Invasive Traits of Symphyotrichum squamatum and S. ciliatum: Insights from Distribution Modeling, Reproductive Success, and Morpho-Structural Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Description
2.2. Ensemble Species Distribution Modeling (ESDM)
2.3. Environmental Context of Populations from Romania
2.4. Reproductive Trait Assessment of S. squamatum
2.5. Comparative Analysis of Anatomical Traits
3. Results
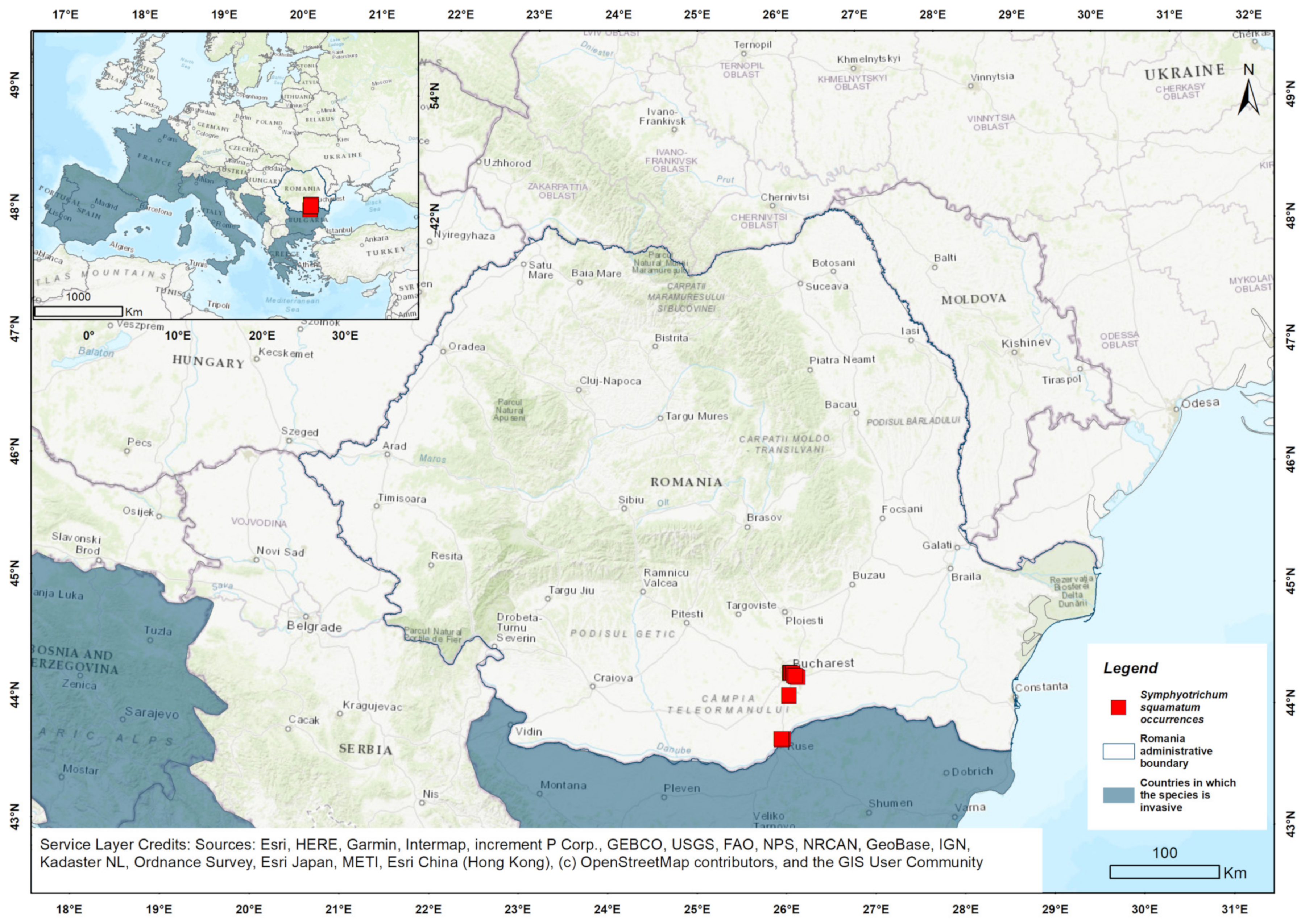
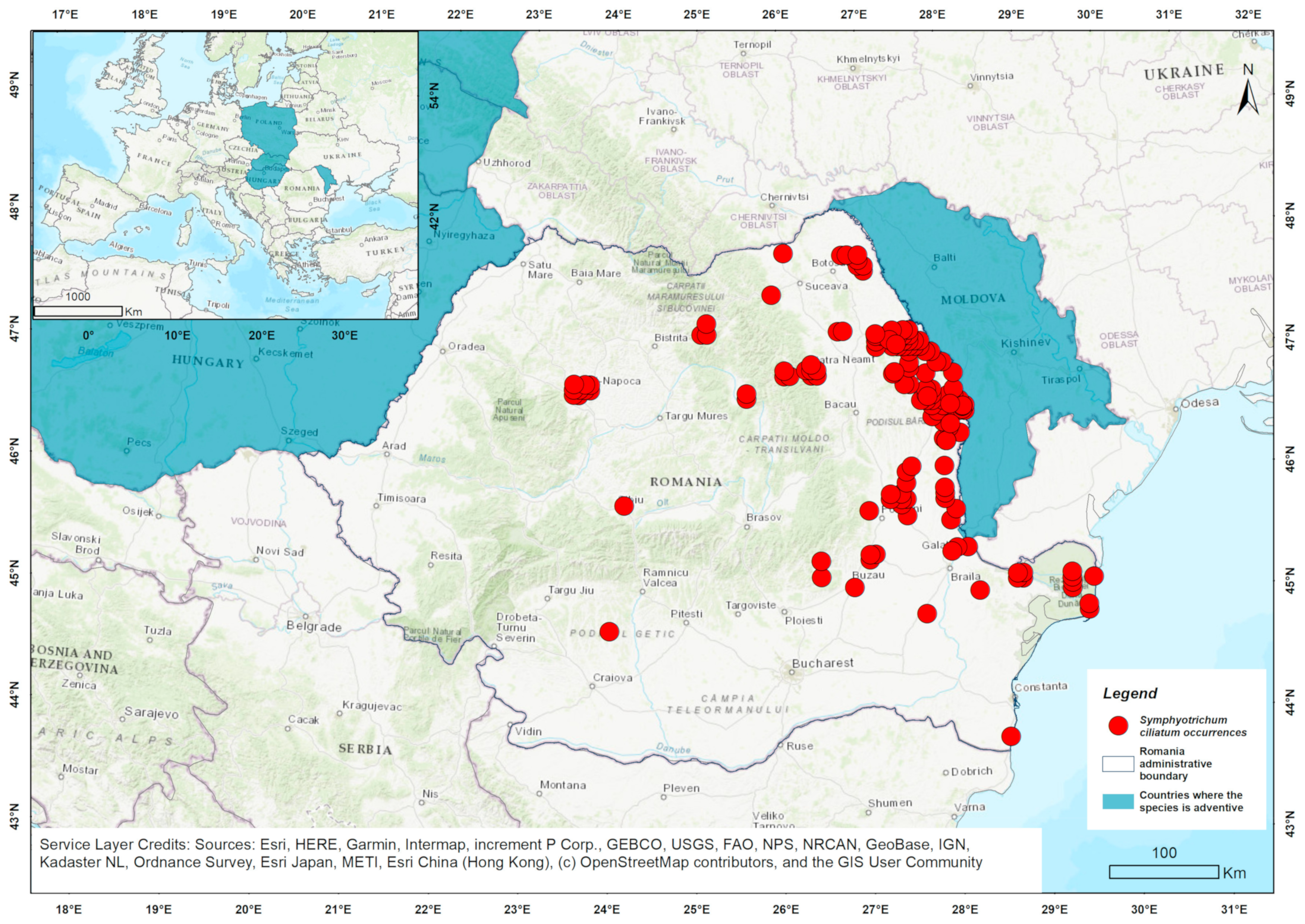
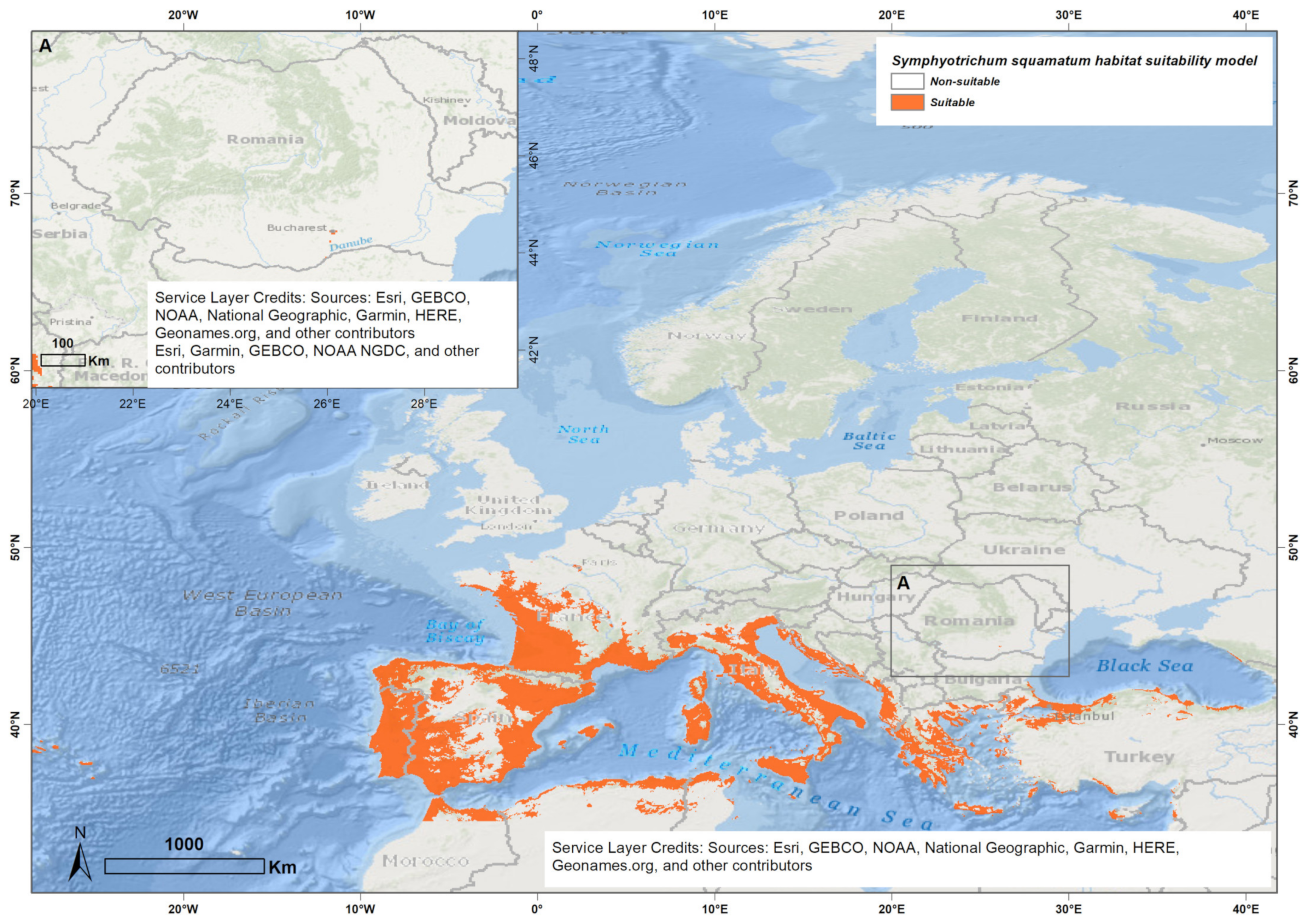
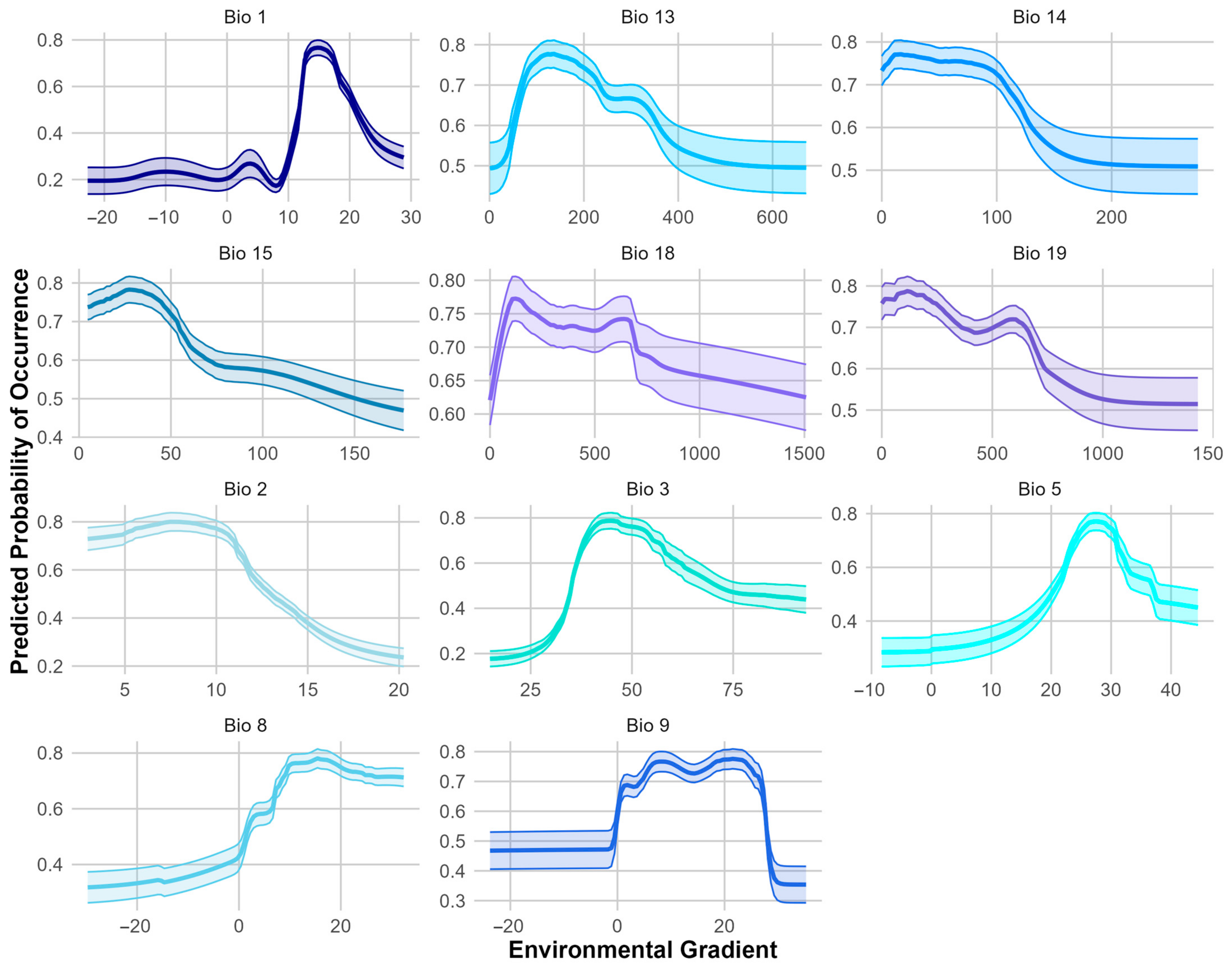
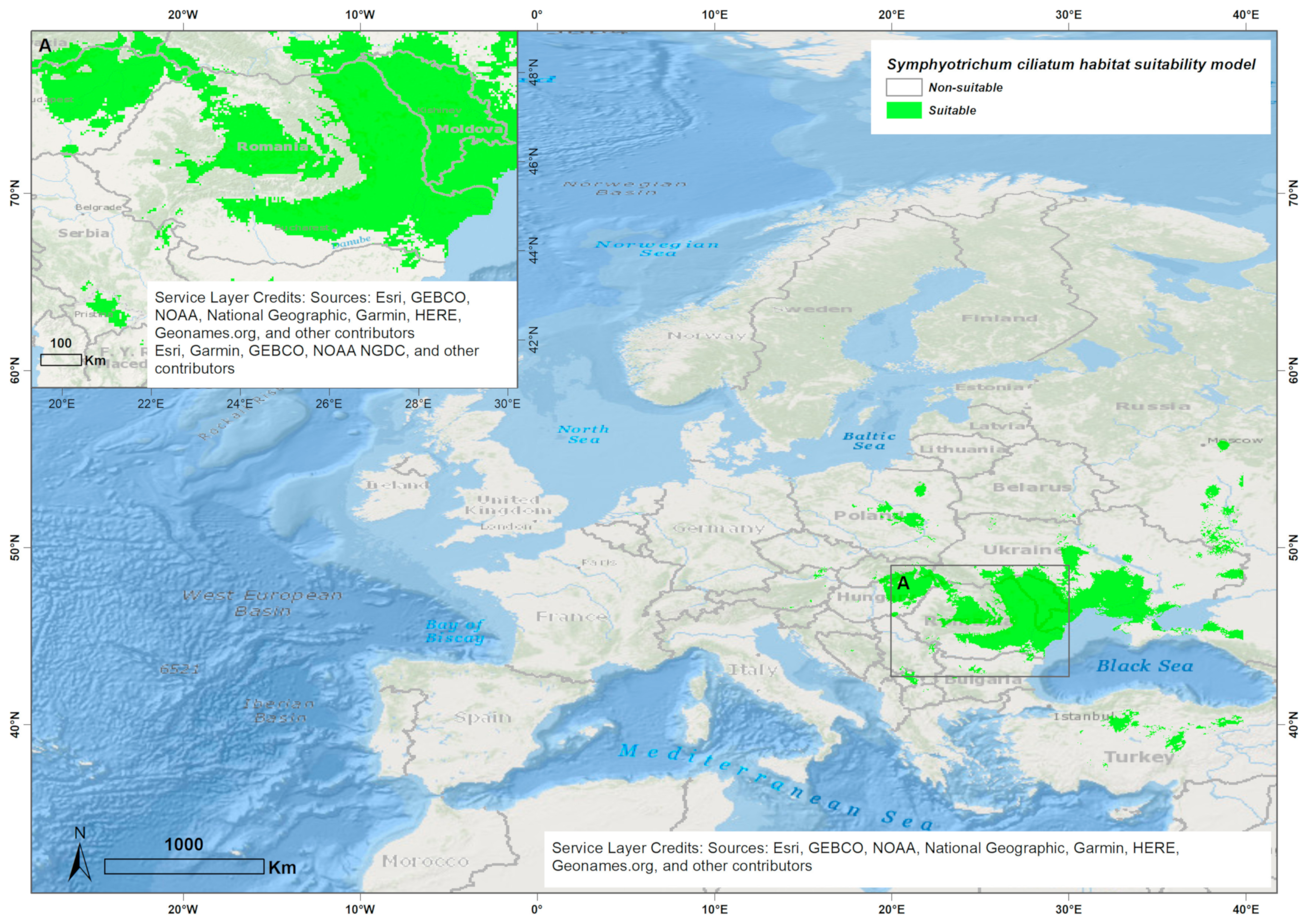
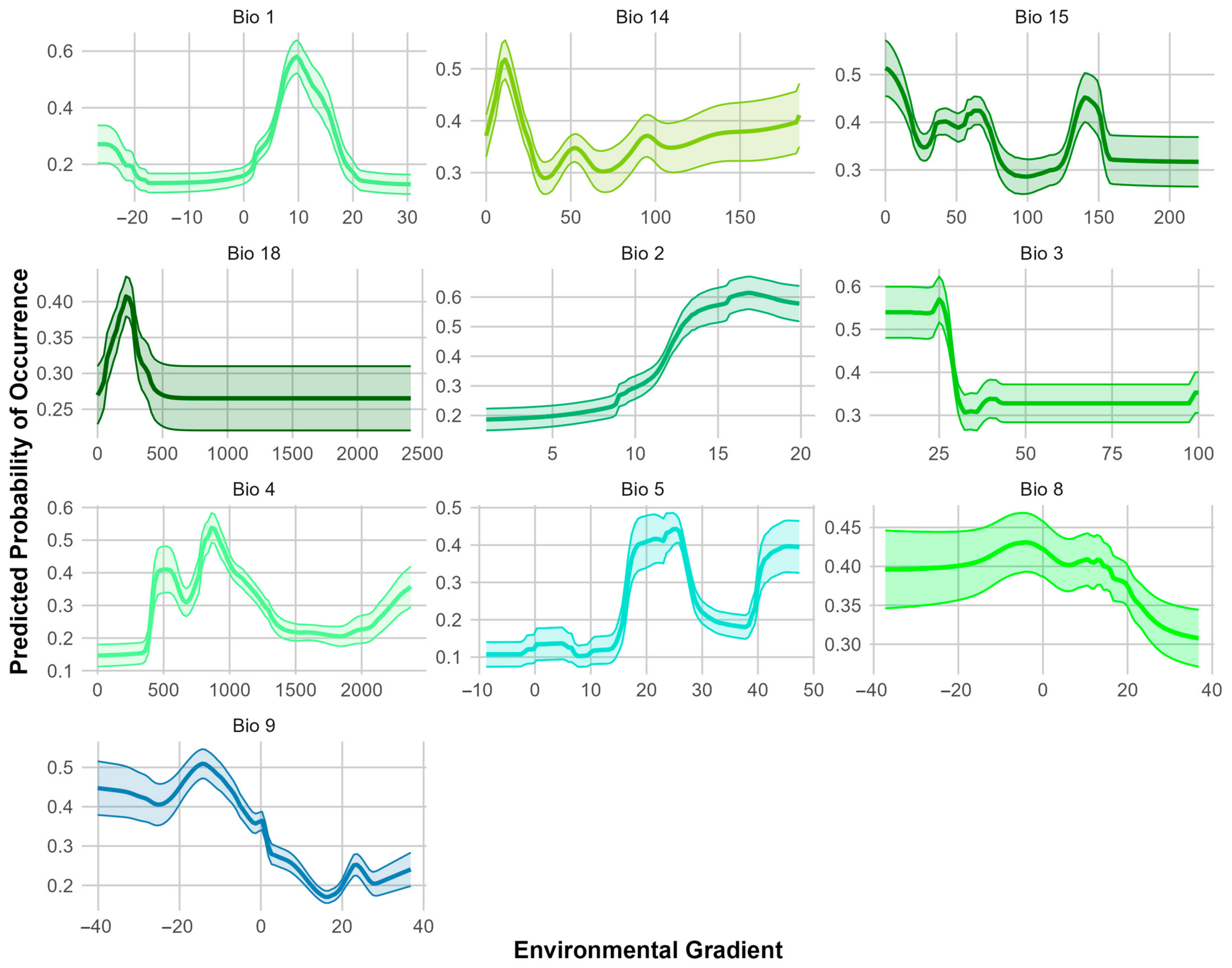
3.1. Ensemble Species Distribution Modeling
3.2. Environmental Context of Populations from Romania
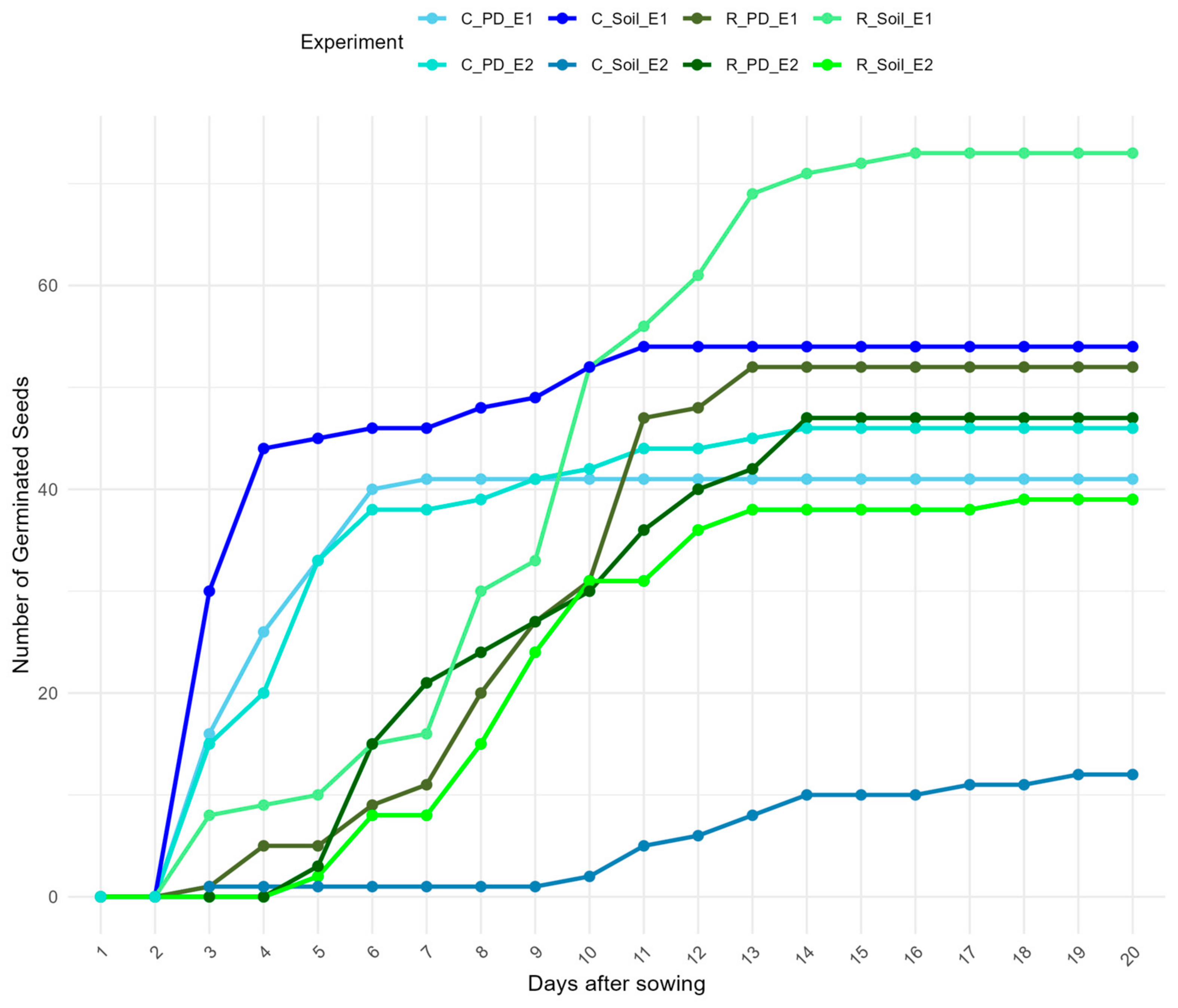
3.3. Reproductive Success of S. squamatum
3.4. Comparative Analysis of Anatomical Traits
4. Discussion
4.1. Ensemble Species Distribution Modeling
