Chemical Composition, Antioxidant Capacity, and Anticancerous Effects against Human Lung Cancer Cells of a Terpenoid-Rich Fraction of Inula viscosa
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Identification of Plant Material
2.2. Preparation of Methanolic and Aqueous Crude Extracts
2.3. Preparation of Terpenoid-Rich Fraction (IVLM DCM)
2.4. Determination of Total Phenols and Flavonoids Contents
2.5. Gas Chromatography/Mass Spectrometry (GC/MS) Analysis
2.6. DPPH Free Radical Scavenging Assay
2.7. ABTS Radical Scavenging Assay
2.8. Cell Culture and MTT Cell Cytotoxicity Assay
2.9. DAPI Staining
2.10. Crystal Violet Staining
2.11. Western Blotting Analysis
2.12. Scratch/Wound-Healing Assay
2.13. Statistical Analysis
3. Results
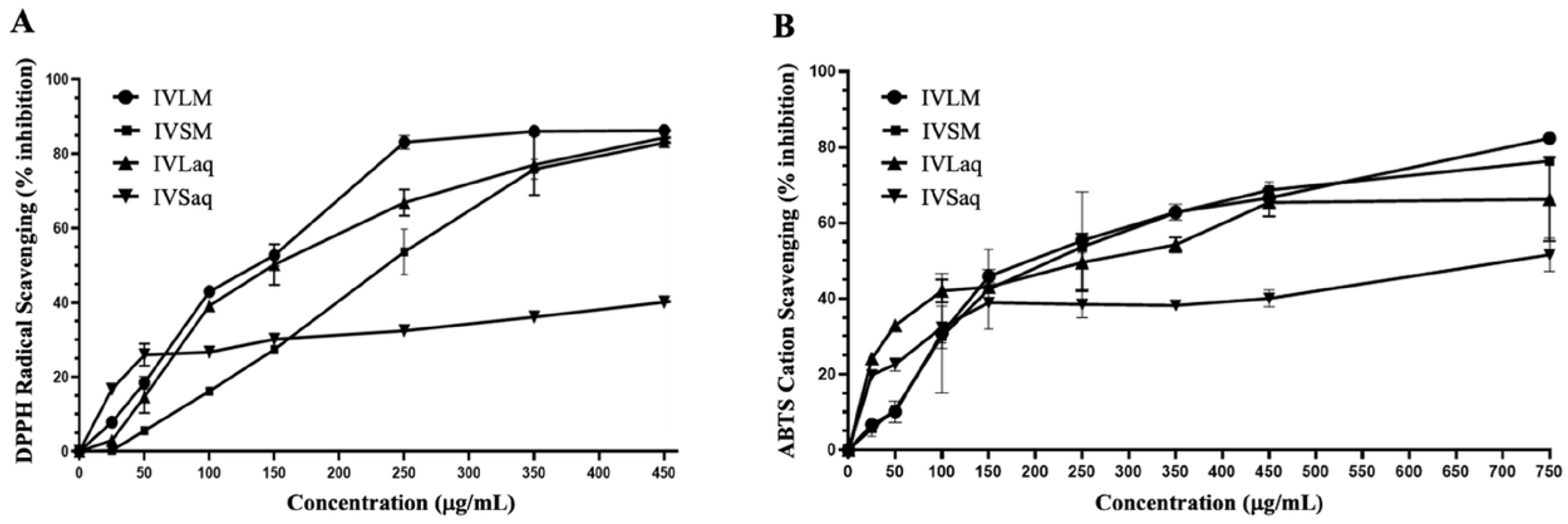
3.1. Extraction Yield, Total Phenolic Content (TPC), Total Flavonoid Content (TFC), and Antioxidant Capacity of Inula viscosa Leaves and Stems Extracts
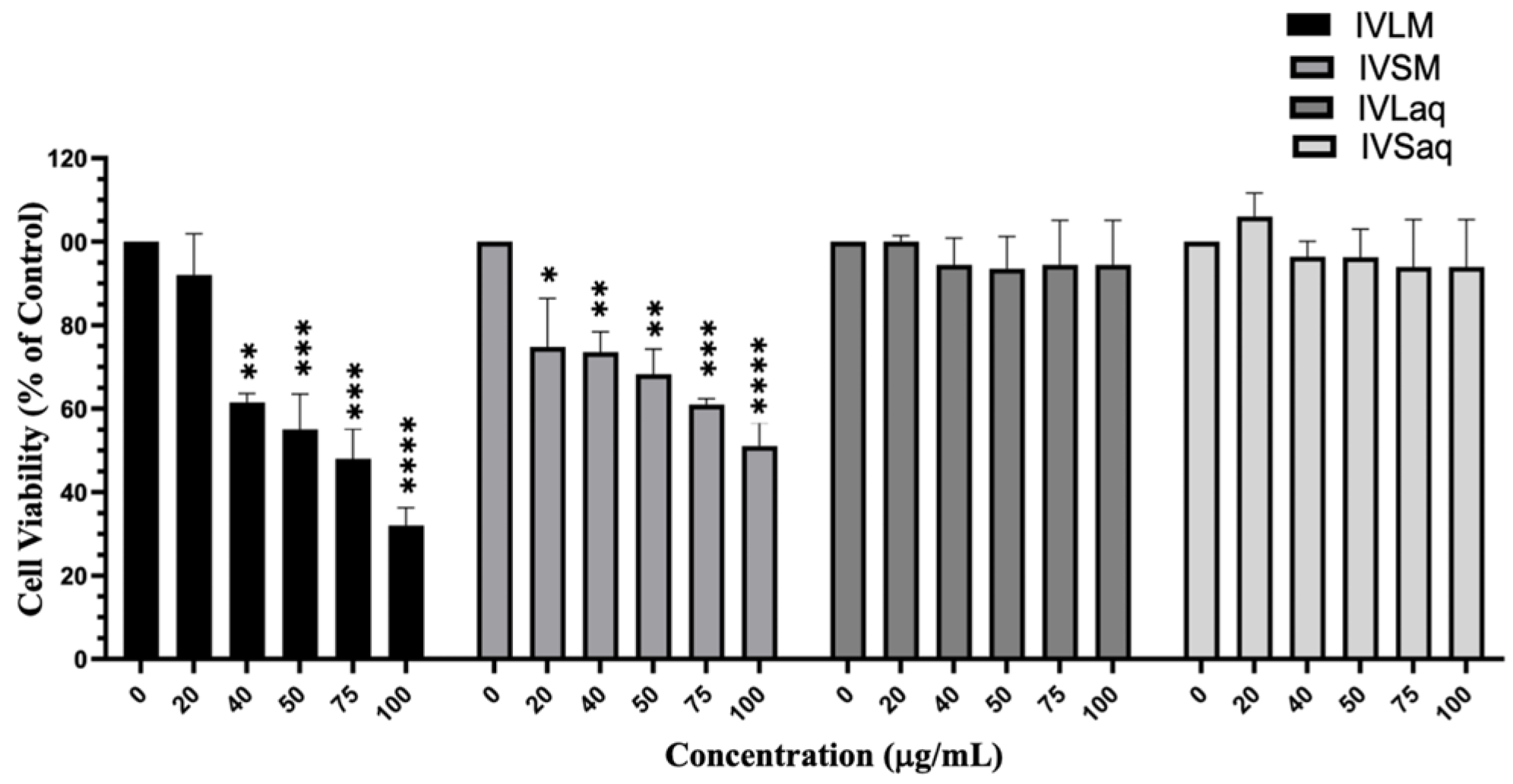
3.2. Inula viscosa Methanolic Extracts Reduced the Viability of A549 Lung Cancer Cells
3.3. Dichloromethane Fraction of Inula viscosa Leaves Methanolic Extract (IVL DCM) Reduced the Viability of A549 Lung Cancer Cells
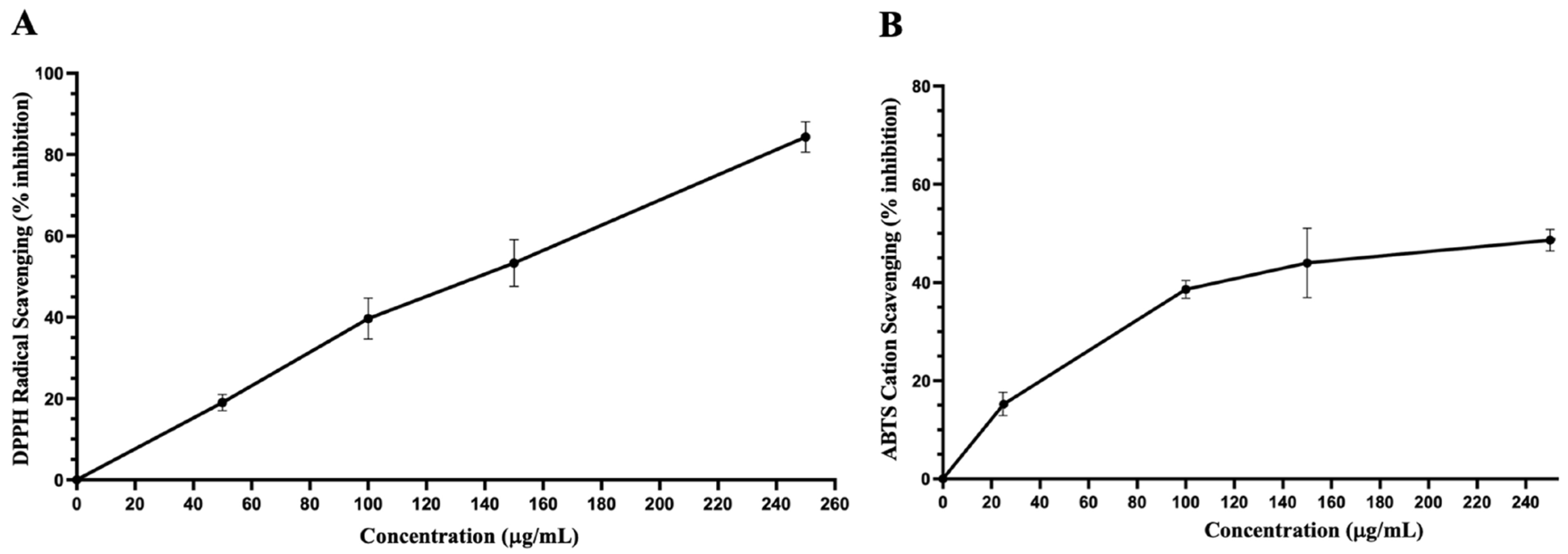
3.4. Antioxidant Capacity and Total Phenolic and Flavonoid Contents of Dichloromethane Fraction of Inula viscosa Leaves Methanolic Extract (IVL DCM)
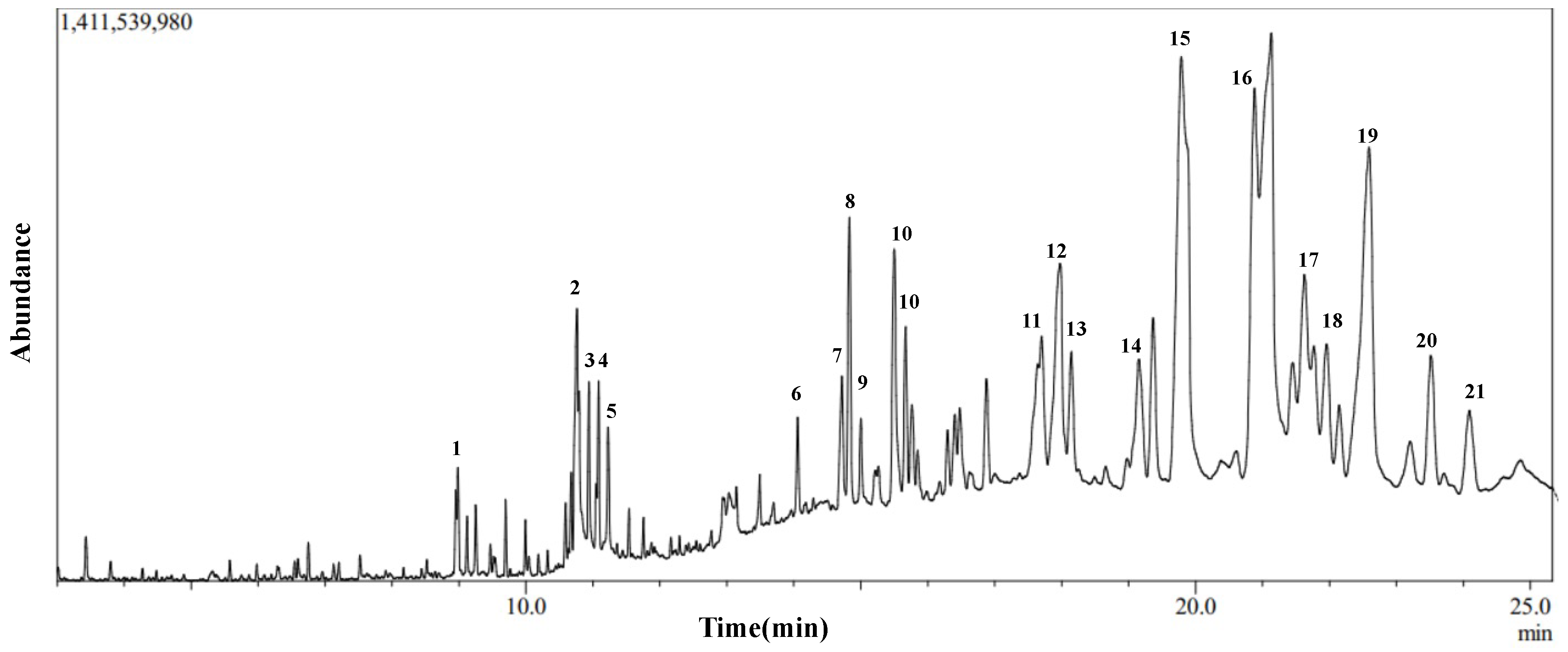
3.5. Gas Chromatography-Mass Spectroscopy (GC-MS) of Dichloromethane Fraction of Inula viscosa Leaves Methanolic Extract (IVL DCM)
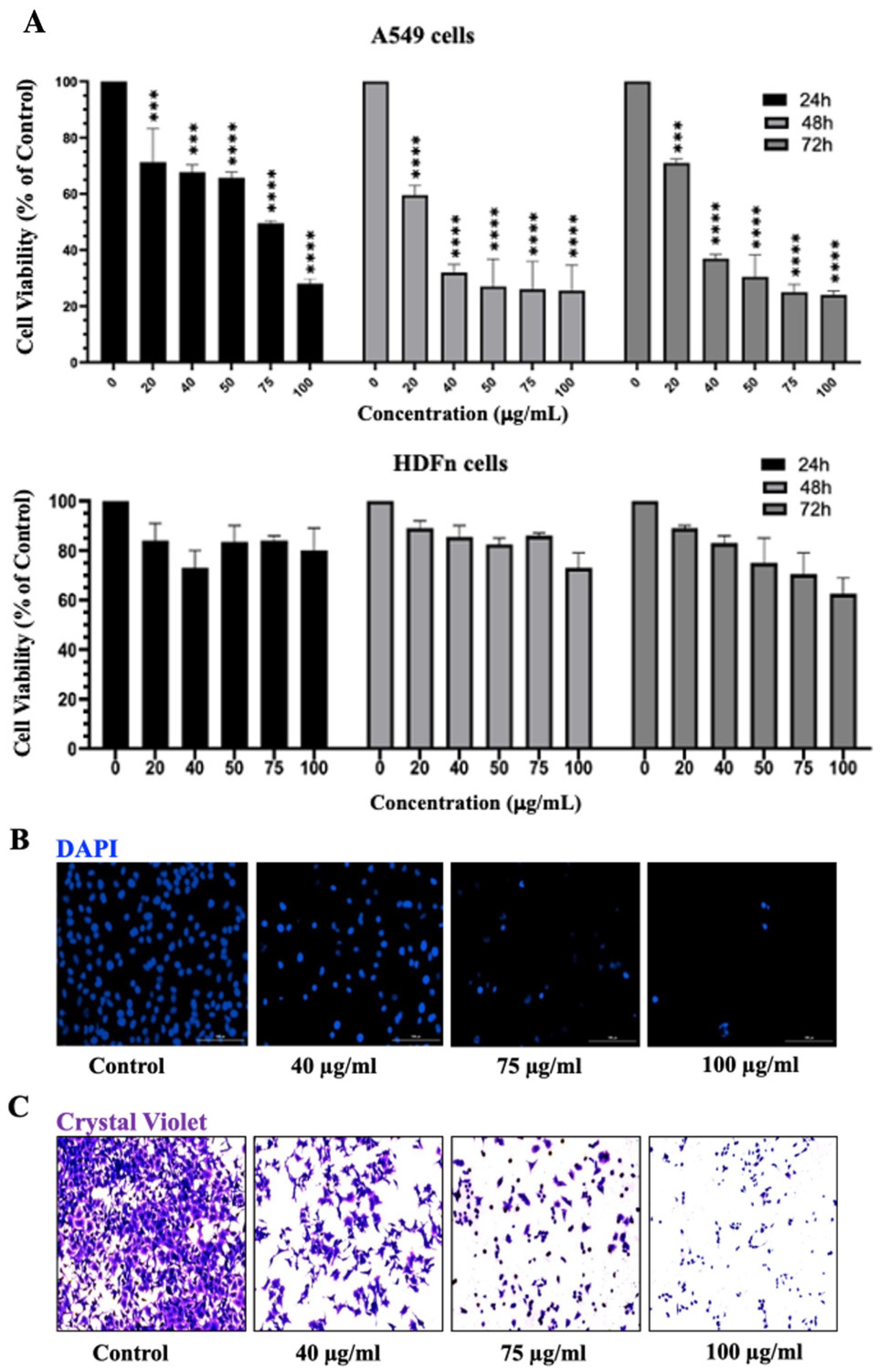
3.6. Inula viscosa Leaves Terpenoid-Rich Fraction (IVL DCM) Inhibited the Proliferation of A549 Lung Cancer Cells
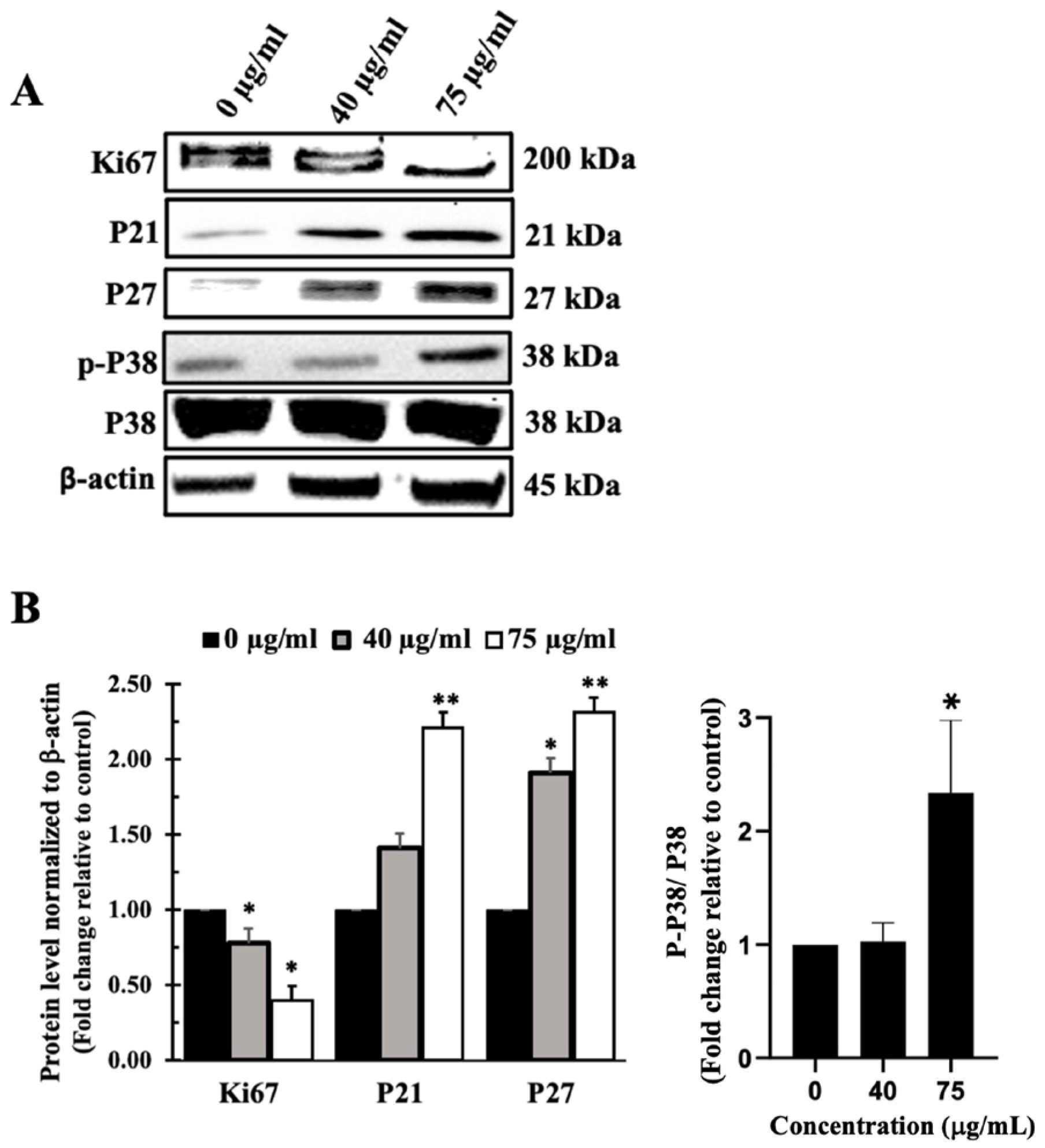
3.7. I. viscosa Leaves Terpenoid-Rich Fraction (IVL DCM) Induced Apoptosis of A549 Cells
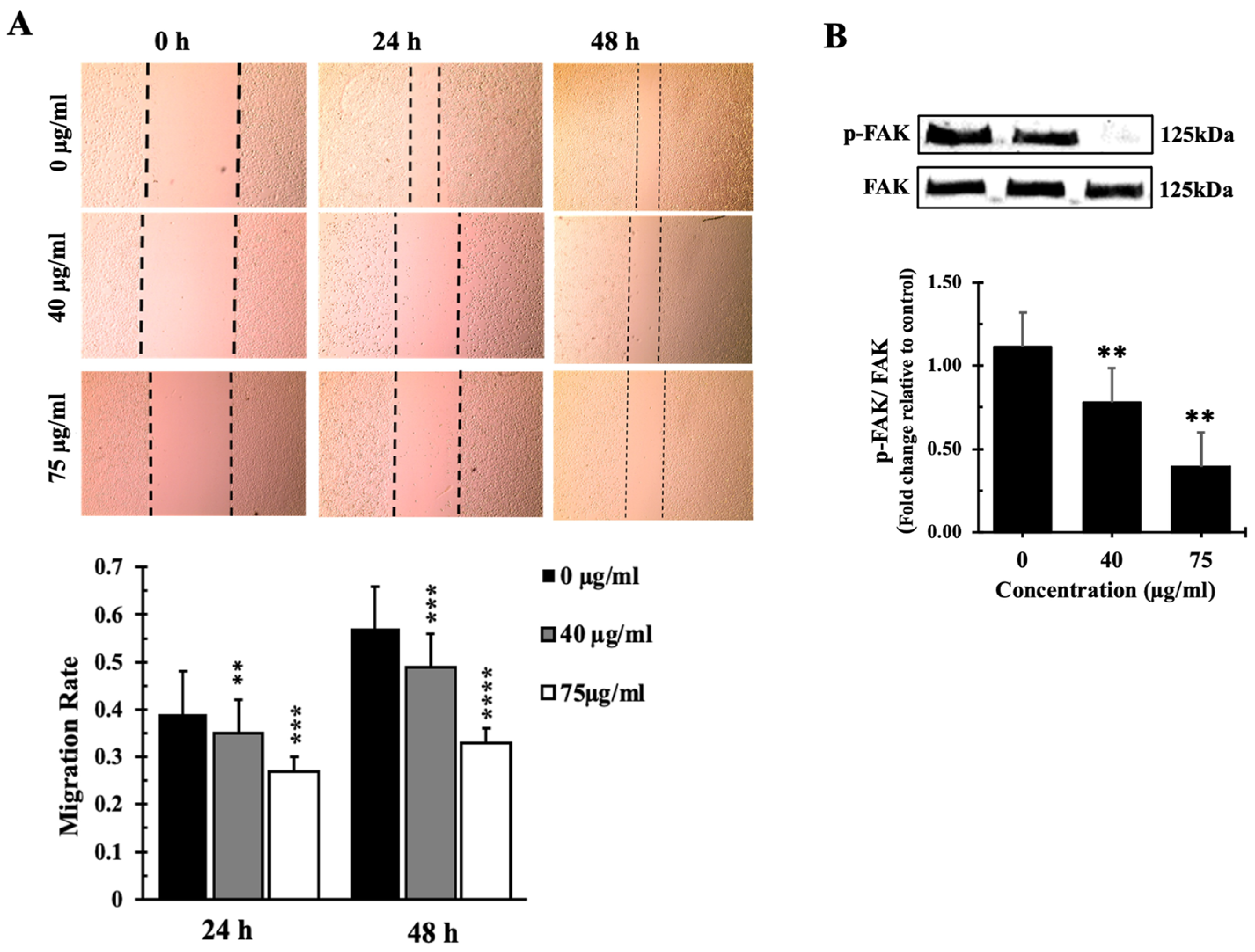
3.8. Inula viscosa Leaves Terpenoid Enriched Fraction (IVL DCM) Reduced the Migration of A549 Lung Cancer Cells through Reduction of FAK Activation
4. Discussion
4.1. Phenolic and Flavonoid Contents and Antioxidant Capacity of I. viscosa Parts
4.2. Inula viscosa Leaves Extracts Reduced the Viability of A549 Lung Cancer Cells
4.3. A Terpenoid-Rich Fraction of I. viscosa (IVL DCM)
4.4. The Terpenoid-Rich Fraction of I. viscosa Reduced the Proliferation and Migration and Induced Apoptosis of A549 Lung Cancer Cells
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung cancer statistics. In Lung Cancer and Personalized Medicine: Current Knowledge and Therapies; Springer: Cham, Sweiterland, 2016; pp. 1–19. [Google Scholar]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non–small cell lung cancer: Epidemiology, screening, diagnosis, and treatment. Mayo Clinic Proceedings. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- Mamdani, H.; Matosevic, S.; Khalid, A.B.; Durm, G.; Jalal, S.I. Immunotherapy in lung cancer: Current landscape and future directions. Front. Immunol. 2022, 13, 823618. [Google Scholar] [CrossRef]
- Zhou, K.; Li, S.; Zhao, Y.; Cheng, K. Mechanisms of drug resistance to immune checkpoint inhibitors in non-small cell lung cancer. Front. Immunol. 2023, 14, 1127071. [Google Scholar] [CrossRef]
- Talib, W.H.; Daoud, S.; Mahmod, A.I.; Hamed, R.A.; Awajan, D.; Abuarab, S.F.; Odeh, L.H.; Khater, S.; Al Kury, L.T. Plants as a Source of Anticancer Agents: From Bench to Bedside. Molecules 2022, 27, 4818. [Google Scholar] [CrossRef]
- Ju, H.; Yu, C.; Zhang, X.-D.; Liu, W.; Wu, Y.-C.; Gong, P.-X.; Li, H.-H.; Liu, Y.; Li, H.-J. Recent trends in anti-cancer activities of terrestrial plants-based polysaccharides: A review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100341. [Google Scholar] [CrossRef]
- Ullah, S.; Khan, T.; Khan, T.; Ali, M.; Khaliq Jan, A.; Khan Shinwari, Z.; Khan, A.; Al-Farsi, M.; Waqas, M. Phytochemicals for Cancer Treatment: An Update on Plant-Derived Anti-Cancer Compounds and their Mechanisms of Action. Curr. Top. Med. Chem. 2024, 24, 1–20. [Google Scholar] [CrossRef]
- Shaito, A.A.; Omairi, I.; Al-Thani, N.; Seglab, F.; Ad-Darwish, E.; Kobeissy, F.; Nasreddine, S. Determination of Medicago orbicularis Antioxidant, Antihemolytic, and Anti-Cancerous Activities and Its Augmentation of Cisplatin-Induced Cytotoxicity in A549 Lung Cancer Cells. Plants 2024, 13, 442. [Google Scholar] [CrossRef]
