Simple Summary
In vivo microdialysis applied to rat submandibular glands showed that an increase in the norepinephrine and serotonin levels in the dialysate was primarily dependent on their release from the nerve endings. Either infusion of imipramine into interstitial fluids in rat submandibular glands through a dialysis probe or intraperitoneal administration of imipramine significantly and dose-dependently increased both norepinephrine and serotonin concentrations in the interstitial fluids.
Abstract
Xerostomia induced by antidepressants such as imipramine has long been thought to be due to their anticholinergic effects. However, even antidepressants with low anticholinergic effects may have a high incidence of xerostomia. In salivary glands, norepinephrine activates alpha-adrenergic receptors in blood vessels and beta-adrenergic receptors in acinar cells, respectively, causing a decrease in the blood flow and an increase in the protein secretion, resulting in the secretion of viscous saliva with low water content and high protein content. A previous study demonstrated that perfusion of the submandibular glands of rats with serotonin significantly decreased saliva secretion. The results of the present study revealed the following: (1) that norepinephrine and serotonin, but not epinephrine nor dopamine, were detected in the interstitial fluids in rat submandibular glands; (2) that norepinephrine and serotonin concentrations in the dialysate was 4.3 ± 2.8 nM and 32.3 ± 19.6 nM at stable level, respectively; (3) that infusion with imipramine, a reuptake inhibitor of norepinephrine and serotonin, significantly and dose-dependently increased both norepinephrine and serotonin concentrations in the dialysate; and (4) that intraperitoneal administration of imipramine significantly increased both norepinephrine and serotonin concentrations in the dialysate. These results suggested that one of the mechanisms of xerostomia induced by reuptake inhibitors of norepinephrine and serotonin involves the activation of adrenergic and serotonin receptors in the salivary glands, respectively.
1. Introduction
As opposed to many organs, where the sympathetic and parasympathetic nerves work antagonistically, both nerves cooperate to enhance salivary gland function. Acetylcholine, released from postganglionic nerve endings in the salivary glands by parasympathetic excitation, stimulates the secretion of water and ions, i.e., serous saliva [1,2,3].
On the other hand, norepinephrine (NE), released from postganglionic sympathetic nerve endings within the salivary glands, has an effect on the salivary glands primarily stimulating protein secretion. Norepinephrine activates alpha-adrenergic receptors in blood vessels and beta-adrenergic receptors in acinar cells, respectively, causing a decrease in the blood flow and an increase in the protein secretion, resulting in the secretion of viscous saliva with low water content and high protein content [1,2,4].
Xerostomia or dry mouth is a collective term for diseases characterized by decreased salivary secretion. Saliva has various physiological effects, leading to caries and periodontitis in patients with xerostomia. In addition, xerostomia also contributes to decreased sensitivity to taste, difficulty in chewing and swallowing, and difficulty in speaking. Inflammation of the salivary glands and systemic diseases such as Sjogren’s syndrome are known as causes of xerostomia [5]. Xerostomia occurs even as a side effect of certain medical treatments, including irradiation for cancer treatment on the head and neck and drugs used to treat other diseases [4,5,6,7]. One particular problem with drug-induced xerostomia is that antidepressants are often administered over a long period of time, which leads to increased susceptibility to xerostomia [8]. Xerostomia induced by antidepressants has long been thought to be due to their anticholinergic effects. However, even antidepressants with low anticholinergic effects may have a high incidence of xerostomia [9].
Norepinephrine transporters (NET) and serotonin transporters (SERT) reuptake NE and serotonin (5-HT) in neuronal membranes as members of the Solute Carrier Family 6 (SLC6) that are widely distributed throughout the brain, respectively. Antidepressants such as imipramine inhibit NET and SERT in the brain and improve the function of these neurotransmitters, thereby exerting an antidepressant effect [10,11,12]. Adverse effects of inhibitors of NET and SERT are known to cause xerostomia.
Previous studies have shown that perfusion of the submandibular glands of rats with 5-HT decreased saliva and increased the protein content of saliva [13,14]. However, the effects of inhibitors of NET and SERT on the transporters of nerve endings in the salivary glands have not been elucidated.
The purpose of the present study is to evaluate changes in NE and 5-HT levels following injection of imipramine into the interstitial fluids of salivary glands of rats via dialysis probes or systemic administration of imipramine, an inhibitor of NET and SERT.
