1. Introduction
Renowned as the “giant panda in water”, the Yangtze sturgeon (
Acipenser dabryanus) represents a rare and precious freshwater fish unique to China, which has extremely high ecological, economic, and cultural significance [
1]. Nevertheless, due to multiple factors such as habitat loss, environmental pollution, overfishing, and the construction of water conservancy projects, the population of Yangtze sturgeon has experienced a drastic decline [
2]. The species was officially listed as critically endangered in the International Union for Conservation of Nature (IUCN) Red List in 2010 [
3], by the time of this study (summer 2024), 14 years had passed since this endangered classification. To mitigate population decline, targeted conservation measures have been implemented over this period, with artificial breeding, stock enhancement, and release programs emerging as core strategies to safeguard and restore wild populations.
Unfortunately, bacterial diseases remain a major threat in artificial breeding systems, frequently endangering Yangtze sturgeon’s health and survival, and thus limiting effective population recovery [
4,
5]. However, previous studies on the pathogenicity of the Yangtze sturgeon were very limited, and they mainly focused on the infection of a single type of bacteria (such as
Aeromonas hydrophila and
Edwardsiella tarda) [
4,
5]. This failed to elucidate the mechanism of disease outbreaks characterized by “complex symptoms and high antibiotic resistance’’ in aquaculture, nor did it address the ecological risks of multi-bacterial cross-host transmission.
Bacterial co-infection, defined as the simultaneous infection of a single host by two or more bacterial species (including pathogenic agents and/or opportunistic colonizers), is prevalent in aquaculture [
6]. Such co-infections not only increase the complexity of the diseases and the challenges in diagnostics, but also potentiate the development of multidrug resistance (MDR) in bacteria, thereby impeding effective disease control [
7]. During the cultivation of Yangtze sturgeon, there exists a wide variety of pathogenic bacteria triggering bacterial diseases, with mixed, cross, secondary, and co-infections being common [
5]. However, the etiology of severe outbreaks remains poorly understood.
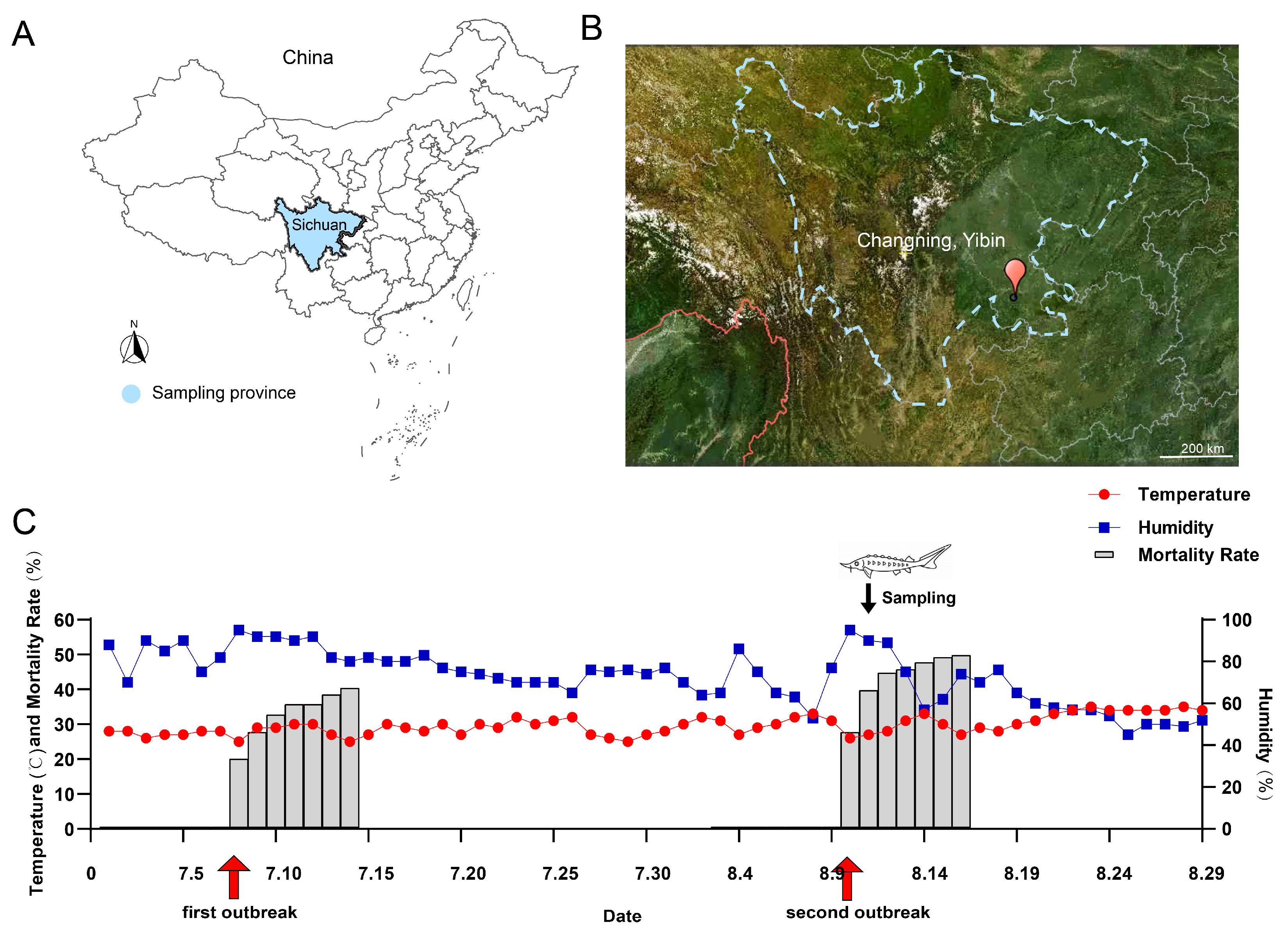
In the summer of 2024, two unexplained deaths of the Yangtze sturgeon population occurred in Sichuan (on 8th July and 10th August, respectively; this study only obtained samples from the second incident), with mortality rates of 40.7% and 50.1%, respectively. The dying fish showed symptoms such as lethargy, loss of appetite, irregular swimming, anoxemia, and acute death. The disease outbreaks have had a significant impact on the health maintenance of the Yangtze sturgeon population in this base, and it is urgent to further study the etiology and develop targeted prevention and control strategies. Therefore, this study aims to elucidate the etiology and pathology of the disease outbreak, so as to provide theoretical support for the healthy breeding and disease prevention and control of Yangtze sturgeon, as well as provide reference for the protection of other endangered fish species.
