Spatio-Temporal Niche Differentiation of Alpine Musk Deer, Chinese Serow, and Tufted Deer in Changdu Prefecture, Tibet, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Spatial Niche Analysis
2.2.1. Occurrence Points of Species
2.2.2. Environmental Variables and Data Processing
2.2.3. Mapping the Suitable Habitat of Species
2.2.4. Niche Overlap Analysis
2.3. Temporal Niche Analysis
2.3.1. Data Processing
2.3.2. Analysis of Daily Activity Rhythm
2.4. Consideration of Sample Size Limitations
3. Results
3.1. Spatial Niche Analysis
3.1.1. Prediction of Suitable Habitat
3.1.2. Impact of Environmental Factors on Habitat Quality
3.1.3. Habitat Overlaps
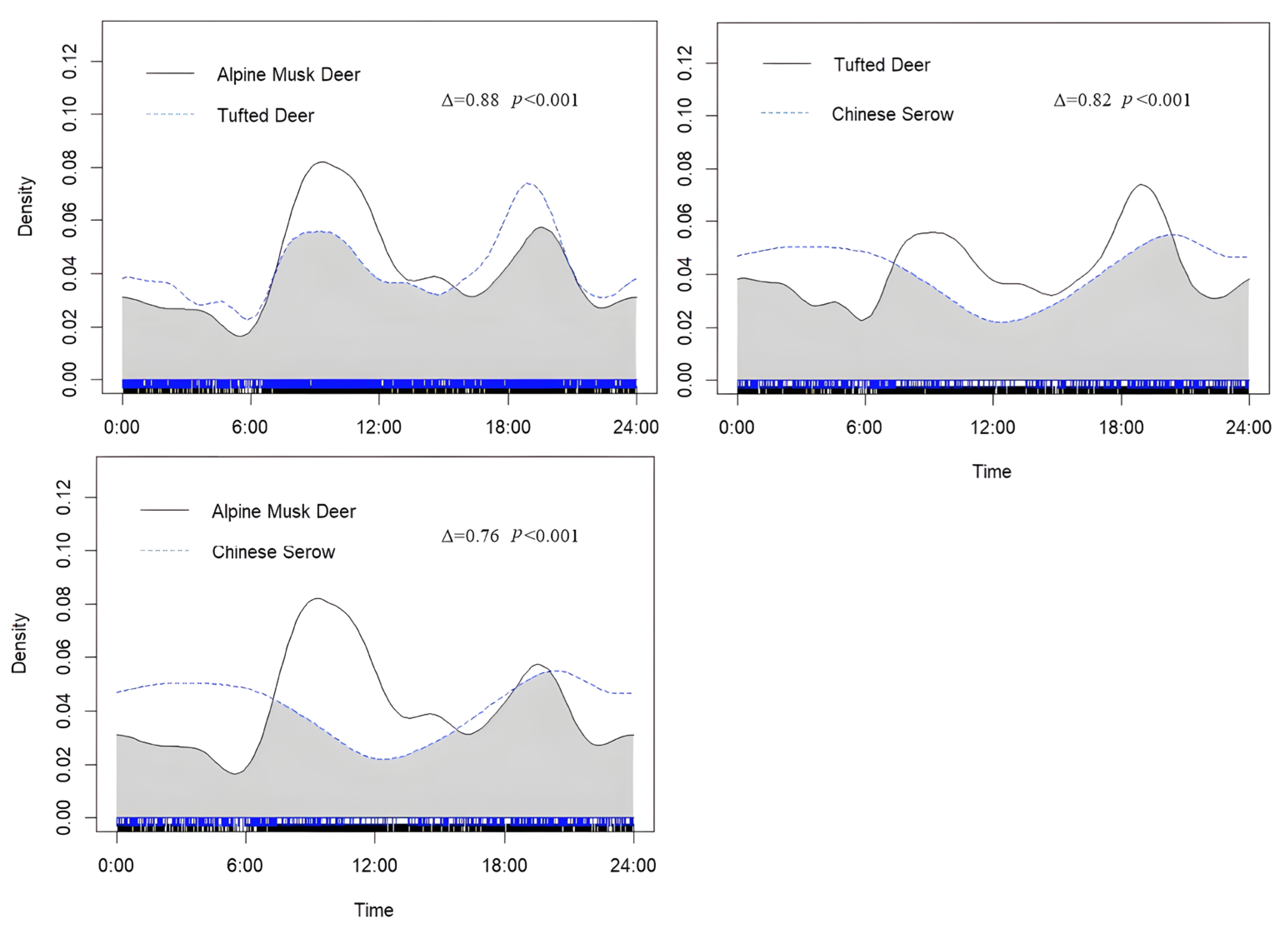
3.2. Temporal Niche Analysis
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amarasekare, P. Competitive Coexistence in Spatially Structured Environments: A Synthesis. Ecol. Lett. 2003, 6, 1109–1122. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of Maintenance of Species Diversity. Annu. Rev. Ecol. Evol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Frey, S.; Fisher, J.T.; Burton, A.C.; Volpe, J.P. Investigating Animal Activity Patterns and Temporal Niche Partitioning Using Camera-Trap Data: Challenges and Opportunities. Remote Sens. Ecol. Conserv. 2017, 3, 123–132. [Google Scholar] [CrossRef]
- Di Carvalho, J.A.; Wickham, S.A. Does Spatiotemporal Nutrient Variation Allow More Species to Coexist? Oecologia 2020, 194, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Yang, Q.; Wu, Y.; Jiang, X.; Liu, S.; Hu, Y.; Ge, D.; Li, B.; Yang, G.; Li, M.; et al. Catalogue of Mammals in China (2024). Acta Theriol. 2025, 45, 1–16. [Google Scholar]
- Panyaboriban, S.; Singh, R.P.; Songsasen, N.; Padilla, L.; Brown, J.; Reed, D.; Techakumphu, M.; Pukazhenthi, B. Reproductive Seasonality and Sperm Cryopreservation in the Male Tufted Deer (Elaphodus cephalophus). Theriogenology 2016, 86, 914–923. [Google Scholar] [CrossRef]
- Harris, R.B.; Jiang, Z. Elaphodus cephalophus. In The IUCN Red List of Threatened Species 2015: E.T7112A22159620 2014; IUCN: Geneva, Switzerland, 2015. [Google Scholar]
- Zhang, Z.; Wang, X.; Su, Y.; Hu, T.; Duo, H.; Yang, Y.; Aorigele; Zhang, J.; Teng, L.; Liu, Z. Assessing Population Status and Influencing Factors of Alpine Musk Deer in Patchy Habitats: Implications for Conservation Strategies. Glob. Ecol. Conserv. 2024, 54, e03134. [Google Scholar] [CrossRef]
