Characteristics of Floral Volatiles and Their Effects on Attracting Pollinating Insects in Three Bidens Species with Sympatric Distribution
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampled Species and Sites
2.2. Pollinator Observations
2.3. Sample Collections
2.4. Identification of Floral Volatile Compounds
2.5. Olfactometer Behavioral Experiments
2.6. Statistical Analysis
3. Results and Analysis
3.1. Pollinator Composition and Visit Frequency
3.2. Composition and Categories of Floral Volatiles
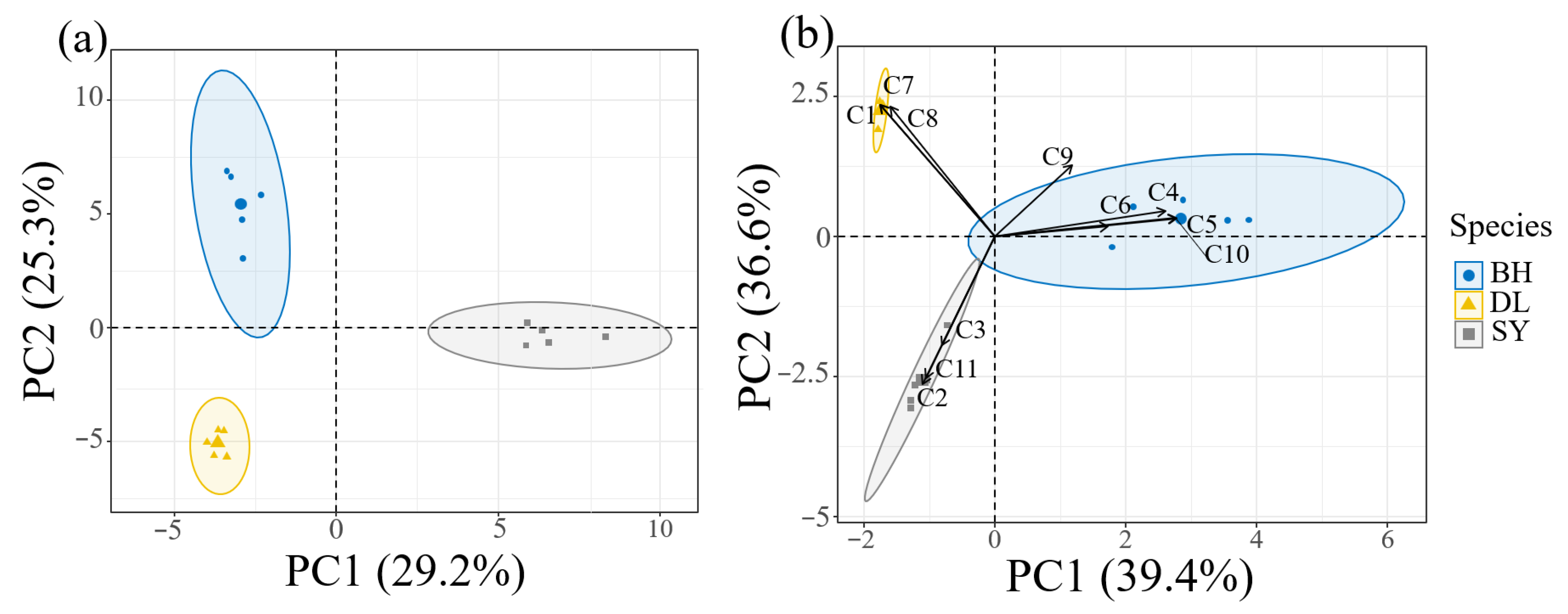
3.3. Analysis of Differential Floral Volatiles
3.4. Behavioral Assays of Floral Volatiles in A. cerana
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stasiak, A.; Latocha, P. Comparative Analysis of Volatile Compounds in Flowers of Different Actinidia Species. Plants 2020, 9, 1675. [Google Scholar] [CrossRef]
- Proffit, M.; Lapeyre, B.; Buatois, B.; Deng, X.; Arnal, P.; Gouzerh, F.; Carrasco, D.; Hossaert-McKey, M. Chemical signal is in the blend: Bases of plant-pollinator encounter in a highly specialized interaction. Sci. Rep. 2020, 10, 10071. [Google Scholar] [CrossRef]
- de Manincor, N.; Andreu, B.; Buatois, B.; Chao, H.L.; Hautekèete, N.; Massol, F.; Piquot, Y.; Schatz, B.; Schmitt, E.; Dufay, M. Geographical variation of floral scents in generalist entomophilous species with variable pollinator communities. Funct. Ecol. 2021, 36, 763–778. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Dötterl, S.; Gershenzon, J. Chemistry, biosynthesis and biology of floral volatiles: Roles in pollination and other functions. Nat. Prod. Rep. 2023, 40, 1901–1937. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yin, G.; Gao, J.; Luo, Y.-B.; Bai, W.-N. Effects of distinct pollinators on the mating system and reproductive success in Incarvillea sinensis, an annual with large floral displays. J. Plant Ecol. 2019, 12, 137–143. [Google Scholar] [CrossRef]
- Parachnowitsch, A.L.; Raguso, R.A.; Kessler, A. Phenotypic selection to increase floral scent emission, but not flower size or colour in bee-pollinated Penstemon digitalis. New Phytol. 2012, 195, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Farré-Armengol, G.; Filella, I.; Llusià, J.; Peñuelas, J. Pollination mode determines floral scent. Biochem. Syst. Ecol. 2015, 61, 44–53. [Google Scholar] [CrossRef]
- Gervasi, D.D.L.; Schiestl, F.P. Real-time divergent evolution in plants driven by pollinators. Nat. Common. 2017, 8, 14691. [Google Scholar] [CrossRef]
- Chapurlat, E.; Ågren, J.; Anderson, J.; Friberg, M.; Sletvold, N. Conflicting selection on floral scent emission in the orchid Gymnadenia conopsea. New Phytol. 2019, 222, 2009–2022. [Google Scholar] [CrossRef]
- Wright, S.I.; Kalisz, S.; Slotte, T. Evolutionary consequences of self-fertilization in plants. Proc. R. Soc. B 2013, 280, 20130133. [Google Scholar] [CrossRef]
- Cutter, A.D. Reproductive transitions in plants and animals: Selfing syndrome, sexual selection and speciation. New Phytol. 2019, 224, 1080–1094. [Google Scholar] [CrossRef]
- Majetic, C.J.; Castilla, A.R.; Levin, D.A. Losing a Scent of One’s Self: Is There a Reduction in Floral Scent Emission in Self-Pollinating Phlox cuspidata versus Outcrossing Phlox drummondii? Int. J. Plant Sci. 2019, 180, 86–92. [Google Scholar] [CrossRef]
