Engineering the Bacterial Laccase CotA for Functional Expression and Dye Decolorization Through Site-Directed Mutagenesis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strains, Plasmids, and Growth Conditions
2.3. Bioinformatics Analysis
2.4. Site-Directed Mutagenesis
2.5. Expression and Purification
2.6. SDS-PAGE and Western Blot
2.7. Effect of Temperature and pH on Laccase Activity and Stability
2.8. Decolorization of Dye Assay
3. Results
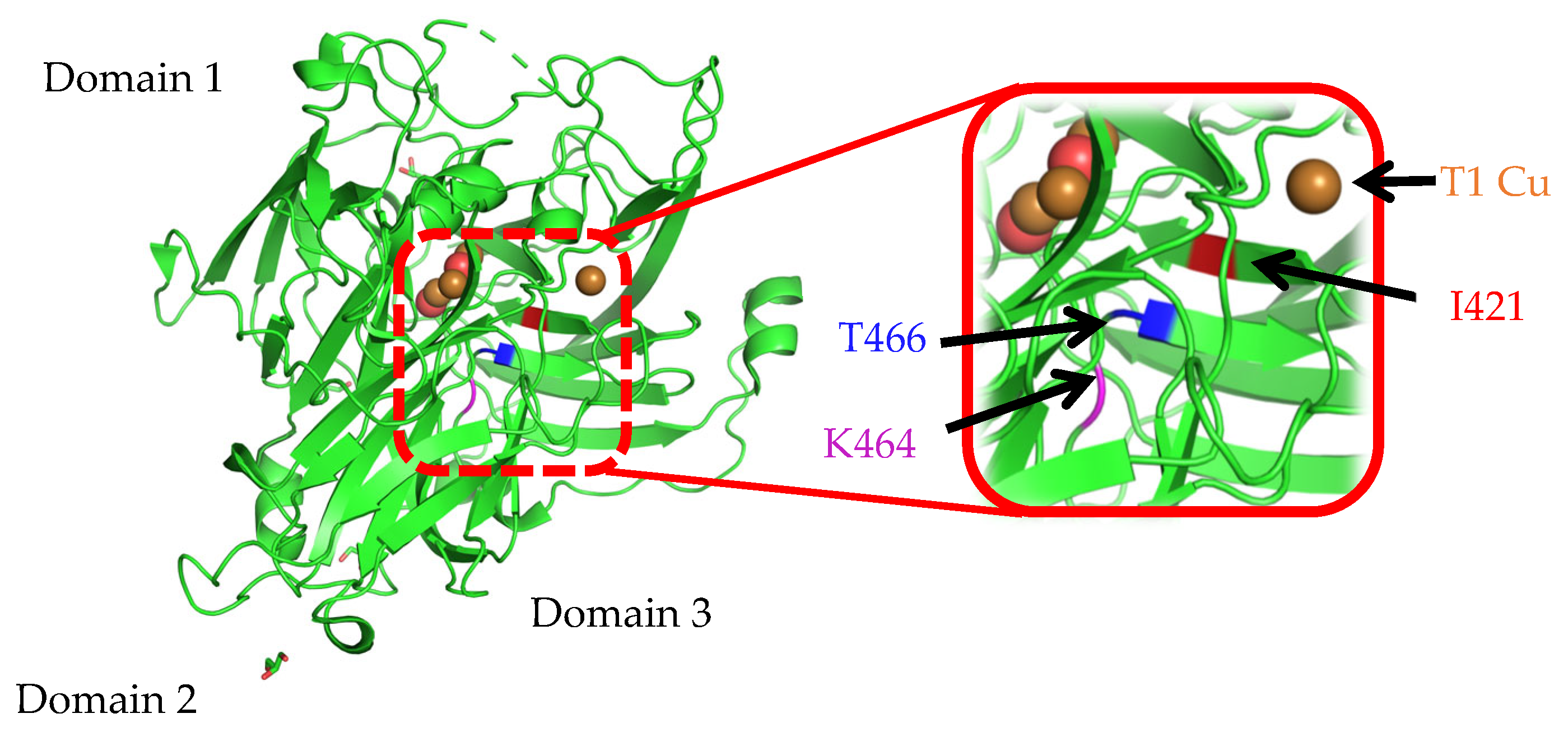
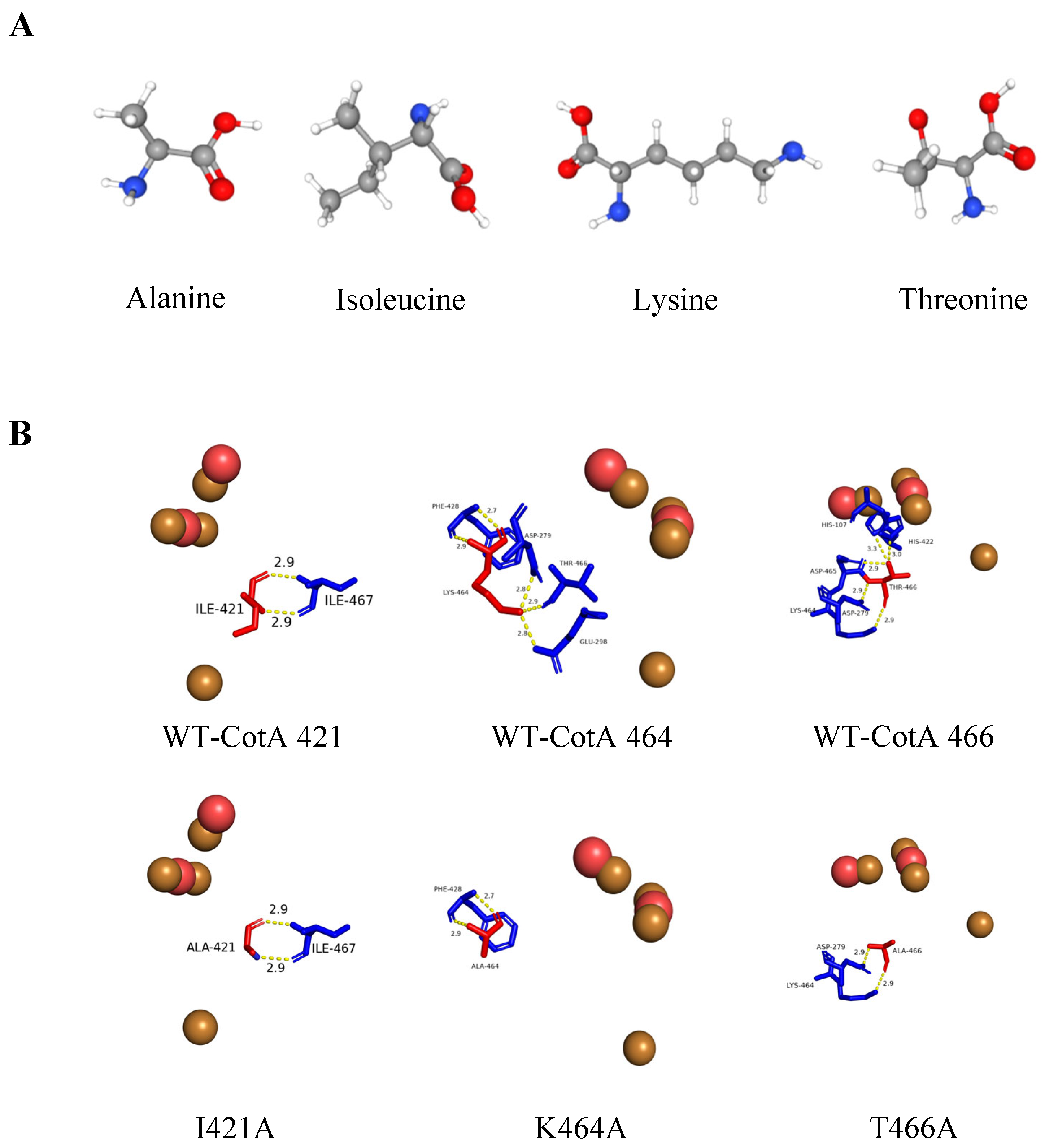
3.1. Mutation Design and Cloning
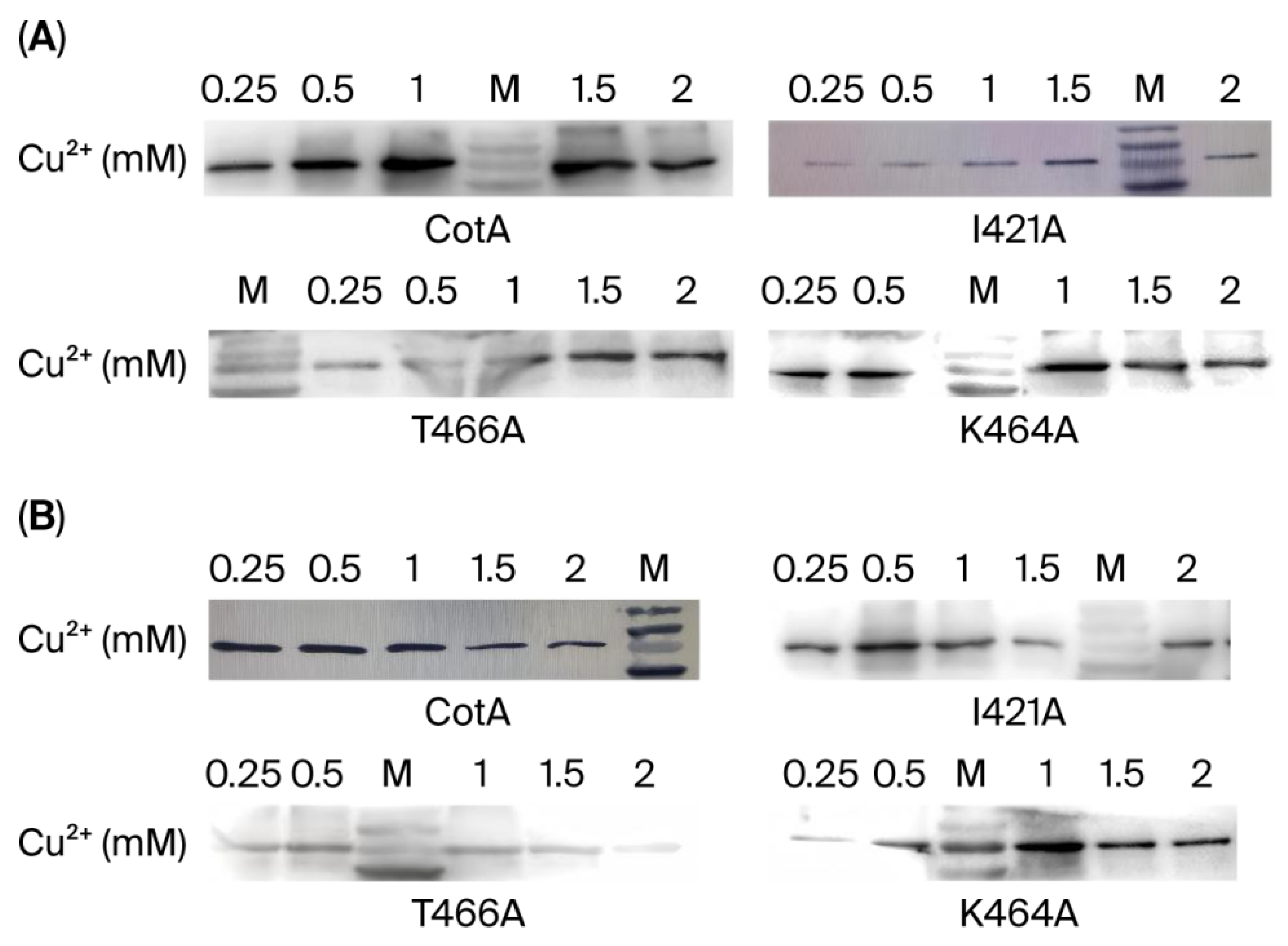
3.2. Expression and Purification
3.3. Enzymatic Property Analysis
3.4. Dye Decolorization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mate, D.M.; Alcalde, M. Laccase engineering: From rational design to directed evolution. Biotechnol. Adv. 2015, 33, 25–40. [Google Scholar] [CrossRef]
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; Herrera De Los Santos, M.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C.; et al. Laccases: Structure, function, and potential application in water bioremediation. Microb. Cell Factories 2019, 18, 200. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, W.; Lv, Z.; Liu, J.; Zhang, W.; Zhou, J.; Xin, F.; Ma, J.; Jiang, M. Surface display of bacterial laccase CotA on Escherichia coli cells and its application in industrial dye decolorization. Mol. Biotechnol. 2018, 60, 681–689. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase properties, physiological functions, and evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Maestre-Reyna, M.; Liu, W.C.; Jeng, W.Y.; Lee, C.C.; Hsu, C.A.; Wen, T.N.; Wang, A.H.J.; Shyur, L.F. Structural and functional roles of glycosylation in fungal laccase from Lentinus sp. PLoS ONE 2015, 10, e0120601. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Gao, Y.; Jiang, M.; Li, L. Deletion and site-directed mutagenesis of laccase from Shigella dysenteriae results in enhanced enzymatic activity and thermostability. Enzym. Microb. Technol. 2009, 44, 274–280. [Google Scholar] [CrossRef]