2. Materials and Methods
This study was conducted in accordance with the “Guidelines for the Care and Use of Animals for Scientific Purposes” of Tokai University. Approval for the protocol of this animal experiments was obtained from Tokai University (Approval No: 211051, 22204, 233018, 244005).
2.1. Animals
Male Wistar rats (from 8 to 9 weeks old, 230–280 g; CLEA Japan Inc., Tokyo, Japan) were reared under a 12 h light/dark cycle (light on: 7:00 a.m.) at a room temperature of 24–26 °C and a humidity 50–60%, with free access to food and water. Animals were acclimated to their new housing environment for at least one week.
2.2. Chemicals
The following were obtained from the sources indicated: imipramine hydrochloride (Tokyo Chemical Industry Co., Tokyo, Japan) and standard solution of monoamine for analysis (MA11-STD, Eicom, Kyoto, Japan). Unless otherwise indicated, all chemicals were purchased from the FUJIFILM Wako Chemical Co. (Osaka, Japan).
2.3. In Vivo Microdialysis in Anesthetized Rats
The interstitial fluids of the submandibular glands of rats were obtained by using in vivo microdialysis in the same manner as described previously [15]. Each sampling interval of 25 min (sample volume = 50 µL) was sufficient to collect enough volume for quantitative measurements of NE and 5-HT levels.
2.4. Quantification of Monoamines in Dialysates
The levels of monoamines in dialysates were measured using high performance liquid chromatography (HPLC) with electrochemical detection (HTEC-500, Eicom, Kyoto, Japan). The samples were separated on an octadecyl silyl (ODS) column (φ 3.0 mm × 150 mm; Eicompak SC-5ODS; Eicom, Kyoto, Japan). The quantification of NE and 5-HT was evaluated by using a peak area ratio relative to that of the following standards: norepinehrine hydrochloride (NE), serotonin hydrochloride (5-HT), 3-methoxy-4-hydroxyphenylglycol (MHPG), epinephrine hydrochloride (EPI), dihydroxyphenylacetic acid (DOPAC), normethanephrine hydrochloride (NM), dopamine hydrochloride (DA), 5-hydroxyindoleaceticacid (5-HIAA), isoprenaline hydrochloride (ISO), homovanillic acid (HVA), 3-methoxytyramine hydrochloride (3-MT).
2.5. Statistical Analyses
The results obtained are presented as means and standard deviations (SDs). Statistical analysis was conducted using GraphPad Prism 9.5.0 (GraphPad Software, San Diego, CA, USA). Nonparametric statistical comparisons were performed using a Mann–Whitney test or Friedman test followed by a Dunn’s multiple comparison test. A p-value of less than 0.05 was considered a statistically significant difference.
3. Results
3.1. Conditions for Optimal Dialysis Perfusion Rate In Vitro
Dialysates were obtained from five independent dialysis probes soaked in Ringer’s solution with 10 nM of NE and 5-HT at 37 °C at various perfusion rates (1 to 5 µL/min) (Figure 1). As the perfusion rate was increased from 1 µL/min to 5 µL/min, the relative recovery rate [(concentration in dialysate)/(concentration in test solution)] of NE and 5-HT decreased. The absolute recovery rate [(concentration in dialysate) × (perfusion rate)], on the other hand, showed a nonlinear increase, reaching an almost steady state at 2 µL/min. At 2 µL/min, the mean relative recoveries of NE and 5-HT were 54.8% ± 4.8% and 61.5% ± 2.9%, respectively, even when the NE and 5-HT concentrations in the test solution were varied (0.5–100 nM).
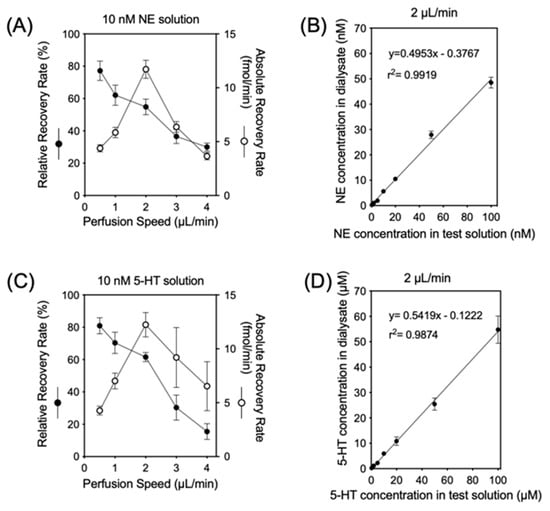
Figure 1.