- Harris, R. Moschus chrysogaster. In The IUCN Red List of Threatened Species 2016: E.T13895A61977139 2014; IUCN: Geneva, Switzerland, 2016. [Google Scholar]
- Li, A.; Yang, Q.; Li, R.; Cai, K.; Zhu, L.; Wang, X.; Cheng, G.; Wang, X.; Lei, Y.; Jiang, Y.; et al. Chromosome-Level Genome Assembly for the Chinese Serow (Capricornis milneedwardsii) Provides Insights Into Its Taxonomic Status and Evolution. Ecol. Evol. 2024, 14, e70400. [Google Scholar] [CrossRef]
- Phan, T.D.; Nijhawan, S.; Li, S.; Xiao, L. Capricornis sumatraensis. In The IUCN Red List of Threatened Species 2020: E.T162916735A162916910 2020; IUCN: Geneva, Switzerland, 2020. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Alanís-Méndez, J.L.; Soto, V.; Limón-Salvador, F. Effects of Climate Change on the Distribution of Prosthechea Mariae (Orchidaceae) and within Protected Areas in Mexico. Plants 2024, 13, 839. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, T.; Li, J.-J.; Kang, Z.-J.; Yu, G.-Q.; Yang, C.-C. Assessment of the Suitable Habitat for the Tufted Deer (Elaphodus cephalophus) in the Hupingshan National Nature Reserve. China Environ. Sci. 2024, 44, 2619–2629. [Google Scholar]
- Moo-Llanes, D.A. Inferring Distributional Shifts of Asian Giant Hornet Vespa Mandarinia Smith in Climate Change Scenarios. Neotrop. Entomol. 2021, 50, 673–676. [Google Scholar] [CrossRef]
- Rodriguez-Burgos, A.M.; Briceño-Zuluaga, F.J.; Ávila Jiménez, J.L.; Hearn, A.; Peñaherrera-Palma, C.; Espinoza, E.; Ketchum, J.; Klimley, P.; Steiner, T.; Arauz, R.; et al. The Impact of Climate Change on the Distribution of Sphyrna Lewini in the Tropical Eastern Pacific. Mar. Environ. Res. 2022, 180, 105696. [Google Scholar] [CrossRef]
- Werenkraut, V.; Arbetman, M.P.; Fergnani, P.N. The Oriental Hornet (Vespa orientalis): A Threat to the Americas? Neotrop. Entomol. 2022, 51, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-Y.; Ding, G.-Y.; Tian, X.-J. Research Progress on the Application of the MaxEnt Model in Species Habitat Prediction. Chin. J. Appl. Ecol. 2025, 36, 614–624. [Google Scholar]
- Defalque, C.; Laeremans, J.; Drugmand, J.; Tcheutchoua, C.F.; Meng, Y.; Zhou, M.; Zhang, K.; Quinet, M. Drought and High Temperatures Impact the Plant–Pollinator Interactions in Fagopyrum Esculentum. Plants 2025, 14, 131. [Google Scholar] [CrossRef]