- Yan, N.; Ma, H.; Yang, C.X.; Liao, X.R.; Guan, Z.B. Improving the decolorization activity of Bacillus pumilus W3 CotA-laccase to Congo Red by rational modification. Enzyme Microb. Technol. 2022, 155, 109977. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.; Farooq, R.; Shaheen, A. Removal of Reactive Blue 19 from wastewaters by physicochemical and biological processes-a review. J. Chem. Soc. Pak. 2011, 33, 284–293. [Google Scholar]
- Rápó, E.; Posta, K.; Csavdári, A.; Vincze, B.É.; Mara, G.; Kovács, G.; Haddidi, I.; Tonk, S. Performance comparison of Eichhornia crassipes and Salvinia natans on azo-dye (Eriochrome Black T) phytoremediation. Crystals 2020, 10, 565. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.; Lin, Y.; Ng, T.B.; Lin, J.; Ye, X. Laccase-catalyzed decolorization of malachite green: Performance optimization and degradation mechanism. PLoS ONE 2015, 10, e0127714. [Google Scholar] [CrossRef]
- Li, T.; Huang, L.; Li, Y.; Xu, Z.; Ge, X.; Zhang, Y.; Wang, N.; Wang, S.; Yang, W.; Lu, F.; et al. The heterologous expression, characterization, and application of a novel laccase from Bacillus velezensis. Sci. Total Environ. 2020, 713, 136713. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.M.; Patel, A.K.; Singhania, R.R.; Tsai, C.H.; Chen, S.Y.; Chen, C.W.; Di Dong, C. Heterologous expression of bacterial CotA-laccase, characterization and its application for biodegradation of malachite green. Bioresour. Technol. 2021, 340, 125708. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, L.; Wang, X.; Shen, Z.; Zheng, L. Comprehensive analysis of rice laccase gene (OsLAC) family and ectopic expression of OsLAC10 enhances tolerance to copper stress in Arabidopsis. Int. J. Mol. Sci. 2017, 18, 209. [Google Scholar] [CrossRef] [PubMed]
- Durán-Sequeda, D.; Suspes, D.; Maestre, E.; Alfaro, M.; Perez, G.; Ramírez, L.; Pisabarro, A.G.; Sierra, R. Effect of nutritional factors and copper on the regulation of laccase enzyme production in Pleurotus ostreatus. J. Fungi 2021, 8, 7. [Google Scholar] [CrossRef]
