Molluscs from South America to the World: Who and Where Are They?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Native Mollusk Species Detected Outside of South America
- 1.
- Pomacea glauca (Linnaeus, 1758) a gastropod native to South America and non-native to the Dominican Republic [111], was classified as Least Concern by Pastorino and Darrigan [112] and, due to this status and the lack of conservation concern, was not included in the final list of species in Table 2 (S. Thiengo, personal communication).
- 2.
- Naesiotus quitensis (L. Pfeiffer, 1848) (=Bulimus quitensis L. Pfeiffer, 1848), a terrestrial gastropod native to South America, was erroneously cited for Spain due to the presence of empty shells found in urban parks in Madrid [113]). This gastropod is native to Ecuador, where it is used in a traditional national dish called “ceviche de churos”, in which the snails are cooked and seasoned. Ecuadorians have actively exported this dish every year between October and December, typically with dead and cooked individuals of N. quitensis (M. Correoso, personal communication). Therefore, it is an accidental transport of shells via food exports.
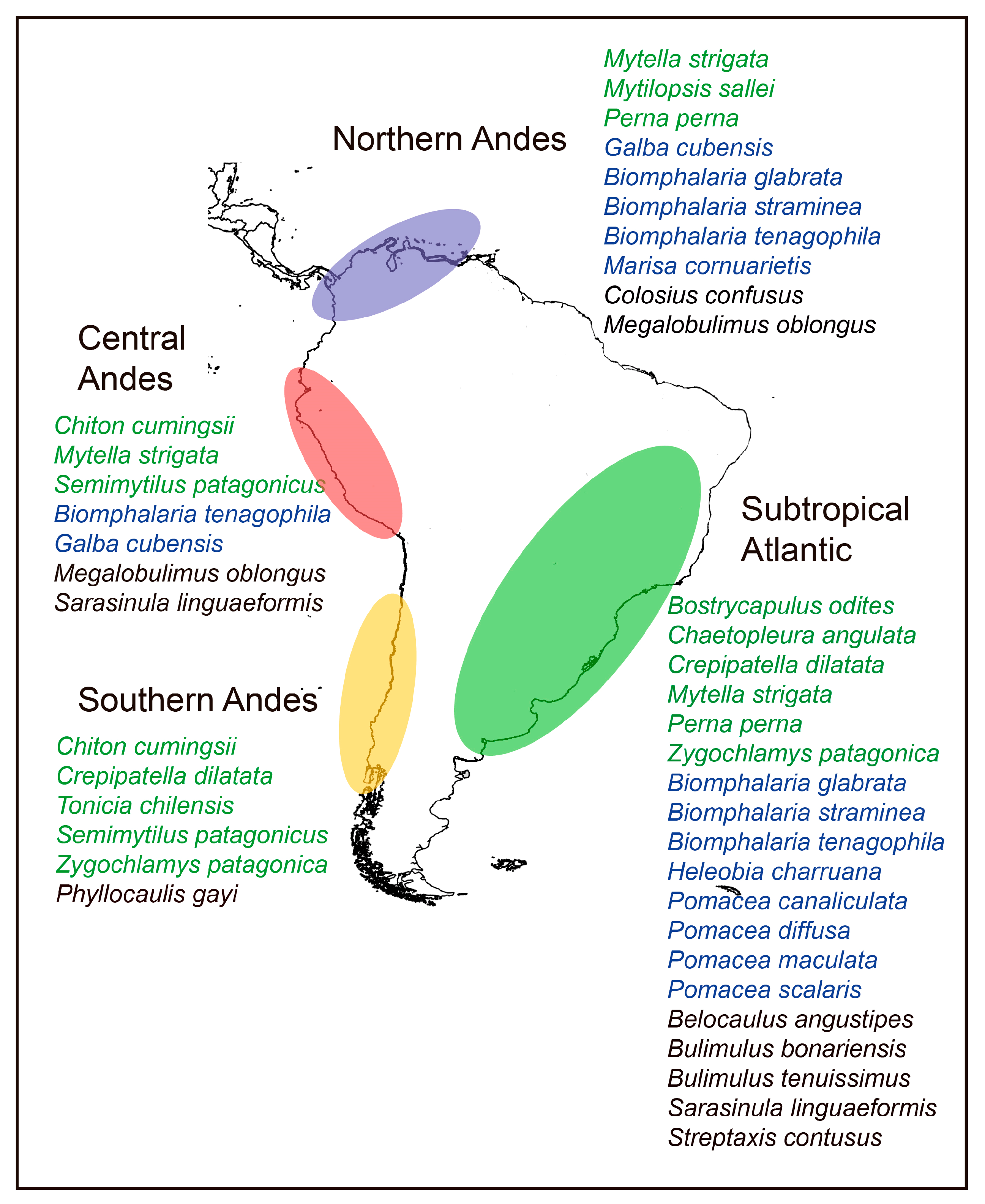
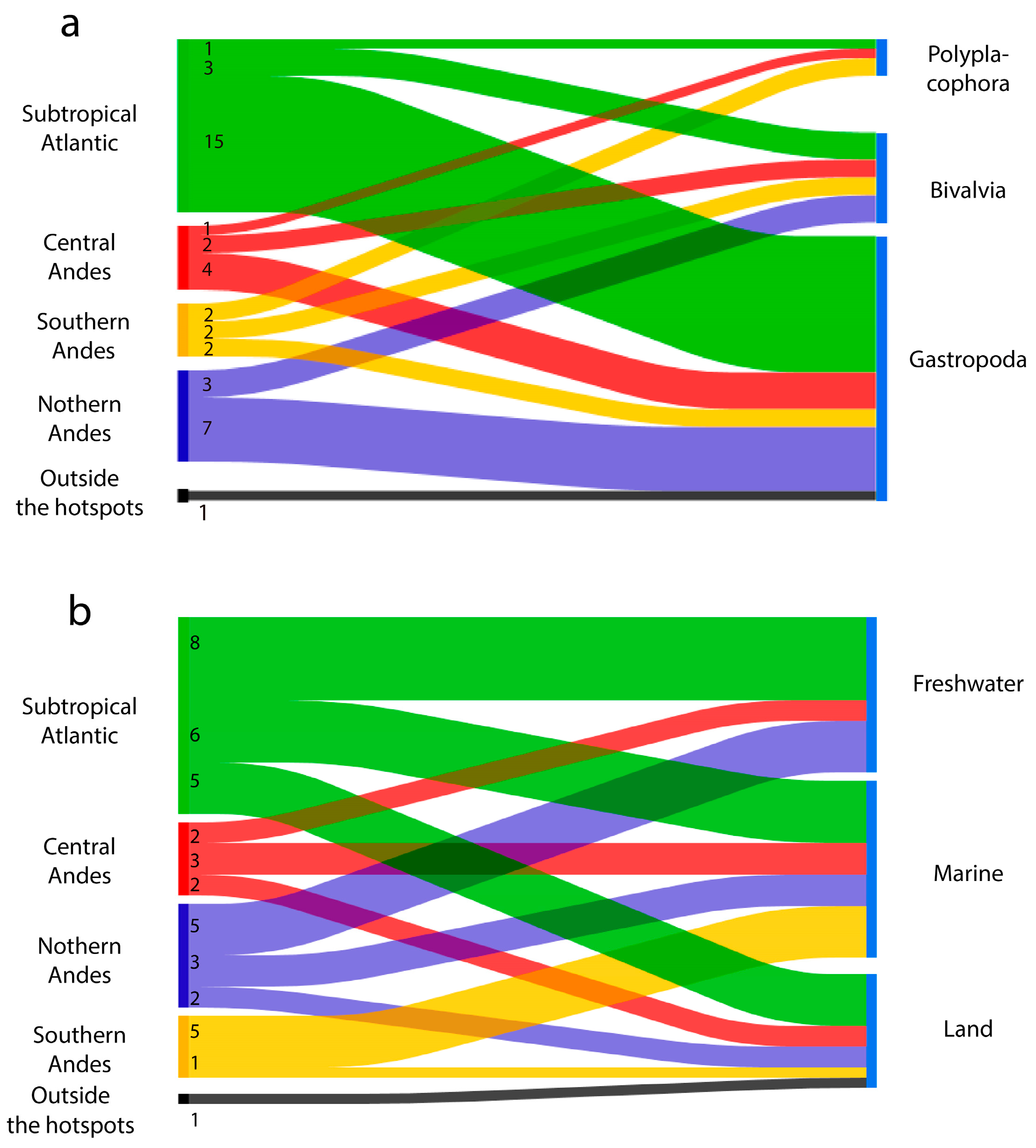
3.2. Areas of Origin of the Species
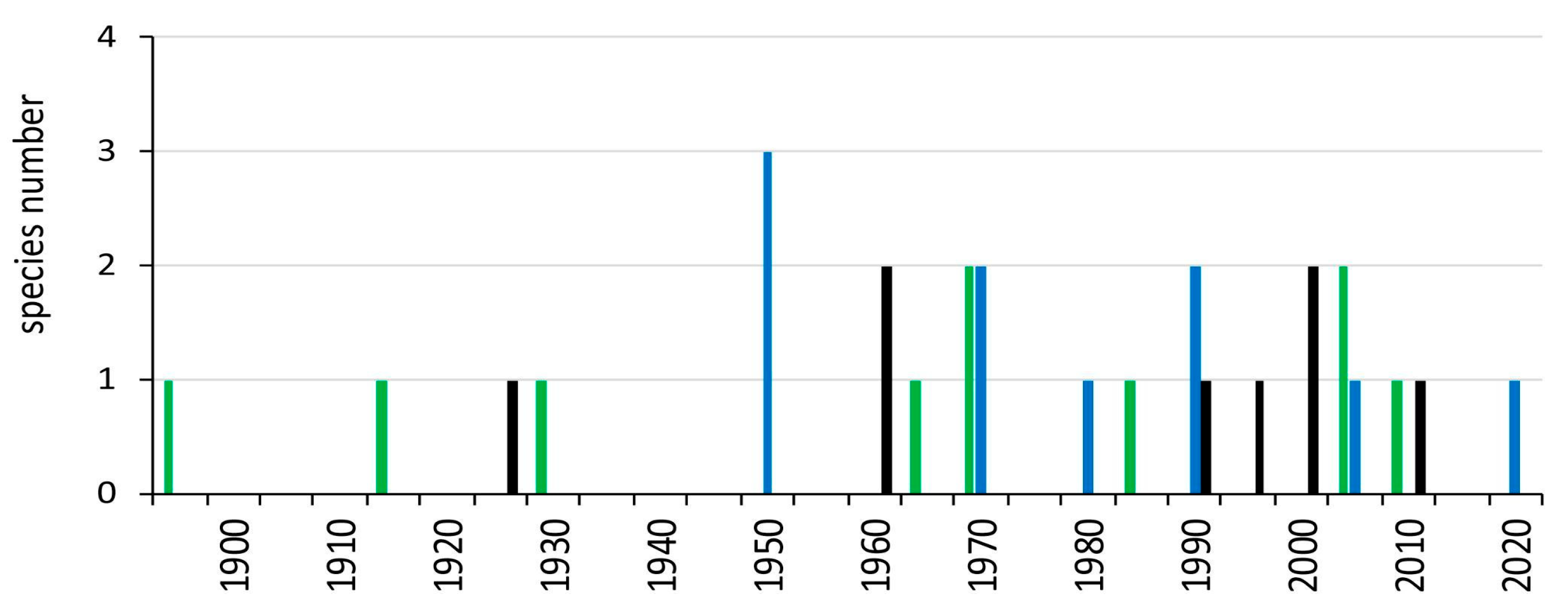
3.3. Detection Outside of South America
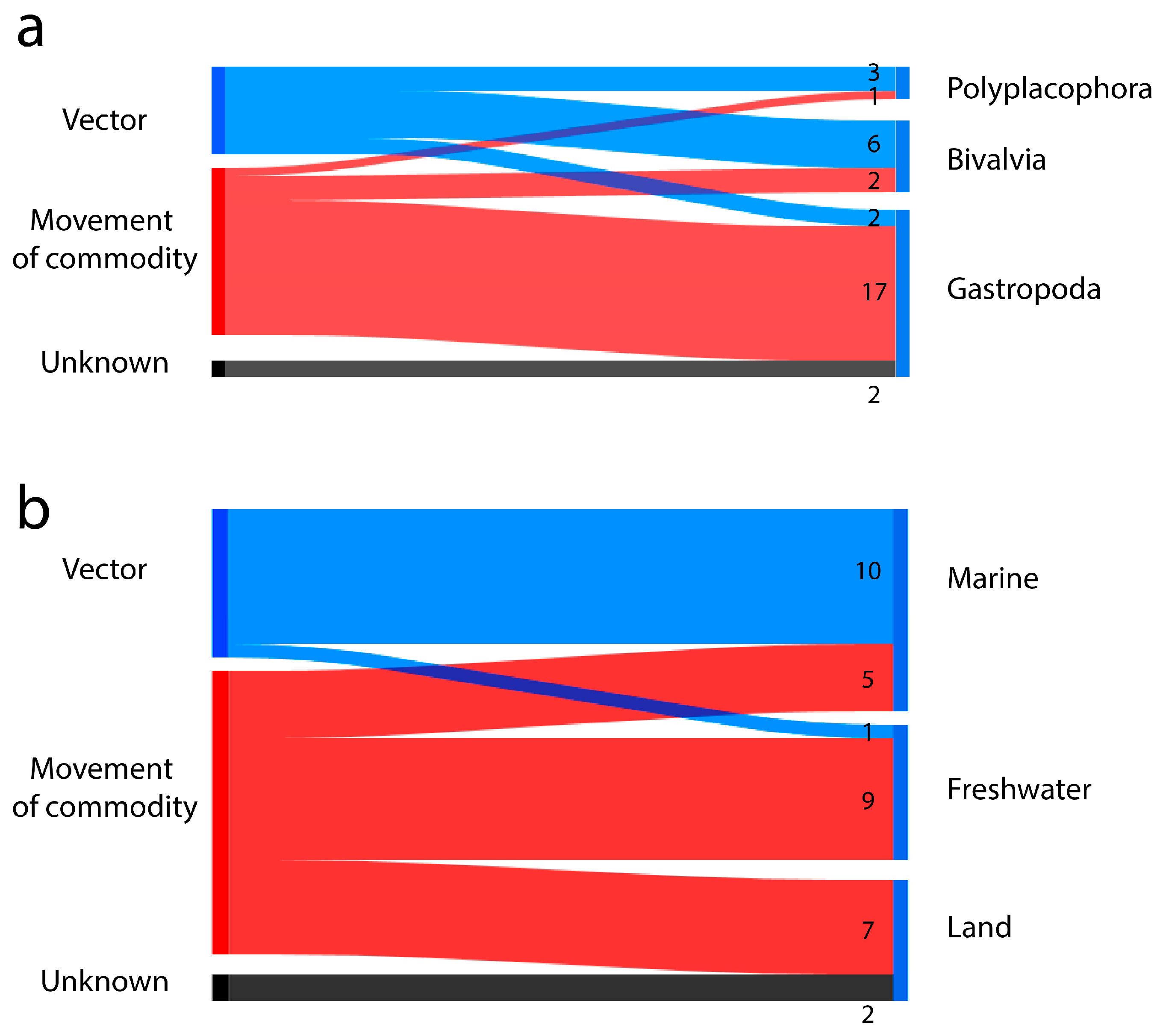
3.4. Introduction Mechanisms
3.5. Relationship Between Biological Characteristics and the Mechanisms of Introduction, Pathway Categories, and Subcategories
3.6. Areas of Introduction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dolan, E.J.; Soto, I.; Dick, J.T.A.; He, F.; Cuthbert, R.N. Riverine Barrier Removals Could Proliferate Biological Invasions. Glob. Change Biol. 2025, 31, e70093. [Google Scholar] [CrossRef]
- IPBES. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Díaz, S., Settele, J., Brondízio, E.S., Ngo, H.T., Guèze, M., Agard, J., Arneth, A., Balvanera, P., Brauman, K.A., Butchart, S.H.M., et al., Eds.; IPBES Secretariat: Bonn, Germany, 2019; p. 56. [Google Scholar]
- Van Dyke, F.; Lamb, R.L. The Anthropocene: Conservation in a Human-Dominated Nature. In Conservation Biology; Springer: Cham, Switzerland, 2020; pp. 45–65. [Google Scholar] [CrossRef]
- Moore, J.; Xerxenesky, A.; Silva, F.S.; Breda, T.; Veronesi, A.; Massunari, L. Antropoceno ou Capitaloceno? 1st ed.; Editora Elefante: São Paulo, Brazil, 2022. [Google Scholar]
- Porto-Gonçalves, C.W. Antropoceno ou Capitaloceno: A trama da vida na sociedade/a sociedade da trama da vida. Rev. Tamoios 2024, 20, 10–22. [Google Scholar] [CrossRef]
- Gauff, R.P.M.; Mugnai, F.; Mancuso, F.P.; Porri, F.; Costantini, F.; Airoldi, L. Distribution of native and nonindigenous bivalves and their settlers along an urban gradient. Front. Mar. Sci. 2024, 11, 1401552. [Google Scholar] [CrossRef]
- Nota, A.; Bertolino, S.; Tiralongo, F.; Santovito, A. Adaptation to bioinvasions: When does it occur? Glob. Change Biol. 2024, 30, e17362. [Google Scholar] [CrossRef] [PubMed]
- Bacher, S.; Ryan-Colton, E.; Coiro, M.; Cassey, P.; Galil, B.S.; Nuñez, M.A.; Ansong, M.; Dehnen-Schmutz, K.; Fayvush, G.; Fernandez, R.D.; et al. Global Impacts Dataset of Invasive Alien Species (GIDIAS). Sci. Data 2025, 12, 832. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, C.; Frossard, V.; Sharaf, N.; Bazin, S.; Bruel, R.; Sentis, A. Climate Impacts on Lake Food-Webs Are Mediated by Biological Invasions. Glob. Change Biol. 2025, 31, e70144. [Google Scholar] [CrossRef] [PubMed]
- Salinas, P.; Carlton, J.T.; Thiel, M.; Santibáñez, J.; Sáez, R.; Puga, A.; Munizaga, M.; Brante, A. Marine bioinvasions in Chile: A national research and conservation management agenda. Manag. Biol. Invasions 2023, 14, 595–618. [Google Scholar] [CrossRef]
- Robinson, D.G. Alien invasions: The effects of the global economy on non-marine gastropod introduction into the United States. Malacol. Phila. 1999, 4, 413–438. [Google Scholar]
