Evaluating the Response to Cryopreservation of Ovine Fibroblast Spheroids
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
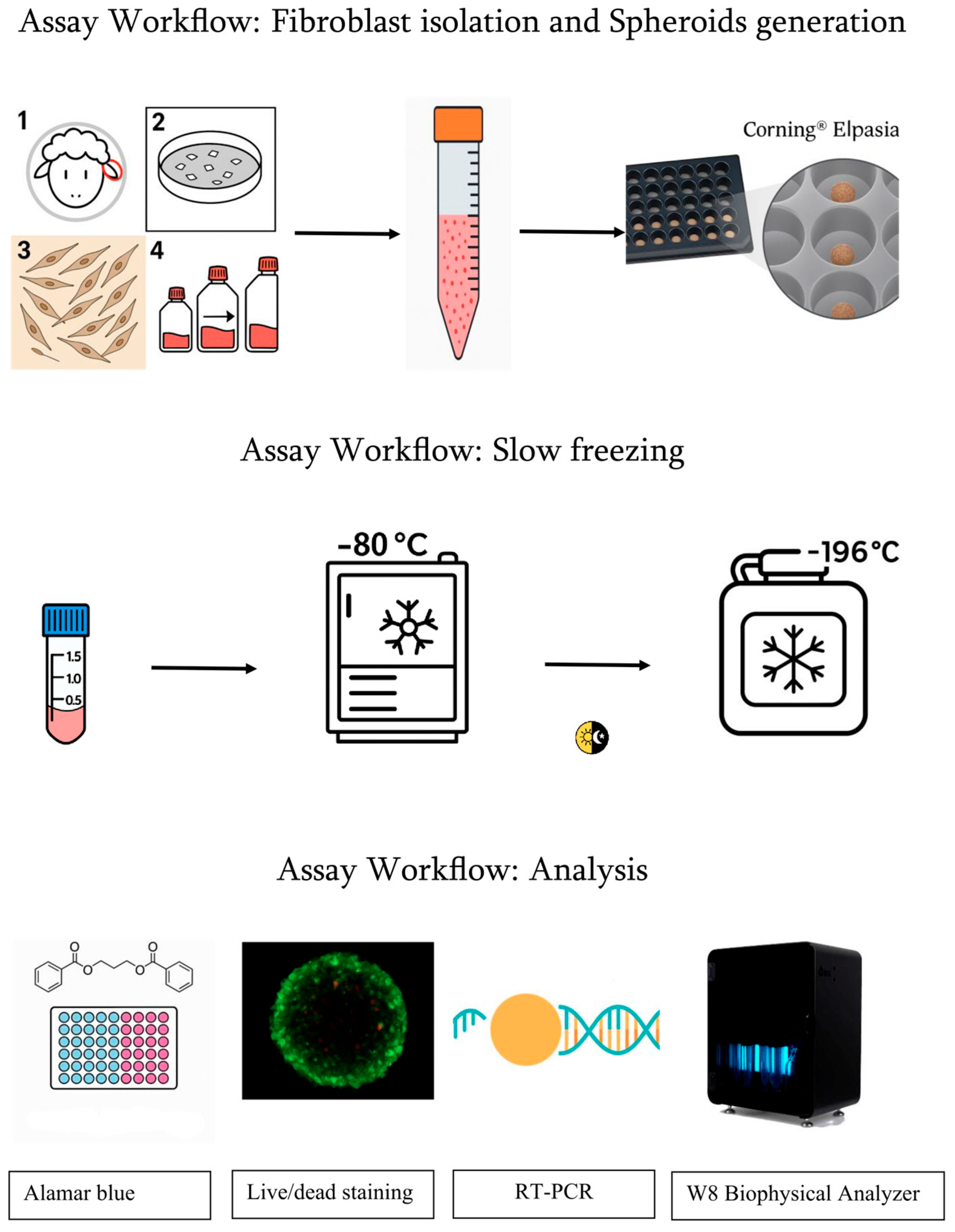
2.1. Fibroblast Isolation
2.2. Spheroid Generation
- 1 × 105 cells per microplate (≈2000 cells per microwell) 140-micron group.
- 1 × 106 cells per microplate (≈4000 cells per microwell) 220-micron group.
2.3. Cryopreservation by Slow Freezing and Thawing
- On day 6, the first third of CTRL spheroids was harvested and assayed, providing the 24 h control data set.
- On day 7, the second third was collected for the 48-h control endpoint as the frozen vials were moved from −80 °C to liquid nitrogen.
- On day 8, the final third of CTRL spheroids was harvested for the 72-h control analysis.