The upper left panel (A) and lower left panel (C) indicate the relationship between perfusion speed and relative (●) or absolute (○) recovery rates in vitro with 10 nM of NE or 5-HT in testing solution, respectively. The upper right panel (B) and lower right panel (D) indicate the relative recovery rate with different NE and 5-HT concentrations in testing solution using a 2 µL/min perfusion speed.
3.2. Monoamines in Dialysates from Submandibular Glands of Rats
Figure 2 shows chromatograms from a standard solution at 500 pg of each monoamine and 50 µL of dialysate samples. Norepinephrine and 5-HT, but not EPI nor DA, were detected in the dialysates. The basal concentrations of NE and 5-HT in dialysate samples from anesthetized rats, measured 125 min after probe implantation with a perfusion flow rate of 2 μL/min, were 4.3 ± 2.8 nM and 32.3 ± 19.6 nM, respectively (calculated from peak area, n = 24).
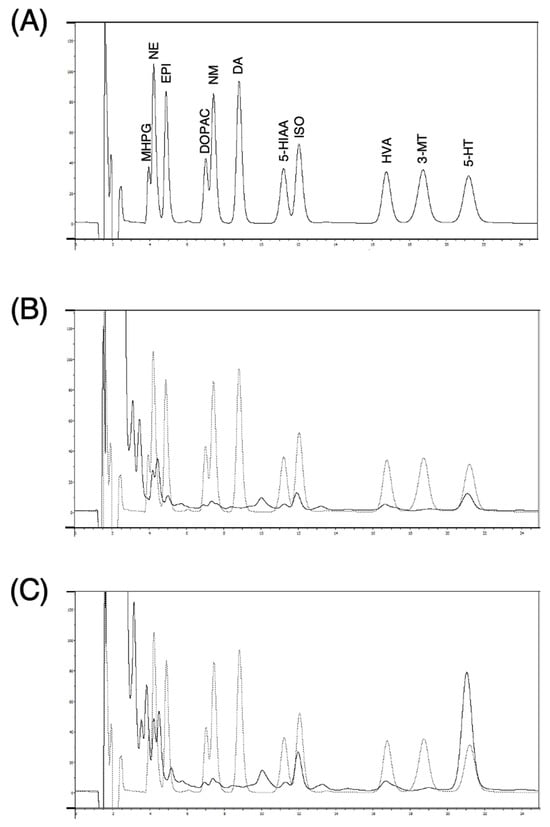
Figure 2.
Representative chromatograms obtained from standard solution containing 500 pg monoamines each (A) and a microdialysis sample before (B) and after (C) infusion of 1 mM imipramine. A standard solution of monoamines for analysis contained 3-methoxy-4-hydroxyphenylglycol (MHPG), norepinehrine hydrochloride (NE), epinephrine hydrochloride (EPI), dihydroxyphenylacetic acid (DOPAC), normethanephrine hydrochloride (NM), dopamine hydrochloride (DA), 5-hydroxyindoleaceticacid (5-HIAA), isoprenaline hydrochloride (ISO), homovanillic acid (HVA), 3-methoxytyramine hydrochloride (3-MT), and serotonin hydrochloride (5-HT).
3.3. Time Course of Norepinephrine and Serotonin Levels in Dialysate after Probe Implantation
Figure 3 shows changes in NE and 5-HT levels in the dialysate of six rats collected at 25 min intervals over a period of 400 min. The levels of both NE and 5-HT were unstable and fluctuating during the first 100 min after probe implantation but decreased gradually to reach basal levels and remained stable up to 400 min.
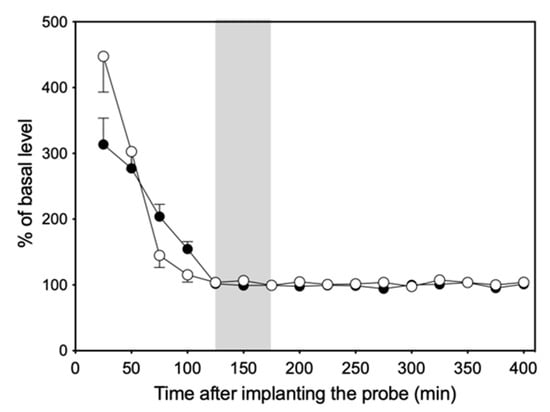
Figure 3.