2. Materials and Methods
2.1. Experimental Fish and Sample Collection
2.1.1. Experimental Fish
The Yangtze sturgeon used in this study originated from the Changning Base of the Fisheries Research Institute of Sichuan Academy of Agricultural Sciences (Agriculture and Rural Affairs Ministry’s Protection Base for Rare and Endemic Fish Species in the Upper Reaches of the Yangtze River). The specific sampling locations are shown in
Figure 1A,B, and the local temperature/humidity is shown in
Figure 1C. The water used for the cultivation of sturgeon was recycled water (the water quality on the sampling day: dissolved oxygen: 5.7 mg/L; pH: 8.83; ammonia nitrogen: 1.07 mg/L; nitrite: 0.41 mg/L) And there were no potential pollution risk points such as farmland, industrial sites, and sewage outlets around the breeding base.
According to the Regulations on the Protection and Management of Aquatic Life in the Yangtze River (Order of the Ministry of Agriculture and Rural Affairs No. 5 of 2021) and the Law of the People’s Republic of China on the Protection of the Yangtze River (Order No. 65 of the President), raising endangered fish like Yangtze sturgeon in recirculatory water with ideal conditions is not subject to ethical review. All animals were treated in accordance with relevant guidelines and regulations given by the Animal Care and Use Committee of the Fisheries Research Institute, Sichuan Academy of Agricultural Sciences. The experimental protocols used for live fish experiment were based on the Animal Welfare Act.
2.1.2. Sample Collection
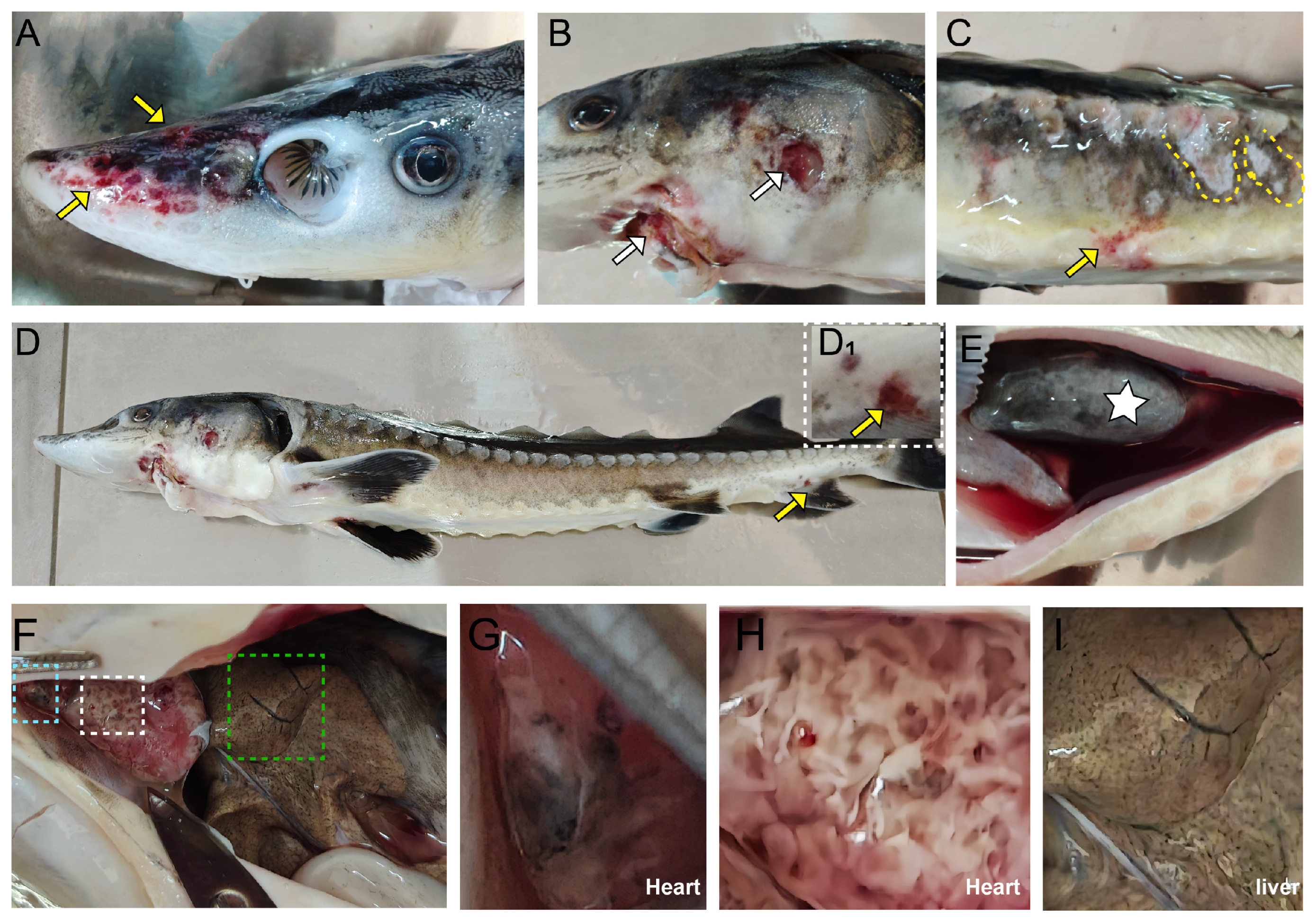
A total of 6 fish were collected in this study, with 3 fish in each group (weight: 1018.34 ± 1.53 g, body length: 47.24 ± 0.47 cm). Prior to tissue sampling, the fish were anesthetized using a neutralized MS222 solution (Aladdin, Shanghai, China) at a final concentration of 200 mg/L. Subsequently, skin and gills from each fish were collected under sterile conditions. Direct Squash and microscopic observation were performed using a Nikon Eclipse E200 microscope (Tokyo, Japan) at 4× and 10×. No obvious parasites were found. Subsequently, ascites, skin ulcer lesions, and organs such as the heart and liver were collected under sterile conditions for bacterial isolation and identification. Additionally, the heart, liver, and kidney of the diseased fish were collected and fixed in 4% paraformaldehyde for histopathological analysis, and stored at −80 °C for molecular biological analysis. This study only obtained samples from the second outbreak.