- Doubleday, L.A.D.; Raguso, R.A.; Eckert, C.G. Dramatic vestigialization of floral fragrance across a transition from outcrossing to selfing in Abronia umbellata (Nyctaginaceae). Am. J. Bot. 2013, 100, 2280–2292. [Google Scholar] [CrossRef]
- Petrén, H.; Toräng, P.; Ågren, J.; Friberg, M. Evolution of floral scent in relation to self-incompatibility and capacity for autonomous self-pollination in the perennial herb Arabis alpina. Ann. Bot. 2021, 127, 737–747. [Google Scholar] [CrossRef]
- Enders, M.; Havemann, F.; Ruland, F.; Bernard-Verdier, M.; Catford, J.A.; Gómez-Aparicio, L.; Haider, S.; Heger, T.; Kueffer, C.; Kühn, I.; et al. A conceptual map of invasion biology: Integrating hypotheses into a consensus network. Glob. Ecol. Biogeogr. 2020, 29, 978–991. [Google Scholar] [CrossRef]
- Parra-Tabla, V.; Alonso, C.; Ashman, T.; Raguso, R.A.; Albor, C.; Sosenski, P.; Carmona, D.; Arceo-Gómez, G.; Heard, M. Pollen transfer networks reveal alien species as main heterospecific pollen donors with fitness consequences for natives. J. Ecol. 2021, 109, 939–951. [Google Scholar] [CrossRef]
- Jakubska-Busse, A.; Dziadas, M.; Gruss, I.; Kobyłka, M.J. Floral Volatile Organic Compounds and a List of Pollinators of Fallopia baldschuanica (Polygonaceae). Insects 2022, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Razanajatovo, M.; Maurel, N.; Dawson, W.; Essl, F.; Kreft, H.; Pergl, J.; Pyšek, P.; Weigelt, P.; Winter, M.; van Kleunen, M. Plants capable of selfing are more likely to become naturalized. Nat. Commun. 2016, 7, 13313. [Google Scholar] [CrossRef] [PubMed]
- Rodger, J.G.; Bennett, J.M.; Razanajatovo, M.; Knight, T.M.; van Kleunen, M.; Ashman, T.-L.; Steets, J.A.; Hui, C.; Arceo-Gómez, G.; Burd, M.; et al. Widespread vulnerability of flowering plant seed production to pollinator declines. Sci. Adv. 2021, 7, eabd3524. [Google Scholar] [CrossRef]
- Pimentel, D.; Lach, L.; Zuniga, R.; Morrison, D. Environmental and Economic Costs of Nonindigenous Species in the United States. BioScience 2000, 50, 53–65. [Google Scholar] [CrossRef]
- Luo, Y.T.; Wang, Z.M.; Cui, X.L.; Zhao, L.K.; Wang, J.H.; Luo, Y.L. The reproductive traits and invasiveness of Bidens pilosa var. radiata. Chin. J. Ecol. 2019, 38, 655–662. [Google Scholar]
- Hao, J.H.; Liu, Q.Q.; Qiang, S. Reproductive Traits Associated with Invasiveness in Bidens pilosa (Asteraceae). Chin. Bull. Bot. 2009, 44, 656–665. [Google Scholar]
- Yan, X.H.; Zhou, B.; Yin, Z.F.; Wang, N.; Zhang, Z.G. Reproductive biological characteristics potentially contributed to invasiveness in an alien invasive plant Bidens frondosa. Plant Species Biol. 2015, 31, 107–116. [Google Scholar] [CrossRef]
- Zeng, J.J.; Zhou, B.; Wang, N. Comparing the reproductive biological characteristics of the alien invasive Coreopsis lanceolata to those of the non-invasive alien congener Coreopsis tinctoria. Plant Species Biol. 2021, 36, 379–389. [Google Scholar] [CrossRef]
- Su, Q.; Zhou, B.; Du, Z.; Sun, D.; Zou, Z. Generalized pollination system provides protection for the invasion of Solidago canadensis. J. Botany 2025, 57, 701–709. [Google Scholar] [CrossRef]
- Wu, G.H.; Cui, L.; Wang, M.X.; Li, H.H.; Han, B.Y. Attraction of aroma from tea flowers and leaves to the Chinese honeybees (Apis cerana cerana). Acta Ecol. Sin. 2020, 40, 4024–4031. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. Available online: https://www.R-project.org/ (accessed on 21 June 2025).