- Yaropolov, A.I.; Skorobogat’Ko, O.V.; Vartanov, S.S.; Varfolomeyev, S.D. Laccase: Properties, catalytic mechanism, and applicability. Appl. Biochem. Biotechnol. 1994, 49, 257–280. [Google Scholar] [CrossRef]
- Sirim, D.; Wagner, F.; Wang, L.; Schmid, R.D.; Pleiss, J. The Laccase Engineering Database: A classification and analysis system for laccases and related multicopper oxidases. Database 2011, 2011, bar006. [Google Scholar] [CrossRef]
- Jones, S.M.; Solomon, E.I. Electron transfer and reaction mechanism of laccases. Cell. Mol. Life Sci. 2015, 72, 869–883. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Competition among metal ions for protein binding sites: Determinants of metal ion selectivity in proteins. Chem. Rev. 2014, 114, 538–556. [Google Scholar] [CrossRef]
- Solomon, E.I.; Heppner, D.E.; Johnston, E.M.; Ginsbach, J.W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M.T.; Kjaergaard, C.H.; Hadt, R.G.; Tian, L. Copper active sites in biology. Chem. Rev. 2014, 114, 3659–3853. [Google Scholar] [CrossRef]
- Sun, X.; Lin, X.; Xian, Y.; Zhang, F.; Zhu, L.; Geng, H.; Wang, W.; Zhang, G. Engineering bacterial laccase with improved catalytic activity and thermostability by rational design. Appl. Biochem. Biotechnol. 2025, 197, 4685–4701. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Wang, X.; Luo, H.; Wang, Y.; Yao, B.; Huang, H.; Tian, J.; Guan, F. Influence of mutations at different distances from the active center on the activity and stability of laccase 13B22. Bioresour. Bioprocess. 2025, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, K.M.; Twarda-Clapa, A.; Białkowska, A.M. Screening of novel laccase producers—Isolation and characterization of cold-adapted laccase from Kabatiella bupleuri G3 capable of synthetic dye decolorization. Biomolecules 2021, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Enguita, F.J.; Marçal, D.; Martins, L.O.; Grenha, R.; Henriques, A.O.; Lindley, P.F.; Carrondo, M.A. Substrate and dioxygen binding to the endospore coat laccase from Bacillus subtilis. J. Biol. Chem. 2004, 279, 23472–23476. [Google Scholar] [CrossRef] [PubMed]