2.2. Bacterial Isolation
The ascites, skin ulcers, liver and heart tissues collected were, respectively, inoculated into LB medium (Hu Kai Microbiology, Guangzhou, China, C28334B). The specific composition of the medium is as shown in
Supplementary Table S1. After culturing in a constant temperature incubator at 30 °C for 48 h [
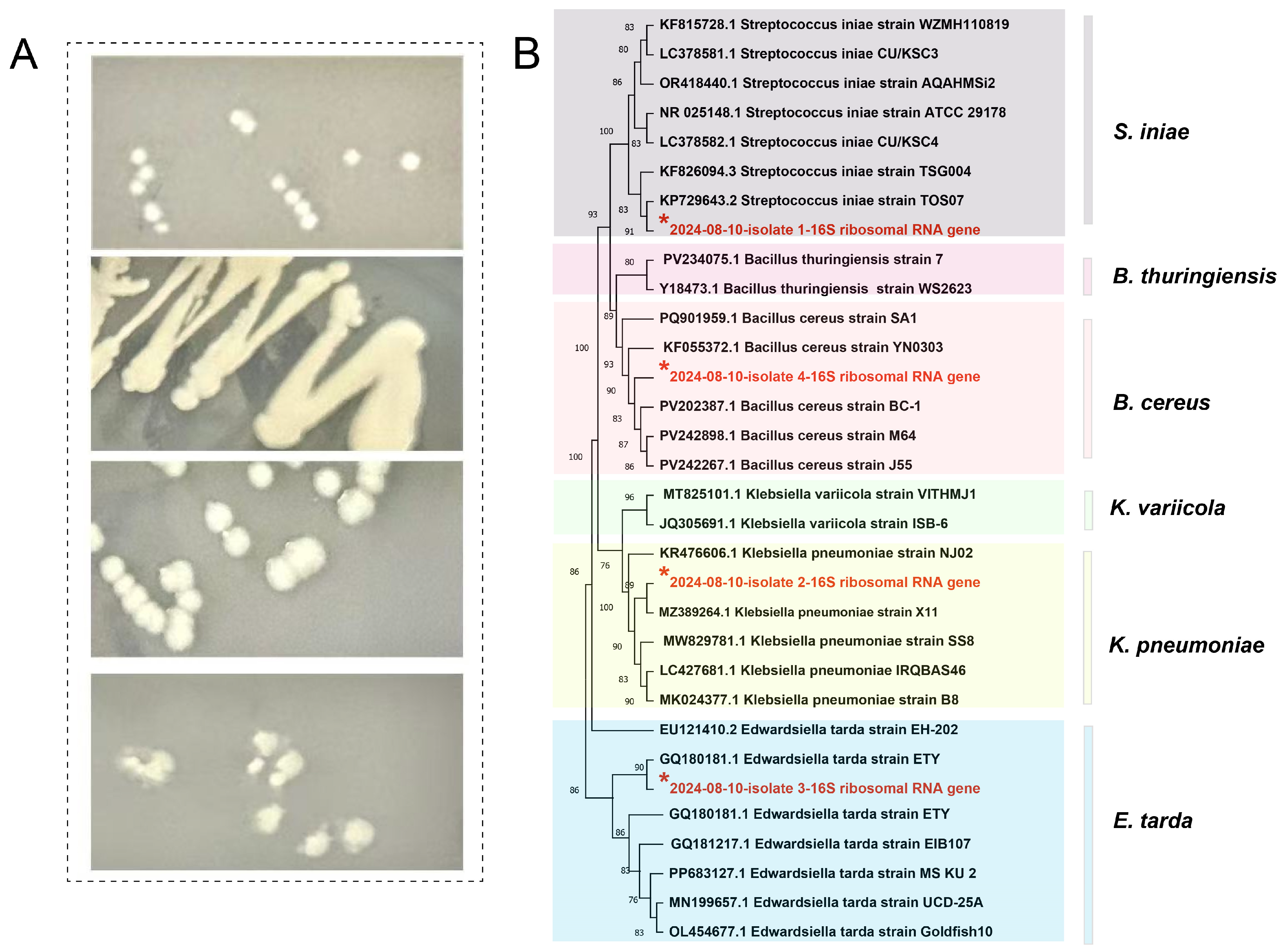
8], the morphological characteristics of the bacteria were observed. Subsequently, the colonies of well-formed and distinct isolated colonies on the LB medium were further selected and re-inoculated on fresh LB medium, and then incubated in a constant temperature incubator at 30 °C for 24 h for purification. Ultimately, 12 single colonies were successfully obtained (3 fish × 4 tissues = 12 isolates).
2.3. Bacterial DNA Extraction and Amplification
Bacterial DNA extraction and purification were carried out using the isolation kit (Foregene, Chengdu, China) in accordance with the manufacturer’s instructions. The bacterial housekeeping gene (16S rRNA gene) was selected as the target for amplification, with specific primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-TACGGCTACCTTGTTACGACTT-3′) [
9]. The PCR reaction system and thermal cycling conditions were set based on the methodologies established by Dunbar et al. [
10].