- Qneibi, M.; Hanania, M.; Jaradat, N.; Emwas, N.; Radwan, S. Inula viscosa (L.) Greuter, phytochemical composition, antioxidant, total phenolic content, total flavonoids content and neuroprotective effects. Eur. J. Integr. Med. 2021, 42, 101291. [Google Scholar] [CrossRef]
- Ouari, S.; Benzidane, N. Chemical composition, biological activities, and molecular mechanism of Inula viscosa (L.) bioactive compounds: A review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 3857–3865. [Google Scholar] [CrossRef] [PubMed]
- Sladonja, B.; Poljuha, D.; Krapac, M.; Uzelac, M.; Mikulic-Petkovsek, M. Dittrichia viscosa: Native-Non Native Invader. Diversity 2021, 13, 380. [Google Scholar] [CrossRef]
- Jerada, R.; Er-Rakibi, A.; Hassani, A.C.; Benzeid, H.; El Ouardi, A.; Harhar, H.; Goh, B.H.; Yow, Y.-Y.; Ser, H.L.; Bouyahya, A. A comprehensive review on ethnomedicinal uses, phytochemistry, toxicology, and pharmacological activities of Dittrichia viscosa (L.) Greuter. J. Tradit. Complement. Med. J. Tradit. Complement. Med. 2024, 14, 355–380. [Google Scholar] [CrossRef] [PubMed]
- Ouahchia, C.; Hamaidi-Chergui, F.; Cherif, H.-S.; Hemma, R.; Negab, I.; Azine, K.; Saidi, F. Total Phenolic Content, Anti-Inflammatory, Analgesic, and Antipyretic Activities of Some Extracts of Inula viscosa (L.) from Algeria. Phytothérapie 2020, 18, 81–91. [Google Scholar] [CrossRef]
- Ben Sassi, A.; Harzallah-Skhiri, F.; Bourgougnon, N.; Aouni, M. Antiviral activity of some Tunisian medicinal plants against Herpes simplex virus type 1. Nat. Prod. Res. 2008, 22, 53–65. [Google Scholar] [CrossRef]
- Kurz, H.; Karygianni, L.; Argyropoulou, A.; Hellwig, E.; Skaltsounis, A.L.; Wittmer, A.; Vach, K.; Al-Ahmad, A. Antimicrobial effects of Inula viscosa extract on the in situ initial oral biofilm. Nutrients 2021, 13, 4029. [Google Scholar] [CrossRef]
- Zeggwagh, N.A.; Ouahidi, M.L.; Lemhadri, A.; Eddouks, M. Study of hypoglycaemic and hypolipidemic effects of Inula viscosa L. aqueous extract in normal and diabetic rats. J. Ethnopharmacol. 2006, 108, 223–227. [Google Scholar] [CrossRef]
- Migheli, R.; Virdis, P.; Galleri, G.; Arru, C.; Lostia, G.; Coradduzza, D.; Muroni, M.R.; Pintore, G.; Podda, L.; Fozza, C. Antineoplastic Properties by Proapoptotic Mechanisms Induction of Inula viscosa and Its Sesquiterpene Lactones Tomentosin and Inuviscolide. Biomedicines 2022, 10, 2739. [Google Scholar] [CrossRef]
- Virdis, P.; Migheli, R.; Galleri, G.; Fancello, S.; Cadoni, M.P.L.; Pintore, G.; Petretto, G.L.; Marchesi, I.; Fiorentino, F.P.; di Francesco, A. Antiproliferative and proapoptotic effects of Inula viscosa extract on Burkitt lymphoma cell line. Tumor Biol. 2020, 42, 1010428319901061. [Google Scholar] [CrossRef]
- Mrid, R.B.; Bouchmaa, N.; Kabach, I.; Zouaoui, Z.; Chtibi, H.; Maadoudi, M.E.; Kounnoun, A.; Cacciola, F.; Majdoub, Y.O.E.; Mondello, L.; et al. Dittrichia viscosa L. Leaves: A Valuable Source of Bioactive Compounds with Multiple Pharmacological Effects. Molecules 2022, 27, 2108. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Zarga, M.H.; Mahasneh, A.M. Antiproliferative, antimicrobial and apoptosis inducing effects of compounds isolated from Inula viscosa. Molecules 2012, 17, 3291–3303. [Google Scholar] [CrossRef]
- Bar-Shalom, R.; Bergman, M.; Grossman, S.; Azzam, N.; Sharvit, L.; Fares, F. Inula viscosa extract inhibits growth of colorectal cancer cells in vitro and in vivo through induction of apoptosis. Front. Oncol. 2019, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Kheyar, N.; Bellik, Y.; Serra, A.T.; Kheyar, F.; Bedjou, F. Inula viscosa phenolic extract suppresses colon cancer cell proliferation and ulcerative colitis by modulating oxidative stress biomarkers. BioTechnologia 2022, 103, 269–281. [Google Scholar] [CrossRef]
- Anglana, C.; Rojas, M.; Girelli, C.R.; Barozzi, F.; Quiroz-Troncoso, J.; Alegría-Aravena, N.; Montefusco, A.; Durante, M.; Fanizzi, F.P.; Ramírez-Castillejo, C.; et al. Methanolic Extracts of D. viscosa Specifically Affect the Cytoskeleton and Exert an Antiproliferative Effect on Human Colorectal Cancer Cell Lines, According to Their Proliferation Rate. Int. J. Mol. Sci. 2023, 24, 14920. [Google Scholar] [CrossRef] [PubMed]
- Benbacer, L.; Merghoub, N.; El Btaouri, H.; Gmouh, S.; Attaleb, M.; Morjani, H.; Amzazi, S.; El Mzibri, M. Antiproliferative effect and induction of apoptosis by Inula viscosa L. and Retama monosperma L. extracts in human cervical cancer cells. In Topics on Cervical Cancer With an Advocacy for Prevention; Rajkumar, R., Ed.; InTech: Houston, TX, USA, 2012. [Google Scholar] [CrossRef]