- Zhukhlistova, N.E.; Zhukova, Y.N.; Lyashenko, A.V.; Zaĭtsev, V.N.; Mikhaĭlov, A.M. Three-dimensional organization of three-domain copper oxidases: A review. Crystallogr. Rep. 2008, 53, 92–109. [Google Scholar] [CrossRef]
- Ladurner, A.G.; Fersht, A.R. Glutamine, alanine or glycine repeats inserted into the loop of a protein have minimal effects on stability and folding rates. J. Mol. Biol. 1997, 273, 330–337. [Google Scholar] [CrossRef]
- Chen, Z.; Durao, P.; Silva, C.S.; Pereira, M.M.; Todorovic, S.; Hildebrandt, P.; Bento, I.; Lindley, P.F.; Martins, L.O. The role of Glu498 in the dioxygen reactivity of CotA-laccase from Bacillus subtilis. Dalton Trans. 2010, 39, 2875–2882. [Google Scholar] [CrossRef]
- Chakrabartty, A.; Kortemme, T.; Baldwin, R.L. Helix propensities of the amino acids measured in alanine-based peptides without helix-stabilizing side-chain interactions. Protein Sci. 1994, 3, 843–852. [Google Scholar] [CrossRef]
- Polaina, J.; MacCabe, A.P. Industrial Enzymes; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper oxidases and oxygenases. Chem. Rev. 1996, 96, 2563–2606. [Google Scholar] [CrossRef]
- Lehninger, A.L. Role of metal ions in enzyme systems. Physiol. Rev. 1950, 30, 393–429. [Google Scholar] [CrossRef]
- Palm-Espling, M.E.; Niemiec, M.S.; Wittung-Stafshede, P. Role of metal in folding and stability of copper proteins in vitro. BBA-Mol. Cell Res. 2012, 1823, 1594–1603. [Google Scholar] [CrossRef]
- Decembrino, D.; Girhard, M.; Urlacher, V.B. Use of copper as a trigger for the in vivo activity of E. coli laccase CueO: A simple tool for biosynthetic purposes. ChemBioChem 2021, 22, 1470–1479. [Google Scholar] [CrossRef]
- Buddhika, U.V.; Savocchia, S.; Steel, C.C. Copper induces transcription of BcLCC2 laccase gene in phytopathogenic fungus, Botrytis cinerea. Mycology 2020, 12, 48–57. [Google Scholar] [CrossRef]
- Zeng, J.; Zhu, Q.; Wu, Y.; Lin, X. Oxidation of polycyclic aromatic hydrocarbons using Bacillus subtilis CotA with high laccase activity and copper independence. Chemosphere 2016, 148, 1–7. [Google Scholar] [CrossRef]