2.4. Sequencing and Phylogenetic Tree Analysis
The PCR products were collected and submitted to Sangon Biotech (Shanghai, China) for purification and sequencing. The sequences were subjected to BLAST+ (v2.15.0) alignment in the NCBI database. Phylogenetic analysis was conducted using the mega software (v11.0) and the maximum likelihood (ML) method [
11]
2.5. Quantification Determination of Bacterial Load
Specific primers were designed based on the conserved sequences of the target virulence genes of different bacteria (primer sequences and annealing temperatures are shown in
Supplementary Table S2). Using the method established by Xue et al. [
12], we quantitatively determined the bacterial load in liver, kidney, and heart tissues using the real-time fluorescence quantitative PCR system (Bio-Rad, Pleasanton, CA, USA). Briefly, standard curves were constructed by 10-fold serial dilution (10
9–10
4 copies/μL) of genomic DNA from pure cultures of each target bacterium, with copy numbers calculated based on DNA concentration (measured by Qubit) and bacterial genome size. Total DNA was extracted from liver, kidney, and heart tissues of healthy and diseased sturgeons (50 mg per sample) using a tissue Animal Tissue DNA Isolation Kit (Foregene, Chengdu, China), and adjusted to 10 ng/μL. The qPCR reaction system (20 μL) contained 10 μL SYBR Green qPCR Mix (with ROX reference dye), 0.8 μL of each primer (10 μM), 2 μL template DNA, and 6.4 μL nuclease-free water. Bacterial load (copies/g tissue) was calculated using the standard curve (R
2 ≥ 0.99, amplification efficiency 90–105%) and normalized to tissue weight using the formula as follows:
Log10 (bacterial load, copies/g) = Log10 [(Copies/μL from qPCR) × (Total DNA elution volume, μL)/(Tissue sample weight, g)]
2.6. Staining
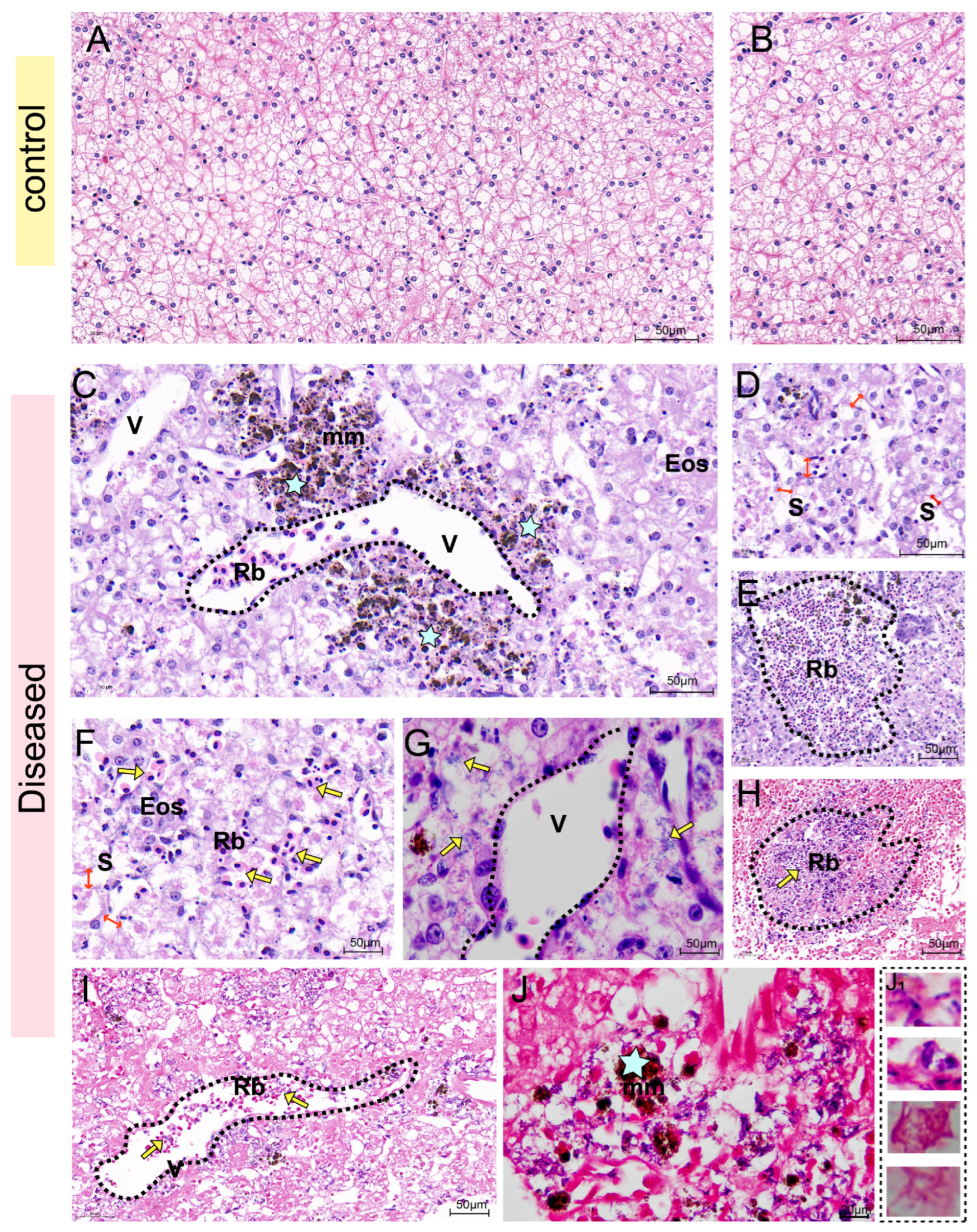
Two groups of heart, liver, and kidney samples fixed in 4% paraformaldehyde were paraffin-embedded. The paraffin-embedded eye samples were then sectioned using a Leica RM2235 microtome (Wetzlar, Hesse, Germany) to prepare tissue sections with a thickness of 5 µm. Subsequent to sectioning, the tissue sections were subjected to Hematoxylin-Eosin (H&E) Staining [
13] and Gram Staining [
14] in accordance with relevant methods, followed by sealing with neutral resin. Finally, the sealed tissue sections were observed and photographed under a Nikon Eclipse E200 (Nikon, Tokyo, Japan) with 4×, 40× and 100× objectives.