Change in NE (●) and 5-HT (○) levels in dialysate over time after probe implantation. The gray portion represents the three fractions at the basal level.
3.4. Infusion of High-K+ Solution
To enhance monoamine release from nerve endings, Ringer’s solution containing 100 mM KCl (high-K+ solution) was infused in the interstitial fluids in the submandibular glands of five rats in each group through a microdialysis probe (Figure 4). Infusion with high-K+ solution significantly and transiently increased NE and 5-HT levels in the dialysate by 302 ± 102% and 167 ± 56%, respectively. The NE and 5-HT levels in the dialysate returned to the basal level at 100 min.
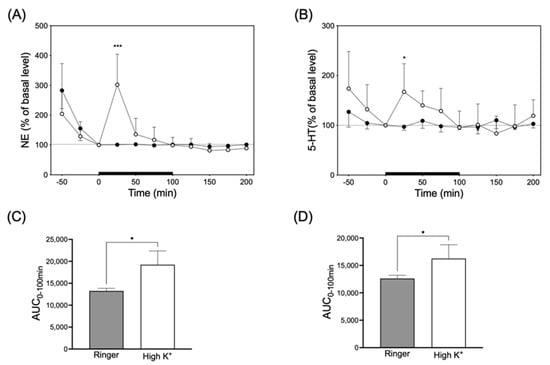
Figure 4.
Left panel (A) and right panel (B) indicates NE and 5-HT release in response to infusion of high-K+ (○) or Ringer’s solution (●), respectively. Significantly different from basal level at each time according to Dunn’s post hoc test following Friedman test; * p < 0.05 and *** p < 0.001. Lower left (C) and right panel (D) indicate the AUC0–100min for values of NE and 5-HT released in the upper panel (A) and (B), respectively. Significantly different from Ringer’s solution according to Mann–Whitney test; * p < 0.05.
3.5. Infusion of Imipramine
Infusion of imipramine via dialysis probes significantly increased both the NE and 5-HT levels in the dialysates of six rats in each group in a dose-dependent manner (Figure 5). Imipramine had a median effective dose (ED50) of 1.86 × 10−3 M and 1.98 × 10−3 M in the value of area under the curve over 200 min (AUC0–200min) for the time course of the changes in NE and 5-HT levels, respectively (Figure 5C,D).
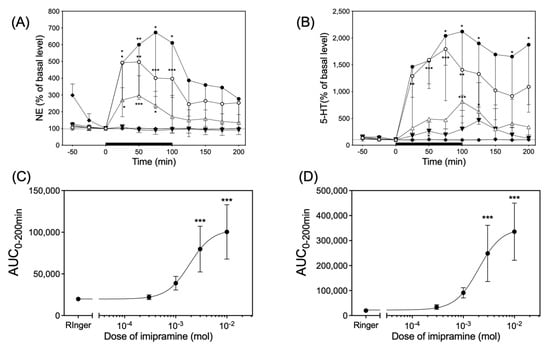
Figure 5.
The upper left panel (A) and right panel (B) indicates time course of the NE and 5-HT release in response to infusion of 0.3 mM (▼), 1 mM (△), 3 mM (○), and 10 mM (●) of imipramine or Ringer’s solution (◆), respectively. The solid black bar indicates the length of application of imipramine solution through the dialysis probe. Significantly different from basal level at each time according to Dunn’s post hoc test following Friedman test; * p < 0.05, ** p < 0.01, and *** p < 0.001. The lower left (C) and right panel (D) indicate the AUC0–200min for the values of NE and 5-HT released in upper panel (A) and (B), respectively. Significantly different from Ringer’s solution according to Dunn’s post hoc test following Friedman test; *** p < 0.001.
3.6. Intraperitoneal Administration of Imipramine
At 10 mg/kg of imipramine, which was considered to be clinically appropriate [16,17,18], significant increases in NE and 5-HT levels were observed in the dialysate of six rats (Figure 6).