- Cuthbert, R.N.; Bodey, T.W.; Briski, E.; Capellini, I.; Dick, J.T.A.; Kourantidou, M.; Ricciardi, A.; Pincheira-Donoso, D. Harnessing Traits to Predict Economic Impacts from Biological Invasions. Trends Ecol. Evol. 2025, 40, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; He, X.-Q.; Tu, Z.-Y.; Sun, D.; Wang, C.-S.; Shen, H.-B.; Pan, X. Bridging the gap between the public’s knowledge and detection of marine non-indigenous species through developing automated image classification applications for marine species. Front. Mar. Sci. 2025, 12, 1508851. [Google Scholar] [CrossRef]
- Haubrock, P.J.; Soto, I.; Cuthbert, R.N.; Kurtul, I.; Briski, E. Analysing factors underlying the reporting of established non-native species. Sci. Rep. 2025, 15, 12337. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Essl, F.; Evans, T.; Hulme, P.E.; Jeschke, J.M.; Kühn, I.; Kumschick, S.; Marková, S.; Mrugała, A.; Nentwig, W.; et al. A Unified Classification of Alien Species Based on the Magnitude of Their Environmental Impacts. PLoS Biol. 2014, 12, e1001850. [Google Scholar] [CrossRef]
- Mugnai, F. Exploring the Distribution and Underlying Drivers of Native and Non-Native Mussel and Oyster Species in Harbour Environment. Master’s Thesis, Università di Bologna, Bologna, Italy, 2017. [Google Scholar]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.A.; Sousa, R.; Bortolus, A.; Aldemir, C.; Angeli, N.F.; Błońska, D.; Briski, E.; Britton, J.R.; Cano-Barbacil, C.; Clark-Ginsberg, A.; et al. Parallels and discrepancies between non-native species introductions and human migration. Biol. Rev. 2025, 100, 1365–1395. [Google Scholar] [CrossRef]
- Carneiro, L.; Miiller, N.O.R.; Cuthbert, R.N.; Vitule, J.R.S. Biological Invasions Negatively Impact Global Protected Areas. Sci. Total Environ. 2024, 948, 174823. [Google Scholar] [CrossRef]
- Elton, C.S. The Ecology of Invasions by Animals and Plants; Methuen & Co. Ltd.: Methuen, UK, 1958; p. 181. [Google Scholar]
- Hicks, G. Turning the Tide: Is aquatic bioinvaders research heading in the right direction? Aquat. Invaders 2004, 15, 9–20. [Google Scholar]
- Briski, E.; Kotronaki, S.G.; Cuthbert, R.N.; Bortolus, A.; Campbell, M.L.; Dick, J.T.A.; Fofonoff, P.; Galil, B.S.; Hewitt, C.L.; Lockwood, J.L.; et al. Does Non-Native Diversity Mirror Earth’s Biodiversity? Glob. Ecol. Biogeogr. 2024, 33, 48–62. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Lopes-Lima, A.; Burlakova, L.; Douda, K.; Alonso, A.; Karatayev, A.; Ng, T.H.; Vinarski, M.; Zieritz, A.; Sousa, R. Non-native freshwater molluscs: A brief global review of species, pathways, impacts and management strategies. Hydrobiologia 2025, 852, 1005–1028. [Google Scholar] [CrossRef]
- Darrigran, G.; Agudo-Padrón, I.; Baez, P.; Belz, C.; Cardoso, F.; Carranza, A.; Collado, G.; Correoso, M.; Cuezzo, M.G.; Fabres, A.; et al. Non-native mollusks throughout South America: Emergent patterns in an understudied continent. Biol. Invasions 2020, 22, 853–871. [Google Scholar] [CrossRef]
- Darrigran, G.; Agudo-Padrón, I.; Baez, P.; Belz, C.; Cardoso, F.; Collado, G.; Correoso, M.; Cuezzo, M.G.; Damborenea, C.; Fabres, A.; et al. Species movements within biogeographic regions: Exploring the distribution of transplanted mollusc species in South America. Biol. Invasions 2022, 25, 673–691. [Google Scholar] [CrossRef]
- Carranza, A.; Agudo-Padrón, I.; Collado, G.A.; Damborenea, C.; Fabres, A.; Gutiérrez Gregoric, D.E.; Lodeiros, C.; Ludwig, S.; Pastorino, G.; Penchaszadeh, P.; et al. Socio-Environmental Impacts of Non-Native and Transplanted Aquatic Mollusc Species in South America: What Do We Really Know? Hydrobiologia 2023, 850, 1001–1020. [Google Scholar] [CrossRef]
- Darrigran, G.; Belz, C.; Carranza, A.; Collado, G.A.; Correoso, M.; Fabres, A.A.; Gutiérrez Gregoric, D.E.; Lodeiros, C.; Pastorino, G.; Penchaszadeh, P.E.; et al. Know About Non-Native, Invasive, and Transplanted Aquatic Mollusks in South America? Biology 2025, 14, 151. [Google Scholar] [CrossRef]
- MolluscaBase Eds. MolluscaBase. Available online: https://www.molluscabase.org (accessed on 2 September 2025).