2.7. Transmission Electron Microscope (TEM) Observation
We selected the areas where the accumulation of bacteria was observed, melted the wax within that area and immersed it in pure acetone. Subsequently, the liver, kidney and heart tissues were washed with PBS, fixed with 1% osmic acid, dehydrated with gradient acetone, replaced and embedded. Then, the samples were stained with 1% uranyl acetate for 20 min, rinsed with ddH2O twice, followed by staining with 1% lead citrate for 20 min, and another two washes with ddH2O. Finally, the ultrastructure of the bacteria was observed by HITACH type I transmission electron microscope under 80 kV. Images were captured and collected for subsequent analysis.
2.8. Antibiotic Sensitive Test
The Kirby–Bauer disk diffusion method was used to assess the sensitivity of isolated strains to 14 common antibiotics. Strains were cultured in LB broth at 30 °C for 24 h, adjusted to 1.5 × 10
8 CFU/mL (0.5 McFarland standard) in sterile PBS, and uniformly swabbed onto LB plates. Antibiotic disks (Oxoid Ltd., Basingstoke, Hampshire, UK) were aseptically placed, and plates were incubated at 30 °C for 48 h. Inhibition zones were measured with a vernier caliper, and antibiotic sensitivity was regarded as susceptible (S), intermediate (I), or resistant (R) according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (M100-S26, 2016) [
15]. All tests were conducted in triplicate, and
Escherichia coli ATCC 25922 was the control strain.
2.9. Data Analysis and Statistics
In this study, all data were presented as mean ± SD (standard deviation). Charts were drawn by GraphPad Prism (v8.0, GraphPad Software, Inc., San Diego, CA, USA) and Adobe lllustrator (v 28.7.8, Adobe Inc., San Jose, CA, USA). The experimental data were first subjected to normality tests. Then, independent sample t-tests were used to evaluate the significance of the differences, and the significance level was set as p < 0.05.
4. Discussion
4.1. The Transition from Single Pathogen to a Multi-Pathogen Disease Pattern
During the second outbreak in 2024, four types of bacteria were isolated from the tissues of diseased Yangtze sturgeon: S. iniae, K. pneumoniae, E. tarda, and B. cereus. Through histopathological and ultrastructural pathological analyses, the co-localization of these four bacteria in the necrotic lesions was determined. Additionally, bacterial load analysis indicated that all four pathogenic bacteria were not only present in the lesion tissues, but their detection rates were highly consistent with the severe clinical symptoms exhibited by the diseased sturgeons (hemorrhagic septicemia, organ necrosis, acute death). These findings collectively suggest a strong association between these four bacterial species and the disease outbreak.
Furthermore, our research revealed the phenomenon of severe polymicrobial infection in the Yangtze sturgeon, marking a significant shift in the traditional understanding of the Yangtze sturgeon’s bacterial ecology. Previous studies mostly documented pathological and physiological conditions related to a single pathogen, such as
A. hydrophila and
E. tarda [
4,
5]. Although these studies were very valuable, they essentially focused on one-on-one “host–pathogen” conflicts. We discovered a co-infection involving
S. iniae,
K. pneumoniae,
E. tarda, and
B. cereus, which provides a more complex and potentially more accurate explanation for the observed acute, multi-systemic damage.
4.2. The Isolated Strains in This Study Pose a Serious Threat to Aquatic Ecosystems
The four bacteria isolated in this study are not only significant pathogens in aquaculture but also zoonotic agents [
16]. They frequently trigger sudden disease outbreaks in farmed fish, posing a serious threat to the aquatic ecosystems. Therefore, appropriate measures must be implemented to prevent and control the infection and spread of these pathogenic bacteria [
17,
18].
Among the identified pathogenic bacteria,
S.iniae is an important primary pathogen that can infect a variety of wild and farmed fish. Due to its high pathogenicity and fatality rate, it has caused huge economic losses to the global aquaculture industry [
19]. Although
S. iniae typically induces chronic infections in fish (such as exophthalmia or corneal opacity) [
20], it can also provoke acute manifestations such as acute septicemia [
21,
22]. It has also been reported to be susceptible to cefotaxime, erythromycin, ofloxacin, penicillin, and vancomycin, but resistant to florfenicol [
23]. In the present study, infected sturgeon exhibited acute systemic symptoms, including ulcerative skin lesions, hemorrhagic septicemia, diffuse organ necrosis, and acute death. Thus, under high-temperature stress,
S. iniae likely triggered acute infection in this species.
Notably,
K. pneumoniae [
24],
E. tarda [
25], and
B. cereus [
26] are opportunistic pathogens in aquaculture, capable of causing severe disease under compromised host conditions. Recently,
K. pneumoniae has been proven capable of infecting a variety of aquatic animals, such as causing renal necrosis, structural changes and tubular swelling in
Labeo rohita [
27], as well as hepatocyte vacuolization and systemic infection in
Amphiprion nigripes [
28]. Moreover, it has been reported to be highly resistant to carbapenem antibiotics [
24].
E. tarda triggers Edwardsiellosis, which is usually associated with sudden outbreaks and high mortality rates of fish diseases, leading to symptoms such as surface ulcers, visceral necrosis and systemic infections [
17]. It has also been reported to be sensitive to chloramphenicol, ciprofloxacin, and streptomycin, but resistant to amoxicillin, erythromycin, and flumequine [
29]. In recent years, although the application of
B. cereus as a probiotic has also witnessed a gradual upsurge [
30], its pathogenic potential has been confirmed: this bacterium can be isolated from the internal organs or lesions of diseased fish, causing hemorrhagic septicemia, skin ulcers, and red head in fish [
31]. Furthermore, reports on the antibiotic sensitivity of its pathogenic strains remain relatively scarce. In this study, all three species co-occurred in diseased sturgeon, manifesting hemorrhagic septicemia, cutaneous ulcers, and systemic infections. This consistent with their opportunistic nature where environmental stress or host immunodeficiency enables virulence.