- Colak, D.K.; Egeli, U.; Eryilmaz, I.E.; Aybastier, O.; Malyer, H.; Cecener, G.; Tunca, B. The Anticancer Effect of Inula viscosa Methanol Extract by miRNAs’ Re-regulation: An in vitro Study on Human Malignant Melanoma Cells. Nutr. Cancer 2022, 74, 211–224. [Google Scholar] [CrossRef]
- El Yaagoubi, O.M.; Lahmadi, A.; Bouyahya, A.; Filali, H.; Samaki, H.; El Antri, S.; Aboudkhil, S. Antitumor Effect of Inula viscosa Extracts on DMBA-Induced Skin Carcinoma Are Mediated by Proteasome Inhibition. Biomed. Res. Int. 2021, 2021, 6687589. [Google Scholar] [CrossRef] [PubMed]
- Rechek, H.; Haouat, A.; Hamaidia, K.; Pinto, D.; Boudiar, T.; Válega, M.; Pereira, D.M.; Pereira, R.B.; Silva, A.M.S. Inula viscosa (L.) Aiton Ethanolic Extract Inhibits the Growth of Human AGS and A549 Cancer Cell Lines. Chem. Biodivers. 2023, 20, e202200890. [Google Scholar] [CrossRef] [PubMed]
- Abu Zarga, M.H.; Hamed, E.M.; Sabri, S.S.; Voelter, W.; Zeller, K.-P. New sesquiterpenoids from the Jordanian medicinal plant Inula viscosa. J. Nat. Prod. 1998, 61, 798–800. [Google Scholar] [CrossRef]
- Lauro, L.; Rolih, C. Observations and research on an extract of Inula viscosa Ait. Boll. Della Soc. Ital. Biol. Sper. 1990, 66, 829–834. [Google Scholar]
- Eruygur, N.; Tuzcu, N.; Tugay, O.; Yilmaz, M.A.; Cakir, O. Phytochemical characterization and biological activities of Inula viscosa L. Aiton: A promising plant from Turkey. Int. J. Environ. Health Res. 2024, 34, 3334–3347. [Google Scholar] [CrossRef]
- Kheyar-Kraouche, N.; Boucheffa, S.; Bellik, Y.; Farida, K.; Brahmi-Chendouh, N. Exploring the potential of Inula viscosa extracts for antioxidant, antiproliferative and apoptotic effects on human liver cancer cells and a molecular docking study. Biotechnologia 2023, 104, 183. [Google Scholar] [CrossRef]
- Nikolakaki, A.; Christodoulakis, N. Leaf structure and cytochemical investigation of secretory tissues in Inula viscosa. Bot. J. Linn. Soc. 2004, 144, 437–448. [Google Scholar] [CrossRef][Green Version]
- Grande, M.; Torres, P.; Piera, F.; Bellido, I.S. Triterpenoids from Dittrichia viscosa. Phytochemistry 1992, 31, 1826–1828. [Google Scholar] [CrossRef]
- Santos, S.A.P.; Mota, L.; Malheiro, R.; Silva, F.; Campos, M.; de Pinho, P.G.; Pereira, J.A. Changes in volatile compounds of Dittrichia viscosa caused by the attack of the gall-forming dipteran Myopites stylatus. Ind. Crops Prod. 2016, 87, 71–77. [Google Scholar] [CrossRef]
- González-Hernández, R.A.; Valdez-Cruz, N.A.; Trujillo-Roldán, M.A. Factors that influence the extraction methods of terpenes from natural sources. Chem. Pap. 2024, 78, 2783–2810. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Lage, H.; Duarte, N.; Coburger, C.; Hilgeroth, A.; Ferreira, M.J.U. Antitumor activity of terpenoids against classical and atypical multidrug resistant cancer cells. Phytomedicine 2010, 17, 441–448. [Google Scholar] [CrossRef]
- Kamran, S.; Sinniah, A.; Abdulghani, M.A.; Alshawsh, M.A. Therapeutic potential of certain terpenoids as anticancer agents: A scoping review. Cancers 2022, 14, 1100. [Google Scholar] [CrossRef]
- El-Baba, C.; Baassiri, A.; Kiriako, G.; Dia, B.; Fadlallah, S.; Moodad, S.; Darwiche, N. Terpenoids’ anti-cancer effects: Focus on autophagy. Apoptosis 2021, 26, 491–511. [Google Scholar] [CrossRef]
- Ismail, N.I.M.; Chua, L.S. Solvent Partition for Terpenoid Rich Fraction From Crude Extract of Eurycoma longifolia. In Advances in Engineering Research, Proceedings of the Third International Conference on Separation Technology 2020, Johor, Malaysia, 15–16 August 2020; Atlantis Press: Amsterdam, The Netherlands, 2020; Volume 200, pp. 62–67. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Araghi, M.; Mannani, R.; Heidarnejad Maleki, A.; Hamidi, A.; Rostami, S.; Safa, S.H.; Faramarzi, F.; Khorasani, S.; Alimohammadi, M.; Tahmasebi, S.; et al. Recent advances in non-small cell lung cancer targeted therapy; an update review. Cancer Cell Int. 2023, 23, 162. [Google Scholar] [CrossRef]
- Prisa, D. Possible use of Inula viscosa (Dittrichia viscosa L.) for biostimulation of Oscularia deltoides and Corpuscolaria lehmanii plants and protection against Aphis nerii. GSC Biol. Pharm. Sci. 2019, 9, 69–75. [Google Scholar] [CrossRef]