- Lipiec, J.; Doussan, C.; Nosalewicz, A.; Kondracka, K. Effect of Drought and Heat Stresses on Plant Growth and Yield: A Review. Int. Agrophysics 2013, 27, 463–477. [Google Scholar] [CrossRef]
- Hamann, E.; Blevins, C.; Franks, S.J.; Jameel, M.I.; Anderson, J.T. Climate Change Alters Plant–Herbivore Interactions. New Phytol. 2021, 229, 1894–1910. [Google Scholar] [CrossRef]
- Felton, A.M.; Wam, H.K.; Borowski, Z.; Granhus, A.; Juvany, L.; Matala, J.; Melin, M.; Wallgren, M.; Mårell, A. Climate Change and Deer in Boreal and Temperate Regions: From Physiology to Population Dynamics and Species Distributions. Glob. Change Biol. 2024, 30, e17505. [Google Scholar] [CrossRef]
- Litvinchuk, S.N.; Skorinov, D.V.; Ivanov, A.Y.; Ermakov, O.A. Detection of Glacial Refugia and Post-Glacial Colonization Routes of Morphologically Cryptic Marsh Frog Species (Anura: Ranidae: Pelophylax) Using Environmental Niche Modeling. Diversity 2024, 16, 94. [Google Scholar] [CrossRef]
- Xiang, Z.-F.; Huo, S.; Wang, L.; Cui, L.-W.; Xiao, W.; Quan, R.-C.; Tai, Z. Distribution, Status and Conservation of the Black-and-White Snub-Nosed Monkey Rhinopithecus bieti in Tibet. Oryx 2007, 41, 525–531. [Google Scholar] [CrossRef]
- Feng, B.; Bai, W.; Fan, X.; Fu, M.; Song, X.; Liu, J.; Qin, W.; Zhang, J.; Qi, D.; Hou, R. Species Coexistence and Niche Interaction between Sympatric Giant Panda and Chinese Red Panda: A Spatiotemporal Approach. Ecol. Evol. 2023, 13, e9937. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hu, Y.; Taukenova, A.; Tian, G.; Mu, B. Identification of Wetland Conservation Gaps in Rapidly Urbanizing Areas: A Case Study in Zhengzhou, China. Land 2023, 12, 221. [Google Scholar] [CrossRef]
- Vignali, S.; Barras, A.G.; Arlettaz, R.; Braunisch, V. SDMtune: An R Package to Tune and Evaluate Species Distribution Models. Ecol. Evol. 2020, 10, 11488–11506. [Google Scholar] [CrossRef]
- Chang, Y.; Bertola, L.V.; Hoskin, C.J. Species Distribution Modelling of the Endangered Mahogany Glider (Petaurus gracilis) Reveals Key Areas for Targeted Survey and Conservation. Austral Ecol. 2023, 48, 289–312. [Google Scholar] [CrossRef]
