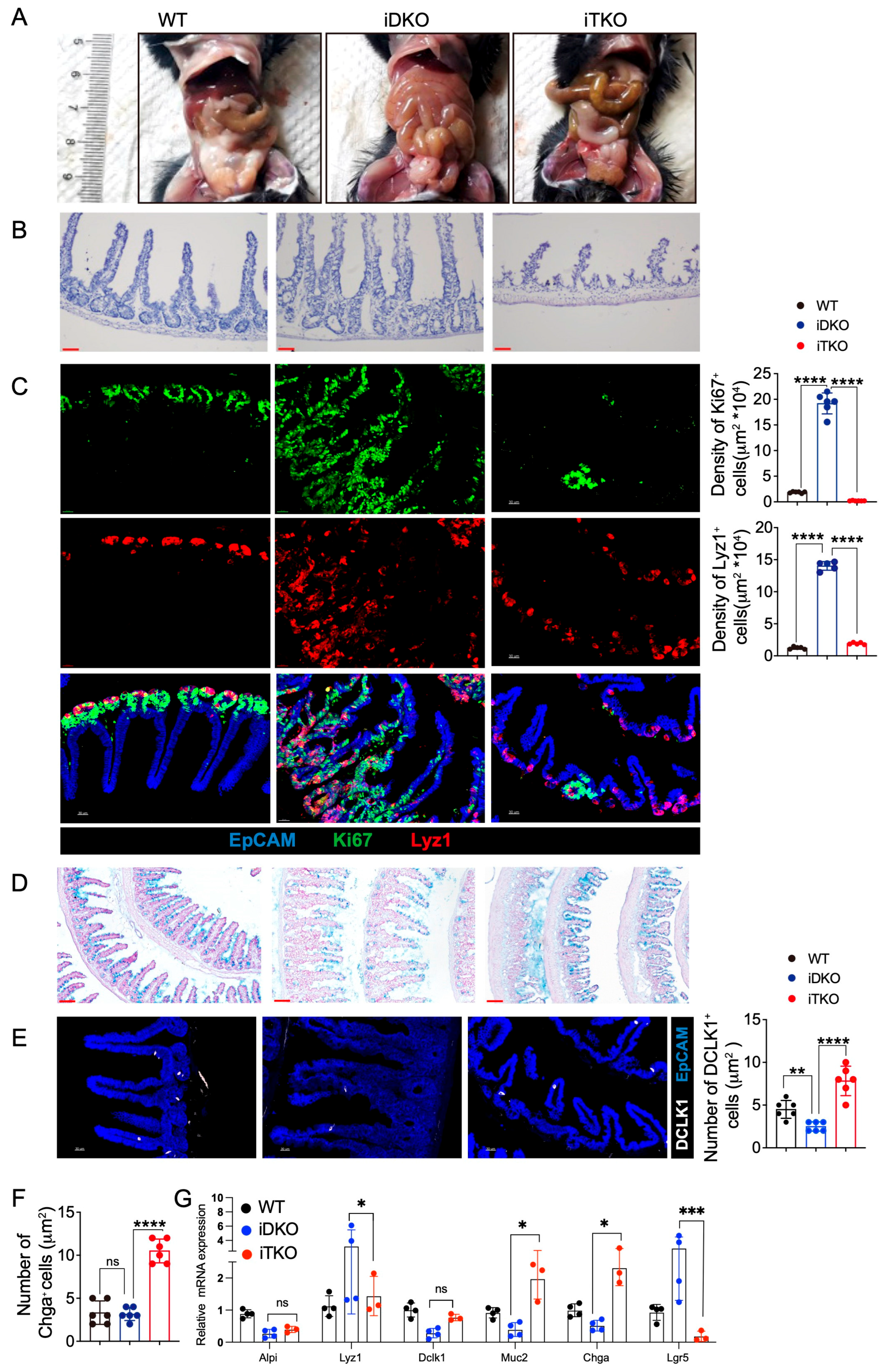
3.3. Inducible Deletion of GSK3 in Adult Mice Results in Intestinal Abnormalities and Lethality
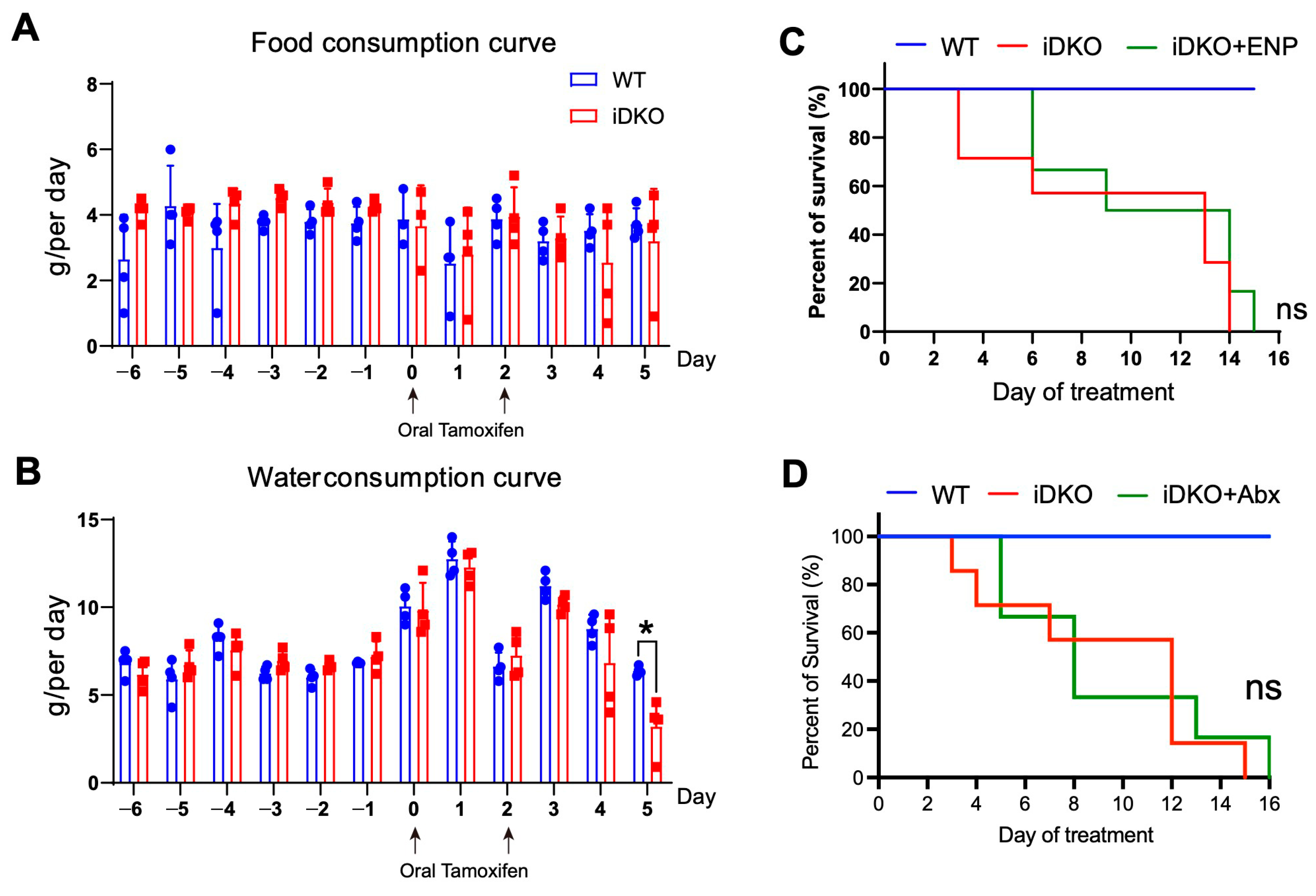
3.4. Intestinal GSK3 Deficiency Dampens Multi-Organs and Slightly Affects Feeding Capacity
3.5. GSK3 Deficiency Affects the Intestinal Cell Niche and Absorptive and Peristaltic Function
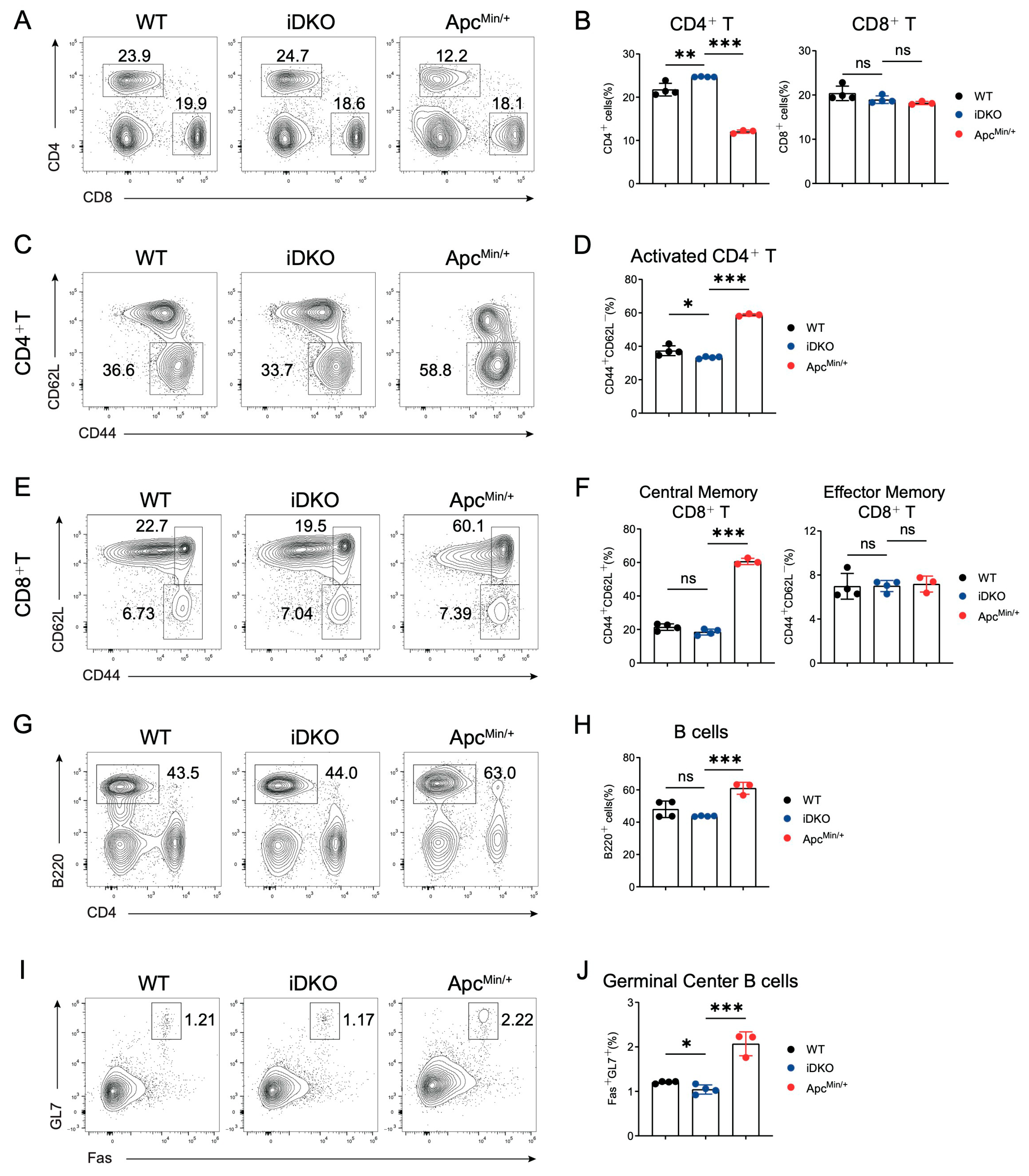
3.6. GSK3 Deficiency Does Not Affect Immune Homeostasis in Mesenteric Lymph Nodes
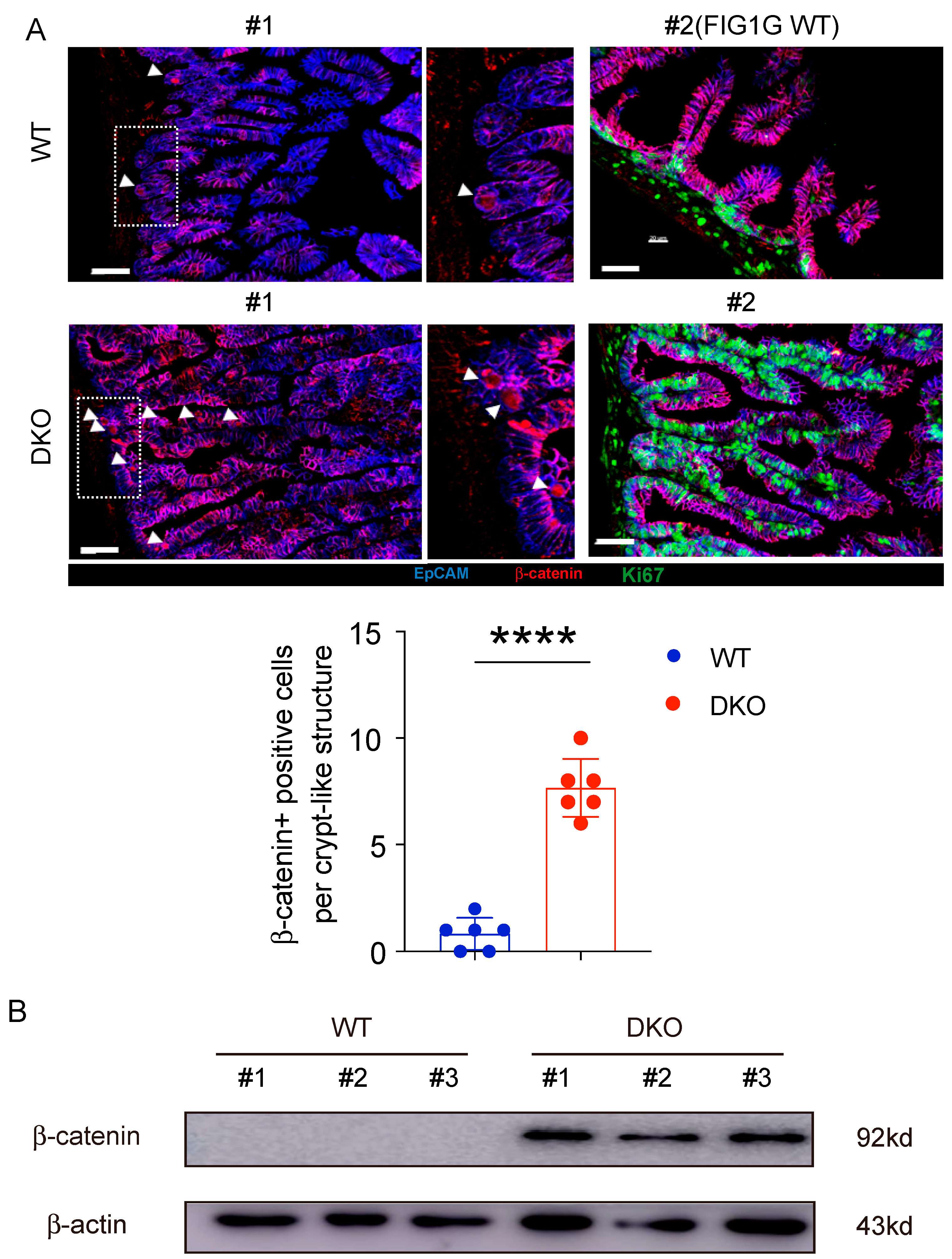
3.7. GSK3 Deficiency Promotes an Increase and Redistribution of β-Catenin-Positive Cells
3.8. β-Catenin Deletion Ameliorates Intestinal Cell Proliferation and Restores the Intestinal Niche by GSK3 Deletion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patricia, J.J.; Dhamoon, A.S. Physiology, Digestion. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sun, M.; He, C.; Cong, Y.; Liu, Z. Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol. 2015, 8, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Michalowski, C.B.; Koehler, J.; Darwish, T.; Guccio, N.; Alcaino, C.; Domingues, I.; Zhang, W.; Marotti, V.; Van Hul, M.; et al. Smart control lipid-based nanocarriers for fine-tuning gut hormone secretion. Sci. Adv. 2024, 10, eadq9909. [Google Scholar] [CrossRef] [PubMed]
- Beumer, J.; Clevers, H. Cell fate specification and differentiation in the adult mammalian intestine. Nat. Rev. Mol. Cell Biol. 2021, 22, 39–53. [Google Scholar] [CrossRef]
- Yu, J.; Liu, D.; Sun, X.; Yang, K.; Yao, J.; Cheng, C.; Wang, C.; Zheng, J. CDX2 inhibits the proliferation and tumor formation of colon cancer cells by suppressing Wnt/β-catenin signaling via transactivation of GSK-3β and Axin2 expression. Cell Death Dis. 2019, 10, 26. [Google Scholar] [CrossRef]