- De Laurentis, N.; Losacco, V.; Milillo, M.A.; Lai, O. Chemical investigations of volatile constituents of Inula viscosa (L.) Aiton (Asteraceae) from different areas of Apulia, Southern Italy. Delpinoa 2002, 44, 115–119. [Google Scholar]
- Hwija, E.; Mossa, Y.; Hasan, M. Chemical composition of essential oils extracted from flowers of Taion plant (Inula viscosa L.) from two different regions of Lattakia–Syria. Tishreen Univ. J.-Basic Sci. Ser. 2017, 39. [Google Scholar]
- Mahmoudi, H.; Hosni, K.; Zaouali, W.; Amri, I.; Zargouni, H.; Hamida, N.B.; Kaddour, R.; Hamrouni, L.; Nasri, M.B.; Ouerghi, Z. Comprehensive phytochemical analysis, antioxidant and antifungal activities of Inula viscosa Aiton leaves. J. Food Saf. 2016, 36, 77–88. [Google Scholar] [CrossRef]
- Haoui, I.E.; Derriche, R.; Madani, L.; Oukali, Z. Analysis of the chemical composition of essential oil from Algerian Inula viscosa (L.) Aiton. Arab. J. Chem. 2015, 8, 587–590. [Google Scholar] [CrossRef]
- Ainseba, N.; Soulimane, A.; DIB, M.E.A.; Djabou, N.; Muselli, A. Comparative Study of the Antioxidant, Antimicrobial and Anti-Inflammatory Activity between Essential Oil and Hydrosol Extract of the Aerial Parts of Inula viscosa L. J. Appl. Biotechnol. Rep. 2023, 10, 1169–1175. [Google Scholar]
- Sriti Eljazi, J.; Selmi, S.; Zarroug, Y.; Wesleti, I.; Aouini, B.; Jallouli, S.; Limam, F. Essential oil composition, phenolic compound, and antioxidant potential of Inulaviscosa as affected by extraction process. Int. J. Food Prop. 2018, 21, 2309–2319. [Google Scholar] [CrossRef]
- Ounoughi, A.; Ramdani, M.; Lograda, T.; Chalard, P. Chemotypes and antibacterial activities of Inula viscosa essential oils from Algeria. Biodiversitas. 2020, 21, 1504–1517. [Google Scholar] [CrossRef]
- Karamenderes, C.; Zeybek, U. Composition of the essential oils of IAJULA viscosa, I. graveolens and I. helenium subsp. turcoracemosa. J. Fac. Pharm. Istanb. Univ. 2000, 33, 1–6. [Google Scholar]
- Hammal, A.; Al-Duihi, H.A.-H.; Alchab, L. Preparation of Nano Hydroxyapatite Loaded with Syrian Inula Extract Against Dental Caries. SSRN Soc. Sci. Netw. 2024. [Google Scholar] [CrossRef]
- Blanc, M.-C.; Bradesi, P.; Gonçalves, M.J.; Salgueiro, L.; Casanova, J. Essential oil of Dittrichia viscosa ssp. viscosa: Analysis by 13C-NMR and antimicrobial activity. Flavour Fragr. J. 2006, 21, 324–332. [Google Scholar] [CrossRef]
- Madani, L.; Derriche, R.; Haoui, I.E. Essential oil of Algerian Inula viscosa leaves. J. Essent. Oil Bear. Plants 2014, 17, 164–168. [Google Scholar] [CrossRef]
- Faria, J.M.; Barbosa, P.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Bioactivity against Bursaphelenchus xylophilus: Nematotoxics from essential oils, essential oils fractions and decoction waters. Phytochemistry 2013, 94, 220–228. [Google Scholar] [CrossRef]
- Rhimi, W.; Salem, I.B.; Iatta, R.; Chaabane, H.; Saidi, M.; Boulila, A.; Cafarchia, C. Dittrichia viscosa L. leaves lipid extract: An unexploited source of essential fatty acids and tocopherols with antifungal and anti-inflammatory properties. Ind. Crops Prod. 2018, 113, 196–201. [Google Scholar] [CrossRef]
- Bentarhlia, N.; Kartah, B.E.; Fadil, M.; El Harkaoui, S.; Matthäus, B.; Abboussi, O.; Abdelmoumen, H.; Bouhnik, O.; El Monfalouti, H. Exploring the wound-healing and antimicrobial potential of Dittrichia viscosa L lipidic extract: Chemical composition and in vivo evaluation. Fitoterapia 2024, 172, 105707. [Google Scholar] [CrossRef]
- Abdallah, R.; Shaito, A.A.; Badran, A.M.; Baydoun, S.; Sobeh, M.; Ouchari, W.; Sahri, N.; Eid, A.H.; Mesmar, J.E.; Baydoun, E. Fractionation and phytochemical composition of an ethanolic extract of Ziziphus nummularia leaves: Antioxidant and anticancerous properties in triple negative human breast cancer cells. Front. Pharmacol. 2024, 15, 1331843. [Google Scholar] [CrossRef] [PubMed]
- Brahmi-Chendouh, N.; Piccolella, S.; Crescente, G.; Pacifico, F.; Boulekbache, L.; Hamri-Zeghichi, S.; Akkal, S.; Madani, K.; Pacifico, S. A nutraceutical extract from Inula viscosa leaves: UHPLC-HR-MS/MS based polyphenol profile, and antioxidant and cytotoxic activities. J. Food Drug Anal. 2019, 27, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Kheyar-Kraouche, N.; da Silva, A.B.; Serra, A.T.; Bedjou, F.; Bronze, M.R. Characterization by liquid chromatography-mass spectrometry and antioxidant activity of an ethanolic extract of Inula viscosa leaves. J. Pharm. Biomed. Anal. 2018, 156, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Kaczorová, D.; Karalija, E.; Dahija, S.; Bešta-Gajević, R.; Parić, A.; Ćavar Zeljković, S. Influence of Extraction Solvent on the Phenolic Profile and Bioactivity of Two Achillea Species. Molecules 2021, 26, 1601. [Google Scholar] [CrossRef]