- Yan, C.; Hao, H.; Sha, S.; Wang, Z.; Huang, L.; Kang, Z.; Wang, L.; Feng, H. Comparative Assessment of Habitat Suitability and Niche Overlap of Three Cytospora Species in China. J. Fungi 2024, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R Package for Conducting Spatially Independent Evaluations and Estimating Optimal Model Complexity for Maxent Ecological Niche Models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for Customizable and Reproducible Modeling of Species’ Niches and Distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Wang, P.; Feng, B.; Zhang, L.; Fan, X.; Tang, Z.; Dong, X.; Zhang, J.; Zhou, C.; Bai, W. Assessment of Habitat Suitability and Connectivity across the Potential Distribution Landscape of the Sambar (Rusa unicolor) in Southwest China. Front. Conserv. Sci. 2023, 3, 2022. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.; Silander, J. A Practical Guide to MaxEnt for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.-J.; Randin, C.; Zimmermann, N.E.; et al. Measuring Ecological Niche Overlap from Occurrence and Spatial Environmental Data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Zhang, G.; Zhang, X.; Miao, Y.; Zhang, M.; Feng, Z.; Zeng, R.; Pei, J.; Huang, L. Potential Global Distribution of the Habitat of Endangered Gentiana rhodantha Franch: Predictions Based on MaxEnt Ecological Niche Modeling. Sustainability 2023, 15, 631. [Google Scholar] [CrossRef]
- Niedballa, J.; Sollmann, R.; Courtiol, A.; Wilting, A. camtrapR: An R Package for Efficient Camera Trap Data Management. Methods Ecol. Evol. 2016, 7, 1457–1462. [Google Scholar] [CrossRef]
- Scotson, L.; Johnston, L.R.; Iannarilli, F.; Wearn, O.R.; Mohd-Azlan, J.; Wong, W.M.; Gray, T.N.E.; Dinata, Y.; Suzuki, A.; Willard, C.E.; et al. Best Practices and Software for the Management and Sharing of Camera Trap Data for Small and Large Scales Studies. Remote Sens. Ecol. Conserv. 2017, 3, 158–172. [Google Scholar] [CrossRef]
- O’Brien, T.; Kinnaird, M.; Wibisono, H. Crouching Tigers, Hidden Prey: Sumatran Tiger and Prey Populations in a Tropical Forest Landscape. Anim. Conserv. 2003, 6, 131–139. [Google Scholar] [CrossRef]
- Ridout, M.S.; Linkie, M. Estimating Overlap of Daily Activity Patterns from Camera Trap Data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Rowcliffe, M. Activity: Animal Activity Statistics. 2023. Available online: https://CRAN.R-project.org/package=activity (accessed on 6 September 2025).
- Meredith, M.; Ridout, M.; Campbell, L.A.D. Overlap: Estimates of Coefficient of Overlapping for Animal Activity Patterns. 2024. Available online: https://CRAN.R-project.org/package=overlap (accessed on 6 September 2025).