- Barrett, S.C.H. Theevolution of plant reproductive systems: Howoften are transitions irreversible? Proc. R. Soc. B 2013, 280, 20130913. [Google Scholar] [CrossRef] [PubMed]
- Sicard, A.; Kappel, C.; Lee, Y.W.; Woźniak, N.J.; Marona, C.; Stinchcombe, J.R.; Wright, S.I.; Lenhard, M. Standing genetic variation in a tissue-specific enhancer underlies selfing-syndrome evolution in Capsella. Proc. Natl. Acad. Sci. USA 2016, 113, 13911–13916. [Google Scholar] [CrossRef]
- Fujikura, U.; Jing, R.; Hanada, A.; Takebayashi, Y.; Sakakibara, H.; Yamaguchi, S.; Kappel, C.; Lenhard, M. Variation in Splicing Efficiency Underlies Morphological Evolution in Capsella. Dev. Cell 2018, 44, 192–203.e195. [Google Scholar] [CrossRef] [PubMed]
- Tsuchimatsu, T.; Fujii, S. The selfing syndrome and beyond: Diverse evolutionary consequences of mating system transitions in plants. Philos. Trans. R Soc. Lond. B Biol. Sci. 2022, 377, 20200510. [Google Scholar] [CrossRef]
- Smith-Huerta, N.L.; Huerta, A.J. Floral biology and the evolution of selfing in natural populations of Clarkia tembloriensis Vasek (Onagraceae). J. Torrey Bot. Soc. 2015, 142, 240–248. [Google Scholar] [CrossRef]
- Tedder, A.; Carleial, S.; Gołębiewska, M.; Kappel, C.; Shimizu, K.K.; Stift, M.; Sassa, H. Evolution of the Selfing Syndrome in Arabis alpina (Brassicaceae). PLoS ONE 2015, 10, e0126618. [Google Scholar] [CrossRef]