4.2. Environmental Context of Populations from Romania
4.3. Reproductive Success of S. squamatum
4.4. Comparative Analysis of Anatomical Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Vilá, M.; Hulme, P.E. Impact of Biological Invasions on Ecosystem Services, 1st ed.; Springer International Publishing: Cham, Switzerland, 2017; p. 354. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Glob. Change Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- Gigante, D.; Acosta, A.T.R.; Agrillo, E.; Armiraglio, S.; Assini, S.; Attorre, F.; Bagella, S.; Buffa, G.; Casella, L.; Giancola, C.; et al. Habitat conservation in Italy: The state of the art in the light of the first European red list of terrestrial and freshwater habitats. Rend. Lincei. Sci. Fis. Nat. 2018, 29, 251–265. [Google Scholar] [CrossRef]
- Daehler, C.C. Performance comparisons of co-occurring native and alien invasive plants: Implications for conservation and restoration. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 183–211. [Google Scholar] [CrossRef]
- Jackson, J. Is there a relationship between herbaceous species richness and buffel grass (Cenchrus ciliaris)? Austral. Ecol. 2005, 30, 505–517. [Google Scholar] [CrossRef]
- Gooden, B.; French, K.; Turner, P.J.; Downey, P.O. Impact threshold for an alien plant invader, Lantana camara L., on native plant communities. Biol. Conserv. 2009, 142, 2631–2641. [Google Scholar] [CrossRef]
- Richardson, D.M.; van Wilgen, B.W. Invasive alien plants in South Africa: How well do we understand the ecological impacts? S. Afr. J. Sci. 2004, 100, 45–52. [Google Scholar]
- Ens, E.-J.; French, K. Exotic woody invader limits the recruitment of three indigenous plant species. Biol. Conserv. 2008, 141, 590–595. [Google Scholar] [CrossRef]
- Inderjit; Seastedt, T.R.; Callaway, R.M.; Pollock, J.L.; Kaur, J. Allelopathy and plant invasions: Traditional, congeneric, and bio-geographical approaches. Biol. Invasions 2008, 10, 875–890. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Callaway, R.M. Soil biota and invasive plants. New Phytol. 2006, 170, 445–457. [Google Scholar] [CrossRef]
- Traveset, A.; Richardson, D.M. Mutualistic interactions and biological invasions. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 89–113. [Google Scholar] [CrossRef]
- Kolar, C.S.; Lodge, D.M. Progress in invasion biology: Predicting invaders. Trends Ecol. Evol. 2001, 16, 199–204. [Google Scholar] [CrossRef]
- Bourdeau, P.; Stanners, D.; European Environment Agency. The Dobříš Assessment—Europe’s Environment; Bourdeau, P., Stanners, D., Eds.; Office for Official Publications of the European Communities: Luxembourg, 1995; Available online: https://op.europa.eu/en/publication-detail/-/publication/76df2cdf-6577-4edb-87a9-524e13b06960/language-en (accessed on 15 November 2024).