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.; Underwood, E.C.; D’Amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on earth. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdana, Z.A.; Finlayson, M.A.; Halpern, B.S.; Jorge, M.A.; Lombana, A.L.; Lourie, S.A.; et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W.; et al. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. Bioscience 2008, 58, 403–414. [Google Scholar] [CrossRef]
- One Earth. One Earth Bioregions Framework. 2025. Available online: https://www.oneearth.org/bioregions-2023/ (accessed on 16 May 2019).
- Faulkner, K.T.; Hulme, P.E.; Pagad, S.; Wilson, J.R.U.; Robertson, M.P. Classifying the Introduction Pathways of Alien Species: Are We Moving in the Right Direction? NeoBiota 2020, 62, 143–159. [Google Scholar] [CrossRef]
- Kaas, P.; Knudsen, J. Lorentz Spengler’s descriptions of chitons (Mollusca: Polyplacophora). Zool. Meded. 1992, 66, 49–90. [Google Scholar]
- Hidalgo, J.G. Fauna Malacológica de España, Portugal y las Baleares: Moluscos Testáceos Marinos; Junta Para Ampliación de Estudios e Investigaciones Científicas: Madrid, Spain, 1917; Volume 30. [Google Scholar]
- Kaas, P.; Van Belle, R. Monograph of Living Chitons (Mollusca: Polyplacophora); 3 Suborder Ischnochitonina, Ischnochitonidae: Chaetopleurinae, & Ischnochitoninae (Pars) Additions to Vols 1 & 2; Brill Academic: Leiden, The Netherlands, 1987. [Google Scholar]
- Rolán, E. Moluscos de la Ría de Vigo 1. Gasterópodos; Thalassas, Anex. 1: Vigo, Spain; Universidade de Santiago de Compostela: Galicia, Spain, 1983; pp. 1–383. [Google Scholar]
- Carmona Zalvide, M.P.; García, F.J. Moluscos Poliplacóforos del litoral atlántico del sur de la Península Ibérica. Graellsia 2000, 56, 5–14. [Google Scholar] [CrossRef]
- Arias, A.; Richter, A.; Anadón, N. Estado actual de los moluscos marinos no autóctonos en aguas del Cantábrico. In Proceedings of the Notas Científicas EEI, 4º Congreso Nacional Sobre EEI, Pontevedra, Spain, 10–11 September 2012; pp. 99–103. [Google Scholar]
- Anadón, N.A. Poliplacóforos de las costas asturianas I: Estudios taxonómicos. Bol. Cienc. Nat. 1979, 24, 119–130. [Google Scholar]
- Chainho, P.; Fernandes, A.; Amorim, A.; Ávila, S.P.; Canning-Clode, J.; Castro, J.J.; Costa, M.J.; Cruz, T.; Gollasch, S.; Grazziotin-Soares, C.; et al. Non-Indigenous Species in Portuguese Coastal Areas, Coastal Lagoons, Estuaries and Islands. Estuar. Coast. Shelf Sci. 2015, 167, 199–211. [Google Scholar] [CrossRef]
- Arias, A.; Anadón, N. Tonicia atrata and Chiton cumingsii (Polyplacophora: Chitonidae): First records in European waters. Zootaxa 2013, 3626, 593–596. [Google Scholar] [CrossRef]
- Ibáñez, C.M.; Pardo-Gandarillas, M.C.; Méndez, M.A.; Sellanes, J.; Sigwart, J.D.; Sirenko, B. Phylogenetic position and morphological descriptions of Chiton species from the south-eastern Pacific. Zool. J. Linn. Soc. 2021, 191, 695–719. [Google Scholar] [CrossRef]
- Rubal, M.; Fernández-Gutiérrez, J.; Carreira-Flores, D.; Gomes, P.T.; Veiga, P. Abundance and distribution of non-indigenous Calyptraeidae gastropods along north and central Atlantic shores of Portugal. Cont. Shelf. Res. 2023, 269, 105138. [Google Scholar] [CrossRef]
- Rolán, E.; Trigo, J. Especies introducidas en Galicia: Algunos nuevos datos. Not. SEM 2007, 47, 37–38. [Google Scholar]
- Paredes, C.; Cardoso, F. La familia Calyptraidae en el Perú (Gastropoda: Caenogastropoda). Rev. Peru Biol. 2007, SN13, 177–184. [Google Scholar]
- Veliz, D.; Winkler, F.M.; Guisado, C.; Collin, R. A new species of Crepipatella (Gastropoda: Calyptraeidae) from northern Chile. Molluscan Res. 2012, 32, 145–153. [Google Scholar] [CrossRef]
- Collin, R.; Ramos-Esplá, A.; Izquierdo, A. Identification of the South Atlantic Spiny Slipper Limpet Bostrycapulus odites Collin, 2005 (Caenogastropoda: Calyptraeidae) on the Spanish Mediterranean Coast. Aquat. Invasions 2010, 5, 277–280. [Google Scholar] [CrossRef]
- López-Soriano, J.; Quiñonero-Salgado, S. Los moluscos alóctonos del Mediterráneo occidental: Estatus actual y tendencias futuras. Spira 2022, 8, 83–94. [Google Scholar]
- Galil, B.; Bogi, C. Mytilopsis sallei (Mollusca: Bivalvia: Dreissenidae) established on the Mediterranean coast of Israel. Mar. Biodivers. Rec. 2009, 2, E73. [Google Scholar] [CrossRef]
- Muhtadi, A.; Leidonald, R.; Rahmawati, A.; Kautsari, N. New record and population dynamics of the invasive bivalve Mytilopsis sallei (Récluz, 1849) in a tropical coastal lake from Indonesia. BioInvasions Rec. 2024, 13, 453–467. [Google Scholar] [CrossRef]
- Klangnurak, W.; Wangkulangkul, K. Mitochondrial DNA Analysis Suggests Invasion of Thailand Coast by a Single Species of Dreissenidae. Mytilopsis sallei. J. Fish. Environ. 2001, 45, 64–76. [Google Scholar]
- Cai, L.Z.; Hwang, J.S.; Dahms, H.U.; Fu, S.J.; Zhuo, Y.; Guo, T. Effect of the Invasive Bivalve Mytilopsis sallei on the Macrofaunal Fouling Community and the Environment of Yundang Lagoon, Xiamen, China. Hydrobiologia 2014, 741, 101–111. [Google Scholar] [CrossRef]
- Fernández-Garcés, R.; Rolán, E. Primera cita de Mytilopsis sallei (Bivalvia, Mytilidae) en aguas cubanas. Not. SEM 2006, 45, 50–51. [Google Scholar]
- Global Invasive Species Database (GISD). Species Profile Mytilopsis sallei. 2024. Available online: https://www.iucngisd.org/gisd/species.php?sc=1047 (accessed on 15 September 2025).