- Quintanar, L.; Yoon, J.; Aznar, C.P.; Palmer, A.E.; Andersson, K.K.; Britt, R.D.; Solomon, E.I. Spectroscopic and electronic structure studies of the trinuclear Cu cluster active site of the multicopper oxidase laccase: Nature of its coordination unsaturation. J. Am. Chem. Soc. 2005, 127, 13832–13845. [Google Scholar] [CrossRef]
- Galli, C.; Gentili, P.; Jolivalt, C.; Madzak, C.; Vadalà, R. How is the reactivity of laccase affected by single-point mutations? Engineering laccase for improved activity towards sterically demanding substrates. Appl. Microbiol. Biotechnol. 2011, 91, 123–131. [Google Scholar] [CrossRef]
- Nasoohi, N.; Khajeh, K.; Mohammadian, M.; Ranjbar, B. Enhancement of catalysis and functional expression of a bacterial laccase by single amino acid replacement. Int. J. Biol. Macromol. 2013, 60, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Khodakarami, A.; Goodarzi, N.; Hoseinzadehdehkordi, M.; Amani, F.; Khodaverdian, S.; Khajeh, K.; Ghazi, F.; Ranjbar, B.; Amanlou, M.; Dabirmanesh, B. Rational design toward developing a more efficient laccase: Catalytic efficiency and selectivity. Int. J. Biol. Macromol. 2018, 112, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Brander, S.; Mikkelsen, J.D.; Kepp, K.P. Characterization of an alkali-and halide-resistant laccase expressed in E. coli: CotA from Bacillus clausii. PLoS ONE 2014, 9, e99402. [Google Scholar] [CrossRef]
- Durao, P.; Chen, Z.; Fernandes, A.T.; Hildebrandt, P.; Murgida, D.H.; Todorovic, S.; Pereira, M.M.; Melo, E.P.; Martins, L.O. Copper incorporation into recombinant CotA laccase from Bacillus subtilis: Characterization of fully copper loaded enzymes. J. Biol. Inorg. Chem. 2008, 13, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Mollania, N.; Khajeh, K.; Ranjbar, B.; Hosseinkhani, S. Enhancement of a bacterial laccase thermostability through directed mutagenesis of a surface loop. Enzyme Microb. Technol. 2011, 49, 446–452. [Google Scholar] [CrossRef]