- Barrett, S.C.H.; Harder, L.D. The Ecology of Mating and Its Evolutionary Consequences in Seed Plants. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 135–157. [Google Scholar] [CrossRef]
- Sas-Nowosielska, H.; Bernas, T. Spatial relationship between chromosomal domains in diploid and autotetraploid Arabidopsis thaliana nuclei. Nucleus 2016, 7, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.-M.; Benfodda, Z.; Molinié, R.; Meffre, P. Volatile Organic Compounds Emitted by Flowers: Ecological Roles, Production by Plants, Extraction, and Identification. Plants 2024, 13, 417. [Google Scholar] [CrossRef]
- Mozaffari, E.; Abai, M.R.; Khanavi, M.; Vatandoost, H.; Sedaghat, M.M.; Moridnia, A.; Saber-Navaei, M.; Sanei-Dehkordi, A.; Rafi, F. Chemical Composition, Larvicidal and Repellency Properties of Cionura erecta (L.) Griseb. Against Malaria Vector, Anopheles stephensi Liston (Diptera: Culicidae). J. Arthropod Borne Dis. 2014, 8, 147–155. [Google Scholar]
- Takemoto, H.; Takabayashi, J. Parasitic Wasps Aphidius ervi are More Attracted to a Blend of Host-Induced Plant Volatiles than to the Independent Compounds. J. Chem. Ecol. 2015, 41, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wei, C.; Zhang, C.; Han, C.; Kuchkarova, N.; Shao, H. Chemical Composition, Phytotoxic, Antimicrobial and Insecticidal Activity of the Essential Oils of Dracocephalum integrifolium. Toxins 2019, 11, 598. [Google Scholar] [CrossRef]