- Tan, K.; Morton, B. The invasive bivalve Mytilopsis sallei (Dreissenidae) introduced to Singapore and Johor Bahru, Malaysia. Raffles Bull. Zool. 2006, 54, 429–434. [Google Scholar] [CrossRef]
- Willan, R.C.; Russell, B.C.; Murfet, N.B.; Moore, K.L.; McEnnulty, F.R.; Horner, S.K.; Hewitt, C.L.; Dally, G.M.; Campbell, M.L.; Bourke, S.T. Outbreak of Mytilopsis sallei (Récluz, 1849) (Bivalvia: Dreissenidae) in Australia. Molluscan Res. 2000, 20, 25–30. [Google Scholar] [CrossRef]
- Joyce, P.W.S.; Lee, S.Y.; Falkenberg, L.J. First record of the alien invasive mussel Mytella strigata (Hanley, 1843) in Hong Kong. BioInvasions Rec. 2023, 12, 385–391. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, Q.; Liu, M.; Li, J.; Wang, S.; Zhang, J. Report on the invasive American brackish-water mussel Mytella strigata (Hanley, 1843) (Mollusca: Mytilidae) in Beibu Gulf. BioInvasions Rec. 2023, 12, 196–207. [Google Scholar] [CrossRef]
- Ravinesh, R.; Laju, R.L.; Edward, J.K.P.; Biju Kumar, A. Invasion of Alien Mussel Mytella strigata (Bivalvia: Mytilidae) in the Gulf of Mannar, India and Possible Threats to the Native Biodiversity. J. Aquat. Biol. Fish. 2023, 11, 28–34. [Google Scholar]
- Valentich-Scott, P.; Coan, E.V.; Zelaya, D. Bivalve Seashells of Western South America. Marine Bivalve Mollusks from Northern Perú to Isla Chiloé; Monographs 8; Santa Barbara Museum of Natural History: Santa Barbara, CA, USA, 2020; p. 594. [Google Scholar]
- Gardner, J.P.A.; Patterson, J.; George, S.; Edward, J.K.P. Combined evidence indicates that Perna indica Kuriakose and Nair 1976 is Perna perna (Linnaeus, 1758) from the Oman region introduced into southern India more than 100 years ago. Biol. Invasions 2016, 18, 1375–1390. [Google Scholar] [CrossRef]
- Galil, B.S.; Mienis, H.K.; Mendelson, M.; Gayer, K.; Goren, M. Here today, gone tomorrow—The Levantine population of the Brown mussel Perna perna obliterated by unprecedented heatwave. Aquat. Invasions 2022, 17, 174–185. [Google Scholar] [CrossRef]
- Douek, J.; Paz, G.; Gayer, K.; Mendelson, M.; Rinkevich, B.; Galil, B.S. An outbreak of Perna perna (Linnaeus, 1758) (Mollusca, Bivalvia, Mytilidae) in the Eastern Mediterranean. BioInvasions Rec. 2021, 10, 136–148. [Google Scholar] [CrossRef]
- Hicks, D.W.; Tunnell, J.W.; McMahon, R.F. Population dynamics of the nonindigenous brown mussel Perna perna in the Gulf of Mexico compared to other world-wide populations. Mar. Ecol. Prog. Ser. 2001, 211, 181–192. [Google Scholar] [CrossRef]
- Lourenço, C.R.; Nicastro, K.R.; Serrão, E.A.; Zardi, G.I. First record of the brown mussel (Perna perna) from the European Atlantic coast. Mar. Biodivers. Rec. 2012, 5, e39. [Google Scholar] [CrossRef]
- Sokołowski, A.; Bawazir, A.S.; Sokołowska, E.; Wołowicz, M. Seasonal variation in the reproductive activity, physiological condition and biochemical components of the brown mussel Perna perna from the coastal waters of Yemen (Gulf of Aden). Aquat. Living Resour. 2010, 23, 177–186. [Google Scholar] [CrossRef]
- Ma, K.C.K.; Zardi, G.I.; McQuaid, C.D.; Nicastro, K.R. Historical and contemporary range expansion of an invasive mussel, Semimytilus algosus, in Angola and Namibia despite data scarcity in an infrequently surveyed region. PLoS ONE 2020, 15, e0239167. [Google Scholar] [CrossRef]
- Zeeman, Z.; Branch, G.M.; Pillay, D. Comparisons of life-history traits of the alien invasive Semimytilus algosus and three other mytilid mussels on the West Coast of South Africa. Mar. Ecol. Prog. Ser. 2018, 607, 113–127. [Google Scholar] [CrossRef]
- Signorelli, J.H.; Pastorino, G. Correction of the authorship and date of Semimytilus patagonicus (Hanley, 1843). Arch. Molluskenkd. 2021, 150, 173. [Google Scholar] [CrossRef] [PubMed]
- Repetto, G. Nuovo ritrovamento in Mediterraneo di Chlamys lischkei (Bunker, 1850). Boll. Malacol. 1989, 25, 261–262. [Google Scholar]
- Crocetta, F.; Renda, W. Further Record of Chlamys lischkei (Bivalvia: Pectinoidea) in the Mediterranean Sea. Mar. Biodivers. Rec. 2008, 1, e57. [Google Scholar] [CrossRef]
- Mannino, A.M.; Parasporo, M.; Crocetta, F.; Balistreri, P. An updated overview of the marine alien and cryptogenic species from the Egadi Islands Marine Protected Area (Italy). Mar. Biodivers. 2017, 47, 469–480. [Google Scholar] [CrossRef]
- Cumming, R.A.; Nikula, R.; Spencer, H.G.; Waters, J.M. Transoceanic Genetic Similarities of Kelp-Associated Sea Slug Populations: Long-Distance Dispersal via Rafting? J. Biogeogr. 2014, 41, 2357–2370. [Google Scholar] [CrossRef]
- Pastorino, G. Revision of the genera Pareuthria Strebel, 1905, Glypteuthria Strebel, 1905 and Meteuthria Thiele, 1912 (Gastropoda: Buccinulidae) with the description of three new genera and two new species from Southwestern Atlantic waters. Zootaxa 2016, 4179, 301–344. [Google Scholar] [CrossRef]
- Schniebs, K.; Glöer, P.; Quiñonero-Salgado, S.; Lopez-Soriano, J.; Hundsdoerfer, A.K. The first record of Galba cubensis (L. Pfeiffer, 1839) (Mollusca: Gastropoda: Lymnaeidae) from open fields of Europe. Folia Malacol. 2018, 26, 3–15. [Google Scholar] [CrossRef]
- van Haaren, T.; Worsfold, T.M.; Stelbrink, B.; Collado, G.A.; Gonçalves, I.C.; Serra, W.S.; Scarabino, F.; Gittenberger, A.; Gittenberger, E. Heleobia charruana (Gastropoda, Truncatelloidea, Cochliopidae), a South American brackish water snail in northwest European estuaries. Basteria 2021, 85, 82–91. [Google Scholar]
- Yousif, F.; Haroun, N.; Ibrahim, A.; El-Bardicy, S. Biomphalaria glabrata: A new threat for schistosomiasis transmission in Egypt. J. Egypt. Soc. Parasitol. 1996, 26, 191–205. [Google Scholar]
- Majoros, G.; Fehér, Z.; Deli, T.; Földvári, G. Establishment of Biomphalaria tenagophila Snails in Europe. Emerg. Infect. Dis. 2008, 14, 1012–1814. [Google Scholar] [CrossRef] [PubMed]
- Pointier, J.P.; David, P.; Jarne, P. Biological invasions: The case of planorbid snails. J. Helminthol. 2005, 79, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Meier-Brook, C. A snail intermediate host of Schistosoma mansoni introduced into Hong Kong. Bull. World Health Organ. 1974, 51, 661. [Google Scholar] [PubMed]
- Liu, Y.Y.; Wang, Y.X.; Zhang, W.Z. The discovery of Biomphalaria straminea (Dunker), an intermediate host of Schistosoma mansoni, from China. Acta Zootaxonomica Sin. 1982, 7, 256. (In Chinese) [Google Scholar]
- Arias, A.; Torralba-Burrial, A. First European record of the giant ramshorn snail Marisa cornuarietis (Linnaeus, 1758) (Gastropoda: Ampullariidae) from northern Spain. Limnetica 2014, 33, 65–72. [Google Scholar] [CrossRef]
- Frisóczki, B.; Vig, Z.S.; Horotán, K.; Varga, J. A new alien snail species from the Eger stream, Hungary (Mollusca, Ampullariidae). Opusc. Zool. Budapest 2016, 47, 197–201. [Google Scholar] [CrossRef]
- Cowie, R.H.; Hayes, K.A.; Strong, E.E.; Thiengo, S.C. Non-Native Apple Snails: Systematics, Distribution, Invasion History and Reasons for Introduction. In Biology and Management of Invasive Apple Snails; Joshi, R.J., Cowie, R.H., Sebastián, L.S., Eds.; Philippine Rice Research Institute: Nueva Ecija, Philippines, 2017; pp. 3–32. [Google Scholar]
- Barker, G.M. Marisa cornuarietis (giant ramshorn). CABI Agric. Biosci. Compend. 2022, 32526. [Google Scholar] [CrossRef]
- Horgan, F.G.; Stuart, A.M.; Kudavidanage, E.P. Impact of invasive apple snails on the functioning and services of natural and managed wetlands. Acta Oecol. 2014, 54, 90–100. [Google Scholar] [CrossRef]
- Al-Khafaji, K.K.; Al-Maliki, G.M.; Al-Shemary, A.J. The ecological habitats and density of invasive apple snail Pomacea canaliculata Lamarck, 1822 (Gastropoda: Ampullariidae) at the banks of Shatt Al-Arab River, Basrah, Iraq. J. Basrah Res. 2016, 42, 10–20. [Google Scholar]
- Buddie, A.G.; Rwomushana, I.; Offord, L.C.; Kibet, S.; Makale, F.; Djeddour, D.; Cafa, G.; Vincent, K.K.; Muvea, A.M.; Chacha, D.; et al. First Report of the Invasive Snail Pomacea canaliculata in Kenya. CABI Agric. Biosci. 2021, 2, 11. [Google Scholar] [CrossRef]
- Komalamisra, C.; Nuamtanong, S.; Dekumyoy, P. Pila ampullacea and Pomacea canaliculata, as new paratenic hosts of Gnathostoma spinigerum. Southeast Asian J. Trop. Med. Public Health 2009, 40, 243–246. [Google Scholar] [PubMed]
- Yang, T.-B.; Wu, Z.-D.; Lun, Z.-R. The apple snail Pomacea canaliculata, a novel vector of the rat lungworm, Angiostrongylus cantonensis: Its introduction, spread, and control in China. Hawai’i J. Med. Public Health 2013, 72, 23–25. [Google Scholar]
- Mochida, O. Spread of freshwater snails (Pilidae, Mollusca) from Argentina to Asia. Micrones. Suppl. 1991, 3, 51–62. [Google Scholar]
- Bohatá, L.; Patoka, J. List of Pet-Traded Terrestrial Gastropods Based on Data from the Czech Republic. In Proceedings of the 11th Workshop on Biodiversity, Jevany, Czech Republic, 10 July 2019. [Google Scholar]
- Bohatá, L.; Patoka, J. Invasion Potential of Ornamental Terrestrial Gastropods in Europe Based on Climate Matching. Diversity 2023, 15, 272. [Google Scholar] [CrossRef]
- Rosenberg, G.; Muratov, I.V. Status Report on the Terrestrial Mollusca of Jamaica. Proc. Acad. Nat. Sci. Phila. 2006, 155, 117–161. [Google Scholar] [CrossRef]
- Rabelo, M.M.; Dimase, M.; Paula-Moraes, S.V. Ecology and management of the invasive land snail Bulimulus bonariensis (Rafinesque, 1833) (Stylommatophora: Bulimulidae) in row crops. Front. Insect Sci. 2022, 2, 1056545. [Google Scholar] [CrossRef]
- Robinson, D.G.; Slapcinsky, J. Recent introductions of alien land snails into North America. Am. Malacol. Bull. 2005, 20, 89–93. [Google Scholar]
- Hoffmann, H. Die Vaginuliden. Ein Beitrag zur Kenntnis ihre Biologie, Anatomie, Systematik, geographischen Verbreitung und Phylogenie. Jenaische Z. Naturwiss. 1925, 61, 1–374. [Google Scholar]
- Naranjo-García, E.; Thomé, J.W.; Castillejo, J. A review of the Veronicellidae from Mexico (Gastropoda: Soleolifera). Rev. Mex. Biodivers. 2007, 78, 41–50. [Google Scholar]
- Baker, H.B. North American Veronicellidae. Proc. Acad. Nat. Sci. Phila. 1925, 77, 157–184. [Google Scholar]
- Gomes, S.R.; Robinson, D.G.; Zimmerman, F.J.; Obregón, O.; Barr, N.B. Morphological and molecular analyses of the Andean slugs Colosius confusus n. sp., a newly recognized pest of cultivated flowers and coffee from Colombia, Ecuador and Peru. Malacologia 2013, 56, 1–30. [Google Scholar] [CrossRef]
- Dundee, D.S. Observation on the Veronicellid slugs of the Southern United Stated. Nautilus 1977, 91, 108–114. [Google Scholar]
- Walls, J.G. Just a plain black slug: Belocaulus angustipes. Am. Conchol. 2009, 37, 28–29. [Google Scholar]
- Caballero, R.; Thomé, J.W.; Andrews, K.L.; Rueda, A. Babosas de Honduras (Soleolifera: Veronicellidae): Biología, ecología, distribución, descripción, importancia económica, y claves para su identificación. Ceiba 1991, 32, 107–126. [Google Scholar]
- Rangel-Ruiz, L.J.; Gamboa-Aguilar, J. Estructura de la comunidad y dinámica poblacional de gasterópodos en una zona enzoótica de fasciolosis en Tabasco, México. Acta Zool. Mex. 2005, 21, 79–85. [Google Scholar] [CrossRef]
- Gutiérrez Gregoric, D.E.; Daglio, E.D.; de Lucía, M.; Robinson, D.G.; Darrigran, G. Land slugs in plant nurseries, a potential cause of dispersal in Argentina. Arx. Miscel·Lània Zoològica 2020, 18, 173–181. [Google Scholar] [CrossRef]
- Rambo, P.R.; Agostini, A.A.; Graeff-Teixeira, C. Abdominal angiostrongylosis in southern Brazil-prevalence and parasitic burden in mollusc intermediate hosts from eighteen endemic foci. Mem. Inst. Oswaldo Cruz 1997, 92, 9–14. [Google Scholar] [CrossRef]
- Thomé, J.W. Annotated and illustrated preliminary list of the Veronicellidae (Mollusca: Gastropoda) of the Antilles, and Central and North America. J. Med. Appl. Malacol. 1989, 1, 11–28. [Google Scholar]
- Cowie, R.H. Patterns of Introduction of Non-Indigenous Non-Marine Snails and Slugs in the Hawaiian Islands. Biodivers. Conserv. 1998, 7, 349–368. [Google Scholar] [CrossRef]
- Chase, R.; Robinson, D.G. The Uncertain History of Land Snails on Barbados: Implications for Conservation. Malacologia 2001, 43, 33–57. [Google Scholar]
- Agudo-Padrón, I. Moluscos exóticos no marinos “introducidos” en la isla caribeña de La Española (Hispaniola), Grandes Antillas: Una aproximación a su conocimiento. Rev. Minerva 2023, 3, 129–138. [Google Scholar] [CrossRef]
- Pastorino, G.; Darrigran, G. Pomacea glauca; The IUCN Red List of Threatened Species 2011: E.T189046A8684331; Calling All Nature Lovers: Somerset, UK, 2011. [Google Scholar]
- Ramos Sánchez, J.M.; Quiñonero-Salgado, S.; López-Soriano, J. First citation for an exotic Bulimulidae species in Europe. Folia Conchyliol. 2018, 47, 11–14. [Google Scholar]
- Marcolin, F.; Mammola, S.; Alba, R.; Segurado, P.; Reino, L.; Chamberlain, D. Socio-Economic Status and Non-Native Species Drive Bird Ecosystem Service Provision in Urban Areas. Glob. Change Biol. 2025, 31, e70311. [Google Scholar] [CrossRef] [PubMed]
- IPCC, 2014: Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)]. IPCC, Geneva, Switzerland, 151p. Available online: https://archive.ipcc.ch/pdf/assessment-report/ar5/syr/SYR_AR5_FINAL_full_wcover.pdf (accessed on 31 October 2025).