- Gonzalez-Perez, D.; Das, S.; Antfolk, D.; Ahsan, H.S.; Medina, E.; Dundes, C.E.; Jokhai, R.T.; Egan, E.D.; Blacklow, S.C.; Loh, K.M.; et al. Affinity-matured DLL4 ligands as broad-spectrum modulators of Notch signaling. Nat. Chem. Biol. 2023, 19, 9–17. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.G. BMP signaling in homeostasis, transformation and inflammatory response of intestinal epithelium. Sci. China Life Sci. 2018, 61, 800–807. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Huang, M.; Wang, X.; Li, C.; Li, S.; Tang, Y.; Yu, S.; Wang, Y.; Song, W.; et al. BMP suppresses Wnt signaling via the Bcl11b-regulated NuRD complex to maintain intestinal stem cells. EMBO J. 2024, 43, 6032–6051. [Google Scholar] [CrossRef]
- Xiao, F.; Zhu, C.; Wei, X.; Chen, G.; Xu, X. Shenhuang plaster enhances intestinal anastomotic healing in rabbits through activation of the TGF-β and Hippo/YAP signaling pathways. J. Appl. Biomed. 2023, 21, 208–217. [Google Scholar] [CrossRef]
- Yekkala, K.; Baudino, T.A. Inhibition of intestinal polyposis with reduced angiogenesis in ApcMin/+ mice due to decreases in c-Myc expression. Mol. Cancer Res. 2007, 5, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, J.; Zhao, X.; Wu, T.; Huang, Z.; Chen, D.; Liu, Y.; Ouyang, G. Periostin Promotes Colorectal Tumorigenesis through Integrin-FAK-Src Pathway-Mediated YAP/TAZ Activation. Cell Rep. 2020, 30, 793–806.e796. [Google Scholar] [CrossRef]
- Lichtinghagen, R.; Huber, R. GSK3 as a Master Regulator of Cellular Processes. Int. J. Mol. Sci. 2023, 24, 15503. [Google Scholar] [CrossRef]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef]