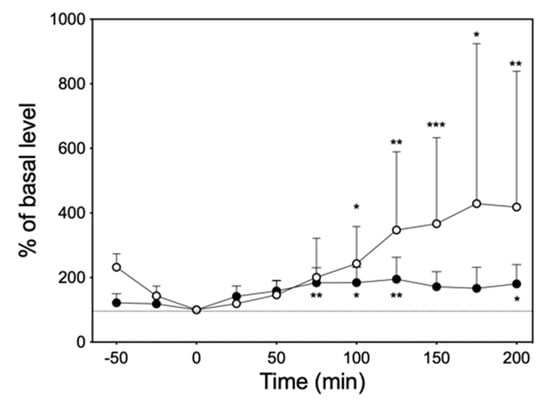
Figure 6.
The time course of changes in NE (●) and 5-HT (○) levels in the dialysate after intraperitoneal administration of 10 mg/kg imipramine. The dashed line represents the 100% of basal level. Significantly different from basal level at each time according to Dunn’s post hoc test following Friedman test; * p < 0.05, ** p < 0.01, and *** p < 0.001.
4. Discussion
The present study demonstrated that imipramine significantly and dose-dependently increased the levels of both NE and 5-HT in the interstitial fluids in the submandibular glands of rats by either infusion in situ or intraperitoneal administration.
Excitation of sympathetic nerves releases NE from the endings, which causes contraction of the blood vessels via the alfa1-adrenergic receptor [1,2,19]. Protein secretion in salivary glands is regulated by cAMP production via beta-adrenergic receptors, resulting in activation of protein kinase A. In salivary glands, beta1-adrenergic receptors are highly expressed and respond well to NE released by the sympathetic nervous system. As a result, protein-rich viscous saliva with low water content is secreted. This is the reason why the sympathetic nervous system is excited by tense and stress, resulting xerostomia [4].
It is thought that 5-HT1, 5-HT2, 5-HT3, and 5-HT4 receptors are the primary 5-HT receptors involved in intestinal motility [20,21,22,23,24,25,26]. Of these, stimulation of 5-HT2A receptors on intestinal smooth muscle enhances intestinal motility [27]. The other three 5-HT receptors are located on the cholinergic nerves in the intestinal tract. Stimulation of the 5-HT1A receptor decreases acetylcholine release from the cholinergic nerves and inhibits intestinal motility, whereas stimulation of the 5-HT3 or 5-HT4 receptor increases acetylcholine release and promotes intestinal motility [28]. In the present study, a significant amount of 5-HT, approximately seven times more than NE at a steady state, was detected in the interstitial fluids in the submandibular glands of rats. The effects of 5-HT on the autonomic nervous system in the digestive tract suggest that potentiation of serotonergic effects affect autonomic activity of the salivary gland. Gene expressions of 5-HT4B and 5-HT7A, which increase cAMP, as well as 5-HT3, an ionotropic receptor with permeability to calcium ions, has been shown in the salivary glands of rats [13,29]. Previous studies have shown that perfusion of the submandibular glands of rats with 5-HT through the external mandibular arteries activates 5-HT receptors, which increases cAMP production, resulting in a decrease in acetylcholine-induced salivation and an increase in the protein content of saliva [13,14].
Xerostomia induced by antidepressants has long been thought to be due to their anticholinergic effects. In general, tricyclic antidepressants have the strongest anticholinergic effects, followed by tetracyclic antidepressants with some anticholinergic effects, while selective serotonin reuptake inhibitors (SSRI), serotonin noradrenergic reuptake inhibitors (SNRI) and noradrenergic and specific serotonergic antidepressants (NaSSA) have little anticholinergic activity [8,9,30]. The stronger the anticholinergic effect, the more likely it has been thought to cause xerostomia. However, even antidepressants with low anticholinergic effects may have high incidence of xerostomia, in which case the mechanism of action is thought to be a decrease in autonomic activity of the salivary glands via the central nervous system [9]. The present study demonstrated that infusion of 10 mM of imipramine into interstitial fluids in the submandibular glands of rats and intraperitoneal administration of 10 mg/kg of imipramine to rats significantly increased te 5-HT contents approximately 22 and four times more than at steady state in the interstitial fluids, respectively. The results of the present study, together with those of previous studies, suggest that one of the mechanisms of xerostomia induced by inhibitors of NET and SERT involves activation of NE and 5-HT receptors in the salivary glands, respectively, in response to elevated levels of NE and 5-HT in their interstitial fluids. Further studies are needed to examine the relationship among saliva secretion, the pharmacokinetics of antidepressants in the salivary glands, and the amount of NE and 5-HT released into their intercellular fluids. In vivo microdialysis will be a powerful tool for these studies.