- Darrigran, G.; Damborenea, C. A bioinvasion history in South America. Limnoperna fortunei (Dunker, 1857), the golden mussel. Am. Malacol. Bull. 2005, 20, 105–112. [Google Scholar]
- Raya, A. ¿Qué es la Globalización? El Orden Mundial (EOM). 2021. Available online: https://elordenmundial.com/que-es-globalizacion/ (accessed on 31 October 2025).
- Mahapatra, B.B.; Das, N.K.; Jadhav, A.; Roy, A.; Aravind, N.A. Global freshwater mollusc invasion: Pathways, potential distribution, and niche shift. Hydrobiologia 2025, 852, 1431–1450. [Google Scholar] [CrossRef]
- Bonfim, M.; Bunson, S.L.; Sellers, A.J.; Torchin, M.E.; Ruiz, G.M.; Freestone, A.L. Biogeographic Variation in Environmental and Biotic Resistance Modifies Predicted Risk of Marine Invasions by Ships. Front. Mar. Sci. 2024, 11, 1374887. [Google Scholar] [CrossRef]
- Keck, F.T.; Alther Romano, C.; Barouillet, R.; Blackman, E.; Capo, T.; Chonova, T.; Couton, M.; Fehlinger, L.; Kirschner, D.; Knüsel, M.; et al. The global human impact on biodiversity. Nature 2025, 641, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Carlton, J.; Ruiz, G. The Vectors of Invasions by Alien Species. 2000. Available online: https://www.researchgate.net/publication/242078026_THE_VECTORS_OF_INVASIONS_BY_ALIEN_SPECIES (accessed on 31 October 2025).
- Dalton, M.C.; Penchaszadeh, P.E. Direct development in the intertidal South American shell-less Onchidella marginata (Couthouy in Gould, 1852) (Gastropoda: Heterobranchia: Onchidiidae). Molluscan Res. 2019, 39, 373–376. [Google Scholar] [CrossRef]
- Jayachandran, P.R.; Aneesh, B.P.; Oliver, P.G.; Philomina, J.; Jima, M.; Harikrishnan, K.; Nandan, S.B. First record of the alien invasive biofouling mussel Mytella strigata (Hanley, 1843) (Mollusca: Mytilidae) from Indian waters. BioInvasions Rec. 2019, 8, 4. [Google Scholar] [CrossRef]
- Rolán, E.; Horro, J. Crepipatella dilatata (Gastropoda, Calyptraeidae) nueva especie introducida en aguas gallegas. Noticiario de la Sociedad Española de Malacología 2005, 44, 60–63. [Google Scholar]
- Vallejo, B., Jr.; Conejar-Espedido, J.; Manubag, L.; Artiaga, K.C.C.; Damatac II, A.M.; Imperial, I.C.V.J.; Itong, T.A.B.; Fontanilla, I.K.; Cao, E.P. First record of the Charru mussel Mytella charruana d’Orbigny, 1846 (Bivalvia: Mytilidae) from Manila Bay, Luzon, Philippines. BioInvasions Rec. 2017, 6, 49–55. [Google Scholar] [CrossRef]
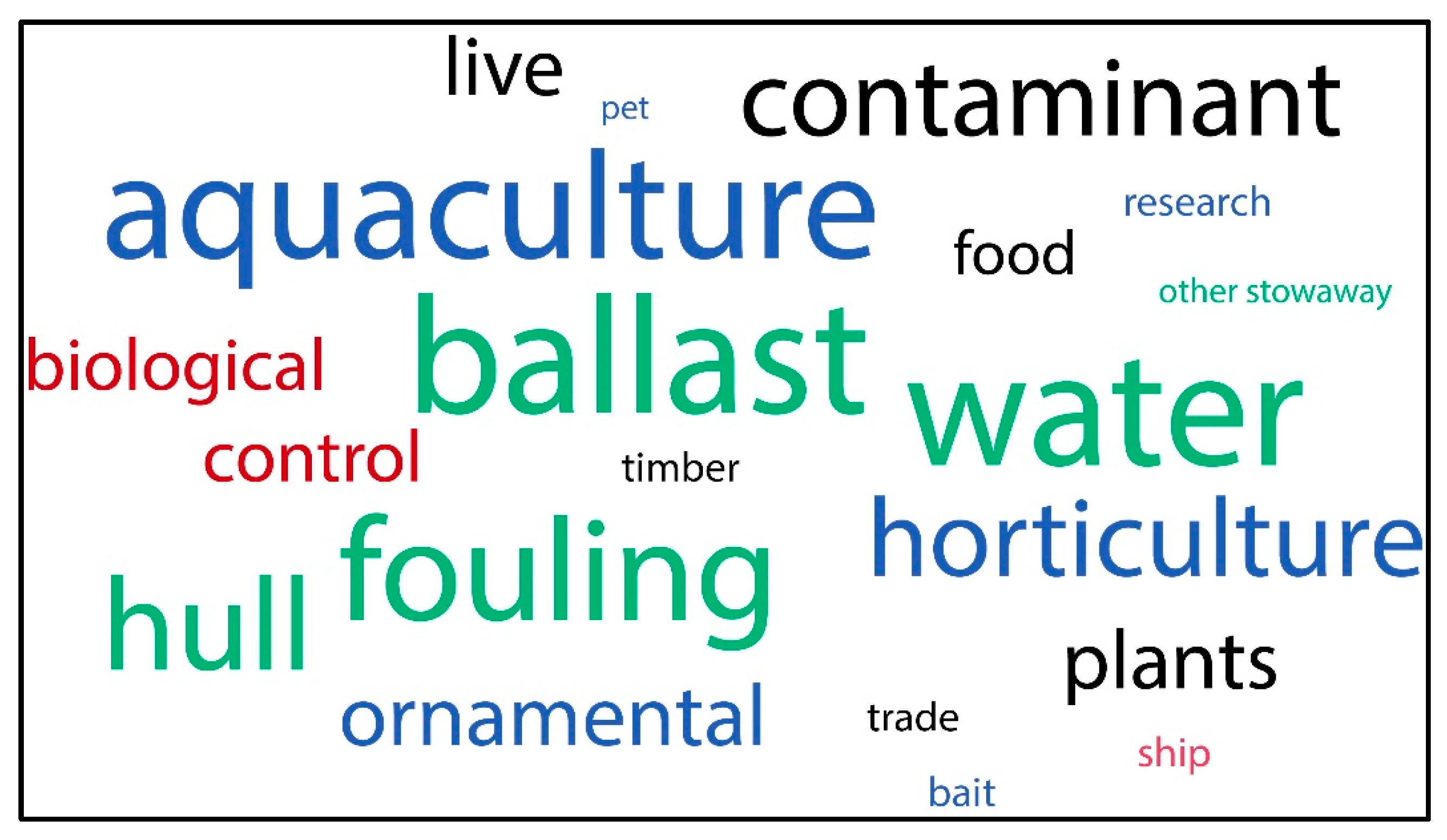
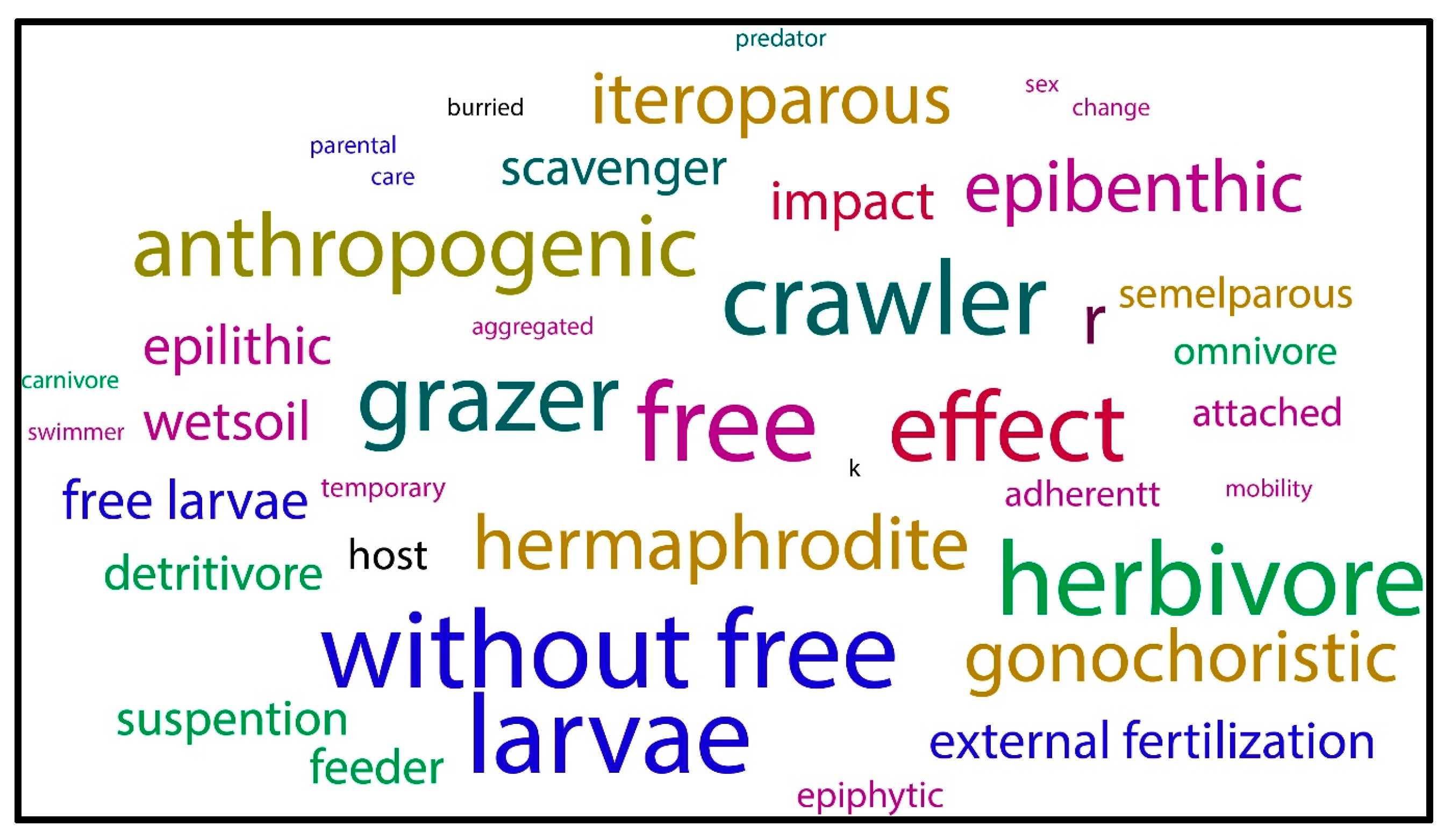
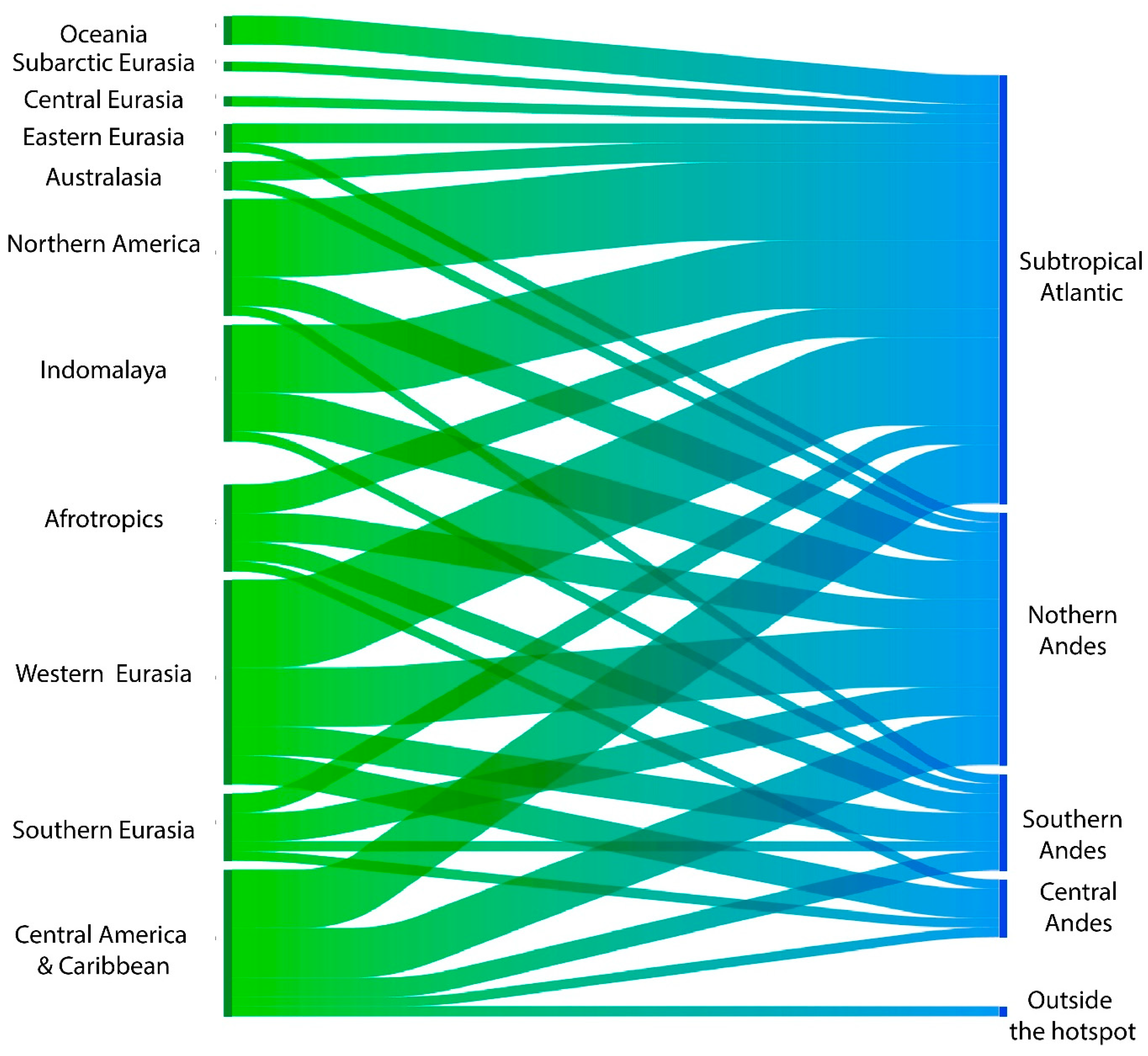
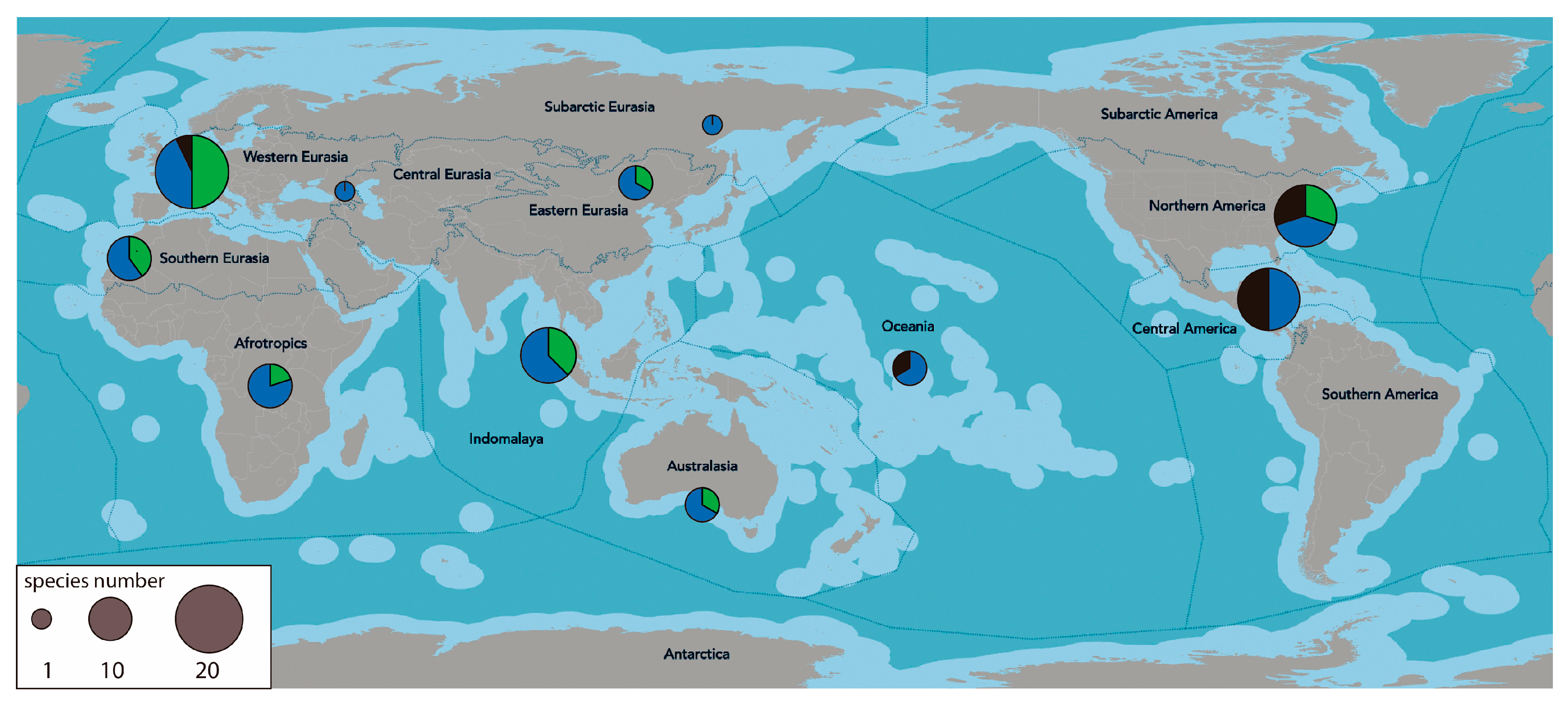
| Species and Species ID | Ecoregions in South America | Place and Date of Entry and Reference | Introduction Mechanism, Pathway Category, and Subcategory | Impacts/Effects/Remarks | |
|---|---|---|---|---|---|
| Polyplacophora | Chitonida | Chaetopleuridae | |||||
| Chaetopleura angulata (Spengler, 1797) https://www.molluscabase.org/aphia.php?p=taxdetails&id=848025, accessed on 1 April 2025 | 18, 19, 21, 22 | Atlantic coast of the Iberian Peninsula, XIX century or before [34,35,36]; Bay of Biscay and south of Portugal [36] to the Straits of Gibraltar [35,37,38]; Cantabrian [39] and Asturias [40] | Vector, stowaway, ship excluding ballast water and hull fouling [34] Ballast water? [41] | Impacts: - Effects: - Remarks: Possibly transported by Spanish or Portuguese merchant vessels to the Iberian coasts, due to the animal’s ability to climb anchor chains rather rapidly [34]. | |
| Polyplacophora | Chitonida | Chitonidae | |||||
| Chiton cumingsii (Frembly, 1827) https://www.molluscabase.org/aphia.php?p=taxdetails&id=386774, accessed on 1 April 2025 | 9–14 | Las Palmas Port, Canary Islands, 28°06′ N, 15°25′ W, 2012 [42,43] | Vector, stowaway, hull fouling [42] | Impacts: - Effects: - Remarks: - | |
| Tonicia chilensis (Frembly, 1827) https://www.molluscabase.org/aphia.php?p=taxdetails&id=386329, accessed on 1 April 2025 | 12–14, 17 | Asturias and Galicia, around 1970 (Eo estuary, 43°28′ N, 7°03′ W; Sado estuary, 1985, 43°28′ N, 7°03′ W, Aviles Port 43°33′ N, 5.55′ W) [42] | Vector, stowaway, hull fouling, ballast water (?) Movement of commodity, escape, aquaculture (?) [41,42] | Impacts: - Effects: - Remarks: The northern ecotype of T. chilensis inhabits the coast of central Chile (~33–39°S), whereas the southern is found from Puerto Mont (~41°S) to Tierra del Fuego (~53°S). | |
| Gastropoda | Littorinimorpha | Calyptraeidae | |||||
| Crepipatella dilatata (Lamarck, 1822) https://www.molluscabase.org/aphia.php?p=taxdetails&id=234137, accessed on 1 April 2025 | 12–19 | Galician in Ría de Aldán, 2005; north coast of Iberian Peninsula, 2018; in Ebro delta (Mediterranean), 2014 [44] | Movement of commodity, escape, aquaculture [44] | Impacts: - Effects: Fouling (at a shellfish treatment plant in Palmeiras [45]). Remarks: The name Crepipatella dilatata was commonly used in Peru [46], but according to Veliz et al. [47], it refers to C. peruviana. | |
| Bostrycapulus odites (Collin, 2005) https://www.molluscabase.org/aphia.php?p=taxdetails&id=457048, accessed on 1 April 2025 | 18, 19, 21, 22 | Alicante Harbour, 1970s [48] | Vector, stowaway, ballast water [49] | Impacts: - Effects: - Remarks: Referred to as Crepidula calyptraeformis and C. aculeata [48]. Common and widely distributed in Alicante Harbour in 2002 and 2007, but has not expanded outside the harbour [48]. | |
| Bivalvia | Mytilida | Dreissenidae | |||||