2.4. Alamar Blue Assay
2.5. Replating Assay
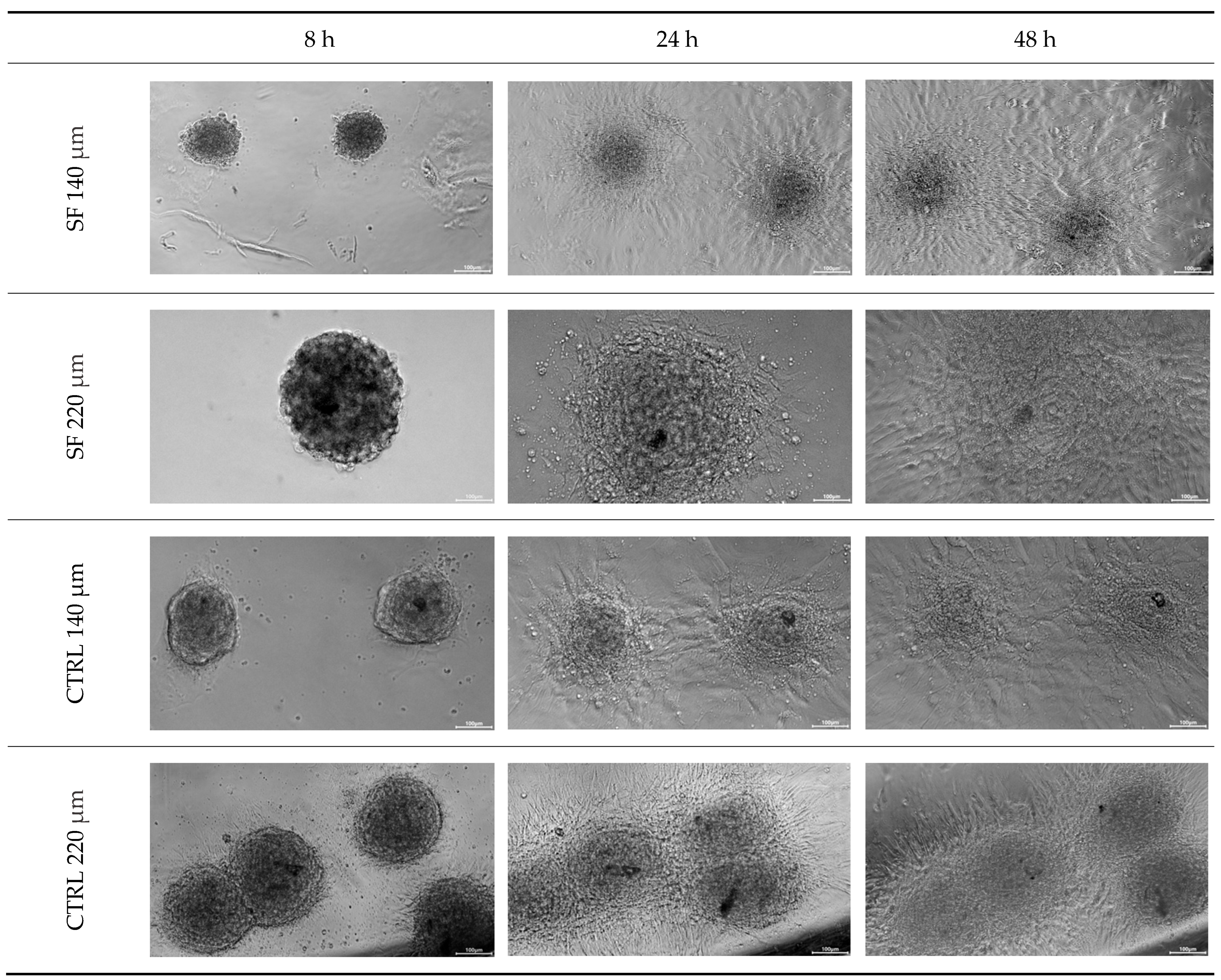
- Time points: 8, 24 and 48 h post-seeding.
- Scoring criteria: a spheroid was counted as “re-plated” when a radial monolayer had spread ≥ 1 spheroid diameter beyond the aggregate edge.
2.6. Live/Dead Staining
2.7. W8 Biophysical Analyzer
2.8. Gene Expression Analysis
2.8.1. RNA Isolation and Reverse Transcription
2.8.2. Real-Time Polymerase Chain Reaction
- 7.5 µL of 2× Quantinova SYBR Green PCR Master Mix (Qiagen);
- 200 nM of each primer (forward and reverse; Table 1);
- 1 µL of cDNA equivalent to ~1 spheroids.
2.9. Statistical Analysis
- Alamar Blue® assay and W8 Analyzer measurements: differences between CTRL and SF spheroids at each time point (24, 48, 72 h) were analyzed by two-tailed, unpaired Student’s t-test. Outliers were excluded when K > 1.5.
- Gene expression data: after verification of distribution (Kolmogorov–Smirnov test), comparisons were performed with a General Linear Model ANOVA.
- Significance threshold: p < 0.05.
- Figure notation: statistical significance is indicated by * (p < 0.05), ** (p < 0.01), *** (p < 0.001).
- Replicates: For each experimental condition, three independent biological replicates were included, with multiple spheroids analyzed per replicate as specified in the Materials and Methods subsections.
3. Results
3.1. Replating
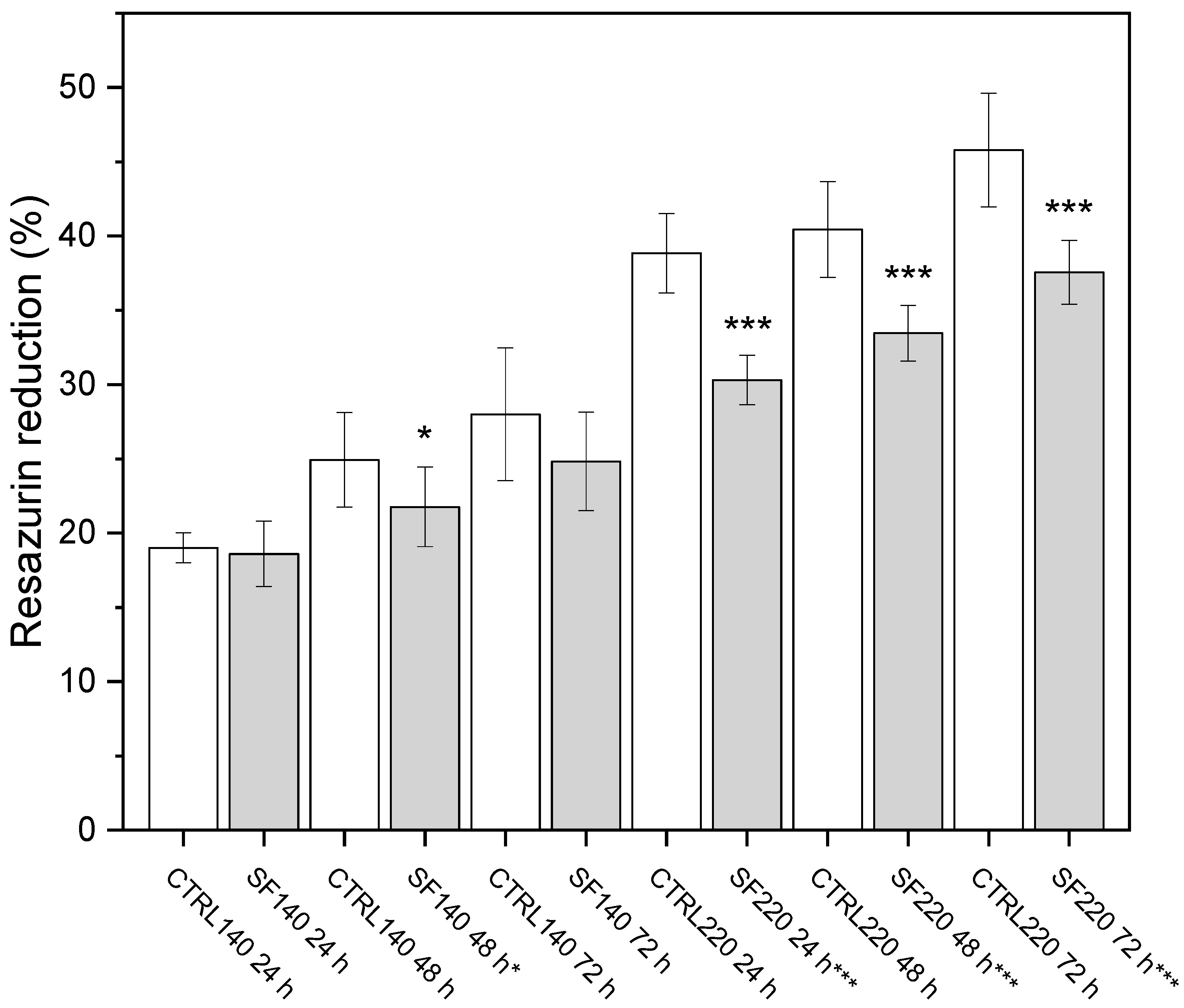
3.2. Alamar Blue
- (A) 140 µm spheroids: a modest decline becomes significant only at 48 h (p < 0.05).