- Hoeflich, K.P.; Luo, J.; Rubie, E.A.; Tsao, M.S.; Jin, O.; Woodgett, J.R. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 2000, 406, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Kaidanovich-Beilin, O.; Lipina, T.V.; Takao, K.; van Eede, M.; Hattori, S.; Laliberté, C.; Khan, M.; Okamoto, K.; Chambers, J.W.; Fletcher, P.J.; et al. Abnormalities in brain structure and behavior in GSK-3alpha mutant mice. Mol. Brain 2009, 2, 35. [Google Scholar] [CrossRef]
- Hu, X.; Gan, L.; Tang, Z.; Lin, R.; Liang, Z.; Li, F.; Zhu, C.; Han, X.; Zheng, R.; Shen, J.; et al. A Natural Small Molecule Mitigates Kidney Fibrosis by Targeting Cdc42-mediated GSK-3β/β-catenin Signaling. Adv. Sci. 2024, 11, e2307850. [Google Scholar] [CrossRef]
- Severson, E.A.; Kwon, M.; Hilgarth, R.S.; Parkos, C.A.; Nusrat, A. Glycogen Synthase Kinase 3 (GSK-3) influences epithelial barrier function by regulating occludin, claudin-1 and E-cadherin expression. Biochem. Biophys. Res. Commun. 2010, 397, 592–597. [Google Scholar] [CrossRef]
- Liu, C.; Ma, L.; Wang, Y.; Zhao, J.; Chen, P.; Chen, X.; Wang, Y.; Hu, Y.; Liu, Y.; Jia, X.; et al. Glycogen synthase kinase 3 drives thymocyte egress by suppressing β-catenin activation of Akt. Sci. Adv. 2021, 7, eabg6262. [Google Scholar] [CrossRef]
- You, S.; Li, S.; Zeng, L.; Song, J.; Li, Z.; Li, W.; Ni, H.; Xiao, X.; Deng, W.; Li, H.; et al. Lymphatic-localized Treg-mregDC crosstalk limits antigen trafficking and restrains anti-tumor immunity. Cancer Cell 2024, 42, 1415–1433.e2. [Google Scholar] [CrossRef]