- Autor, E.; Cornejo, A.; Bimbela, F.; Maisterra, M.; Gandía, L.M.; Martínez-Merino, V. Extraction of Phenolic Compounds from Populus Salicaceae Bark. Biomolecules 2022, 12, 539. [Google Scholar] [CrossRef]
- Karimi, E.; Jaafar, H.Z.; Ghasemzadeh, A.; Ibrahim, M.H. Light intensity effects on production and antioxidant activity of flavonoids and phenolic compounds in leaves, stems and roots of three varieties of Labisia pumila Benth. Aust. J. Crop Sci. 2013, 7, 1016. [Google Scholar]
- Kumari, R.; Singh, S.; Agrawal, S. Effects of supplemental ultraviolet-B radiation on growth and physiology of Acorus calamus L.(sweet flag). Acta Biol. Cracoviensia Ser. Bot. 2009, 51, 19–27. [Google Scholar]
- Nguyen, K.Q.; Scarlett, C.J.; Vuong, Q.V. Assessment and comparison of phytochemicals and antioxidant properties from various parts of the Australian maroon bush (Scaevola spinescens). Heliyon 2021, 7, e06810. [Google Scholar] [CrossRef] [PubMed]
- Larbat, R.; Paris, C.; Le Bot, J.; Adamowicz, S. Phenolic characterization and variability in leaves, stems and roots of Micro-Tom and patio tomatoes, in response to nitrogen limitation. Plant Sci. 2014, 224, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Martin-Puzon, J.J.R.; Rivera, W.L. Free-radical scavenging activity and bioactive secondary metabolites from various extracts of Glinus oppositifolius (L.) Aug. DC. (Molluginaceae) roots, stems and leaves. Asian Pac. J. Trop. Dis. 2015, 5, 711–715. [Google Scholar] [CrossRef]
- Kuntorini, E.M.; Nugroho, L.H.; Maryani, M.; Nuringtyas, T. R. Anatomical structure, flavonoid content, and antioxidant activity of Rhodomyrtus tomentosa leaves and fruits on different age and maturity level. Biodiversitas J. Biol. Divers. 2019, 20, 3619. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz. J. Pharm. Sci. 2020, 56, e17129. [Google Scholar] [CrossRef]
- Seglab, F.; Hamia, C.; Khacheba, I.; Djeridane, A.; Yousfi, M. High in vitro antioxidant capacities of Algerian Cleome arabica leaves’ extracts. Phytothérapie 2021, 19, 16–24. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chem. 2006, 99, 835–841. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2—Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Duxford, UK, 2019; pp. 33–50. [Google Scholar] [CrossRef]
- Asraoui, F.; Kounnoun, A.; Cacciola, F.; El Mansouri, F.; Kabach, I.; Oulad El Majdoub, Y.; Alibrando, F.; Arena, K.; Trovato, E.; Mondello, L. Phytochemical profile, antioxidant capacity, α-amylase and α-glucosidase inhibitory potential of wild Moroccan inula viscosa (L.) aiton leaves. Molecules 2021, 26, 3134. [Google Scholar] [CrossRef]
- Chahmi, N.; Anissi, J.; Jennan, S.; Farah, A.; Sendide, K.; Hassouni, M.E. Antioxidant activities and total phenol content of Inula viscosa extracts selected from three regions of Morocco. Asian Pac. J. Trop. Biomed. 2015, 5, 228–233. [Google Scholar] [CrossRef]
- Gökbulut, A.; Ozhan, O.; Satilmiş, B.; Batçioğlu, K.; Günal, S.; Sarer, E. Antioxidant and antimicrobial activities, and phenolic compounds of selected Inula species from Turkey. Nat. Prod. Commun. 2013, 8, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Hepokur, C.; Budak, Y.; Karayel, H.B.; Selvi, B.; Yaylım, İ. Investigation of cytotoxic effects of Inula viscosa extract. Cumhur. Sci. J. 2019, 40, 578–582. [Google Scholar] [CrossRef][Green Version]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef] [PubMed]
- Mesmar, J.; Abdallah, R.; Hamade, K.; Baydoun, S.; Al-Thani, N.; Shaito, A.; Maresca, M.; Badran, A.; Baydoun, E. Ethanolic extract of Origanum syriacum L. leaves exhibits potent anti-breast cancer potential and robust antioxidant properties. Front. Pharmacol. 2022, 13, 994025. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- AlKahlout, A.; Fardoun, M.; Mesmar, J.; Abdallah, R.; Badran, A.; Nasser, S.A.; Baydoun, S.; Kobeissy, F.; Shaito, A.; Iratni, R.; et al. Origanum syriacum L. Attenuates the Malignant Phenotype of MDA-MB231 Breast Cancer Cells. Front. Oncol. 2022, 12, 922196. [Google Scholar] [CrossRef]
- Bonta, R.K. Dietary Phenolic Acids and Flavonoids as Potential Anti-Cancer Agents: Current State of the Art and Future Perspectives. Anti-Cancer Agents Med. Chem. 2020, 20, 29–48. [Google Scholar] [CrossRef]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014, 8, 122–146. [Google Scholar] [CrossRef]