- (B) 220 µm spheroids: activity is significantly lower at every time point (** p < 0.001). Scale bar not applicable.
3.3. W8 Biophysical Analysis
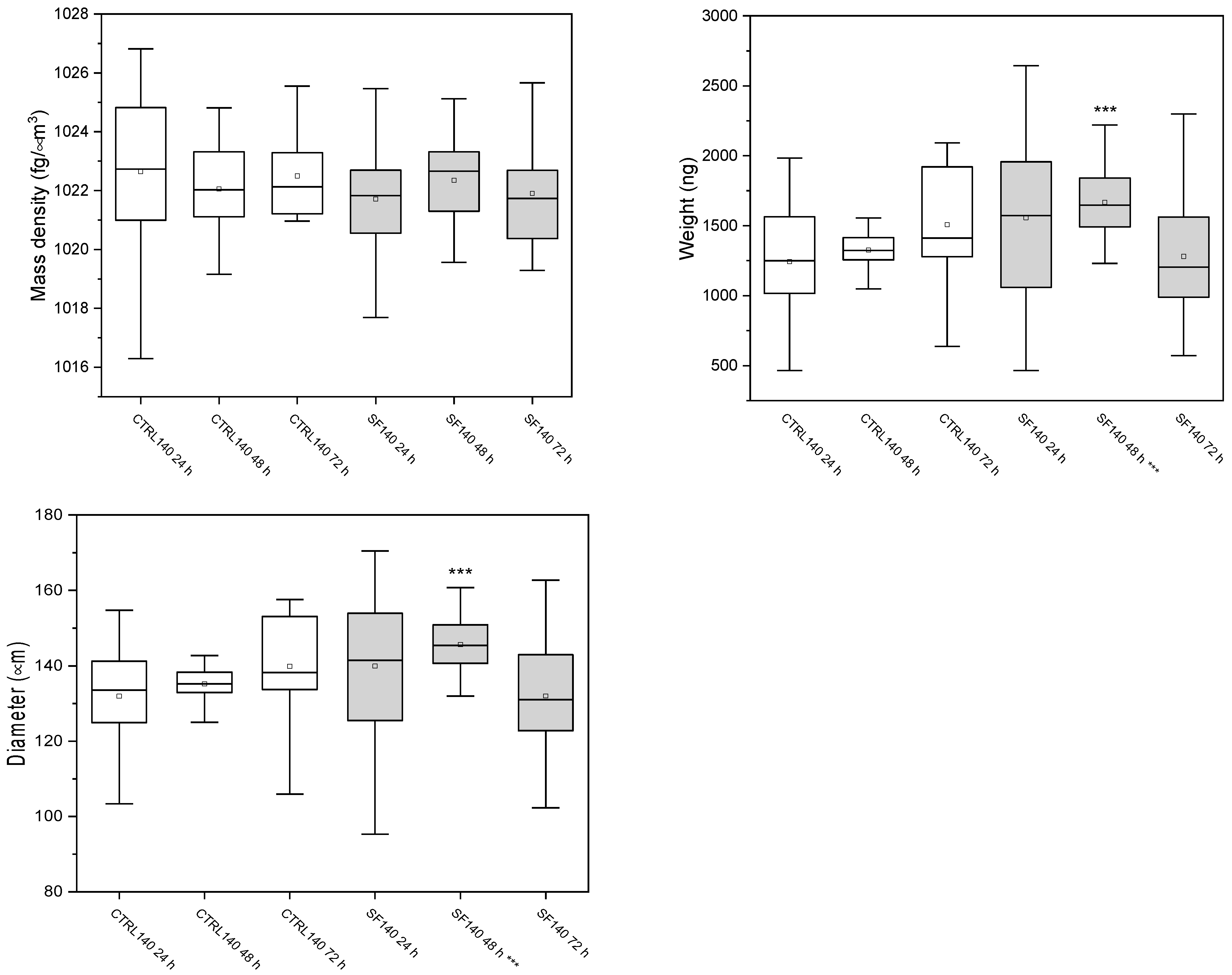
3.3.1. Spheroids of 140 μm
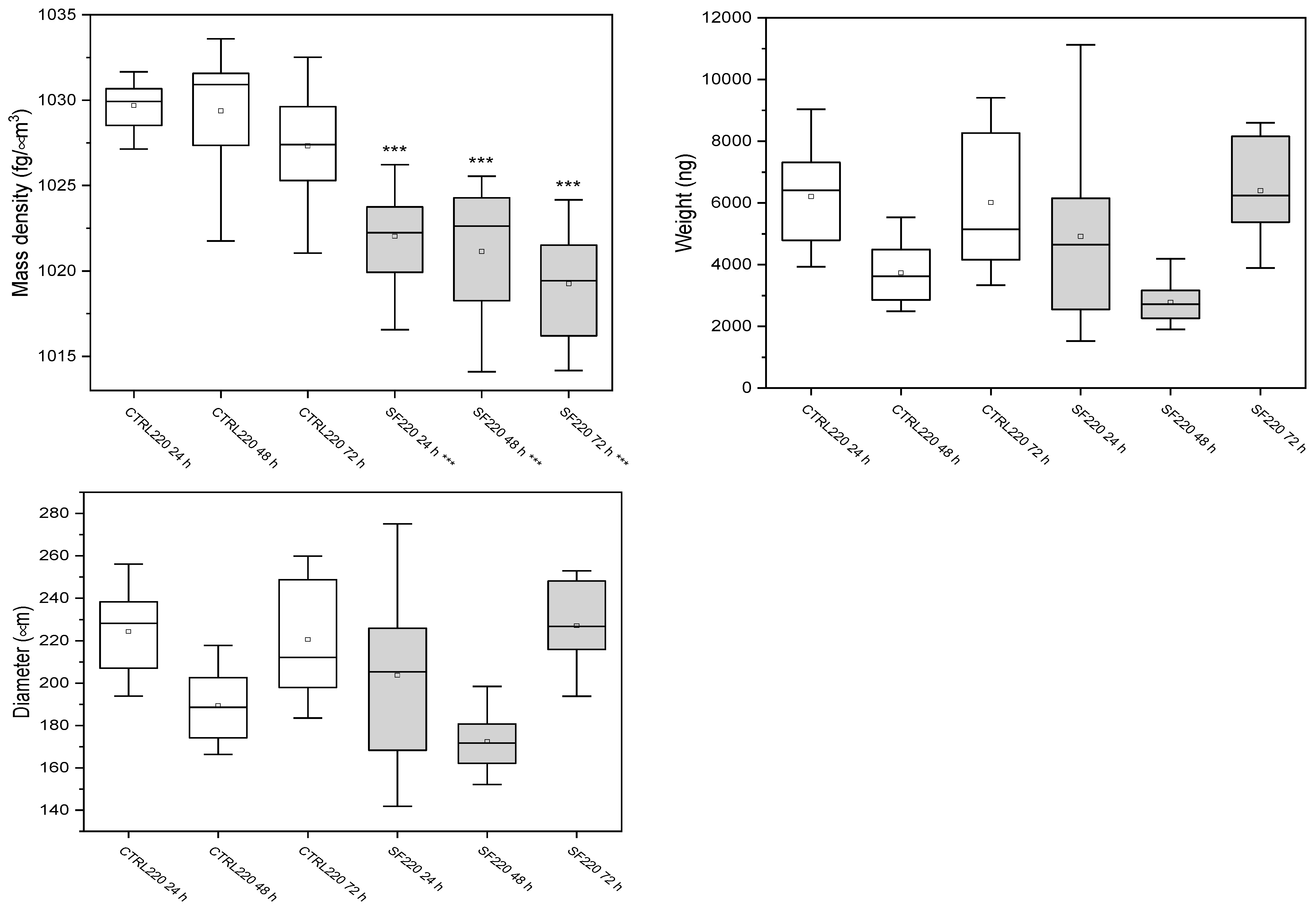
3.3.2. Spheroids of 220 μm
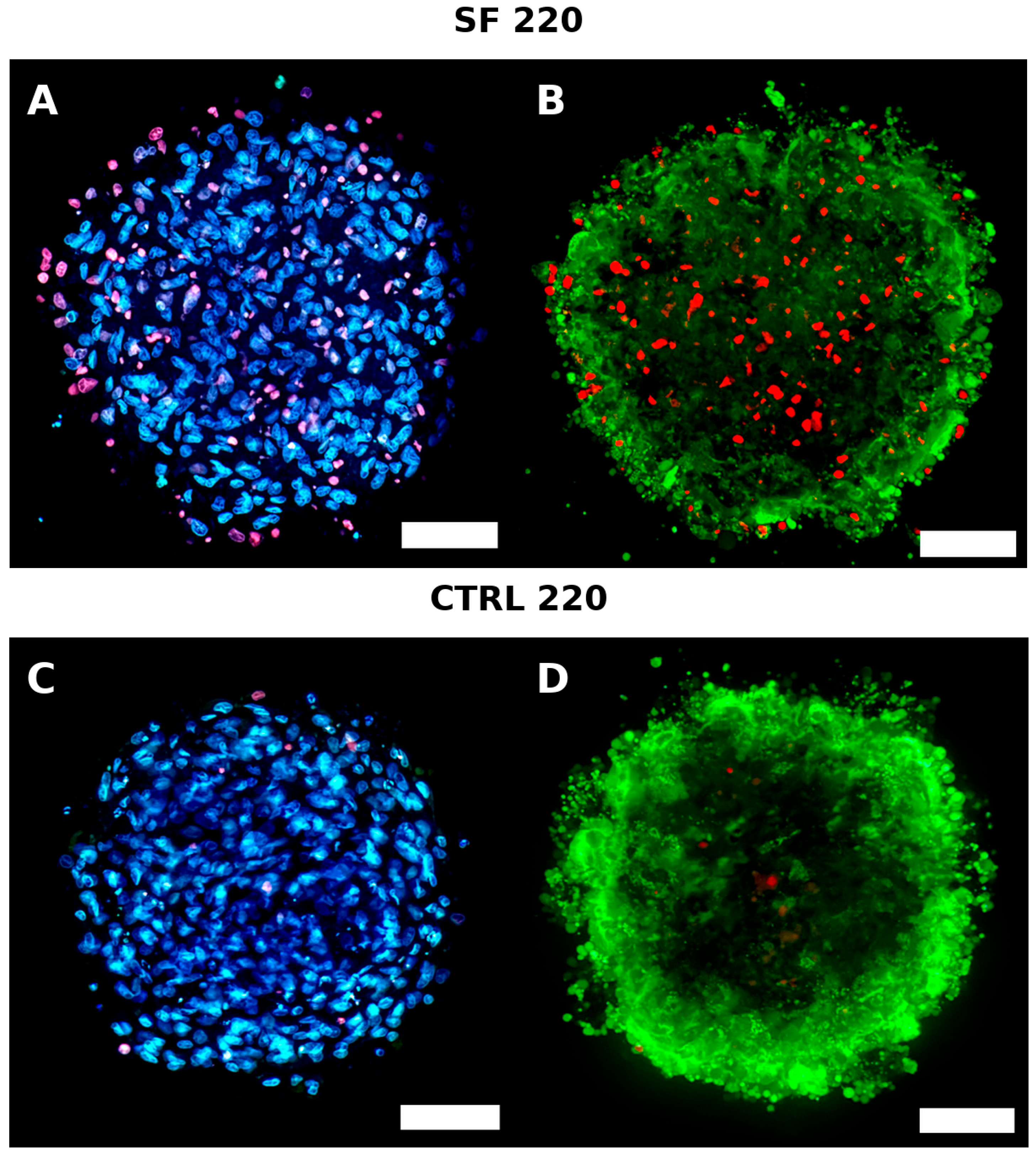
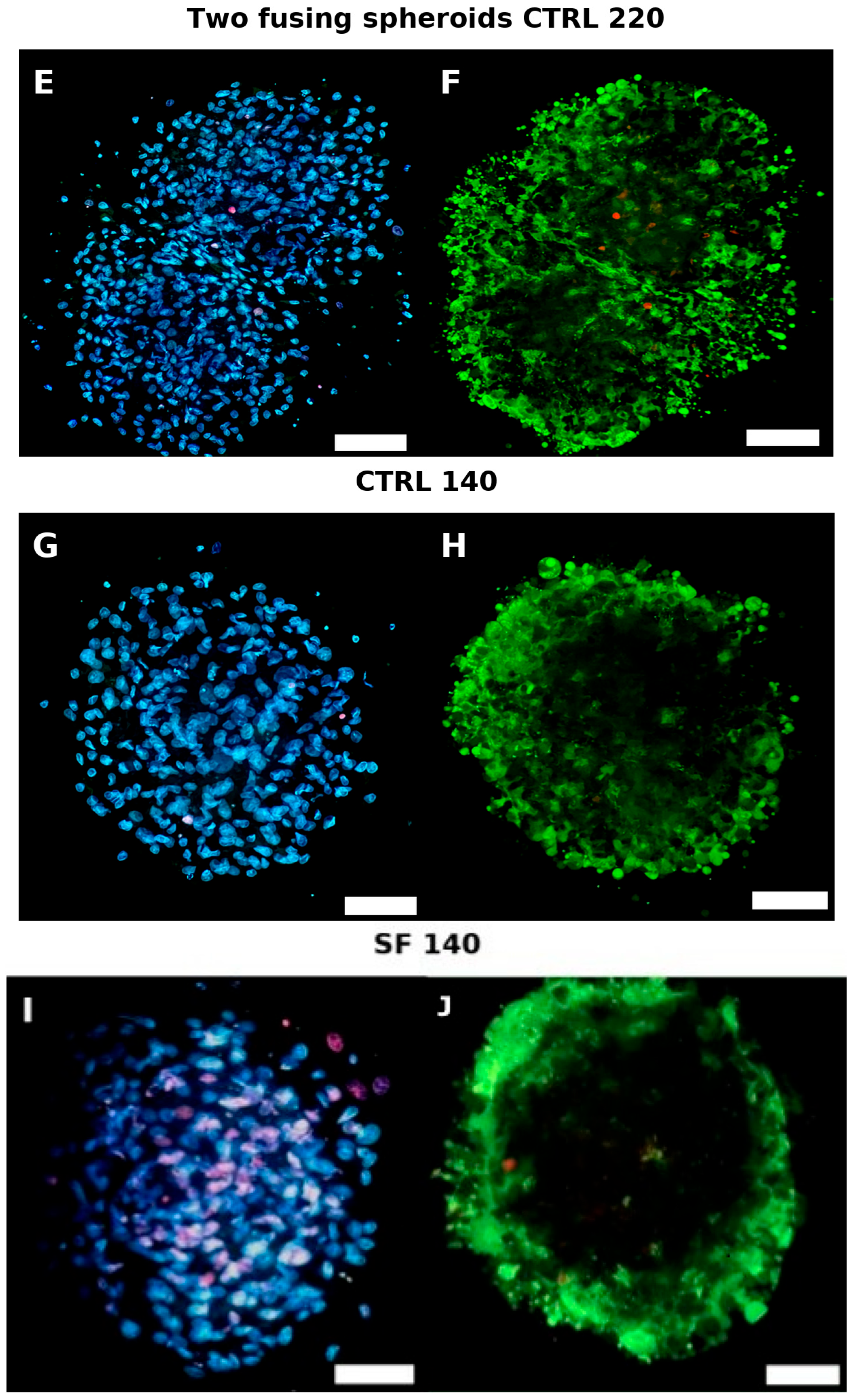
4. Live Dead
4.1. Live/Dead Imaging
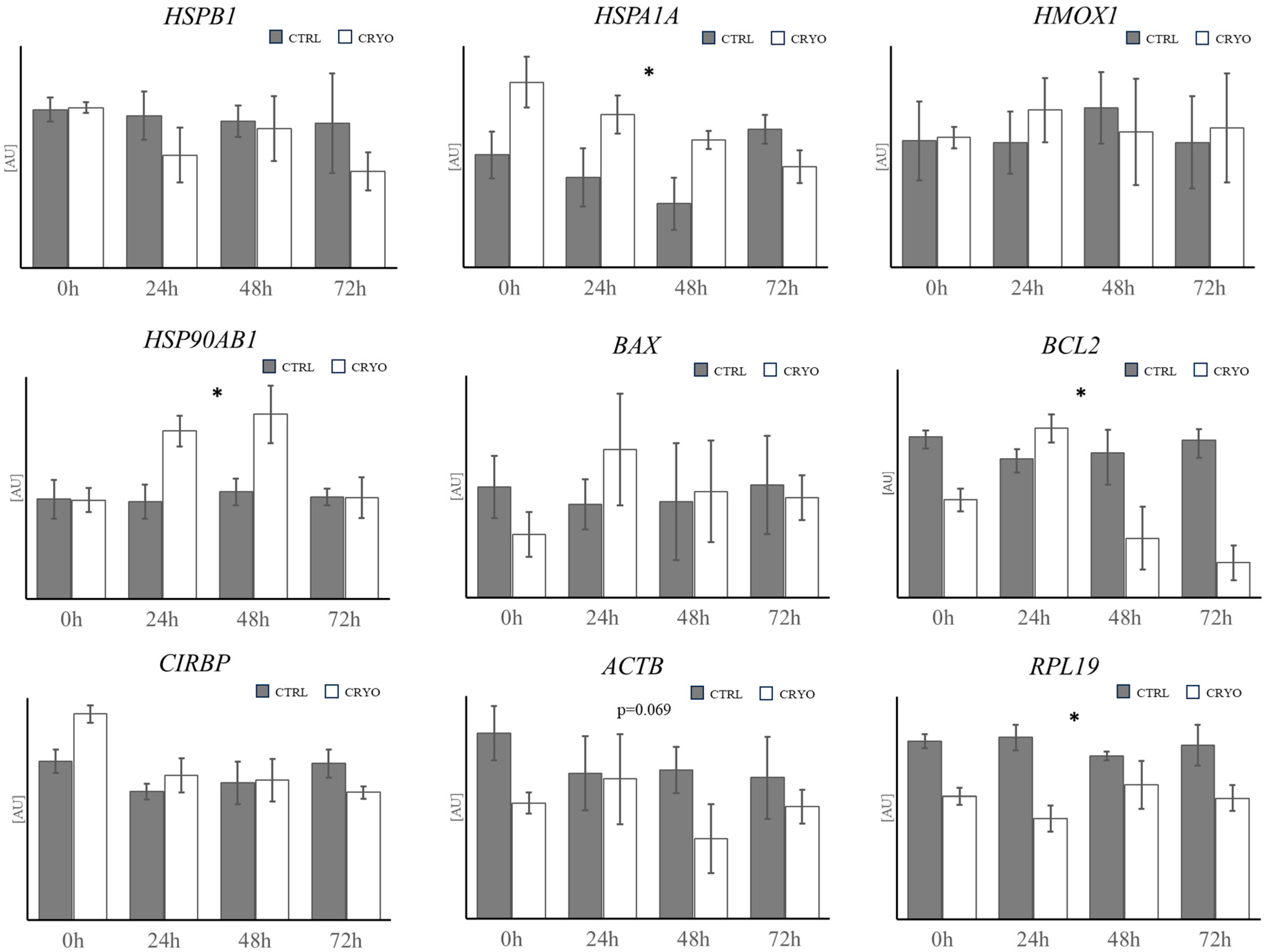
4.2. Gene Expression Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTRL | Control |
| SF | Slow frozen |
| DMSO | Dimethyl sulfoxide |
| RT-PCR | Real-time polymerase chain reaction |
| SD | Standard deviation |
| SEM | Standard error of the mean |
References
- Pennarossa, G.; Arcuri, S.; De Iorio, T.; Gandolfi, F.; Brevini, T.A.L. Current Advances in 3D Tissue and Organ Reconstruction. Int. J. Mol. Sci. 2021, 22, 830. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D Cell Cultures—A Comparison of Different Types of Cancer Cell Cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The Third Dimension Bridges the Gap between Cell Culture and Live Tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the Third Dimension—How 3D Culture Microenvironments Alter Cellular Cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Scaffold-Based 3D Cell-Culture Models in Cancer Research. Biomater. Sci. 2019, 7, 1187–1205. [Google Scholar] [CrossRef]
- Marx, U.; Sandig, V. Drug Testing In Vitro: Breakthroughs and Trends in Cell Culture Technology; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Day, J.G.; Stacey, G.N. Cryopreservation of Animal and Human Cell Lines. In Cryopreservation and Freeze-Drying Protocols; Walker, J.M., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2007; Volume 368, pp. 227–236. [Google Scholar]
- Finger, E.B.; Bischof, J.C. Cryopreservation by Vitrification: A Promising Approach for Transplant Organ Banking. Curr. Opin. Organ Transplant. 2018, 23, 353–360. [Google Scholar] [CrossRef]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and Its Clinical Applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef]