- Satyal, P.; Hieu, H.V.; Chuong, N.T.H.; Hung, N.H.; Sinh, L.H.; Van The, P.; Tai, T.A.; Hien, V.T.; Setzer, W.N. Chemical composition, Aedes mosquito larvicidal activity, and repellent activity against Triatoma rubrofasciata of Severinia monophylla leaf essential oil. Parasitol. Res. 2019, 118, 733–742. [Google Scholar] [CrossRef]
- Woźniak, N.J.; Sartori, K.; Kappel, C.; Tran, T.C.; Zhao, L.; Erban, A.; Gallinger, J.; Fehrle, I.; Jantzen, F.; Orsucci, M.; et al. Convergence and molecular evolution of floral fragrance after independent transitions to self-fertilization. Curr. Biol. 2024, 34, 2702–2711.e2706. [Google Scholar] [CrossRef] [PubMed]
- Farré-Armengol, G.; Filella, I.; Llusià, J.; Peñuelas, J. β-Ocimene, a Key Floral and Foliar Volatile Involved in Multiple Interactions between Plants and Other Organisms. Molecules 2017, 22, 1148. [Google Scholar] [CrossRef] [PubMed]
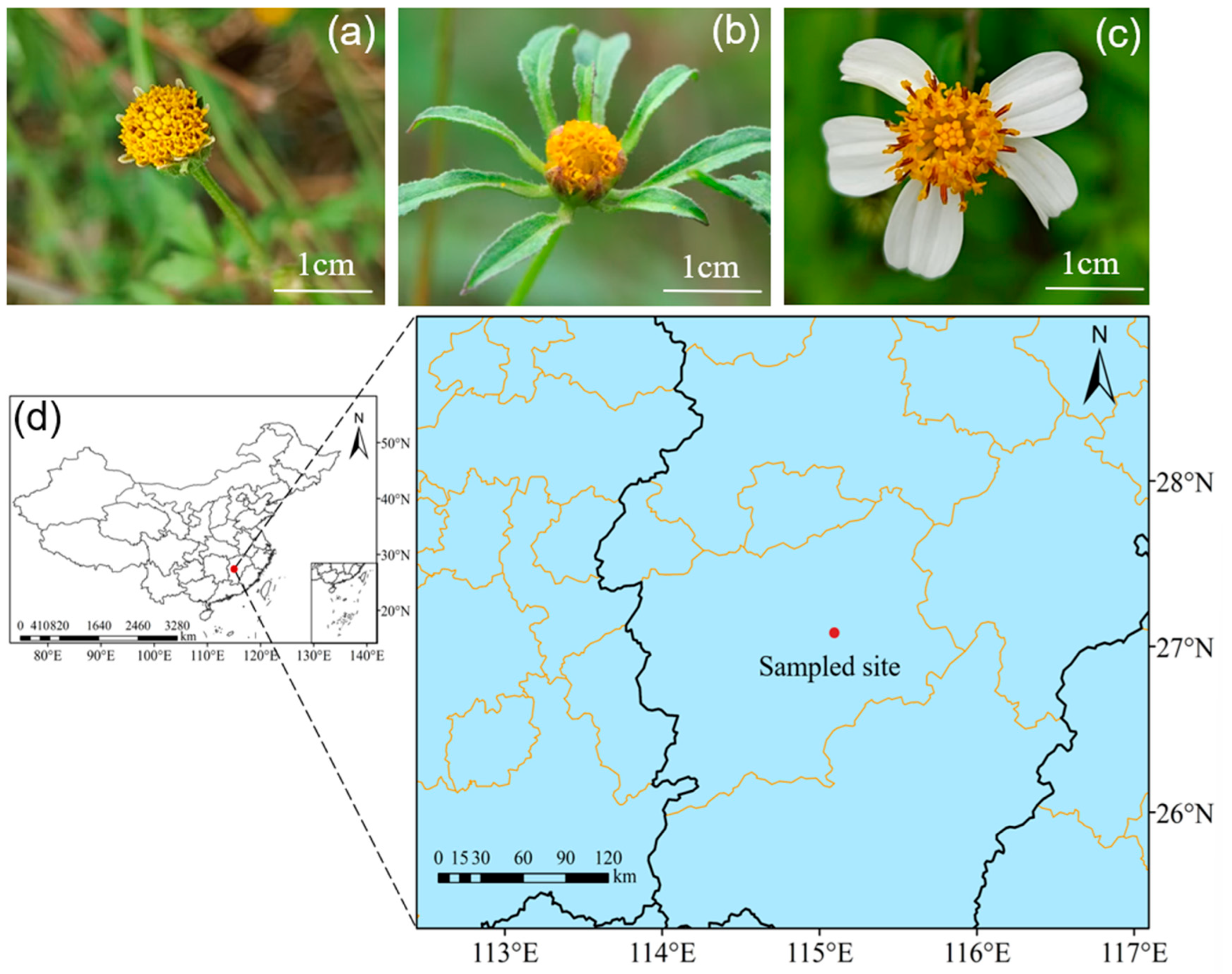
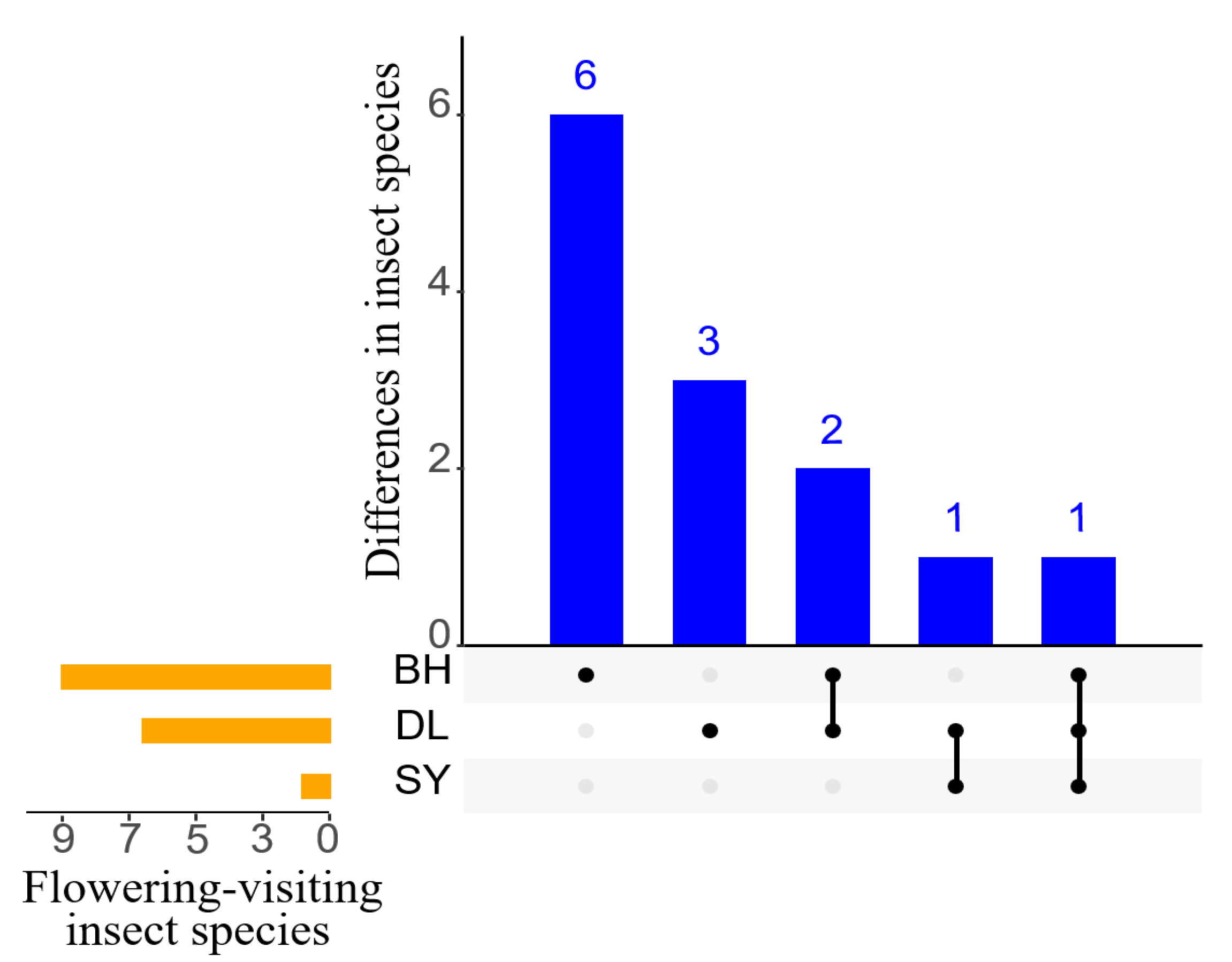
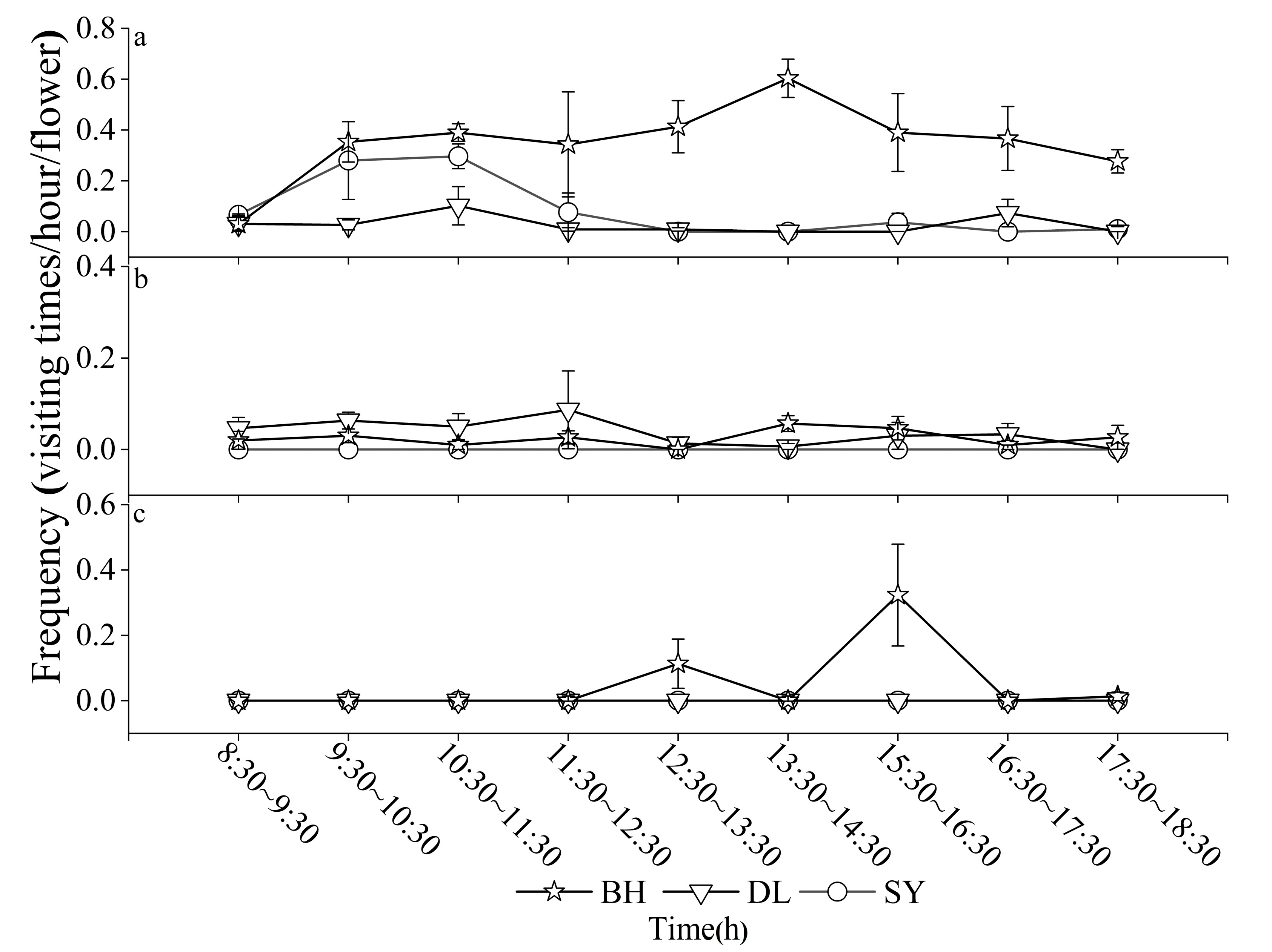
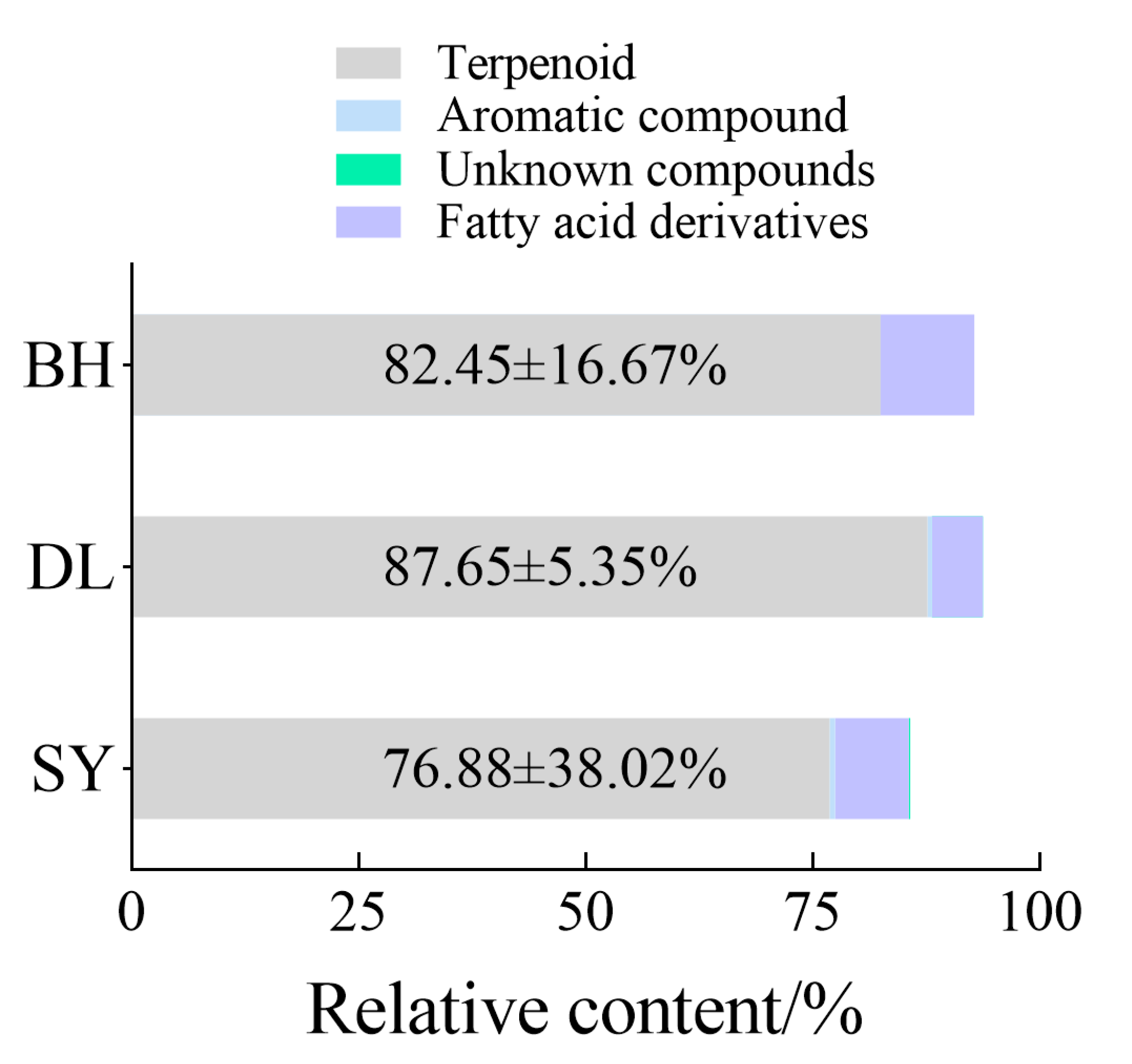
| Order | Family | Genus | Species | Relative Abundance/% | ||
|---|---|---|---|---|---|---|
| BH | DL | SY | ||||
| Hymenoptera | Halictidae | Lasioglossum | L. occidens | 0.02 | 0.13 | 1.00 |
| L. resurgens | 0.34 | — | — | |||
| Megachile | Megachile sp.1 | 0.01 | 0.02 | — | ||
| Apidae | Apis | A. cerana | 0.48 | — | — | |
| Diptera | Phoridae | Pseudacteon | P. tricuspis | — | 0.26 | — |