| Mytilopsis sallei (Récluz, 1849) https://www.molluscabase.org/aphia.php?p=taxdetails&id=397147, accessed on 1 April 2025 | 1, 7, 4 | India (approx. 1967); Japan, 1974; Taiwan, 1977; Hong Kong, approx. 1980; Singapore and Malaysia, 1984?; China, approx. 1990; Thailand, between 1990 and 2000; Australia (1999); Egypt (2006); Philippines, 2008; Israel, approx. 2009 [50,51,52,53,54,55,56,57] | Vector, stowaway, hull fouling | Impacts: Displacement of native species. Effects: On aquaculture (of shrimp, by water clarification). Remarks: Highly opportunistic species and can survive under extreme and wide-ranging conditions. | |
| Bivalvia | Mytilida | Mytilidae | |||||
| Mytella strigata Hanley, 1843 [= Mytella charruana (d’Orbigny, 1846)] https://www.molluscabase.org/aphia.php?p=taxdetails&id=1458663, accessed on 10 April 2025 | 1–4, 7–10, 19, 21–23, 25–27 | Florida, 2006; Philippines, 2014; Singapore 2016; Thailand, 2018; Cochin and Mannar Golf India and Sri Lanka, 2019; southwest coast of Taiwan, 2021; Beibu Gulf, 2021; Hong Kong, 2022 [58,59] | Vector, stowaway, ballast water | Impacts: Displacement of native species (compete with native species for resources, alter habitat structures, and disrupt local food chains). Effects: On aquaculture (of shrimp, by water clarification) [59]. Remarks: Often thriving in new environments without natural predators or competitors [60]. For native distribution, see Valentich-Scott et al. [61]. | |
| Perna perna (Linnaeus, 1758) https://www.molluscabase.org/aphia.php?p=taxdetails&id=140483, accessed on 12 April 2025 | 1, 4, 7, 15, 19, 21–23 | South India and Sri Lanka, approx. 1917 [62]; Israel, 1965 [63]; Mozambique, 1970 [64]; Arkansas, Texas, 1990; Golf of Mexico, approx. 1990 [65]; Portugal, 2011 [66]; from Ludertz to Gibraltar Strait, Tunisia, 2020; Israel, 2020 [64]; Yemen [67] | Vector, movement of commodity, stowaway, escape, hull fouling, ballast water, aquaculture [64] | Impacts: - Effects: Fouling (on navigation buoys and pipelines); food resource. Remarks: - | |
| Semimytilus patagonicus (Hanley, 1843) https://www.molluscabase.org/aphia.php?p=taxdetails&id=1518612, accessed on 15 April 2025 | 9–13, 18, 26, 28 | Namibia, Walvis Bay 1928–1929; Northern Angola, 1969 Moçâmedes [68] to Elands Bay Northern South Africa, 2009 or sometime earlier [69] | Vector, stowaway, hull fouling? movement of commodity, escape, aquaculture? | Impacts: - Effects: Potential on aquaculture. Remarks: Originally described from Río Negro, Northern Patagonia, Argentina, where the population is no longer present. Alternatively, the name S. algosus has been used instead, particularly in Chile and Africa. Recently, Signorelli and Pastorino [70] resurrected the older name, Semimytilus patagonicus. For native distribution see [61] | |
| Bivalvia | Pectinida | Pectinidae | |||||
| Zygochlamys patagonica (P. P. King, 1832) https://www.molluscabase.org/aphia.php?p=taxdetails&id=236717, accessed on 1 April 2025 | 14–21 | Mediterranean Sea, 1985 [71] | Movement of commodity, escape, live food and live bait [72] | Impacts: - Effects: Potential on aquaculture. Remarks: Unsure whether all Mediterranean records are based on discarded dead specimens imported with shrimps, or whether specimens obtained from fishermen may actually originate from the Atlantic trawling by fleets based in the Mediterranean Sea; Mannino et al. [73] suggest excluding this taxon from all Mediterranean lists until confirmation of living specimens with reliable distributional data. | |
| Species that spread unaimed | |||||
| Species and Species ID | Ecoregions in South America | Place and Date of Entry and Reference | Introduction Mechanism, Pathway Category, and Subcategory | Impacts/Effects/Remarks | |
| Gastropoda | Systellommatophora | Onchidiidae | |||||
| Onchidella marginata (Couthouy, 1852) https://www.molluscabase.org/aphia.php?p=taxdetails&id=509919, accessed on 21 April 2025 | 12, 13, 14, 15, 27, 28 | Campbell Is., New Zealand [74] | Spread, unaimed, natural dispersal rafting on kelps | Impacts: - Effects: - Remarks: - | |
| Gastropoda | Neogastropoda | Cominellidae | |||||
| Pareuthria fuscata (Bruguière, 1789) https://www.molluscabase.org/aphia.php?p=taxdetails&id=491214, accessed on 1 April 2025 | 15, 16, 17, 18, 19 | Campbell Is., New Zealand, Gough and Tristan da Cunha Is. [75] | Spread, unaimed, natural dispersal, rafting on kelps | Impacts: - Effects: - Remarks: - | |
| Species and Species ID | Ecoregions in South America | Place and Date of Entry and Reference | Introduction Mechanism, Pathway Category, and Subcategory | Impacts/Effects/Remarks | |
|---|---|---|---|---|---|
| Gastropoda | Hygrophila | Lymnaeidae | |||||
| Galba cubensis (L. Pfeiffer, 1839) https://www.molluscabase.org/aphia.php?p=taxdetails&id=724475, accessed on 21 April 2025 | 1–5, 12 | Eastern Iberia, 2018 [76] | Movement of commodity, escape, horticulture; apparently originating from releases from horticultural facilities | Impacts: - Effects: Intermediate host (of Fasciola hepatica Linnaeus, 1758) Remarks: Non-native in anthropogenic environments, native in natural environments. | |
| Gastropoda | Littorinimorpha | Cochliopidae | |||||
| Heleobia charruana (d’Orbigny, 1841) https://www.molluscabase.org/aphia.php?p=taxdetails&id=760578, accessed on 14 April 2025 | 37–39, 41–43 | Barking Creek (Thames, England, U.K.), 2003; Antwerp, Belgium, May 2014; The Netherlands, Noord Holland, North Sea Canal and surroundings [77]) | Vector, stowaway, ballast water | Impacts: Potential displacement of native species. Effects: - Remarks: H. charruana is often a dominant species where it occurs. | |
| Gastropoda | Hygrophila| Planorbidae | |||||