- European Environment Agency. Europe’s Environment: The Second Assessment; Office for Official Publications of the European Communities: Luxembourg, 1998. [Google Scholar]
- European Environment Agency. Europe’s Environment: The Third Assessment; Environmental Assessment Report No 10; Office for Official Publications of the European Communities: Luxembourg, 2003. [Google Scholar]
- Commision of the European Communities. Communication from the Commision to the Council and the European Parliament on A European Community Biodiversity Strategy; COM (98) 42 final; Office for Official Publications of the European Communities: Luxembourg, 1998; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:51998DC0042 (accessed on 15 November 2024).
- European Commission. Thematic Report on Alien Invasive Species; Second report of the European Community to the Conference of the Parties of the Convention on Biological Diversity; Office for Official Publications of the European Communities: Luxembourg, 2002. [Google Scholar]
- Council of Europe. European Strategy on Invasive Alien Species; Council of Europe Publishing: Strasbourg, France, 2002; ISBN 92-871-5488-0. [Google Scholar]
- Kurtul, I.; Haubrock, P.I. The need of centralized coordination to counter biological invasions in the European Union. Environ. Sci. Eur. 2024, 36, 129. [Google Scholar] [CrossRef]
- Nesom, G.L. Taxonomy of the Symphyotrichum (Aster) subulatum group and Symphyotrichum (Aster) tenuifolium (Asteraceae: Astereae). Sida 2005, 21, 2125–2140. [Google Scholar]
- Arianoutsou, M.; Bazos, I.; Delipetrou, P.; Kokkoris, Y. The alien flora of Greece: Taxonomy, life traits and habitat preferences. Biol. Invasions 2010, 12, 3525–3549. [Google Scholar] [CrossRef]
- Uludağ, A.; Aksoy, N.; Yazlık, A.; Arslan, Z.F.; Yazmış, E.; Üremiş, I.; Cossu, T.A.; Groom, Q.; Pergl, J.; Pyšek, P.; et al. Alien flora of Turkey: Checklist, taxonomic composition, and ecological attributes. NeoBiota 2017, 35, 61–85. Available online: https://neobiota.pensoft.net/article/12460/ (accessed on 25 October 2024). [CrossRef]
- Mokni, R.E.; Iamonico, D. A new record for the non-native flora of Tunisia, Eclipta prostrata (Asteraceae), and a note on the national status of Erigeron bonariensis, Symphyotrichum squamatum (Asteraceae), and Lepidium didymium (Brassicaceae). Flora Mediterr. 2018, 28, 145–153. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Sharma, J.K. Occurrence of Symphyotrichum squamatum (Spreng.) G.L. Nesom in Uttar Pradesh India: A new record. Mod. Phytomorphol. 2019, 13, 26–29. [Google Scholar]
- Sîrbu, C.; Ferus, P.; Eliaš, P., Jr.; Samuil, C.; Oprea, A. Symphyotrichum ciliatum in Romania: Trends of spread and invaded plant communities. Open Life Sci. 2015, 10, 147–164. [Google Scholar] [CrossRef]
- Lazzaro, L.; Bolpagni, R.; Buffa, G.; Gentili, R.; Lonati, M.; Stinca, A.; Acosta, A.T.R.; Adorni, M.; Aleffi, M.; Allegrezza, M.; et al. Impact of invasive alien plants on native plant communities and Natura 2000 habitats: State of the art, gap analysis and perspectives in Italy. J. Environ. Manag. 2020, 274, 111140. [Google Scholar] [CrossRef]
- Hoffman, A.A.; Parsons, P.A. Evolutionary Genetics and Environmental Stress; Oxford University Press: Oxford, UK, 1991; p. 284. [Google Scholar]
- Reichard, S.H. Assessing the Potential of Invasiveness in Woody Plants Introduced in North America. Ph.D. Thesis, University of Washington, Seattle, WA, USA, 1994. [Google Scholar]
- Pyšek, P.; Richardson, D.M. The biogeography of naturalization in alien plants. J. Biogeogr. 2006, 33, 2040–2050. [Google Scholar] [CrossRef]
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef]
- Higgins, S.I.; Richardson, D.M. Pine invasions in the southern hemisphere: Modelling interactions between organism environment and disturbance. Plant Ecol. 1998, 135, 79–93. [Google Scholar] [CrossRef]
- Rejmánek, M.; Richardson, D.M.; Higgins, S.I.; Pitcairn, M.J.; Grotkopp, E. Ecology of invasive plants: State of the art. In Invasive Alien Species: Searching for Solutions; Mooney, H.A., Mack, R.M., McNeeley, J.A., Neville, L., Schei, P., Waage, J., Eds.; Island Press: Washington, DC, USA, 2005; pp. 104–161. [Google Scholar]
- Pieterse, A.H.; Murphy, K.J. Aquatic Weeds, 1st ed.; Oxford University Press: Oxford, UK, 1990; p. 612. [Google Scholar]
- Auld, B.A.; Hosking, J.; McFadyen, R.E. Analysis of the spread of tiger pear and parthenium weed in Australia. Aust. Weeds 1983, 2, 56–60. [Google Scholar]
- Henderson, L. Alien invasive Salix spp. (willows) in the grassland biome of South Africa. S. Afr. For. J. 1991, 157, 91–95. [Google Scholar]
- Barrett, S.C.H. Genetics of weed invasions. In Applied Population Biology; Jain, S.K., Botsford, L.W., Eds.; Kluwer Academic Publishers: Dordrech, The Netherlands, 1992; pp. 91–119. [Google Scholar]
- Forcella, F.; Wood, J.T. Colonization potentials of alien weeds are related to their ’native’ distributions: Implications for plant quarantine. J. Aust. Inst. Agric. Sci. 1984, 50, 36–40. [Google Scholar]
- Elith, J.; Kearney, M.; Phillips, S.J. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Elith, J.; Franklin, J. Species distribution modelling. Encycl. Biodivers. 2013, 6, 692–705. [Google Scholar]
- Bazzichetto, M.; Malavasi, M.; Barták, V.; Acosta, A.T.R.; Rocchini, D.; Carranza, M.L. Plant invasion risk: A quest for invasive species distribution modelling in managing protected areas. Ecol. Indic. 2018, 95, 311–319. [Google Scholar] [CrossRef]
- Lazano, V.; Di Febbraro, M.; Brundu, G.; Carranza, M.L.; Alessandrini, A.; Ardenghi, N.M.G.; Barni, E.; Bedeni, G.; Celesti-Grapow, L.; Cianfaglione, K.; et al. Plant invasion risk inside and outside protected areas: Propagule pressure, abiotic and biotic factors definitively matter. Sci. Total Environ. 2023, 877, 162993. [Google Scholar] [CrossRef] [PubMed]
- Addison, P.F.; Rumpff, L.; Bau, S.S.; Carey, J.M.; Chee, Y.E.; Jarrad, F.C.; McBride, M.F.; Burgman, M.A. Practical solutions for making models indispensable in conservation decision-making. Divers. Distrib. 2013, 19, 490–502. [Google Scholar] [CrossRef]
- Crall, A.W.; Jarnevich, C.S.; Panke, B.; Young, N.; Renz, M.; Morisette, J. Using habitat suitability models to target invasive plant species surveys. Ecol. Appl. 2013, 23, 60–72. [Google Scholar] [CrossRef]
- Underwood, E.C.; Klinger, R.; Moore, P.E. Predicting patterns of non-native plant invasions in Yosemite National Park, California, USA. Divers. Distrib. 2004, 10, 447–459. [Google Scholar] [CrossRef]
- Vinogradova, K.Y.; Grygorieva, O.V.; Vergun, E.N. Stomatal Structure in Symphyotrichum Nees Species as an Additional Index of Invasiveness. Russ. J. Biol. Invasions 2021, 12, 27–35. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, S.; Wang, S. What structural traits enduring Solidago canadensis L. to invade heterogeneous habitats successfully? Pak. J. Bot. 2022, 54, 345–353. [Google Scholar] [CrossRef]
- Rejmánek, M.; Richardson, D.M.; Barbour, M.G.; Crawley, M.J.; Hrusa, G.F.; Moyle, P.B.; Randall, J.M.; Simberloff, D.; Williamson, M. Biological invasions: Politics and the discontinuity of ecological terminology. Bull. Ecol. Soc. Am. 2002, 83, 131–133. [Google Scholar]
- Moravcová, L.; Pyšek, P.; Jarošík, V.; Havlíčková, V.; Zákravský, P. Reproductive characteristics of neophytes in the Czech Republic: Traits of invasive and non-invasive species. Preslia 2010, 82, 365–390. [Google Scholar]
- Plants of the World Online. Royal Botanic Garden Kew. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:981785-1 (accessed on 10 September 2024).