- Arai, K.; Murata, D.; Takao, S.; Verissimo, A.R.; Nakayama, K. Cryopreservation Method for Spheroids and Fabrication of Scaffold-Free Tubular Constructs. PLoS ONE 2020, 15, e0230428. [Google Scholar]
- Dutta, D.; Daugherty, M.D.; He, L.; Klim, J.D.; Zhang, J. Comparison of Vitrification and Conventional Freezing for the Cryopreservation of Human Pluripotent Stem-Cell-Derived Cardiomyocytes in Spheroids. Cryobiology 2020, 101, 199–207. [Google Scholar]
- Zhang, Y.; Liu, Z.; Chen, Z.; Hu, D. Cryopreservation of Human Pluripotent Stem-Cell-Derived Retinal Pigment Epithelial Cells in Spheroids Using Polyvinyl Alcohol as a Cryoprotectant. Cryobiology 2020, 95, 114–121. [Google Scholar]
- Jeong, Y.H.; Kim, U.; Lee, S.G.; Ryu, B.; Kim, J.; Igor, A.; Kim, J.S.; Jung, C.R.; Park, J.H.; Kim, C.Y. Vitrification for Cryopreservation of 2D and 3D Stem-Cell Cultures Using High Concentrations of Cryoprotective Agents. BMC Biotechnol. 2020, 20, 45. [Google Scholar] [CrossRef]
- Wang, X.; Wang, E.; Zhao, G. Advanced Cryopreservation Engineering Strategies: The Critical Step to Utilise Stem-Cell Products. Stem Cell Res. Ther. 2019, 10, 342. [Google Scholar] [CrossRef]
- Matsumura, K.; Bae, J.Y.; Kim, H.H.; Hyon, S.H. Effective Vitrification of Human Induced Pluripotent Stem Cells Using Carboxylated ε-Poly-L-Lysine. Cryobiology 2011, 63, 76–83. [Google Scholar] [CrossRef]
- Pichugin, Y.; Fahy, G.M.; Morin, R. Cryopreservation of Rat Hippocampal Slices by Vitrification. Cryobiology 2006, 52, 228–240. [Google Scholar] [CrossRef]
- Fahy, G.M.; Wowk, B.; Wu, J.; Paynter, S. Improved Vitrification Solutions Based on the Predictability of Vitrification Solution Toxicity. Cryobiology 2004, 48, 22–35. [Google Scholar] [CrossRef]
- He, X. Thermostability of Biological Systems: Fundamentals, Challenges, and Quantification. Open Biomed. Eng. J. 2011, 5, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Virchow, R. Die Cellularpathologie in Ihrer Begründung auf Physiologische und Pathologische Gewebelehre; Hirschwald: Berlin, Germany, 1858. [Google Scholar]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and Mechano-Regulation of Connective-Tissue Remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.R.; Russo, F.B.; Pignatari, G.C.; Evangelinellis, M.M.; Tavolari, S.; Muotri, A.R.; Beltrão-Braga, P.C. Fibroblast Sources: Where Can We Get Them? Cytotechnology 2016, 68, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Villegas, J.; McPhau, N. Establishment and Culture of Human Skin Fibroblasts. In Current Protocols in Molecular Biology; Ausubel, F.M., Ed.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Tarin, D.; Croft, C.B. Ultrastructural Features of Wound Healing in Mouse Skin. J. Anat. 1969, 105, 189–190. [Google Scholar]
- Parsonage, G.; Filer, A.D.; Haworth, O.; Nash, G.B.; Rainger, G.E.; Salmon, M.; Buckley, C.D. A Stromal Address Code Defined by Fibroblasts. Trends Immunol. 2005, 26, 150–156. [Google Scholar] [CrossRef]
- Singh, M.K.; Shin, Y.; Ju, S.; Han, S.; Choe, W.; Yoon, K.S.; Kim, S.S.; Kang, I. Heat-Shock Response and Heat-Shock Proteins: Current Understanding and Future Opportunities in Human Diseases. Int. J. Mol. Sci. 2020, 21, 5324. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Y.; Jia, Y.; Chen, X.; Niu, T.; Chatterjee, A.; He, P.; Hou, G. Heat-Shock Protein 90: Biological Functions, Diseases, and Therapeutic Targets. Cells 2022, 11, 1293. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Yan, J.; Wang, T.; Xia, X.; Zhuang, X.; Hong, K.; Li, R.; Liu, P.; Jiang, H.; Qiao, J. Up-Regulation of Heme Oxygenase-1 Expression Modulates Reactive Oxygen Species Level during the Cryopreservation of Human Seminiferous Tubules. Fertil. Steril. 2014, 102, 974–980.e4. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.M.; Barbone, D.; Fennell, D.A.; Broaddus, V.C. Bcl-2 Family Proteins Contribute to Apoptotic Resistance in Lung Cancer Multicellular Spheroids. Oncogene 2009, 28, 4169–4178. [Google Scholar] [CrossRef]
- Barbone, D.; Ryan, J.A.; Kolhatkar, N.; Chacko, A.D.; Jablons, D.M.; Sugarbaker, D.J.; Bueno, R.; Letai, A.G.; Coussens, L.M.; Fennell, D.A.; et al. The Bcl-2 Repertoire of Mesothelioma Spheroids Underlies Acquired Apoptotic Multicellular Resistance. Cell Death Dis. 2011, 2, e174. [Google Scholar] [CrossRef][Green Version]