| Biomphalaria glabrata (Say, 1818) https://www.molluscabase.org/aphia.php?p=taxdetails&id=848622, accessed on 13 April 2025 | 2, 5, 7, 8, 10, 16, 25–27, 34–36, 39, 41 | Lesser Antilles, 1970–1979 (Dominica), Haıti, Dominican Republic; canals in Egypt and from the irrigation and drainage systems in the Nile Delta area, around 1981; South Africa, mid-1980s [78] | Movement of commodity, escape, research | Impacts: - Effects: Intermediate host (of Schistosoma mansoni Sambon, 1907). Remarks: B. glabrata was introduced into laboratories in South Africa for the maintenance of cultures Schistosoma mansoni. It was shown experimentally to be slightly susceptible to S. mansoni from Egypt and Israel. Despite several searches in South Africa, it has not been collected in recent years. | |
| Biomphalaria tenagophila (A. d’Orbigny, 1835) https://www.molluscabase.org/aphia.php?p=taxdetails&id=1001488, accessed on 1 April 2025 | 1–5, 7, 10, 12, 14–20, 23–27, 29, 30, 33–35, 41–45, 47 | Congo, early 1970 Romania, 2004 [79,80] | Movement of commodity, escape, contaminant of plants | Impacts: - Effects: Intermediate host (of Schistosoma mansoni Sambon, 1907). Remarks: - | |
| Biomphalaria straminea (Dunker, 1848) https://www.molluscabase.org/aphia.php?p=taxdetails&id=1060816, accessed on 11 April 2025 | 2, 7, 10, 14–16, 21–28, 30, 33–35, 41–44, 47, 48 | Costa Rica, 1976; Lesser Antilles and Martinique, approx. 1950; Grenada, 1970; Hong Kong Special Administrative Region, 1973; Shenzhen city, Guangdong province in China, 1981; Guadeloupe, 1985; St. Lucia, 1992 [80,81,82] | Movement of commodity (?), contaminant (?), contaminant of plants? | Impacts: - Effects: Intermediate host (of Schistosoma mansoni Sambon, 1907). Remarks: It is considered to be the most important intermediate host of Schistosoma mansoni. | |
| Gastropoda | Architaenioglossa | Ampullariidae | |||||
| Marisa cornuarietis (Linnaeus, 1758) https://www.molluscabase.org/aphia.php?p=taxdetails&id=737469, accessed on 20 April 2025 | 1, 3, 7 | Cuba, 1950; St. Kitts, 1950s; Puerto Rico, 1952; near Coral Gables, Florida, USA, 1957; Egypt, 1972; Tanzania, 1977; Texas in the headwaters of the San Marcos River, San Marcos (city), Hays County, 1981; Sudan, 1981; Costa Rica, UD; Panama, UD; Dominican Republic, 1986; Martinique, 1987; Idaho, USA: Outfall of geothermally heated spring waters from a tropical fish hatchery on Deep Creek in the central Snake River drainage, Twin Falls County, 1992; California, USA, 2003; Grenada, 2009; North Spain, Colloto, Río Nora (43°22′ N-5°47′ W), 2012; Hungary, UD (only in an urban section of a stream close to the outflow from a thermal spa [83,84,85] | Movement of commodity, escape, release, ornamental, biological control [86] | Impacts: - Effects: Control (of freshwater weeds and Biomphalaria sp.). Remarks: The species alters the structure of the macrophyte community through selective herbivory, and prey on or compete with other snails [87]. | |
| Pomacea canaliculata (Lamarck, 1822) https://www.molluscabase.org/aphia.php?p=taxdetails&id=741113, accessed on 20 April 2025 | 30, 33, 34, 41–44, 47, 48 | Taiwan, 1979–1981; Philippines, 1980; Indonesia, 1981–1984; China, 1981–1985; Japan, 1981; South Korea, 1981–1986; Thailand, 1982–1990; Russia, 1986 (Kamchatka); Malaysia, 1987–1992; Vietnam, approx.. 1988; Guam, 1989; Hawaii, 1989; Singapore, 1991; South Africa, before 1991; Dominican Republic, 1991; Papua New Guinea, 1991; Laos, 1991–1994; USA (continental), 1997; Spain, 2001; Bangladesh, 2006; Cambodia, 2006; Egypt, 2006; India, 2006; Myanmar, 2008; Mexico, 2009; Iraq, 2013; Trinidad, 2014; Kenya, 2020 [85,88,89] | Movement of commodity, escape, ornamental; aquaculture | Impacts: Displacement of native species. Effects: Intermediate host (of Angiostrongylus cantonensis and Gnathostoma spinigerum [90,91]); negative on crops. Remarks: Possible intermediate host for parasites transmissible to humans, such as. Alters the structure of the macrophyte community through selective herbivory and food chains [87]. | |
| Pomacea maculata (Perry, 1810) https://www.molluscabase.org/aphia.php?p=taxdetails&id=737473, accessed on 20 April 2025 | 15, 16, 20–26, 28–30, 33, 34, 42, 43 | Thailand, 1990; USA, 1989; Cambodia, before 1995; China, 2006–2007; Israel, 2008; Singapore, 2008; South Korea, 2008; Malaysia, 2008; Vietnam, 2008; Japan, 2008–2013; Spain, 2009; Pakistan, 2009; Philippines, 2013 [85] | Movement of commodity, escape, aquaculture [92] | Impacts: On community structure (alters macrophyte and benthic communities and food chains [87]). Effects: Intermediate host (of trematode and human parasitic nematodes); negative on crops. Remarks: - | |
| Pomacea diffusa (Blume, 1957) https://www.molluscabase.org/aphia.php?p=taxdetails&id=848365, accessed on 10 April 2025 | 11, 13, 15, 16, 20, 21–23, 28, 29 | USA (continental), 1950s; Hawaii, 1962; Sri Lanka, early 1980s; Australia, 2004; Panamá, 2008; New Zealand, 2010 [85] | Movement of commodity, escape, pet [87] | Impacts: - Effects: Food resource; aquariums (detritus and algae cleaner). Remarks: - | |
| Pomacea scalaris (d’Orbigny, 1835) https://www.molluscabase.org/aphia.php?p=taxdetails&id=741148, accessed on 20 April 2025 | 28–30, 33, 42, 43 | Taiwan, 1989 [85] | Movement of commodity, escape, live food [87] | Impacts: - Effects: - Remarks: - | |
| Species and Species ID | Ecoregions in South America | Place and Date of Entry and Reference | Introduction Mechanism, Pathway Category, and Subcategory | Impacts/Effects/Remarks | |
|---|---|---|---|---|---|
| Gastropoda | Stylommatophora | Strophocheilidae | |||||