- Zhang, Y.; Tu, S.; Ji, X.; Wu, J.; Meng, J.; Gao, J.; Shao, X.; Shi, S.; Wang, G.; Qiu, J.; et al. Dubosiella newyorkensis modulates immune tolerance in colitis via the L-lysine-activated AhR-IDO1-Kyn pathway. Nat. Commun. 2024, 15, 1333. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Chen, Z.; Li, J.; Luo, J.; Castro-Martinez, F.; Wisniewski, J.; Cui, K.; Wang, Y.; Sun, J.; Ren, X.; et al. Gut-liver axis calibrates intestinal stem cell fitness. Cell 2024, 187, 914–930.e20. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Fan, H.; Zhang, Z.; Jiang, F.; Li, M.; Zhou, H.; Guo, W.; Zhang, Z.; Kang, Z.; Gui, Y.; et al. Berberine ameliorates DSS-induced intestinal mucosal barrier dysfunction through microbiota-dependence and Wnt/β-catenin pathway. Int. J. Biol. Sci. 2022, 18, 1381–1397. [Google Scholar] [CrossRef]
- van Es, J.H.; Jay, P.; Gregorieff, A.; van Gijn, M.E.; Jonkheer, S.; Hatzis, P.; Thiele, A.; van den Born, M.; Begthel, H.; Brabletz, T.; et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat. Cell Biol. 2005, 7, 381–386. [Google Scholar] [CrossRef]
- Yu, B.; Peng, X.H.; Wang, L.Y.; Wang, A.B.; Su, Y.Y.; Chen, J.H.; Zhang, X.W.; Zhao, D.Z.; Wang, H.; Pang, D.X.; et al. Abnormality of intestinal cholesterol absorption in Apc(Min/+) mice with colon cancer cachexia. Int. J. Clin. Exp. Pathol. 2019, 12, 759–767. [Google Scholar]
- Taylor, A.; Harker, J.A.; Chanthong, K.; Stevenson, P.G.; Zuniga, E.I.; Rudd, C.E. Glycogen Synthase Kinase 3 Inactivation Drives T-bet-Mediated Downregulation of Co-receptor PD-1 to Enhance CD8(+) Cytolytic T Cell Responses. Immunity 2016, 44, 274–286. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, J.; Liu, C.; Liao, K.; Gao, X.; Tang, R.; Fan, B.; Hong, Y.; Xiao, N.; Xiao, C.; et al. Glycogen synthase kinase 3 controls T-cell exhaustion by regulating NFAT activation. Cell Mol. Immunol. 2023, 20, 1127–1139. [Google Scholar] [CrossRef]