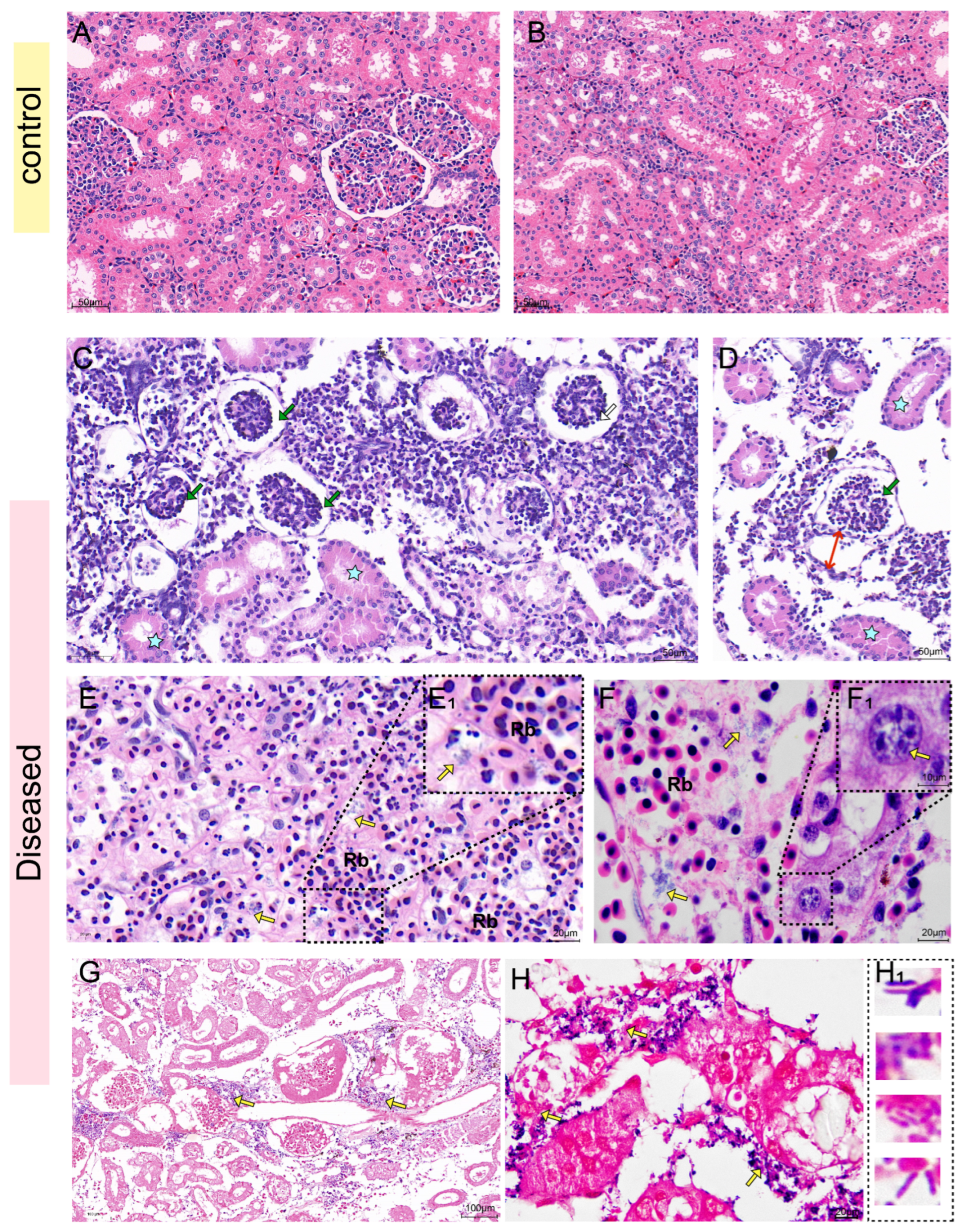
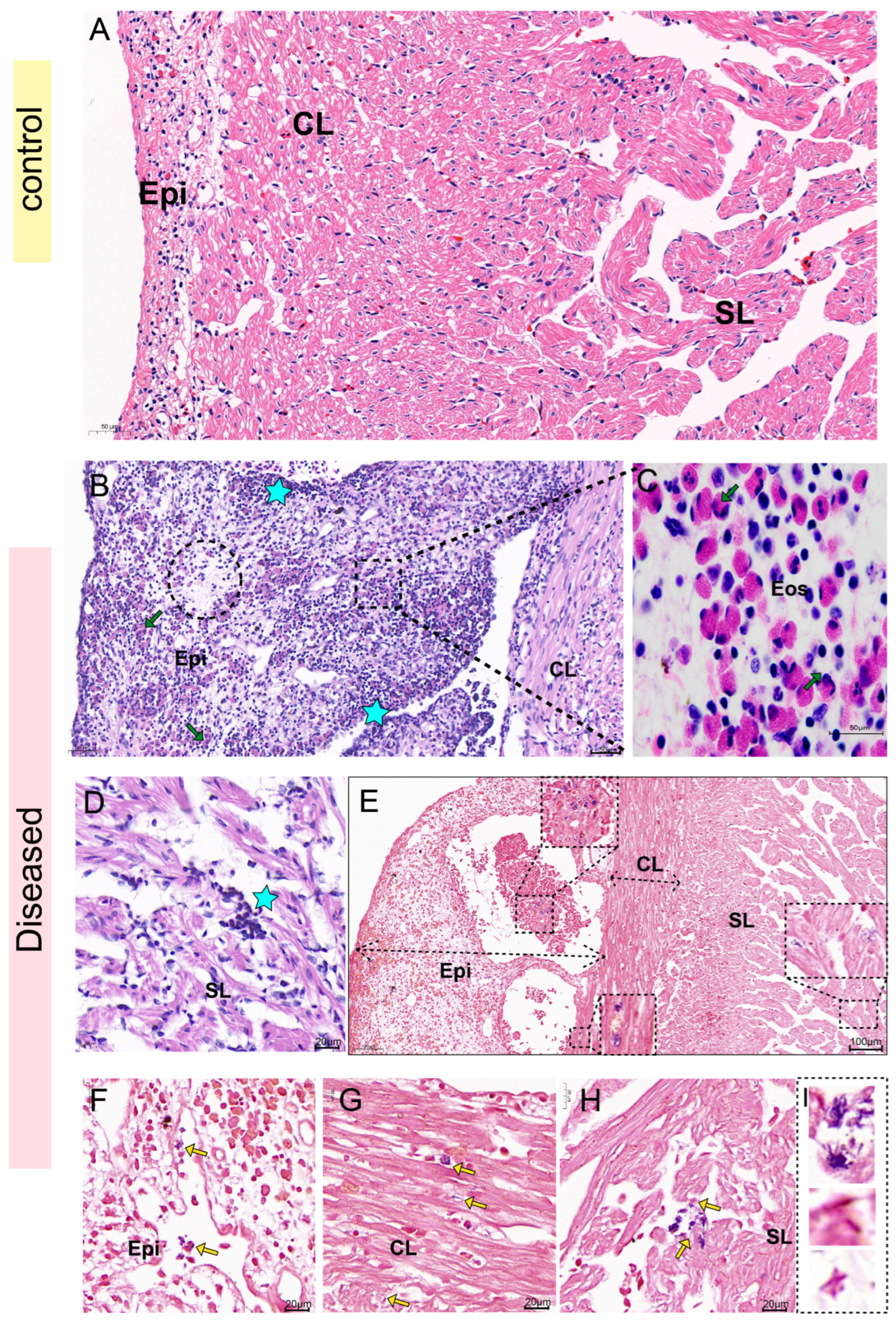
Histopathological examination in this study revealed co-localization of S. iniae with opportunistic pathogens within necrotic lesions. Collectively, these observations lead us to hypothesize that: S. iniae first exploited the mucosal immune system damaged by high temperatures, leading to micro-ulcers and vascular damage, thereby facilitating systemic dissemination. Subsequently, some opportunistic pathogens (such as K. pneumoniae) colonized in the damaged tissues via bacterial synergism, exacerbating the lesion. However, it should be noted that due to the lack of bacterial load analysis during the early stage of the disease, the chronological sequence hypothesis proposed in this study is merely a speculation based on the existing indirect evidence, rather than an absolute conclusion.
4.3. Possible Causes of the Disease Outbreak
The disease outbreak coincided with severe environmental deterioration, which critically increased the host’s susceptibility to infection. As a cold-water species, the Yangtze sturgeon has an optimal growth temperature of 20–22 °C [
32]. The persistently high water- temperature of approximately 30 °C during the outbreak subjected the fish to intense thermal stress, disrupting internal homeostasis and causing dysregulation of the immune system. This physiological compromise significantly heightened the host’s vulnerability to pathogens. From an ecological perspective, the same elevated temperature provided ideal thermodynamic conditions for the rapid proliferation of the four mesophilic bacterial pathogens isolated in this study [
33]. effectively shifting the host–pathogen balance in favor of the latter.
Furthermore, the high temperature is likely to have accelerated the decomposition of the feed, leading to eutrophication and deterioration of water quality. As the water quality data on the sampling day showed (dissolved oxygen: 5.7 mg/L, pH: 8.83, ammonia nitrogen: 1.07 mg/L, nitrite: 0.41 mg/L), it clearly indicates that the environmental conditions have seriously deteriorated, which in turn promotes the reproduction and adaptation of bacteria.
Moreover, high humidity during the outbreak weakened the protective function of the sturgeon’s body surface mucous layer, reducing its viscosity and antibacterial activity, which in turn facilitated bacterial attachment and invasion. Simultaneously, the high-humidity environment favored bacterial survival, reproduction, and virulence. It also expanded the pathogens’ transmission routes (such as diffusion via water flow or aerosol formation) that enables respiratory invasion [
34], which greatly aggravates the complexity of the disease.
Considering the high pathogenicity of
S. iniae and the opportunistic potential of
K. pneumoniae,
E. tarda, and
B. cereus [