| Syrphidae | Eristalinus | E. basalis | — | 0.33 | — | |
| E. arvorum | 0.05 | 0.21 | — | |||
| E. baltea | 0.01 | — | — | |||
| Helophilus | H. affinis | — | 0.05 | — | ||
| Papilionidae | Graphium | G. sarpedon | 0.05 | — | — | |
| Lepidoptera | Lycaenidae | Lampides | L. boeticus | 0.01 | — | — |
| Hesperiidae | Pelopidas | P. sinensis | 0.02 | — | — | |
| Code | VIP Value | Name | CAS | Average Relative Content/% (Mean ± SD) | ||
|---|---|---|---|---|---|---|
| BH | DL | SY | ||||
| C1 | 3.97 | m-Cymene | 535-77-3 | — | 52.23 ± 1.90 | — |
| C2 | 3.00 | Sabinene | 3387-41-5 | — | — | 35.02 ± 6.79 |
| C3 | 2.55 | (1R)-(+)-α-Pinene | 7785-70-8 | — | — | 27.19 ± 24.18 |
| C4 | 2.47 | (Z)-β-Ocimene | 13877-91-3 | 33.93 ± 3.49 | 1.49 ± 0.44 | 0.13 ± 0.05 |
| C5 | 2.06 | (E)-β-Ocimene | 3779-61-1 | 18.31 ± 1.10 | 0.23 ± 0.06 | 0.19 ± 0.14 |
| C6 | 2.04 | D-Limonene | 5989-27-5 | 5.87 ± 4.45 | — | — |
| C7 | 1.82 | α-Phellandrene | 99-83-2 | — | 11.17 ± 0.99 | — |
| C8 | 1.61 | β-Myrcene | 123-35-3 | 3.25 ± 3.22 | 11.97 ± 1.00 | 2.54 ± 0.62 |
| C9 | 1.53 | α-Pinene | 80-56-8 | 8.67 ± 1.47 | 8.41 ± 0.41 | — |
| C10 | 1.48 | Neo-alloocimene | 7216-56-0 | 9.91 ± 0.58 | 0.09 ± 0.04 | — |
| C11 | 1.09 | 3-Carene | 13466-78-9 | — | — | 3.59 ± 1.35 |
| Component | Dosage (g/mL) | Number of Bees to Choose Odors | Number of Bees to Choose Paraffin | Binomial GLMs |
|---|---|---|---|---|
| (Z)-β-Ocimene | 10−2 | 12 | 8 | β = 0.41 ± 0.45, p = 0.374 |