- Neumann, T.; Benajiba, L.; Göring, S.; Stegmaier, K.; Schmidt, B. Evaluation of Improved Glycogen Synthase Kinase-3α Inhibitors in Models of Acute Myeloid Leukemia. J. Med. Chem. 2015, 58, 8907–8919. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Ahmad, F.; Woodgett, J.; Force, T. The GSK-3 family as therapeutic target for myocardial diseases. Circ. Res. 2015, 116, 138–149. [Google Scholar] [CrossRef]
- Umbarkar, P.; Tousif, S.; Singh, A.P.; Anderson, J.C.; Zhang, Q.; Tallquist, M.D.; Woodgett, J.; Lal, H. Fibroblast GSK-3α Promotes Fibrosis via RAF-MEK-ERK Pathway in the Injured Heart. Circ. Res. 2022, 131, 620–636. [Google Scholar] [CrossRef]
- Xu, S.C.; Ma, Z.G.; Wei, W.Y.; Yuan, Y.P.; Tang, Q.Z. Bezafibrate Attenuates Pressure Overload-Induced Cardiac Hypertrophy and Fibrosis. PPAR Res. 2017, 2017, 5789714. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jeong, S.; Kim, S.W.; Kim, H.J.; Kim, D.Y.; Yook, T.H.; Yang, G. Indigo Naturalis in Inflammatory Bowel Disease: Mechanisms of action and insights from clinical trials. J. Pharmacopuncture 2024, 27, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, P.; Deng, J.; Chen, Y.; Wang, F.; Han, Y.; Wang, H.; Li, Y.; Fang, X.; Hui, J.; et al. Gut microbial metabolite TMAO impairs cognitive function and induces hippocampal synaptic plasticity decline through modulation of GSK-3β activity. Alzheimers Res. Ther. 2025, 17, 196. [Google Scholar] [CrossRef]