Assuming that the in vivo recovery rate is comparable to the in vitro recovery rate, the latter (about 55% and 62%) could be used to estimate the concentrations of NE and 5-HT in the interstitial fluids in the submandibular glands of rats. The estimated means of the concentration of NE and 5-HT recorded during the steady state were 7.8 and 52.1 nM, respectively, in the present study. Concentrations of 5-HT in plasma are kept low (<1 nM) by the metabolism of 5-HT to 5-HIAA by monoamine oxidase, as well as by the uptake of 5-HT into platelets via a SERT [31]. The estimated steady-state values of 5-HT concentration in dialysate were approximately 50 times greater than that of plasma. These results suggest that the levels of 5-HT in dialysate correspond to those derived from nerves in the salivary glands. Previous studies have found monoamines in homogenate obtained from the salivary glands of rats or mice [32,33]. The concentrations of NE, 5-HT, and DA in the homogenate from rats were approximately 1870, 410 and 24 nmol/g fresh weight, respectively. In the present study, no EPI nor DA was detected in the dialysate. The detection limit for monoamines, including EPI and DA according to the conditions of the microdialysis method applied in the present study, is approximately 10 pM, which suggests that the amount of EPI and DA in the intercellular spaces may have been below the detection limit.
Despite the rapid increase in NE and 5-HT levels after administration of antidepressants, the therapeutic effect is not observed until several weeks after dosing, indicating that the mechanism of action of antidepressants cannot be explained by the simple monoamine hypothesis and that more detailed mechanisms still need to be elucidated [34]. In fact, it has been reported that, after administration of desipramine, a tetracyclic antidepressant, or sibutramine, an SNRI, for 28 days, the amount of protein in the saliva decreased [35,36]. More detailed mechanistic insights remain to be elucidated in order to understand the possible antidepressant side effects on the salivary glands.
Whereas existing studies have analyzed changes in salivary flow and protein secretion due to the central and systemic effects of antidepressants [35,37,38], this study focused on quantitative changes in neurotransmitters in the salivary glands. Future studies are needed to clarify whether changes in these neurotransmitter levels in the interstitial fluids in the submandibular glands are associated with reduced salivary flow or changes in the biochemical composition of saliva. In addition, the effects of imipramine on NE and 5-HT levels in the submandibular glands, as well as the parotid and sublingual glands, will also be analyzed.
5. Conclusions
The present study demonstrated that imipramine, a monoamine reuptake inhibitor, increased NE and 5-HT levels in the interstitial fluids of the submandibular glands of rats, where the sympathetic nerve endings exist, by either its infusion or intraperitoneal administration.
Author Contributions
Conceptualization, K.S., M.Y., T.K., M.M. (Masaaki Miura), M.M. (Mitsumasa Matsuda) and M.K.; methodology, K.S., M.Y. and T.K.; investigation, K.S., M.Y. and T.K.; writing—original draft preparation, K.S. and M.Y.; supervision, H.K., M.K., K.I. and T.S.; funding acquisition, M.Y. and M.W. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded in part by grants from JPSP KAKENHI; grant number 18K09514, 19K09337 and 22K09102.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Animal Investigation Committee of Tokai University (Approval No: 211051, 22204, 233018 and 244005).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [M.Y.] upon reasonable request.