- Corre, M.; Lebreton, A. Regulation of Cold-Inducible RNA-Binding Protein (CIRBP) in Response to Cellular Stresses. Biochimie 2024, 217, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Corning Life Sciences. Uniform Spheroid Formation Using Elplasia® 12K Flask; Application Note CLS-AN-615; Corning Life Sciences: Corning, NY, USA, 2022. [Google Scholar]
- Ehsan, S.M.; George, S.C. Non-Neoplastic Tumor Models: Multicellular Spheroids and Organoids. Tissue Eng. Part A 2013, 19, 210–222. [Google Scholar]
- Grimes, D.R.; Kelly, C.; Bloch, K.; Partridge, M. The Role of Oxygen in Avascular Tumour Growth. Sci. Rep. 2017, 7, 10638. [Google Scholar]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Kim, W.H.; Kweon, O.K.; Kang, B.J. Heat-Shock Proteins Can Potentiate the Therapeutic Ability of Cryopreserved Mesenchymal Stem Cells for the Treatment of Acute Spinal Cord Injury in Dogs. Vet. Sci. 2018, 5, 81. [Google Scholar] [CrossRef]
- Boyd, R.A.; Majumder, S.; Stiban, J.; Mavodza, G.; Straus, A.J.; Kempelingaiah, S.K.; Reddy, V.; Hannun, Y.A.; Obeid, L.M.; Senkal, C.E. The Heat-Shock Protein Hsp27 Controls Mitochondrial Function by Modulating Ceramide Generation. J. Biol. Chem. 2020, 295, 8398–8411. [Google Scholar] [CrossRef]
- Magalhaes, R.; Wang, X.W.; Gouk, S.S.; Lee, K.H.; Ten, C.M.; Yu, H.; Kuleshova, L.L. Vitrification successfully preserves hepatocyte spheroids. Cell Transplant. 2008, 17, 813–828. [Google Scholar] [CrossRef]
- Truong, T.T.; Lee, Y.B.; Park, K.H.; Shim, H.E.; Song, J.J.; Kim, H.S.; Hwang, J.H.; Kang, S.W.; Huh, K.M. Cryopreservable three-dimensional spheroid culture for ready-to-use systems. Korean J. Chem. Eng. 2023, 40, 390–397. [Google Scholar] [CrossRef]
- Bissoyi, A.; Tomas, R.M.; Gao, Y.; Guo, Q.; Gibson, M.I. Gibson* Cryopreservation of Liver-Cell Spheroids with Macromolecular Cryoprotectants. ACS Appl. Mater. Interfaces 2023, 15, 2630–2638. [Google Scholar] [CrossRef]
- Ishizaki, T.; Takeuchi, Y.; Ishibashi, K.; Gotoh, N.; Hirata, E.; Kuroda, K. Cryopreservation of tissues by slow-freezing using an emerging zwitterionic cryoprotectant. Sci. Rep. 2023, 13, 37. [Google Scholar] [CrossRef]
- Grimes, D.R.; Kelly, C.; Bloch, K.; Partridge, M. A Method for Estimating the Oxygen Consumption Rate in Multicellular Tumour Spheroids. J. Biol. Eng. 2014, 8, 9. [Google Scholar] [CrossRef]