- Fatma, H.; Jameel, M.; Siddiqui, A.J.; Kuddus, M.; Buali, N.S.; Bahrini, I.; Siddique, H.R. Chemotherapeutic Potential of Lupeol Against Cancer in Pre-Clinical Model: A Systematic Review and Meta-Analysis. Phytomedicine 2024. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, X.; Xie, L.; Deng, M.; Chen, H.; Song, J.; Long, J.; Li, X.; Luo, J. Lupeol and its derivatives as anticancer and anti-inflammatory agents: Molecular mechanisms and therapeutic efficacy. Pharmacol. Res. 2021, 164, 105373. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Saha, D.; Das, G.; Mukherjee, R.; Banerjee, S.; Alam, N.; Mustafi, S.M.; Nath, P.; Majumder, A.; Majumder, B.; et al. Lupeol synergizes with 5-fluorouracil to combat c-MET/EphA2 mediated chemoresistance in triple negative breast cancer. iScience 2023, 26, 108395. [Google Scholar] [CrossRef]
- Adepoju, F.O.; Duru, K.C.; Li, E.; Kovaleva, E.G.; Tsurkan, M.V. Pharmacological Potential of Betulin as a Multitarget Compound. Biomolecules 2023, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Król, S.K.; Kiełbus, M.; Rivero-Müller, A.; Stepulak, A. Comprehensive review on betulin as a potent anticancer agent. Biomed. Res. Int. 2015, 2015, 584189. [Google Scholar] [CrossRef] [PubMed]
- Shabana, S.M.; Gad, N.S.; Othman, A.I.; Mohamed, A.F.; El-Missiry, M.A. β-caryophyllene oxide induces apoptosis and inhibits proliferation of A549 lung cancer cells. Med. Oncol. 2023, 40, 189. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Nam, D.; Yun, H.M.; Lee, S.G.; Jang, H.J.; Sethi, G.; Cho, S.K.; Ahn, K.S. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—Natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Hanušová, V.; Caltová, K.; Svobodová, H.; Ambrož, M.; Skarka, A.; Murínová, N.; Králová, V.; Tomšík, P.; Skálová, L. The effects of β-caryophyllene oxide and trans-nerolidol on the efficacy of doxorubicin in breast cancer cells and breast tumor-bearing mice. Biomed. Pharmacother. 2017, 95, 828–836. [Google Scholar] [CrossRef]
- Delgado, C.; Mendez-Callejas, G.; Celis, C. Caryophyllene Oxide, the Active Compound Isolated from Leaves of Hymenaea courbaril L. (Fabaceae) with Antiproliferative and Apoptotic Effects on PC-3 Androgen-Independent Prostate Cancer Cell Line. Molecules 2021, 26, 6142. [Google Scholar] [CrossRef]
- Velu, P. TNF-α regulated inflammatory pathway by Isopulegol in human lung adenocarcinoma (A549) cells through ROS generation. Aust. J. Sci. Technol. 2020, 4, 348–352. [Google Scholar]
- Jaafari, A.; Tilaoui, M.; Mouse, H.A.; M’bark, L.A.; Aboufatima, R.; Chait, A.; Lepoivre, M.; Zyad, A. Comparative study of the antitumor effect of natural monoterpenes: Relationship to cell cycle analysis. Rev. Bras. Farmacogn. 2012, 22, 534–540. [Google Scholar] [CrossRef]
- Jiang, Q. Different Roles of Tocopherols and Tocotrienols in Chemoprevention and Treatment of Prostate Cancer. Adv. Nutr. 2024, 15, 100240. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Ahmed Jum, A.D.; Attallah, Z.S.; Jallad, M.S.; Al Kury, L.T.; Hadi, R.W.; Mahmod, A.I. Role of vitamins A, C, D, E in cancer prevention and therapy: Therapeutic potentials and mechanisms of action. Front. Nutr. 2023, 10, 1281879. [Google Scholar] [CrossRef]
- Orlando, A.; Linsalata, M.; Tutino, V.; D′Attoma, B.; Notarnicola, M.; Russo, F. Vitamin K1 Exerts Antiproliferative Effects and Induces Apoptosis in Three Differently Graded Human Colon Cancer Cell Lines. BioMed Res. Int. 2015, 2015, 296721. [Google Scholar] [CrossRef]
- Linsalata, M.; Orlando, A.; Tutino, V.; Notarnicola, M.; D’Attoma, B.; Russo, F. Inhibitory effect of vitamin K1 on growth and polyamine biosynthesis of human gastric and colon carcinoma cell lines. Int. J. Oncol. 2015, 47, 773–781. [Google Scholar] [CrossRef]
- Pai, J.T.; Hsu, M.W.; Leu, Y.L.; Chang, K.T.; Weng, M.S. Induction of G2/M Cell Cycle Arrest via p38/p21(Waf1/Cip1)-Dependent Signaling Pathway Activation by Bavachinin in Non-Small-Cell Lung Cancer Cells. Molecules 2021, 26, 5161. [Google Scholar] [CrossRef]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. p53 signaling in cancer progression and therapy. Cancer Cell Int. 2021, 21, 703. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Dubey, V.; Luqman, S. Activation of Caspase-3 by terpenoids and flavonoids in different types of cancer cells. Curr. Top. Med. Chem. 2020, 20, 1876–1887. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef]