Acknowledgments
The authors thank Kazuko Ikimura, Eicom Corporation, for their technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Proctor, G.; Carpenter, G. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 2007, 133, 3–18. [Google Scholar] [CrossRef]
- Proctor, G.B. The physiology of salivary secretion. Periodontol. 2000 2016, 70, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Tobin, G.; Giglio, D.; Lundgren, O. Muscarinic receptor subtypes in the alimentary tract. J. Physiol. Pharmacol. 2009, 60, 3–21. [Google Scholar]
- Scully, C. Drug effects on salivary glands: Dry mouth. Oral Dis. 2003, 9, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Ramos Casals, M.; Tzioufas, A.; Stone, J.; Sisó, A.; Bosch, X. Treatment of primary Sjögren syndrome: A systematic review. JAMA J. Am. Med. Assoc. 2010, 304, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Dirix, P.; Nuyts, S.; Van den Bogaert, W. Radiation-induced xerostomia in patients with head and neck cancer: A literature review. Cancer 2006, 107, 2525–2534. [Google Scholar] [CrossRef]
- Stroup, T.S.; Gray, N. Management of common adverse effects of antipsychotic medications. World Psychiatry 2018, 17, 341–356. [Google Scholar] [CrossRef]
- Khawam, E.A.; Laurencic, G.; Malone, D.A., Jr. Side effects of antidepressants: An overview. Clevel. Clin. J. Med. 2006, 73, 351–361. [Google Scholar] [CrossRef]
- Arany, S.; Kopycka-Kedzierawski, D.T.; Caprio, T.V.; Watson, G.E. Anticholinergic medication: Related dry mouth and effects on the salivary glands. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 662–670. [Google Scholar] [CrossRef]
- Huot, P.; Fox, S.H.; Brotchie, J.M. Monoamine reuptake inhibitors in parkinson’s disease. Park. Dis. 2015, 2015, 609428. [Google Scholar] [CrossRef]
- Licinio, J.; Wong, M.L. Pharmacogenomics of antidepressant treatment effects. Dial. Clin. Neurosci. 2011, 13, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Obata, H. Analgesic mechanisms of antidepressants for neuropathic pain. Int. J. Mol. Sci. 2017, 18, 2483. [Google Scholar] [CrossRef]
- Bourdon, D.M.; Camden, J.M.; Landon, L.A.; Levy, F.O.; Turner, J.T. Identification of the adenylyl cyclase-activating 5-hydroxytryptamine receptor subtypes expressed in the rat submandibular gland. Br. J. Pharmacol. 2000, 130, 104–108. [Google Scholar] [CrossRef]
- Turner, J.T.; Sullivan, D.M.; Rovira, I.; Camden, J.M. A regulatory role in mammalian salivary glands for 5-hydroxytryptamine receptors coupled to increased cyclic AMP production. J. Dent. Res. 1996, 75, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Kawaguchi, M. In Vivo Monitoring of Acetylcholine Release from Nerve Endings in Salivary Gland. Biology 2021, 10, 351. [Google Scholar] [CrossRef]
- Kitamura, Y.; Doi, M.; Kuwatsuka, K.; Onoue, Y.; Miyazaki, I.; Shinomiya, K.; Koyama, T.; Sendo, T.; Kawasaki, H.; Asanuma, M.; et al. Chronic treatment with imipramine and lithium increases cell proliferation in the hippocampus in adrenocorticotropic hormone-treated rats. Biol. Pharm. Bull. 2011, 34, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Fenton, E.Y.; Fournier, N.M.; Lussier, A.L.; Romay-Tallon, R.; Caruncho, H.J.; Kalynchuk, L.E. Imipramine protects against the deleterious effects of chronic corticosterone on depression-like behavior, hippocampal reelin expression, and neuronal maturation. Prog Neuropsychopharmacol. Biol. Psychiatry 2015, 60, 52–59. [Google Scholar]
- Habib, M.; Shaker, S.; El-Gayar, N.; Aboul-Fotouh, S. The effects of antidepressants “fluoxetine and imipramine” on vascular abnormalities and Toll like receptor-4 expression in diabetic and non-diabetic rats exposed to chronic stress. PLoS ONE 2015, 10, e0120559. [Google Scholar] [CrossRef]
- Maldonado, J.O.; Riveros, P.P.; Chiorini, J.A. Myoepithelial Cell Function in Salivary Gland Physiology and Disease. In Sjögren’s Syndrome and Oral Health: Disease Characteristics and Management of Oral Manifestations; Cha, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Galligan, J.J.; Parkman, H. Recent advances in understanding the role of serotonin in gastrointestinal motility and functional bowel disorders. Neurogastroenterol. Motil. 2007, 19 (Suppl. S2), 1–4. [Google Scholar]