- Shajib, M.S.; Futrega, K.; Franco, R.A.G.; McKenna, E.; Guillesser, B.; Klein, T.J.; Crawford, R.W.; Doran, M.R. Method for Manufacture and Cryopreservation of Cartilage Microtissues. Tissue Eng. Part C Methods 2023, 29, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Paz-Artigas, L.; González-Lana, S.; Polo, N.; Vicente, P.; Montero-Calle, P.; Martínez, M.A.; Rábago, G.; Serra, M.; Prósper, F.; Mazo, M.M.; et al. Generation of Self-Induced Myocardial Ischemia in Large-Sized Cardiac Spheroids without Alteration of Environmental Conditions Recreates Fibrotic Remodeling and Tissue Stiffening Revealed by Constriction Assays. ACS Biomater. Sci. Eng. 2023, 9, 1715–1729. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, F.; Singh, N.H.; Tan, E.K.F.; Xing, J.; Li, H.; Hissette, S.; Manesh, S.; Fulwood, J.; Gupta, K.; Ng, C.W.; et al. Tethered Primary Hepatocyte Spheroids on Polystyrene Multi-Well Plates for High-Throughput Drug-Safety Testing. Sci. Rep. 2020, 10, 21921. [Google Scholar] [CrossRef] [PubMed]
| Gene | Symbol | Sequence | Accession Number | Annealing T° | Size (bps) |
|---|---|---|---|---|---|
| Actin β | ACTB | F: 5′ttcctgggtatggatcctg3′ R: 5′ggtgatctccttctgcatcc3′ | NM_001009784 | 60 °C | 162 |
| BCL2 associated X Protein | BAX | F: 5′ ctccccgagaggtctttttc 3′ R: 5′ tcgaaggaagtccaatgtcc 3′ | XM_004015363 | 58 °C | 176 |
| BCL2 apoptosis regulator | BCL2 | F: 5′ tggatgaccgagtacctgaa3′ R:5′ gccaggagaaatcaaacagg 3′ | XM_027960877.2 | 60 °C | 118 |
| Cold inducible RNA binding protein | CIRBP | F: 5′ gagggctgagttttgacacc 3′ R: 5′ atgggaagtctgtggatggg 3′ | XM_004008776 | 60 °C | 190 |
| Heme oxygenase 1 | HMOX1 | F: 5′ gtcagaggccctgaaggag 3′ R:5′ agggccacgtagatgtggta 3′ | XM_027967703.2 | 60 °C | 144 |
| Heat shock protein 90 alpha family class B member 1 | HSP90AB1 | F: 5′ tggagatcaaccctgacca 3′ R: 5′ gggatcctcaagcgagaag 3′ | XM_004018854 | 58 °C | 143 |
| Heat shock 70 kDa protein 1A | HSPA1A | F: 5′ gttcgacgtgtccatcctga 3′ R: 5′ cagcctgttgtcgaagtcct 3′ | NM_001267874 | 60 °C | 100 |