- Marinho, P.H.; Fonseca, C.R.; Sarmento, P.; Fonseca, C.; Venticinque, E.M. Temporal Niche Overlap among Mesocarnivores in a Caatinga Dry Forest. Eur. J. Wildl. Res. 2020, 66, 34. [Google Scholar] [CrossRef]
- Marneweck, C.; Marneweck, D.G.; van Schalkwyk, O.L.; Beverley, G.; Davies-Mostert, H.T.; Parker, D.M. Spatial Partitioning by a Subordinate Carnivore Is Mediated by Conspecific Overlap. Oecologia 2019, 191, 531–540. [Google Scholar] [CrossRef]
- Leslie, D.M.; Lee, D.N.; Dolman, R.W. Elaphodus cephalophus (Artiodactyla: Cervidae). Mamm. Species 2013, 904, 80–91. [Google Scholar] [CrossRef]
- Weerman, J.; Li, F. Tufted Deer Elaphodus cephalophus A. Milne-Edwards in A. David, 1871. In Deer of the World: Ecology, Conservation and Management; Melletti, M., Focardi, S., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 457–468. [Google Scholar]
- Schoener, T.W. Resource Partitioning in Ecological Communities. Science 1974, 185, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Farina, A. Distribution and Dynamics of Birds in a Rural Sub-Mediterranean Landscape. Landsc. Urban Plan. 1995, 31, 269–280. [Google Scholar] [CrossRef]
- McCain, C.M.; Grytnes, J.-A. Elevational Gradients in Species Richness. In Encyclopedia of Life Sciences; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Götmark, F.; Blomqvist, D.; Johansson, O.; Bergkvist, J. Nest Site Selection: A Trade-Off between Concealment and View of the Surroundings? J. Avian Biol. 1995, 26, 305–312. [Google Scholar] [CrossRef]
- Leyequién, E.; de Boer, W.F.; Cleef, A. Influence of Body Size on Coexistence of Bird Species. Ecol. Res. 2007, 22, 735–741. [Google Scholar] [CrossRef]
- Suraprasit, K.; Jaeger, J.-J.; Chaimanee, Y.; Chavasseau, O.; Yamee, C.; Tian, P.; Panha, S. The Middle Pleistocene Vertebrate Fauna from Khok Sung (Nakhon Ratchasima, Thailand): Biochronological and Paleobiogeographical Implications. ZooKeys 2016, 613, 1–157. [Google Scholar] [CrossRef] [PubMed]
- Suraprasit, K.; Bocherens, H.; Chaimanee, Y.; Panha, S.; Jaeger, J.-J. Late Middle Pleistocene Ecology and Climate in Northeastern Thailand Inferred from the Stable Isotope Analysis of Khok Sung Herbivore Tooth Enamel and the Land Mammal Cenogram. Quat. Sci. Rev. 2018, 193, 24–42. [Google Scholar] [CrossRef]
- Bagchi, S.; Goyal, S.P.; Sankar, K. Niche Relationships of an Ungulate Assemblage in a Dry Tropical Forest. J. Mammal. 2003, 84, 981–988. [Google Scholar] [CrossRef]
- Linkie, M.; Ridout, M.S. Assessing Tiger–Prey Interactions in Sumatran Rainforests. J. Zool. 2011, 284, 224–229. [Google Scholar] [CrossRef]
- Bu, H.; Wang, F.; McShea, W.J.; Lu, Z.; Wang, D.; Li, S. Spatial Co-Occurrence and Activity Patterns of Mesocarnivores in the Temperate Forests of Southwest China. PLoS ONE 2016, 11, e0164271. [Google Scholar] [CrossRef]
- Moretti, L.; Hentrup, M.; Kotrschal, K.; Range, F. The Influence of Relationships on Neophobia and Exploration in Wolves and Dogs. Anim. Behav. 2015, 107, 159–173. [Google Scholar] [CrossRef]
- Luo, G.; Yang, C.; Zhou, H.; Seitz, M.; Wu, Y.; Ran, J. Habitat Use and Diel Activity Pattern of the Tibetan Snowcock (Tetraogallus tibetanus): A Case Study Using Camera Traps for Surveying High-Elevation Bird Species. Avian Res. 2019, 10, 4. [Google Scholar] [CrossRef]