35], we hypothesize that the high-temperature and high-humidity during the summer were the key factors contributing to water quality deterioration and the outbreak of this disease. It was precisely the deterioration of environmental conditions and the reduced immunity of the host that enabled the bacteria already existing in the aquaculture environment to multiply and trigger serious infections.
4.4. The Risk of Cross-Host–Pathogen Transmission in the Aquaculture Industry
In this study, phylogenetic analysis revealed that the S. iniae isolate from this research clustered with the diseased Trachinotus ovatus (golden pomfret) strain (KP729643.2) from Zhanjiang, Guangdong Province, and exhibited a high degree of homology. This suggests potential cross-species transmission risks of pathogens between the Yangtze sturgeon and marine aquaculture fish species. Within the interaction between southern coastal mariculture and inland freshwater aquaculture in China, frequent seed transportation is often accompanied by the migration of live carriers harboring pathogens, while feed may serve as vectors for pathogen attachment and dissemination during production, transportation, and feeding processes. These factors collectively establish potential pathways for cross-host transmission of S. iniae, posing a threat to the diverse cultured species within the aquaculture industry.
The K. pneumoniae isolate identified in this study demonstrated high homology to a strain (MZ389264.1) originating from Chengdu Giant Panda Breeding Station. Although there is no direct water connection between the two bases, residual K. pneumoniae in water and soil within giant panda habitats may undergo natural dispersal via tributaries of the Yangtze River system and agricultural irrigation networks of the Sichuan Basin (such as the Min River-Dujiangyan water system), consequently manifesting as clustering on the phylogenetic tree. This phenomenon not only reflects the bacterium’s robust environmental adaptability and dispersal capacity but also highlights the potential for cross-transmission of pathogens between aquaculture environments and terrestrial wildlife habitats.
The E. tarda isolate in this study shared high homology with a strain (GQ180181.1) isolated from eel in Fuzhou, Fujian Province. This is presumably attributed to the cross-provincial circulation of seedlings (such as introduction of eel fry into Sichuan) and exchanges of aquaculture technologies in China’s freshwater aquaculture industry. Such cross-provincial seedling circulation enables pathogens to traverse geographical barriers alongside their hosts. Moreover, If the tools and equipment used in the exchange of aquaculture techniques are not thoroughly sterilized, they may also act as transmission vectors. Consequently, E. tarda maintains its potential for transmission and pathogenicity across diverse hosts and geographical regions.