- Randall, R.P. A Global Compendium of Weeds, 3rd ed.; Weeds Society of Western Australia: Perth, WA, Australia, 2017; p. 3336. ISBN 9780646967493. [Google Scholar]
- Wittenberg, R.; Cock, M.J.W. Invasive Alien Species: A Toolkit for Best Prevention and Management Practices; CAB International Publishing: Wallingford, UK, 2001; pp. 1–228. Available online: https://www.cabidigitallibrary.org/doi/book/10.1079/9780851995694.0000 (accessed on 20 October 2024).
- DAISIE. Handbook of Alien Species in Europe; Springer: Dordrecht, The Netherlands, 2009; p. 399. [Google Scholar]
- Greuter, W. Compositae (Pro Parte Majore). In Compositae; Greuter, W., von Raab-Straube, E., Eds.; Euro+Med Plantbase—The Information Resource for Euro-Mediterranean Plant Diversity. 2006. Available online: https://europlusmed.org/cdm_dataportal/taxon/7a7699ea-4cd0-4299-8ca8-059abea98e24 (accessed on 10 September 2024).
- Camen-Comănescu, P.; Negrean, G.; Nagodă, E.; Anastasiu, P. Symphyotrichum squamatum—A new alien plant in Romania. Acta Horti Bot. Bucurest. 2016, 43, 79–84. [Google Scholar]
- Nagodă, E.; Hovaneț, M.V.; Urziceanu, M. Alien plant species in Giurgiu county, Romania an inventory and distribution analysis. Acta Horti Bot. Bucurest. 2023, 49, 77–109. [Google Scholar] [CrossRef]
- Šajna, N.; Kaligarič, M.; Ivajnšič, D. Reproduction biology of an alien invasive plant: A case of drought-tolerant Aster squamatus on the Northern Adriatic seacoast Slovenia. In Managing Protected Areas in Central and Eastern Europe Under Climate Change; Rannow, S., Neubert, M., Eds.; Advances in Global Change Research; Springer: Dordrecht, The Netherlands, 2014; Volume 58, pp. 279–288. [Google Scholar] [CrossRef]
- Schembri, P.J.; Lanfranco, E. Introduced species in the maltese Islands. In Introduction of Alien Species of Flora and Fauna; Baldacchino, A.E., Pizzuto, A., Eds.; Environment Protection Department: Floriana, Malta, 1996; pp. 29–54. [Google Scholar]
- Invasive Plants in Portugal. Aster squamatus (Annual Saltmarsh Aster). Aster Squamatus | Plantas Invasoras em Portugal. Available online: https://invasoras.pt/en/invasive-plant/aster-squamatus (accessed on 10 October 2023).
- Taxon Profile of Symphyotrichum squamatum (Spreng.) G.L.Nesom|Florabase (dbca.wa.gov.au). Available online: https://florabase.dbca.wa.gov.au (accessed on 20 November 2024).
- Brouillet, L.; Semple, C.J.; Allen, A.G.; Chambers, L.K.; Sundberg, D.S. Symphyotrichum ciliatum (Spreng) G. L. Nesom. In Flora of North America; Editorial Committee, Ed.; Flora of North America, Oxford University Press: New York, NY, USA; Oxford, UK, 1995; Volume 20, pp. 465–539. [Google Scholar]
- Nobis, M.; Pliszko, A. New localities of Symphyotrichum ciliatum (Asteraceae) in Poland. Acta Mus. Siles. Sci. Natur. 2016, 65, 283–286. [Google Scholar] [CrossRef]
- Tokaryuk, A.I.; Chorney, I.I.; Budzhak, V.V.; Protopopova, V.V.; Shevera, M.V. Chorological, ecological and coenotic characteristics of Symphyotrichum ciliatum (Lindl.) Nesom (Asteraceae) in the Bukovinian Cis-Carpathian. Biol. Stud. 2017, 11, 103–114. [Google Scholar] [CrossRef]
- Greuter, W. The Euro+Med treatment Senecioneae and theminor Compositae tribes—Generic concepts and required newnames, with an addendum to Cardueae. Willdenowia 2003, 33, 245–250. [Google Scholar] [CrossRef]
- Sîrbu, C.; Oprea, A. Plante Adventive în Flora României; Editura “Ion Ionescu de la Brad”: Iaşi, Romania, 2011; pp. 1–735. [Google Scholar]
- Sârbu, A.; Smarandache, D. Symphyotrichum ciliatum an invasive species in the Romanian flora—Contribution to the knowledge of the vegetative organs structure. Acta Horti Bot. Bucurest. 2015, 42, 5–22. [Google Scholar] [CrossRef]
- Anastasiu, P.; Negrean, G.; Smarandache, D.; Litescu, S.; Basnou, C. Neophytes in protected areas. Case study: The Danube Delta Biosphere Reserve. Acta Horti Bot. Bucurest. 2014, 41, 41–68. [Google Scholar] [CrossRef]
- Global Biodiversity Information Facility. Available online: https://www.gbif.org/ (accessed on 17 May 2024).