- Glatzle, J.; Sternini, C.; Robin, C.; Zittel, T.T.; Wong, H.; Reeve, J.R., Jr.; Raybould, H.E. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology 2002, 123, 217–226. [Google Scholar] [CrossRef]
- Poole, D.P.; Xu, B.; Koh, S.L.; Hunne, B.; Coupar, I.M.; Irving, H.R.; Shinjo, K.; Furness, J.B. Identification of neurons that express 5-hydroxytryptamine4 receptors in intestine. Cell Tissue Res. 2006, 325, 413–422. [Google Scholar] [CrossRef]
- Raybould, H.E.; Glatzle, J.; Robin, C.; Meyer, J.H.; Phan, T.; Wong, H.; Sternini, C. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G367–G372. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R. Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: Alterations in 5-HT signalling and metabolism in human disease. Neurogastroenterol. Motil. 2007, 19 (Suppl. S2), 25–31. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.M.; Sanders, K.M.; Hirst, G.D. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol. Motil. 2004, 16 (Suppl. S1), 112–117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Galligan, J.J. Synaptic activation and properties of 5-hydroxytryptamine(3) receptors in myenteric neurons of guinea pig intestine. J. Pharmacol. Exp. Ther. 1999, 290, 803–810. [Google Scholar]
- Fiorica-Howells, E.; Hen, R.; Gingrich, J.; Li, Z.; Gershon, M.D. 5-HT(2A) receptors: Location and functional analysis in intestines of wild-type and 5-HT(2A) knockout mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G877–G893. [Google Scholar] [CrossRef] [PubMed]
- Guzel, T.; Mirowska-Guzel, D. The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy. Molecules 2022, 27, 1680. [Google Scholar] [CrossRef]
- Johnson, D.S.; Heinemann, S.F. Embryonic expression of the 5-HT3 receptor subunit, 5-HT3R-A, in the rat: An in situ hybridization study. Mol. Cell Neurosci. 1995, 6, 122–138. [Google Scholar] [CrossRef]
- Sadek, J. Antidepressants. In Clinician’s Guide to Psychopharmacology; Sadek, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Zolkowska, D.; Baumann, M.H.; Rothman, R.B. Chronic fenfluramine administration increases plasma serotonin (5-hydroxytryptamine) to nontoxic levels. J. Pharmacol. Exp. Ther. 2008, 324, 791–797. [Google Scholar] [CrossRef]
- Murai, S.; Saito, H.; Masuda, Y.; Itoh, T. Sex-dependent differences in the concentrations of the principal neurotransmitters, noradrenaline and acetylcholine, in the three major salivary glands of mice. Arch. Oral Biol. 1998, 43, 9–14. [Google Scholar] [CrossRef]
- Murai, S.; Saito, H.; Masuda, Y.; Itsukaichi, O.; Itoh, T. Basal levels of noradrenaline, dopamine, 5-hydroxytryptamine, and acetylcholine in the submandibular, parotid, and sublingual glands of mice and rats. Arch. Oral Biol. 1995, 40, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.W.; Kim, Y.K. Molecular Neurobiology and Promising New Treatment in Depression. Int. J. Mol. Sci. 2016, 17, 381. [Google Scholar] [CrossRef]
- Koller, M.M.; Maeda, N.; Scarpace, P.J.; Humphreys-Beher, M.G. Desipramine changes salivary gland function, oral microbiota, and oral health in rats. Eur. J. Pharmacol. 2000, 408, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.R.; Fiais, G.A.; Oliveira, H.A.; Ribas, T.B.; Souza, R.O.; Tsosura, T.V.S.; Matsushita, D.H.; Ervolino, E.; Dornelles, R.C.M.; Nakamune, A.; et al. Assessment of redox state and biochemical parameters of salivary glands in rats treated with anti-obesity drug sibutramine hydrochloride. Clin. Oral Investig. 2022, 26, 5833–5846. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, M.; Winder, M.; Zawia, H.; Lödöen, I.; Tobin, G.; Götrick, B. In vivo studies of effects of antidepressants on parotid salivary secretion in the rat. Arch. Oral Biol. 2016, 67, 54–60. [Google Scholar] [CrossRef]
- Scarpace, P.J.; Koller, M.M.; Rajakumar, G. Desipramine desensitizes β-adrenergic signal transduction in rat salivary glands. Neuropharmacology 1992, 31, 1305–1309. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).