- Lima, S.L.; Bednekoff, P.A. Temporal Variation in Danger Drives Antipredator Behavior: The Predation Risk Allocation Hypothesis. Am. Nat. 1999, 153, 649–659. [Google Scholar] [CrossRef]
- Jiang, F.; Song, P.; Zhang, J.; Wang, D.; Li, R.; Liang, C.; Zhang, T. Assessment Approach for Conservation Effectiveness and Gaps for Endangered Species Based on Habitat Suitability: A Case Study of Alpine Musk Deer in Western China. Ecol. Indic. 2025, 170, 113080. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial Filtering to Reduce Sampling Bias Can Improve the Performance of Ecological Niche Models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Stockwell, D.R.B.; Peterson, A.T. Effects of Sample Size on Accuracy of Species Distribution Models. Ecol. Model. 2002, 148, 1–13. [Google Scholar] [CrossRef]
- Holbrook, J.D.; Olson, L.E.; DeCesare, N.J.; Hebblewhite, M.; Squires, J.R.; Steenweg, R. Functional Responses in Habitat Selection: Clarifying Hypotheses and Interpretations. Ecol. Appl. 2019, 29, e01852. [Google Scholar] [CrossRef]
- Anselmetto, N.; Morresi, D.; Barbarino, S.; Loglisci, N.; Betts, M.G.; Garbarino, M. Species Distribution Models Built with Local Species Data Perform Better for Current Time, but Suffer from Niche Truncation. Agric. For. Meteorol. 2025, 362, 110361. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Q.; Cui, X.; Peng, J.; Zhou, S.; Wang, F.; Zhong, L.; Wang, X.; Zheng, H.; Yang, C.; et al. Prediction of Potential Suitable Habitats for Elaphodus cephalophus in China Under Climate Change Scenarios. Ecol. Evol. 2025, 15, e72194. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ma, L.; Jiang, W.; Wang, L.; Wei, L.; Zhang, H.; Yang, R. Anthropogenic Disturbance and Climate Change Impacts on the Suitable Habitat of Sphenomorphus incognitus in China. Ecol. Evol. 2025, 15, e70848. [Google Scholar] [CrossRef] [PubMed]






| Environmental Variables | Source | Type of Variable | Applied Species |
|---|---|---|---|
| Bio2 | http://www.worldclim.org (accessed on 25 October 2025) | Continuous variables | C |
| Bio3 | http://www.worldclim.org (accessed on 25 October 2025) | Continuous variables | T, A, C |
| Bio4 | http://www.worldclim.org (accessed on 25 October 2025) | Continuous variables | A, C |
| Bio7 | http://www.worldclim.org (accessed on 25 October 2025) | Continuous variables | T, A, C |
| Bio8 | http://www.worldclim.org (accessed on 25 October 2025) | Continuous variables | C |
| Bio10 | http://www.worldclim.org (accessed on 25 October 2025) | Continuous variables | T |
| Bio12 | http://www.worldclim.org (accessed on 25 October 2025) | Continuous variables | T, C |
| Bio14 | http://www.worldclim.org (accessed on 25 October 2025) | Continuous variables | T, A, C |
| Bio15 | http://www.worldclim.org (accessed on 25 October 2025) | Continuous variables | A, C |
| Bio16 | http://www.worldclim.org (accessed on 25 October 2025) | Continuous variables | C |
| Bio18 | http://www.worldclim.org (accessed on 25 October 2025) | Continuous variables | T, A |
| Aspect | http://www.gscloud.cn (accessed on 25 October 2025) | Continuous variables | T, A, C |