- Cao, F.; Chu, C.; Qin, J.-J.; Guan, X. Research progress on antitumor mechanisms and molecular targets of Inula sesquiterpene lactones. Chin. Med. 2023, 18, 164. [Google Scholar] [CrossRef] [PubMed]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Compr. Physiol. 2012, 2, 2369–2392. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef]
- Siraj, M.A.; Islam, M.A.; Al Fahad, M.A.; Kheya, H.R.; Xiao, J.; Simal-Gandara, J. Cancer chemopreventive role of dietary terpenoids by modulating Keap1-Nrf2-ARE signaling system—A comprehensive update. Appl. Sci. 2021, 11, 10806. [Google Scholar] [CrossRef]
- Harmankaya, A.; Çınar, İ.; Yayla, M.; Harmankaya, S.; Beytur, M.; Öziç, C. In vitro evaluation of the effects of Inula viscosa’s different extracts on wound healing and oxidative stress in mouse L929 fibroblast cell line. Fabad J. Pharm. Sci. 2023, 49, 129–142. [Google Scholar] [CrossRef]
- Lee, C.M.; Lee, J.; Nam, M.J.; Choi, Y.S.; Park, S.H. Tomentosin Displays Anti-Carcinogenic Effect in Human Osteosarcoma MG-63 Cells via the Induction of Intracellular Reactive Oxygen Species. Int. J. Mol. Sci. 2019, 20, 1508. [Google Scholar] [CrossRef] [PubMed]
- Virdis, P.; Migheli, R.; Bordoni, V.; Fiorentino, F.P.; Sanna, L.; Marchesi, I.; Pintore, G.; Galleri, G.; Muroni, M.R.; Bagella, L.; et al. Clarifying the molecular mechanism of tomentosin-induced antiproliferative and proapoptotic effects in human multiple myeloma via gene expression profile and genetic interaction network analysis. Int. J. Mol. Med. 2021, 48. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lin, H.H.; Hsu, C.H.; Wang, C.J.; Chiang, T.A.; Chen, J.H. Inhibitory effects of andrographolide on migration and invasion in human non-small cell lung cancer A549 cells via down-regulation of PI3K/Akt signaling pathway. Eur. J. Pharmacol. 2010, 632, 23–32. [Google Scholar] [CrossRef]
- Tai, Y.L.; Chen, L.C.; Shen, T.L. Emerging roles of focal adhesion kinase in cancer. Biomed. Res. Int. 2015, 2015, 690690. [Google Scholar] [CrossRef]
- Shen, J.; Cao, B.; Wang, Y.; Ma, C.; Zeng, Z.; Liu, L.; Li, X.; Tao, D.; Gong, J.; Xie, D. Hippo component YAP promotes focal adhesion and tumour aggressiveness via transcriptionally activating THBS1/FAK signalling in breast cancer. J. Exp. Clin. Cancer Res. 2018, 37, 175. [Google Scholar] [CrossRef]
- Luo, M.; Guan, J.L. Focal adhesion kinase: A prominent determinant in breast cancer initiation, progression and metastasis. Cancer Lett. 2010, 289, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Zhao, X.; Qin, Y.; Ding, Y.; Chen, R.; Li, G.; Labrie, M.; Ding, Z.; Zhou, J.; Hu, J.; et al. FAK-ERK activation in cell/matrix adhesion induced by the loss of apolipoprotein E stimulates the malignant progression of ovarian cancer. J. Exp. Clin. Cancer Res. 2018, 37, 32. [Google Scholar] [CrossRef]
- Weiner, T.M.; Liu, E.T.; Craven, R.J.; Cance, W.G. Expression of focal adhesion kinase gene and invasive cancer. Lancet 1993, 342, 1024–1025. [Google Scholar] [CrossRef]
- Taliaferro-Smith, L.; Oberlick, E.; Liu, T.; McGlothen, T.; Alcaide, T.; Tobin, R.; Donnelly, S.; Commander, R.; Kline, E.; Nagaraju, G.P.; et al. FAK activation is required for IGF1R-mediated regulation of EMT, migration, and invasion in mesenchymal triple negative breast cancer cells. Oncotarget 2015, 6, 4757–4772. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Kim, H.S.; Jin, T.; Hwang, E.H.; Jung, M.; Moon, W.K. Overexpression of the miR-141/200c cluster promotes the migratory and invasive ability of triple-negative breast cancer cells through the activation of the FAK and PI3K/AKT signaling pathways by secreting VEGF-A. BMC Cancer 2016, 16, 570. [Google Scholar] [CrossRef] [PubMed]
- TIAN, R.; LIU, Z.-M.; JIN, H.-W.; ZHANG, L.-R.; LIN, W.-H. Target identification of isomalabaricane terpenes extracted from sponges. Acta Phys.-Chim. Sin. 2011, 27, 1214–1222. [Google Scholar]
- Xu, L.; Bi, Y.; Xu, Y.; Zhang, Z.; Xu, W.; Zhang, S.; Chen, J. Oridonin inhibits the migration and epithelial-to-mesenchymal transition of small cell lung cancer cells by suppressing FAK-ERK1/2 signalling pathway. J. Cell Mol. Med. 2020, 24, 4480–4493. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between Medical Plant-Derived Bioactive Compounds: Focus on Antimicrobial Combination Effects. Antibiotics 2022, 11, 1014. [Google Scholar] [CrossRef]