| Megalobulimus oblongus (OF Müller, 1774) https://www.molluscabase.org/aphia.php?p=taxdetails&id=1446864, accessed on 20 April 2025 | 1, 3, 7, 30, 32, 36, 37, 53–57, 60–66 | Czech Republic, Europe, UD [93,94]; Jamaica, UD [95] | Movement of commodity, escape, ornamental | Impacts: - Effects: Negative potential on crops. Remarks: Species exemplifies the paradox of invaders, at risk of extinction in its native area but successfully invasive in other areas. | |
| Gastropoda | Stylommatophora | Bulimulinae | |||||
| Bulimulus bonariensis (Rafinesque, 1833) = B. sporadicus (d’Orbigny) https://www.molluscabase.org/aphia.php?p=taxdetails&id=1288732, accessed on 25 April 2025 | 75, 85, 87, 89, 96, 97 | South States of USA, before 2009 [96] | UD | Impacts: - Effects: Negative on crops. Remarks: In the United States, solutions are urgently needed to manage growing populations of this species in row crops, particularly peanuts (Arachis hipogaea L.) and citrus. Concern in economic and food safety due to its infestation of commercial crops in southern U.S. states. | |
| Bulimulus tenuissimus (A. Férussac, 1832) https://www.molluscabase.org/aphia.php?p=taxdetails&id=1292059, accessed on 21 April 2025 | 28, 32, 62–67, 69, 70, 73–78, 88, 89, 92, 93 | Wilmington, New Hanover County, North Carolina, USA, 1995 [97] | Movement of commodity, contaminant, contaminant of plants | Impacts: - Effects: Negative on crops; intermediate host. Remarks: - | |
| Gastropoda | Stylommatophora | Veronicellidae | |||||
| Phyllocaulis gayi (P. Fischer, 1871) https://www.molluscabase.org/aphia.php?p=taxdetails&id=1064171, accessed on 10 April 2025 | 101, 102 | Sinaloa Mexico, 1925 [98] | Movement of commodity, escape, horticulture | Impacts: On native plant species. Effects: - Remarks: Naranjo et al. [99] mentioned that the record of this species in Mexico needs confirmation. In Mexico, the record for Sinaloa, nearly 100 years ago [98,100] apparently did not result in establishing breeding populations. | |
| Colosius confusus (Gomes, Robinson, Zimmerman, Obregón & Barr, 2013) https://www.molluscabase.org/aphia.php?p=taxdetails&id=1575338, accessed on 23 April 2025 | 12, 15, 40, 44, 48, 50 | USA-Intercepted-Miami, 2001 [101] | Movement of commodity, escape, horticulture | Impacts: - Effects: Negative on crops (coffee and cultivated flowers). Remarks: - | |
| Belocaulus angustipes (Heynemann, 1885) https://www.molluscabase.org/aphia.php?p=taxdetails&id=1064167 | 75, 88–90, 97 | Seven states on USA between 1960 [102], 2005 [103]; Honduras, 1984 [104]; Mexico, 1989 [105] | Movement of commodity, escape, horticulture | Impacts: - Effects: Intermediate host (of Angiostrongylus costaricensis Morera & Céspedes, 1971). Remarks: It is a synanthropic species. Recently, it was the most frequently recorded native species in Argentine nurseries [106]. Rambo et al. [107] mention it was infected with Angiostrongylus costaricensis Morera & Céspedes, 1971 in the municipalities of Três de Maio and Santa Rosa, in Rio Grande do Sul, Brazil, causing human abdominal angiostrongyliasis. | |
| Sarasinula linguaeformis (Semper, 1885) https://www.molluscabase.org/aphia.php?p=taxdetails&id=1441990, accessed on 20 April 2025 | 42, 75, 76, 90 | Guadalupe Island [108]; Dominican Island; San Pedro (Intercepted by Agricultural Department, USA, lot USDA 20060114-00); Martinique (Intercepted by Agricultural Department, USA, lot USDA BX090708-006, BX090708-007) | Movement of commodity, contaminant, timber trade contaminant | Impacts: - Effects: Negative on crops (soybeans and beans). Remarks: - | |
| Gastropoda | Stylommatophora | Streptaxidae | |||||
| Streptaxis contusus (Férussac, 1821) https://www.molluscabase.org/aphia.php?p=taxdetails&id=1326821, accessed on 26 April 2025 | 76, Southeast and south Brazil | Hawaii, 1961 [109] | Movement of commodity, release, biological control | Impacts: - Effects: - Remarks: Introduced in Hawaii in 1961 as a possible biological control of the giant African snail Lissachatina fulica (Bowdich, 1822), but did not establish as Streptaxis contundata) [109]. | |
| Gastropoda | Stylommatophora | Scolodontidae | |||||
| Tamayoa decolorata (Drouët, 1859) https://www.molluscabase.org/aphia.php?p=taxdetails&id=1442170, accessed on 27 April 2025 | 28 | Barbados, 2001 [110]; Jamaica, 2006 Guadeloupe, Dominica, Saint Vincent [95] | UD | Impacts: - Effects: - Remarks: - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darrigran, G.; Agudo-Padrón, I.; Báez, P.; Belz, C.E.; Cardoso, F.; Carranza, A.; Collado, G.A.; Correoso, M.; Cuezzo, M.G.; Fabres, A.A.; et al. Molluscs from South America to the World: Who and Where Are They? Biology 2025, 14, 1538. https://doi.org/10.3390/biology14111538
Darrigran G, Agudo-Padrón I, Báez P, Belz CE, Cardoso F, Carranza A, Collado GA, Correoso M, Cuezzo MG, Fabres AA, et al. Molluscs from South America to the World: Who and Where Are They? Biology. 2025; 14(11):1538. https://doi.org/10.3390/biology14111538
Chicago/Turabian StyleDarrigran, Gustavo, Ignacio Agudo-Padrón, Pedro Báez, Carlos Eduardo Belz, Franz Cardoso, Alvar Carranza, Gonzalo A. Collado, Modesto Correoso, María Gabriela Cuezzo, Alejandra A. Fabres, and et al. 2025. "Molluscs from South America to the World: Who and Where Are They?" Biology 14, no. 11: 1538. https://doi.org/10.3390/biology14111538
APA StyleDarrigran, G., Agudo-Padrón, I., Báez, P., Belz, C. E., Cardoso, F., Carranza, A., Collado, G. A., Correoso, M., Cuezzo, M. G., Fabres, A. A., Fernandez, M. A., Gomes, S. R., Gutierrez Gregoric, D. E., Letelier, S., Lodeiros, C., Ludwig, S., Mansur, M. C., Oliveira Arruda, J., Pastorino, G., ... Damborenea, C. (2025). Molluscs from South America to the World: Who and Where Are They? Biology, 14(11), 1538. https://doi.org/10.3390/biology14111538