| Elevation | http://www.gscloud.cn (accessed on 25 October 2025) | Continuous variables | A |
| Land cover | https://data.tpdc.ac.cn (accessed on 25 October 2025) | Discrete variables | T, A, C |
| NDVI | https://data.tpdc.ac.cn (accessed on 25 October 2025) | Continuous variables | T, A, C |
| Slope | http://www.gscloud.cn (accessed on 25 October 2025) | Continuous variables | T, A, C |
| Forest tree height | https://www.3decology.org (accessed on 25 October 2025) | Continuous variables | T, A, C |
| Species | Model Evaluation Indices | Habitat Type | HSI Threshold | Area, km2 | Proportion of Study Area, % |
|---|---|---|---|---|---|
| Tufted Deer | AUC = 0.994 | Highly Suitable | >0.1176 | 3147 | 2.86 |
| MTSS = 0.1176 | Sub-suitable | 0.0129–0.1176 | 11,821 | 10.76 | |
| BTPT = 0.0129 | Total Suitable | >0.0129 | 14,968 | 13.62 | |
| Alpine Musk Deer | AUC = 0.950 | Highly Suitable | >0.2709 | 15,016 | 13.67 |
| MTSS = 0.2709 | Sub-suitable | 0.0726–0.2709 | 26,893 | 24.48 | |
| BTPT = 0.0726 | Total Suitable | >0.0726 | 41,909 | 38.14 | |
| Chinese Serow | AUC = 0.957 | Highly Suitable | >0.2402 | 13,860 | 12.61 |
| MTSS = 0.2402 | Sub-suitable | 0.0705–0.2402 | 23,094 | 21.02 | |
| BTPT = 0.0705 | Total Suitable | >0.0705 | 36,954 | 33.63 |
| Species Pair | Total Overlap Area, km2 | Highly Suitable Overlap Area, km2 | Proportion of Own Total Habitat, % | Proportion of Own Suitable Habitat, % | Niche Overlap Indices | |||
|---|---|---|---|---|---|---|---|---|
| Species A | Species B | Species A | Species B | D | I | |||
| Tufted deer–Alpine musk deer | 5102 | 1658 | 34.09 | 12.17 | 11.08 | 3.96 | 0.3098 | 0.5983 |
| Tufted deer–Chinese serow | 6483 | 2455 | 43.31 | 17.54 | 16.4 | 6.64 | 0.3889 | 0.6999 |
| Alpine musk deer–Chinese serow | 26,869 | 11,242 | 64.11 | 72.71 | 26.82 | 26.82 | 0.8266 | 0.9729 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Yu, Y.; Liu, Y.; Zhang, T.; Shu, F.; Chen, Y.; Yu, J.; Chen, Y.; Chen, H.; Quzhen, Z.; et al. Spatio-Temporal Niche Differentiation of Alpine Musk Deer, Chinese Serow, and Tufted Deer in Changdu Prefecture, Tibet, China. Biology 2025, 14, 1536. https://doi.org/10.3390/biology14111536
Wang C, Yu Y, Liu Y, Zhang T, Shu F, Chen Y, Yu J, Chen Y, Chen H, Quzhen Z, et al. Spatio-Temporal Niche Differentiation of Alpine Musk Deer, Chinese Serow, and Tufted Deer in Changdu Prefecture, Tibet, China. Biology. 2025; 14(11):1536. https://doi.org/10.3390/biology14111536
Chicago/Turabian StyleWang, Changjian, Yang Yu, Yang Liu, Tong Zhang, Fu Shu, Yuling Chen, Jiyuan Yu, Yi Chen, Haochun Chen, Zhuoma Quzhen, and et al. 2025. "Spatio-Temporal Niche Differentiation of Alpine Musk Deer, Chinese Serow, and Tufted Deer in Changdu Prefecture, Tibet, China" Biology 14, no. 11: 1536. https://doi.org/10.3390/biology14111536
APA StyleWang, C., Yu, Y., Liu, Y., Zhang, T., Shu, F., Chen, Y., Yu, J., Chen, Y., Chen, H., Quzhen, Z., Krzton, A., Guo, K., & Xiang, Z. (2025). Spatio-Temporal Niche Differentiation of Alpine Musk Deer, Chinese Serow, and Tufted Deer in Changdu Prefecture, Tibet, China. Biology, 14(11), 1536. https://doi.org/10.3390/biology14111536