- Bróz, E.; Podgórska, M. Symphyotrichum ciliatum (Brachyactisciliata) (Asteraceae) w Polsce. Fragm. Flor. Geobot. Pol. 2005, 12, 291–299. [Google Scholar]
- Sârbu, I.; Ştefan, N.; Oprea, A. Plante Vasculare din România. Determinator Ilustrat de Teren; Ed. VictorBVictor: Bucureşti, România, 2013; p. 760. [Google Scholar]
- Dudáš, M.; Eliáš, P.; Eliáš, P., Jr.; Górecki, A.; Hrivnák, M.; Hrivnák, R.; Malovcová-Staníková, M.; Marcinčinová, M.; Pliszko, A. New floristic records from Central Europe 6 (reports 81-98). Thaiszia. J. Bot. 2020, 30, 209–220. [Google Scholar] [CrossRef]
- Naimi, B.; Araújo, M.B. sdm: A reproducible and extensible R platform for species distribution modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef]
- Mau-Crimmins, T.M.; Schussman, H.R.; Geiger, E.L. Can the invaded range of a species be predicted sufficiently using only native-range data? Ecol. Model. 2006, 193, 736–746. [Google Scholar] [CrossRef]
- Trethowan, P.D.; Robertson, M.P.; McConnachie, A.J. Ecological niche modelling of an invasive alien plant and its potential biological control agents. S. Afr. J. Bot. 2011, 22, 137–146. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- WorldClim. Bioclimatic Variables. Available online: https://www.worldclim.org/data/bioclim.html (accessed on 20 October 2024).
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Zizka, A.; Silvestro, D.; Andermann, T.; Azevedo, J.; Ritter, C.D.; Edler, D.; Farooq, H.; Herdean, A.; Ariza, M.; Scharn, R.; et al. CoordinateCleaner: Standardized cleaning of occurrence records from biological collection databases. Methods Ecol. Evol. 2019, 10, 744–751. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudik, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Wisz, M.; Guisan, A. Do pseudo-absence selection strategies influence species distribution models and their predictions? An information-theoretic approach based on simulated data. BMC Ecol. 2009, 9, 8. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martinez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions (MPB-49); Princeton University Press: Princeton NJ, USA, 2011; p. 314. ISBN 9781400840670. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Wildlife Conservation Society—WCS Center for International Earth Science Information Network-CIESIN-Columbia University. Last of the Wild Project Version 2, 2005 (LWP-2): Global Human Footprint Dataset (Geographic); NASA Socioeconomic Data and Applications Center (SEDAC): Palisades, NY, USA, 2005. [Google Scholar] [CrossRef]
- Corine Land Cover. Available online: https://land.copernicus.eu/pan-european/corine-land-cover/clc2018 (accessed on 17 January 2023).
- Florea, B.; Patrichi, M. Harta Solurilor (Generalizare după Harta Solurilor Scara 1:1000000 Atlasul RSRomânia) [Soil Map (Generalised from 1:1000000 Soil Map Atlas of Romania]; Institutul de Cercetări pentru Pedologie și Agrochimie (ICPA): Bucharest, România, 1978; Available online: https://esdac.jrc.ec.europa.eu/content/harta-solurilor-generalizare-dupa-harta-solurilor-scara-11000000-atlasul-rsromania-1978-soil (accessed on 17 January 2023).
- [ISTA] International Seed Testing Association. International Rules for Seed Testing; ISTA: Bassersdorf, Switzerland, 2003. [Google Scholar]
- Ikram, R.M.; Tanveer, A.; Ata, Z.; Saqib, M. Dormancy studies on Euphorbia dracunculoides and Astragalus spp.: Major weeds of arid areas. Planta Daninha 2014, 32, 747–753. Available online: https://www.scielo.br/j/pd/a/f5TjcLBytfvnhyttyPwg5NR/?lang=en (accessed on 25 November 2024). [CrossRef]
- Kader, M.A. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. R. Soc. New South Wales 2005, 138, 65–75. [Google Scholar] [CrossRef]
- Dumitraşcu, M.; Sârbu, A.; Cîşlariu, A.G. The anatomical structure of Symphyotrichum squamatum, an alien plant in Romanian flora. Acta Horti Bot. Bucurest. 2023, 49, 5–16. [Google Scholar] [CrossRef]
- Şerbănescu-Jitariu, G.; Andrei, M.; Rădulescu-Mitroiu, N.; Petria, E. Practicum de Biologie Vegetală; Edit Ceres: Bucureşti, România, 1983; p. 295. [Google Scholar]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettman, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve predictions of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- EPPO Global Database. Available online: https://gd.eppo.int (accessed on 7 February 2024).
- Pace, L.; Tammaro, F. The main invasive alien plants in the protected areas in Central Italy (Abruzzo). In Global Change and Protected Areas; Visconti, G., Beniston, M., Iannorelli, E.D., Barba, D., Eds.; Advances in Global Change Research; Springer: Dordrecht, The Netherlands, 2001; Volume 9, pp. 495–504. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Rivas-Sáenz, S.; Penas, A. Worldwide Bioclimatic Classification System. Glob. Geobot. 2011, 1, 1–634. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Manea, A.; Birsan, M.V.; Dima, V.; Havriş, L.E. Comparative Analysis of Land Air Temperature in Romania since A.D. 1961. Land 2024, 13, 596. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P. Plant invasions: Merging the concepts of species invasiveness and community invasibility. Prog. Phys. Geogr. 2006, 30, 409–431. [Google Scholar] [CrossRef]
- Mack, R.N.; Simberloff, D.; Lonsdale, W.M.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic Invasions: Causes, Epidemiology, Global Consequences, and Control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- van Kleunen, M.; Weber, E.; Fischer, M. A meta analysis of trait differences between invasive and non invasive plant species. Ecol. Lett. 2010, 13, 235–245. [Google Scholar] [CrossRef]
- Hamilton, M.A.; Murray, B.R.; Cadotte, M.W.; Hose, G.C.; Baker, A.C.; Harris, C.J.; Licari, D. Life-history correlates of plant invasiveness at regional and continental scales. Ecol. Lett. 2005, 8, 1066–1074. [Google Scholar] [CrossRef]
- van Kleunen, M.; Dawson, W.; Maurel, N. Characteristics of successful alien plants. Mol. Ecol. 2015, 24, 1954–1968. [Google Scholar] [CrossRef]
- Muoghalu, J.I.; Chuba, D.K. Seed reproductive strategies of Tithonia diversifolia and Tithonia rotundifolia. Appl. Ecol. Environ. Res. 2005, 3, 39–46. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Murray, B.R.; Lovett-Doust, J. Ecological patterns and biological invasions: Using regional species inventories in macroecology. Biol. Invasions 2006, 8, 809–821. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V. Residence time determines the distribution of alien plants. In Invasive Plants: Ecological and Agricultural Aspects; Inderjit, Ed.; Birkhäuser: Basel, Switzerland, 2005; pp. 77–96. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Lovett-Doust, J. Ecological and taxonomic differences between native and introduced plants of southwestern Ontario. Ecoscience 2001, 8, 230–238. [Google Scholar] [CrossRef]
- Lake, J.C.; Leishman, M.R. Invasion success of exotic plants in natural ecosystems: The role of disturbance plant attributes and freedom from herbivores. Biol. Conserv. 2004, 117, 215–226. [Google Scholar] [CrossRef]
- Baker, H.G. Characteristics and modes of origin of weeds. In The Genetics of Colonizing Species; Baker, H.G., Stebbins, G.L., Eds.; Academic Press: New York, NY, USA, 1965; pp. 147–172. [Google Scholar]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic niche shifts are rare among terrestrial plant invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef]
- Brouillet, L.; Semple, J.C.; Allen, G.A.; Chambers, K.L.; Sundberg, S.D. Symphyotrichum Nees. In Flora of North America; Editorial Committee, Ed.; Flora of North America; Oxford University Press: New York, NY, USA; Oxford, UK, 2006; Volume 20, Available online: https://beta.floranorthamerica.org/Symphyotrichum.html (accessed on 10 September 2024).