However, it must be clearly emphasized that phylogenetic relatedness only reflects correlations in strain distribution and does not equate to a clearly demonstrated transmission path. Future work should prioritize the use of advanced techniques such as whole-genome sequencing to conduct in-depth investigations into potential genetic differences between different strains. Concurrently, it is essential to analyze the molecular mechanisms underlying host specificity and the evolutionary characteristics of these strains, thereby enhancing the ability to interpret the transmission chain.
4.5. The Antibiotic Resistance (AMR)
Antibiotic treatment represents one of the primary measures for disease prevention and control in aquaculture industry. However, AMR has become an important threat to the sustainable development of the global aquaculture industry. Due to the intrinsic connection between aquaculture systems and open water bodies such as rivers and lakes, antimicrobial use (AMU) in aquaculture has greater potential for environmental transmission compared to terrestrial animals, thereby having a more significant impact on ecosystems and public health [
36]. In high-density intensive aquaculture, although the preventive use and therapeutic abuse of antibiotics aim to prevent and treat bacterial infections in fish, they form a vicious cycle of “drug use—evolution of drug resistance” in the aquaculture environment [
37]. The current spread of drug resistance genes (ARGs) caused by the abuse of antibiotics not only intensifies the difficulty of disease prevention and control, but also promotes the horizontal transfer of drug resistance genes in the flora, and threatens public health and ecological security through the food chain and environmental pollution [
38].
Our antimicrobial susceptibility testing revealed that the isolated
S. iniae,
K. pneumoniae,
E. tarda, and
B. cereus strains all exhibited MDR, particularly to florfenicol, tetracycline, and ampicillin, which are empirically and frequently used in clinical aquaculture [
39], even when the specific pathogen type or drug sensitivity results are unclear [
40]. This MDR pattern aligns with the historical and widespread use of these antibiotics in Chinese aquaculture, as evidenced by high detection rates of residues in aquatic products [
41]. These findings underscore the urgent need to adopt a “One Health” approach to AMR management in aquaculture.
4.6. Recommendations for Disease Prevention, Management, and Conservation
Notably, a critical gap exists between in vitro lab efficacy and real-world application feasibility of the sensitive antibiotics we identified. While all four pathogens showed high sensitivity to ceftazidime, ceftriaxone, and ciprofloxacin in lab tests, China’s veterinary drug regulations explicitly prohibit the use of the first two in aquaculture. This restriction rendered only ciprofloxacin hydrochloride and berberine hydrochloride premix as viable therapeutic options for the 2024 outbreak.
For a Class I protected species like the Yangtze sturgeon, strict adherence to veterinary drug regulations is non-negotiable, as any unapproved antibiotic use could introduce toxic drug or ARGs into wild populations via stock enhancement programs, endangering the species’ long-term recovery. Consequently, there is an urgent need to prioritize non-antibiotic, preventive strategies aligned with the “One Health” principle.
The following comprehensive measures are recommended: First, conduct proactive monitoring of meteorological trends to anticipate thermal stress events; second, use shading facilities or automated cooling systems to alleviate heat stress; third, strictly disinfect the circulating water (for example, through ultraviolet irradiation) to limit pathogen persistence; fourth, develop Chinese herbal medicine alternative therapies and precise vaccine control [
42]. Such approaches are essential to minimize antibiotic reliance and mitigate the dissemination of ARGs.
4.7. The Limitations of This Study and the Future Directions
Regrettably, as an emergency field investigation addressing the Yangtze sturgeon bacterial disease outbreak, this study has notable limitations that need to be clearly pointed out. First, in terms of sample representativeness: two unexplained mortality events occurred in the Yangtze sturgeon population in Sichuan between July and August 2024 (with similar clinical symptoms). However, access to samples was only granted during the second outbreak. Consequently, our analysis is based solely on the samples collected from that single event, which may affect the generalizability of the findings. We cannot confirm whether the polymicrobial co-infection identified herein (the second outbreak) is consistent with the etiology of the first outbreak, nor can we rule out the potential variations in virulence gene expression or host–pathogen interaction patterns that may have existed between the two events.
Second, legal and ethical regulations protecting this critically endangered species precluded infection experiments to fulfill Koch’s postulates. Consequently, we cannot establish a definitive causal link or infection chronology. Therefore, the proposed roles of the isolated bacteria in the disease outbreak should be interpreted as strong correlations inferred from multiple lines of evidence, rather than proven causality.
Third, our pathogen analysis relied solely on culturable bacteria (the four isolated species bacteria) and lacked metagenomic or NGS-based profiling. This approach failed to capture non-culturable bacteria, low-abundance pathogens, or potential co-existing viruses and fungi, which limits ecological interpretation.
To address these limitations and improve the applicability of future research, our follow-up work will prioritize three directions: First, conduct metagenomic or NGS-based profiling to capture the complete pathogen community and clarify whether these uncaptured microbes interact with the four isolated bacteria to exacerbate disease. Second, select healthy Siberian sturgeon (Acipenser baerii) a non-protected species closely related to the Yangtze sturgeon) to conduct a time-series mixed infection study using the four pathogenic bacteria isolated in the original research, to verify the chronological sequence. Third, select the liver and kidney organs for transcriptome analysis, and conduct correlation analysis between the expression levels of DEGs, the bacterial load and the degree of tissue pathological damage, to clarify the association pattern between “pathogen bacterial load, host immune response, and pathological damage”.
Despite these limitations, the strong correlative evidence obtained in this study, including pathogen co-localization in lesions, bacterial load detection, and alignment with clinical symptoms, still provide valuable insights into the potential causes of the epidemic outbreak. Our study provides the first evidence of the four-pathogen co-infection in Yangtze sturgeon, and indicates that their presence is related to the environmental stress of high temperature and high humidity, offering important reference for emergency disease control and ecological conservation of this critically endangered species.