- Gromiha, M.M.; Thomas, S.; Santhosh, C. Role of cation-π interactions to the stability of thermophilic proteins. Prep. Biochem. Biotechnol. 2002, 32, 355–362. [Google Scholar] [CrossRef]
- Bian, L.; Zhang, S.; Chang, T.; Zhang, J.; Zhu, X.; Zhang, C. Enhanced catalytic performance and pH stability of Streptomyces Laccase Y230R and its degradation of malachite green. Int. J. Biol. Macromol. 2024, 277, 134108. [Google Scholar] [CrossRef] [PubMed]
- Vajanapanich, P.; Nearmnala, P.; Parkbhorn, J.; Nutho, B.; Rungrotmongkol, T.; Hongdilokkul, N. Catalytic residue reprogramming enhances enzyme activity at alkaline pH via phenolate-mediated proton transfer. ACS Synth. Biol. 2025, 14, 3612–3623. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.B.; Shui, Y.; Song, C.M.; Zhang, N.; Cai, Y.J.; Liao, X.R. Efficient secretory production of CotA-laccase and its application in the decolorization and detoxification of industrial textile wastewater. Environ. Sci. Pollut. Res. 2015, 22, 9515–9523. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cui, D.; Lu, L.; Zhang, N.; Yang, H.; Zhao, M.; Dai, S. Cloning and characterization of CotA laccase from Bacillus subtilis WD23 decoloring dyes. Ann. Microbiol. 2016, 66, 461–467. [Google Scholar] [CrossRef]
- Xie, T.; Liu, Z.; Wang, G. Structural insight into the allosteric coupling of Cu1 site and trinuclear Cu cluster in CotA laccase. ChemBioChem 2018, 19, 1502–1506. [Google Scholar] [CrossRef]
- Morley, K.L.; Kazlauskas, R.J. Improving enzyme properties: When are closer mutations better? Trends Biotechnol. 2005, 23, 231–237. [Google Scholar] [CrossRef]
- Yang, X.; Shi, F.; Su, X.; Cavaco-Paulo, A.; Wang, H.; Su, J. In-situ encapsulation and construction of Lac@ HOFs/hydrogel composite for enhancing laccase stability and azo dyes decolorization efficiency. Carbohydr. Polym. 2023, 320, 121157. [Google Scholar] [CrossRef]
- Liu, Y.H.; Ye, M.; Lu, Y.; Zhang, X.; Li, G. Improving the decolorization for textile dyes of a metagenome-derived alkaline laccase by directed evolution. Appl. Microbiol. Biotechnol. 2011, 91, 667–675. [Google Scholar] [CrossRef]