| Heat shock protein family B (small) member 1 | HSPB1 | F: 5′ agctgacggtcaagaccaag 3′ R: 5′ tatttgcgagtgaagcaacg 3′ | XM_027961472.2 | 60 °C | 103 |
| Ribosomal protein L9 | RLP19 | F: 5′ caactcccgccagcagat 3′ R:5′ ccgggaatggacagtcaca 3′ | XM_004012836 | 56 °C | 127 |
| Succinate dehydrogenase | SDHA | F: 5′ catccactacatgacggagca 3′ R: 5′ atcttgccatcttcagttctgcta 3′ | XM_012125144 | 60 °C | 90 |
| Tyrosine 3-Monooxygenase | YWHAZ | F: 5′ tgtaggatcccgtaggtcatc 3′ R: 5′ ttctctctgtattctcgagcca 3′ | NM_001135699 | 60 °C | 168 |
| Samples | Mass Density (fg/μm3) | Weight (ng) | Diameter (μm) |
|---|---|---|---|
| Ctrl 24 h | 1022.6 ± 2.7 | 1260 ± 385 | 132 ± 14 |
| Ctrl 48 h | 1022 ± 2 | 1557 ± 613 | 135 ± 7 |
| Ctrl 72 h | 1021.4 ± 2.3 | 1320 ± 215 | 140 ± 14 |
| SF 24 h | 1022 ± 2 | 1666 ± 260 | 140 ± 20 |
| SF 48 h | 1022 ± 2 | 1508 ± 428 | 146 ± 8 |
| SF 72 h | 1022 ± 2 | 1394 ± 579 | 135 ± 19 |
| Samples | Mass Density (fg/μm3) | Weight (ng) | Diameter (μm) |
|---|---|---|---|
| Ctrl 24 h | 1029.7± 1.3 | 6207 ± 1501 | 224 ± 19 |
| Ctrl 48 h | 1029 ± 4 | 3735 ± 928 | 189 ± 15 |
| Ctrl 72 h | 1027.3 ± 3.4 | 6019 ± 21,170 | 221 ± 27 |
| SF 24 h | 1022.0 ± 2.6 | 4014 ± 2547 | 204 ± 35 |
| SF 48 h | 1020.5 ± 3.8 | 3273 ± 1310 | 180 ± 22 |
| SF 72 h | 1019.3 ± 3.3 | 6397 ± 1719 | 227 ± 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piras, D.; Olia, F.; Cosseddu, C.; Bebbere, D.; Ledda, S. Evaluating the Response to Cryopreservation of Ovine Fibroblast Spheroids. Biology 2025, 14, 1381. https://doi.org/10.3390/biology14101381
Piras D, Olia F, Cosseddu C, Bebbere D, Ledda S. Evaluating the Response to Cryopreservation of Ovine Fibroblast Spheroids. Biology. 2025; 14(10):1381. https://doi.org/10.3390/biology14101381
Chicago/Turabian StylePiras, Davide, Federico Olia, Chiara Cosseddu, Daniela Bebbere, and Sergio Ledda. 2025. "Evaluating the Response to Cryopreservation of Ovine Fibroblast Spheroids" Biology 14, no. 10: 1381. https://doi.org/10.3390/biology14101381
APA StylePiras, D., Olia, F., Cosseddu, C., Bebbere, D., & Ledda, S. (2025). Evaluating the Response to Cryopreservation of Ovine Fibroblast Spheroids. Biology, 14(10), 1381. https://doi.org/10.3390/biology14101381