| Plant Extract | Extraction Yield (%) | TPC (µg GAE/g) | TFC (µg QE/g) | DPPH EC50 (µg/mL) | ABTS EC50 (µg/mL) |
|---|---|---|---|---|---|
| IVLM | 16.5 | 726.4 ± 1.1 | 303.3 ± 8.8 | 145.7 ± 2.6 | 236.9 ± 22.2 |
| IVSM | 4.4 | 532.0 ± 10.3 | 114.0 ± 4.4 | 229.7 ± 3.1 | 239.0 ± 5.5 |
| IVLaq | 3.5 | 212.0 ± 1.5 | 79.8 ± 2.9 | 155.8 ± 6.1 | 268.9 ± 3.7 |
| IVSaq | 2 | 174.3 ± 0.7 | 45.2 ± 0.1 | 693.8 ± 3.4 | 791.0 ± 14.5 |
| L-Ascorbic acid | ─ | ─ | ─ | 27.5 ± 1.3 | 93.4 ± 0.9 |
| Cell Line | IC50 (μg/mL) |
|---|---|
| SK-OV-3 | 80.0 ± 5.7 |
| MCF-7 | 54.0 ± 4.9 |
| HepG2 | 59.9 ± 7.5 |
| HCT116 | 39.2 ± 6.1 |
| IC50 (μg/mL) | |||
|---|---|---|---|
| Cell Line | 24 h | 48 h | 72 h |
| SK-OV-3 | 110.2 ± 6.9 | 96.5 ± 4.0 | 52.95 ± 6.7 |
| MCF-7 | 84.4 ± 6.1 | 42.5 ± 2.9 | 29.32 ± 1.2 |
| MDA-MB-231 | 86.8 ± 4.8 | 69.3 ± 3.3 | 49.09 ± 1.8 |
| HepG2 | NT | 20.2 ± 5.2 | NT |
| HCT116 | NT | 19.7 ± 3.7 | NT |
| TPC (µg GAE/g) | TFC (µg QE/g) | DPPH EC50 (µg/mL) | ABTS EC50 (µg/mL) | |
|---|---|---|---|---|
| IVL DCM | 724.4 ± 12.1 | 235.4 ± 5.1 | 143.0 ± 1.4 | 241.6 ± 9.7 |
| No. | Compound Name | Chemical Nature | RT (min) | Molecular Formula | Molecular Weight | Reference |
|---|---|---|---|---|---|---|
| 1 | 2-Hexyldecan-1-ol | Alcohol | 8.995 | C16H34O | 242 | NR |
| 2 | Pyridine, 1-acetyl-1,2,3,4-tetrahydro-5-(2-piperidinyl)-(Ammodendrine) | Pyridine alkaloid | 10.77 | C12H20N2O | 208 | NR |
| 3 | Isopulegol | Monoterpene | 10.95 | C10H18O | 154 | NR |
| 4 | Linoleic acid ethyl ester | Fatty acid derivative | 11.06 | C20H36O2 | 308 | [51,52,53] |
| 5 | Caryophyllene oxide | Oxygenated sesquiterpene | 11.10 | C15H24O | 220 | [53,54,55,56,57,58,59,60,61,62] |
| 6 | 3,25-bis(acetyloxy)-5-hydroxyergostan-6-one | Steroid | 13.90 | C32H52O6 | 532 | NR |
| 7 | Citronellal | Monoterpene | 14.60 | C10H18O | 154 | [63] |
| 8 | Lup-20(29)-en-3-one (Lupenone) | Triterpenoid | 14.85 | C30H48O | 424 | NR |
| 9 | δ-Tocopherol | Vitamin E | 15.23 | C27H46O2 | 402 | [64,65] |
| 10 | Lupeol, trifluoroacetate | Triterpene | 15.69 | C32H49F3O2 | 522 | NR |
| 11 | Linalyl propionate | Monoterpene | 17.73 | C13H22O2 | 210 | [53,60] |
| 12 | Betulin | Triterpenoid | 18.01 | C30H50O2 | 442 | NR |
| 13 | Phytyl palmitate | Fatty acid/diterpene derivative | 18.20 | C36H70O2 | 534 | NR |
| 14 | Campesterol | Phytosterol | 19.41 | C28H48O | 400 | [65] |
| 15 | 6-Octadecenoic acid derivative | Fatty acid derivative | 19.83 | C22H41NO | 335 | NR |
| 16 | Norcodeine | Alkaloid | 21.10 | C17H19NO3 | 285 | NR |
| 17 | Phytonadione (Phylloquinone) | Vitamin K | 21.50 | C31H46O2 | 450 | NR |
| 18 | Lup-20(29)-en-3beta-ol, acetate (20(29)-(Lupenol acetate) | Triterpenoid | 21.67 | C32H52O2 | 468 | NR |
| 19 | Lupeol | Triterpenoid | 22.63 | C30H50O | 426 | NR |
| 20 | 9,19-Cyclolanostan-3-ol acetate | Triterpenoid | 23.77 | C32H54O2 | 470 | NR |
| 21 | 2-Hexadecyloxirane | Oxirane | 24.16 | C18H36O | 268 | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seglab, F.; Abou Assali, M.; AlYafei, T.; Hassan, H.; Pinto, D.C.G.A.; Baydoun, S.; Al Thani, A.A.; Shaito, A.A. Chemical Composition, Antioxidant Capacity, and Anticancerous Effects against Human Lung Cancer Cells of a Terpenoid-Rich Fraction of Inula viscosa. Biology 2024, 13, 687. https://doi.org/10.3390/biology13090687
Seglab F, Abou Assali M, AlYafei T, Hassan H, Pinto DCGA, Baydoun S, Al Thani AA, Shaito AA. Chemical Composition, Antioxidant Capacity, and Anticancerous Effects against Human Lung Cancer Cells of a Terpenoid-Rich Fraction of Inula viscosa. Biology. 2024; 13(9):687. https://doi.org/10.3390/biology13090687
Chicago/Turabian StyleSeglab, Fatiha, Mazen Abou Assali, Thoraya AlYafei, Hassan Hassan, Diana C. G. A. Pinto, Safaa Baydoun, Asmaa A. Al Thani, and Abdullah A. Shaito. 2024. "Chemical Composition, Antioxidant Capacity, and Anticancerous Effects against Human Lung Cancer Cells of a Terpenoid-Rich Fraction of Inula viscosa" Biology 13, no. 9: 687. https://doi.org/10.3390/biology13090687
APA StyleSeglab, F., Abou Assali, M., AlYafei, T., Hassan, H., Pinto, D. C. G. A., Baydoun, S., Al Thani, A. A., & Shaito, A. A. (2024). Chemical Composition, Antioxidant Capacity, and Anticancerous Effects against Human Lung Cancer Cells of a Terpenoid-Rich Fraction of Inula viscosa. Biology, 13(9), 687. https://doi.org/10.3390/biology13090687