- Bu, T.; Yang, R.; Zhang, Y.; Cai, Y.; Tang, Z.; Li, C.; Wu, Q.; Chen, H. Improving decolorization of dyes by laccase from Bacillus licheniformis by random and site-directed mutagenesis. PeerJ 2020, 8, e10267. [Google Scholar] [CrossRef]
- Mustafa, Z.; Haq, I.U.; Nawaz, A.; Alessa, A.H.; Aftab, M.N.; Alsaigh, A.A.; Rehman, A.U. Laccase production optimization from recombinant E. coli BL21 codon plus containing novel laccase gene from Bacillus megaterium for removal of wastewater textile dye. Molecules 2024, 29, 5514. [Google Scholar] [CrossRef]


| Laccase | Enzyme Activity (U/mL) | Protein Content (mg/mL) | Enzyme Activity (U/mg) |
|---|---|---|---|
| CotA | 1735.9 ± 63.35 | 0.1 | 17,359.4 ± 633.5 |
| I421A | 1842.2 ± 32.67 | 0.1 | 18,421.6 ± 326.7 |
| K464A | 3970.0 ± 102.42 | 0.1 | 39,700.2 ± 1024.2 |
| T466A | 1041.4 ± 89.32 | 0.1 | 10,414.0 ± 893.2 |
| C492A | 10.3 ± 4.82 | 0.1 | 103.1 ± 48.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Yao, S.; Ying, S.; Yu, M.; Song, Z.; Sun, Y.; Qian, L.; Zhang, Y. Engineering the Bacterial Laccase CotA for Functional Expression and Dye Decolorization Through Site-Directed Mutagenesis. Biology 2025, 14, 1335. https://doi.org/10.3390/biology14101335
Zhou Z, Yao S, Ying S, Yu M, Song Z, Sun Y, Qian L, Zhang Y. Engineering the Bacterial Laccase CotA for Functional Expression and Dye Decolorization Through Site-Directed Mutagenesis. Biology. 2025; 14(10):1335. https://doi.org/10.3390/biology14101335
Chicago/Turabian StyleZhou, Zhiguo, Shuyuan Yao, Sitie Ying, Mengyan Yu, Zhihua Song, Yongtao Sun, Lisheng Qian, and Yue Zhang. 2025. "Engineering the Bacterial Laccase CotA for Functional Expression and Dye Decolorization Through Site-Directed Mutagenesis" Biology 14, no. 10: 1335. https://doi.org/10.3390/biology14101335
APA StyleZhou, Z., Yao, S., Ying, S., Yu, M., Song, Z., Sun, Y., Qian, L., & Zhang, Y. (2025). Engineering the Bacterial Laccase CotA for Functional Expression and Dye Decolorization Through Site-Directed Mutagenesis. Biology, 14(10), 1335. https://doi.org/10.3390/biology14101335