| 10−4 | 15 * | 5 | β = 1.10 ± 0.50, p = 0.029 * | |
| 10−6 | 12 | 8 | β = 0.41 ± 0.4, p = 0.374 | |
| (E)-β-Ocimene | 10−2 | 12 | 8 | β = 0.41 ± 0.45, p = 0.374 |
| 10−4 | 16 * | 4 | β = 1.39 ± 0.56, p = 0.013 * | |
| 10−6 | 8 | 12 | β = –0.41 ± 0.45, p = 0.374 | |
| D-Limonene | 10−2 | 13 | 7 | β = 0.62 ± 0.47, p = 0.190 |
| 10−4 | 11 | 9 | β = 0.20 ± 0.45, p = 0.655 | |
| 10−6 | 12 | 8 | β = 0.41 ± 0.45, p = 0.374 | |
| α-Phellandrene | 10−2 | 10 | 10 | β = 0.00 ± 0.45, p = 1.000 |
| 10−4 | 14 | 6 | β = 0.85 ± 0.48, p = 0.074 | |
| 10−6 | 6 | 14 | β = –0.85 ± 0.48, p = 0.074 | |
| Sabinene | 10−2 | 11 | 9 | β = 0.20 ± 0.45, p = 0.655 |
| 10−4 | 10 | 10 | β = 0.00 ± 0.45, p = 1.000 | |
| 10−6 | 9 | 11 | β = –0.20 ± 0.45, p = 0.655 | |
| 3-Carene | 10−2 | 13 | 7 | β = 0.62 ± 0.47, p = 0.190 |
| 10−4 | 13 | 7 | β = 0.62 ± 0.47, p = 0.190 | |
| 10−6 | 10 | 10 | β = 0.00 ± 0.45, p = 1.000 | |
| α-Pinene | 10−2 | 10 | 10 | β = 0.00 ± 0.45, p = 1.000 |
| 10−4 | 9 | 11 | β = –0.20 ± 0.45, p = 0.655 | |
| 10−6 | 7 | 13 | β = –0.62 ± 0.47, p = 0.190 | |
| (1R)-(+)-α-Pinene | 10−2 | 14 | 6 | β = 0.85 ± 0.48, p = 0.074 |
| 10−4 | 12 | 8 | β = 0.41 ± 0.45, p = 0.374 | |
| 10−6 | 13 | 7 | β = 0.62 ± 0.47, p = 0.190 | |
| m-Cymene | 10−2 | 11 | 9 | β = 0.20 ± 0.45, p = 0.655 |
| 10−4 | 8 | 12 | β = –0.41 ± 0.45, p = 0.374 | |
| 10−6 | 9 | 11 | β = –0.20 ± 0.45, p = 0.655 | |
| β-Myrcene | 10−2 | 9 | 11 | β = –0.20 ± 0.45, p = 0.655 |