- Li, B.; Wei, Z.; Wang, Z.; Xu, F.; Yang, J.; Lin, B.; Chen, Y.; Wenren, H.; Wu, L.; Guo, X.; et al. Fusobacterium nucleatum induces oxaliplatin resistance by inhibiting ferroptosis through E-cadherin/β-catenin/GPX4 axis in colorectal cancer. Free Radic. Biol. Med. 2024, 220, 125–138. [Google Scholar] [CrossRef]
- Yan, K.S.; Janda, C.Y.; Chang, J.; Zheng, G.X.Y.; Larkin, K.A.; Luca, V.C.; Chia, L.A.; Mah, A.T.; Han, A.; Terry, J.M.; et al. Non-equivalence of Wnt and R-spondin ligands during Lgr5(+) intestinal stem-cell self-renewal. Nature 2017, 545, 238–242. [Google Scholar] [CrossRef]
- Wilson, C.L.; Heppner, K.J.; Labosky, P.A.; Hogan, B.L.; Matrisian, L.M. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc. Natl. Acad. Sci. USA 1997, 94, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Shen, Z.Y.; Zhan, Y.Z.; Feng, X.C.; Chen, K.L.; Li, Y.S.; Deng, H.J.; Pan, S.M.; Wu, D.H.; Ding, Y. CD36 inhibits β-catenin/c-myc-mediated glycolysis through ubiquitination of GPC4 to repress colorectal tumorigenesis. Nat. Commun. 2019, 10, 3981. [Google Scholar] [CrossRef]
- Moser, A.R.; Shoemaker, A.R.; Connelly, C.S.; Clipson, L.; Gould, K.A.; Luongo, C.; Dove, W.F.; Siggers, P.H.; Gardner, R.L. Homozygosity for the Min allele of Apc results in disruption of mouse development prior to gastrulation. Dev. Dyn. 1995, 203, 422–433. [Google Scholar] [CrossRef]
- Behrens, J. Control of beta-catenin signaling in tumor development. Ann. N. Y. Acad. Sci. 2000, 910, 21–33, Discussion 33–35. [Google Scholar] [CrossRef]
- Brischetto, C.; Krieger, K.; Klotz, C.; Krahn, I.; Kunz, S.; Kolesnichenko, M.; Mucka, P.; Heuberger, J.; Scheidereit, C.; Schmidt-Ullrich, R. NF-κB determines Paneth versus goblet cell fate decision in the small intestine. Development 2021, 148, dev199683. [Google Scholar] [CrossRef] [PubMed]