- Ciocârlan, V. Flora Ilustrată a României: Pteridophyta et Spermatophyta; Editura Ceres: Bucureşti, Romania, 2009; pp. 1–1141. [Google Scholar]
- Dihoru, G. Area limits in the Romanian territory: Brachyactis ciliata (Ledeb) Ledeb 1845. Analele Univ. Bucureşti Biol. 1989, 38, 67–70. [Google Scholar]
- Cousens, R.; Dytham, C.; Law, R. Dispersal in Plants. A Population Perspective; Oxford University Press Inc.: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Vespremeanu-Stroe, A.; Cheval, S.; Tătui, F. The wind regime of Romania—Characteristics trends and North Atlantic oscillation influences. Forum Geogr. Stud. Cerc. Geogr. Prot. Mediu. 2012, 11, 118–126. [Google Scholar] [CrossRef]
- Gioria, M.; Osborne, B.A. Resource competition in plant invasions: Emerging patterns and research needs. Front. Plant Sci. 2014, 5, 501. [Google Scholar] [CrossRef]
- Folorunso, A.E.; Awosode, O.D. Comparative anatomy of invasive and non-invasive species in the family Asteraceae in Nigeria. Int. J. Biol. Chem. Sci. 2013, 7, 1804–1819. [Google Scholar] [CrossRef]
- Hacke, U.G.; Stiller, V.; Sperry, I.S.; Pittterman, I.; Mcculloh, K.A. Cavitation fatigue embolism and refilling cycles can weaken the cavitation resistance of xylem. Plant Physiol. 2001, 125, 779–786. [Google Scholar] [CrossRef]
| Metric | Symphyotrichum squamatum | Symphyotrichum ciliatum |
|---|---|---|
| AUC (Area Under the Curve) | 0.95 | 0.94 |
| COR (Correlation) | 0.7 | 0.65 |
| Deviance | 0.35 | 0.3 |
| TSS (True Skill Statistic) | 0.78 | 0.76 |
| Sensitivity | 0.91 | 0.89 |
| Specificity | 0.86 | 0.87 |
| Variables | Description | Units | Romania (Mean ± SD) | Native Range (Mean ± SD) | European Invaded Range (Mean ± SD) |
|---|---|---|---|---|---|
| Bio 1 | Annual Mean Temperature | °C | 10.9 ± 0.4 | 17.8 ± 3 | 14.3 ± 1.9 |
| Bio 3 | Isothermality | % | 31.9 ± 0.7 | 52.5 ± 8.6 | 38.7 ± 3.7 |
| Bio 5 | Max Temperature of Warmest Month | °C | 29 ± 0.5 | 29.2 ± 3.4 | 28.5 ± 2.8 |
| Bio 9 | Mean Temperature of Driest Quarter | °C | 1.4 ± 0.3 | 14.4 ± 3.4 | 19.9 ± 5.2 |
| Bio 2 | Mean Diurnal Range | °C | 10.9 ± 0.1 | 11.6 ± 1.9 | 9.8 ± 1.6 |
| Variables | Description | Units | Romania (Mean ± SD) | Native Range (Mean ± SD) | European Invaded Range (Mean ± SD) |
|---|---|---|---|---|---|
| Bio 1 | Annual Mean Temperature | °C | 9.4 ± 1.3 | 5.4 ± 3.5 | 9.4 ± 1.2 |
| Bio 5 | Max Temperature of Warmest Month | °C | 26.1 ± 1.5 | 26.7 ± 3.2 | 25.8 ± 1.7 |
| Bio 4 | Temperature Seasonality | °C × 100 | 855.8 ± 33.4 | 1104.3 ± 191.7 | 842.2 ± 66.4 |
| Bio 2 | Mean Diurnal Range | °C | 9.5 ± 0.8 | 12.3 ± 2.4 | 9.4 ± 0.9 |
| Bio 3 | Isothermality | % | 29.8 ± 1.9 | 29.2 ± 6.1 | 30 ± 2 |
| Population | Substrate Type | Temperature Treatment | Germination Percentage (GP) (%) | Germination Energy (GE) (%) | Germination Rate Index (GRI) (%/day) |
|---|---|---|---|---|---|
| Rahova | Soil | Controlled Temperatures: 21 °C/8 °C (Experiment 1) | 73 | 9 | 70.1 |
| Rahova | Soil | Room Temperature: 23 °C (Experiment 2) | 39 | 0 | 35.3 |
| Rahova | Petri dishes with filter paper | Controlled Temperatures: 21 °C/8 °C (Experiment 1) | 52 | 5 | 48.2 |
| Rahova | Petri dishes with filter paper | Room Temperature: 23 °C (Experiment 2) | 47 | 0 | 44.6 |
| Cotroceni | Soil | Controlled Temperatures: 21 °C/8 °C (Experiment 1) | 54 | 44 | 97 |
| Cotroceni | Soil | Room Temperature: 23 °C (Experiment 2) | 12 | 1 | 7.59 |
| Cotroceni | Petri dishes with filter paper | Controlled Temperatures: 21 °C/8 °C (Experiment 1) | 41 | 26 | 72.2 |
| Cotroceni | Petri dishes with filter paper | Room Temperature: 23 °C (Experiment 2) | 46 | 20 | 72.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cîșlariu, A.G.; Mânzu, C.C.; Dumitrașcu, M.; Mihai, D.C.; Andronache, M.N.; Camen-Comănescu, P.; Nagodă, E.; Sârbu, A. Invasive Traits of Symphyotrichum squamatum and S. ciliatum: Insights from Distribution Modeling, Reproductive Success, and Morpho-Structural Analysis. Biology 2025, 14, 47. https://doi.org/10.3390/biology14010047
Cîșlariu AG, Mânzu CC, Dumitrașcu M, Mihai DC, Andronache MN, Camen-Comănescu P, Nagodă E, Sârbu A. Invasive Traits of Symphyotrichum squamatum and S. ciliatum: Insights from Distribution Modeling, Reproductive Success, and Morpho-Structural Analysis. Biology. 2025; 14(1):47. https://doi.org/10.3390/biology14010047
Chicago/Turabian StyleCîșlariu, Alina Georgiana, Ciprian Claudiu Mânzu, Mioara Dumitrașcu, Daniela Clara Mihai, Marius Nicu Andronache, Petronela Camen-Comănescu, Eugenia Nagodă, and Anca Sârbu. 2025. "Invasive Traits of Symphyotrichum squamatum and S. ciliatum: Insights from Distribution Modeling, Reproductive Success, and Morpho-Structural Analysis" Biology 14, no. 1: 47. https://doi.org/10.3390/biology14010047
APA StyleCîșlariu, A. G., Mânzu, C. C., Dumitrașcu, M., Mihai, D. C., Andronache, M. N., Camen-Comănescu, P., Nagodă, E., & Sârbu, A. (2025). Invasive Traits of Symphyotrichum squamatum and S. ciliatum: Insights from Distribution Modeling, Reproductive Success, and Morpho-Structural Analysis. Biology, 14(1), 47. https://doi.org/10.3390/biology14010047