| 10−4 | 9 | 11 | β = –0.20 ± 0.45, p = 0.655 | |
| 10−6 | 8 | 12 | β = –0.41 ± 0.45, p = 0.374 | |
| Neo-alloocimene | 10−2 | 11 | 9 | β = 0.20 ± 0.45, p = 0.655 |
| 10−4 | 8 | 12 | β = –0.41 ± 0.45, p = 0.374 | |
| 10−6 | 11 | 9 | β = 0.20 ± 0.45, p = 0.655 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, J.-W.; Jia, J.-L.; Xiao, Y.-H.; Zhou, J.-H.; Zeng, J.-J. Characteristics of Floral Volatiles and Their Effects on Attracting Pollinating Insects in Three Bidens Species with Sympatric Distribution. Biology 2025, 14, 1310. https://doi.org/10.3390/biology14101310
Ye J-W, Jia J-L, Xiao Y-H, Zhou J-H, Zeng J-J. Characteristics of Floral Volatiles and Their Effects on Attracting Pollinating Insects in Three Bidens Species with Sympatric Distribution. Biology. 2025; 14(10):1310. https://doi.org/10.3390/biology14101310
Chicago/Turabian StyleYe, Jun-Wei, Jing-Lin Jia, Yong-Hong Xiao, Jia-Hui Zhou, and Jian-Jun Zeng. 2025. "Characteristics of Floral Volatiles and Their Effects on Attracting Pollinating Insects in Three Bidens Species with Sympatric Distribution" Biology 14, no. 10: 1310. https://doi.org/10.3390/biology14101310
APA StyleYe, J.-W., Jia, J.-L., Xiao, Y.-H., Zhou, J.-H., & Zeng, J.-J. (2025). Characteristics of Floral Volatiles and Their Effects on Attracting Pollinating Insects in Three Bidens Species with Sympatric Distribution. Biology, 14(10), 1310. https://doi.org/10.3390/biology14101310