- Foerster, E.G.; Mukherjee, T.; Cabral-Fernandes, L.; Rocha, J.D.B.; Girardin, S.E.; Philpott, D.J. How autophagy controls the intestinal epithelial barrier. Autophagy 2022, 18, 86–103. [Google Scholar] [CrossRef]
- Xi, S.; Wang, Y.; Wu, C.; Peng, W.; Zhu, Y.; Hu, W. Intestinal Epithelial Cell Exosome Launches IL-1β-Mediated Neuron Injury in Sepsis-Associated Encephalopathy. Front. Cell Infect. Microbiol. 2021, 11, 783049. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.A.; Davis, B.M.; Albers, K.M. Epithelial-Neuronal Communication in the Colon: Implications for Visceral Pain. Trends Neurosci. 2020, 43, 170–181. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Li, X.; Zhan, J.; Pan, R.; Yang, Z.; Zhou, M.; Ma, L.; Liu, C. Glycogen Synthase Kinase 3 Is Essential for Intestinal Cell Niche and Digestive Function. Biology 2025, 14, 1551. https://doi.org/10.3390/biology14111551
Yang M, Li X, Zhan J, Pan R, Yang Z, Zhou M, Ma L, Liu C. Glycogen Synthase Kinase 3 Is Essential for Intestinal Cell Niche and Digestive Function. Biology. 2025; 14(11):1551. https://doi.org/10.3390/biology14111551
Chicago/Turabian StyleYang, Minggang, Xiaohui Li, Jiajia Zhan, Rui Pan, Ziye Yang, Mengsha Zhou, Lei Ma, and Chenfeng Liu. 2025. "Glycogen Synthase Kinase 3 Is Essential for Intestinal Cell Niche and Digestive Function" Biology 14, no. 11: 1551. https://doi.org/10.3390/biology14111551
APA StyleYang, M., Li, X., Zhan, J., Pan, R., Yang, Z., Zhou, M., Ma, L., & Liu, C. (2025). Glycogen Synthase Kinase 3 Is Essential for Intestinal Cell Niche and Digestive Function. Biology, 14(11), 1551. https://doi.org/10.3390/biology14111551



