Reproductive Strategies and Embryonic Development of Autumn-Spawning Bitterling (Acheilognathus rhombeus) within the Mussel Host
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
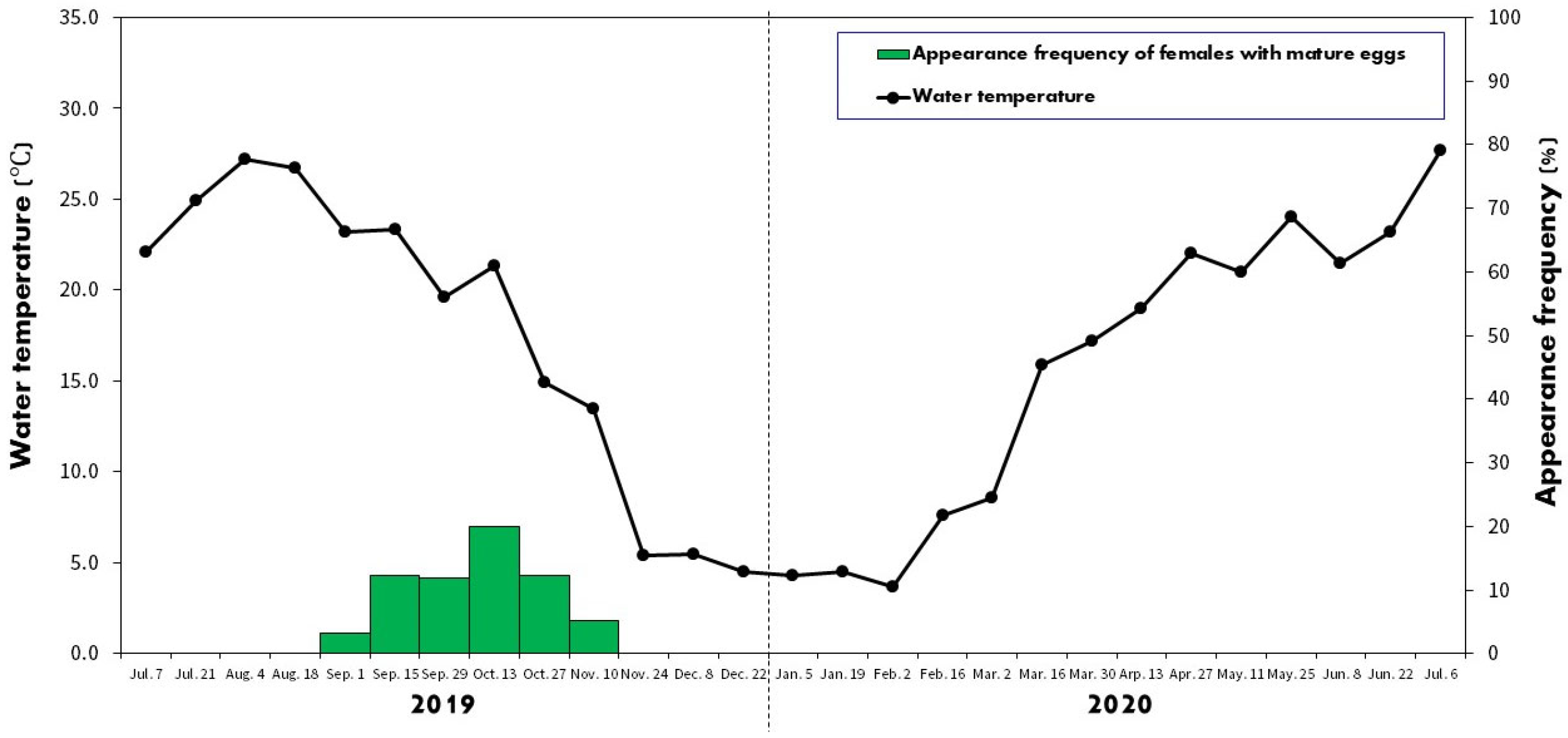
2.2. Spawning Period
2.3. Developmental Stage of the Embryos inside Mussels
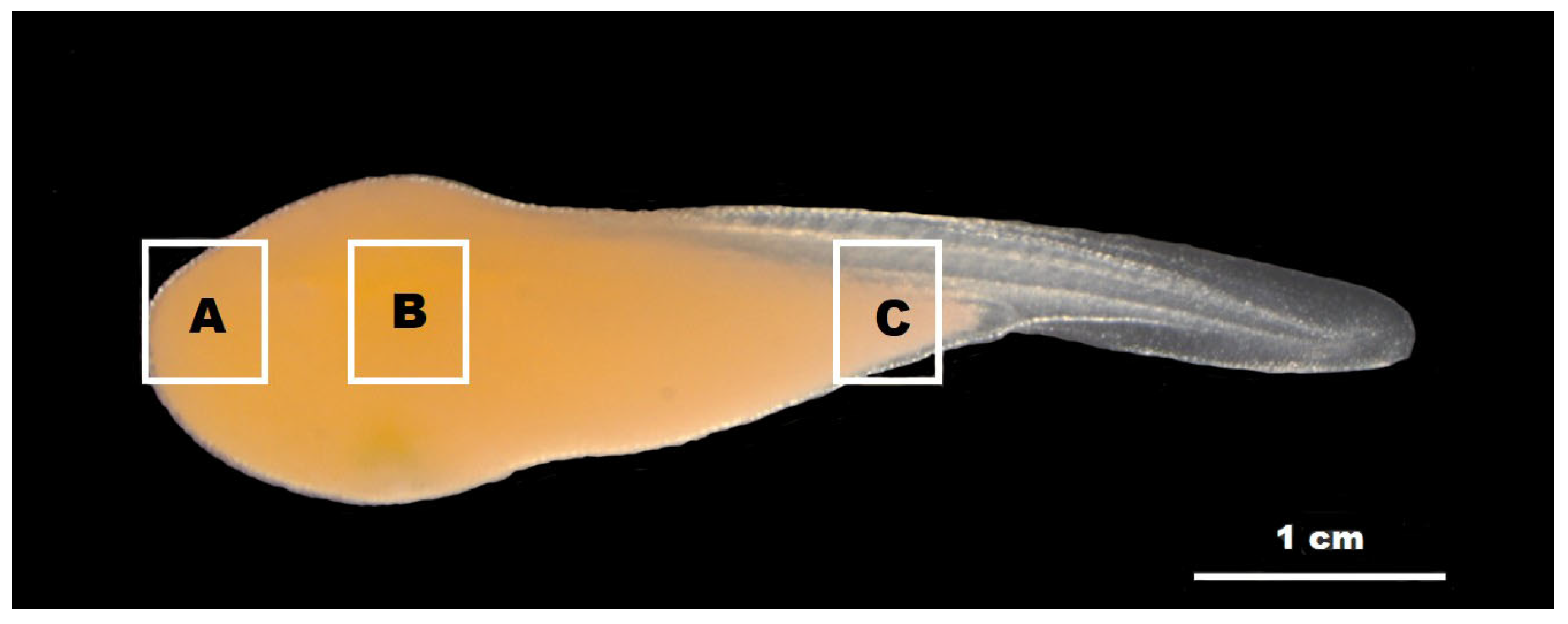
2.4. Observation of the Minute Tubercles on the Embryos
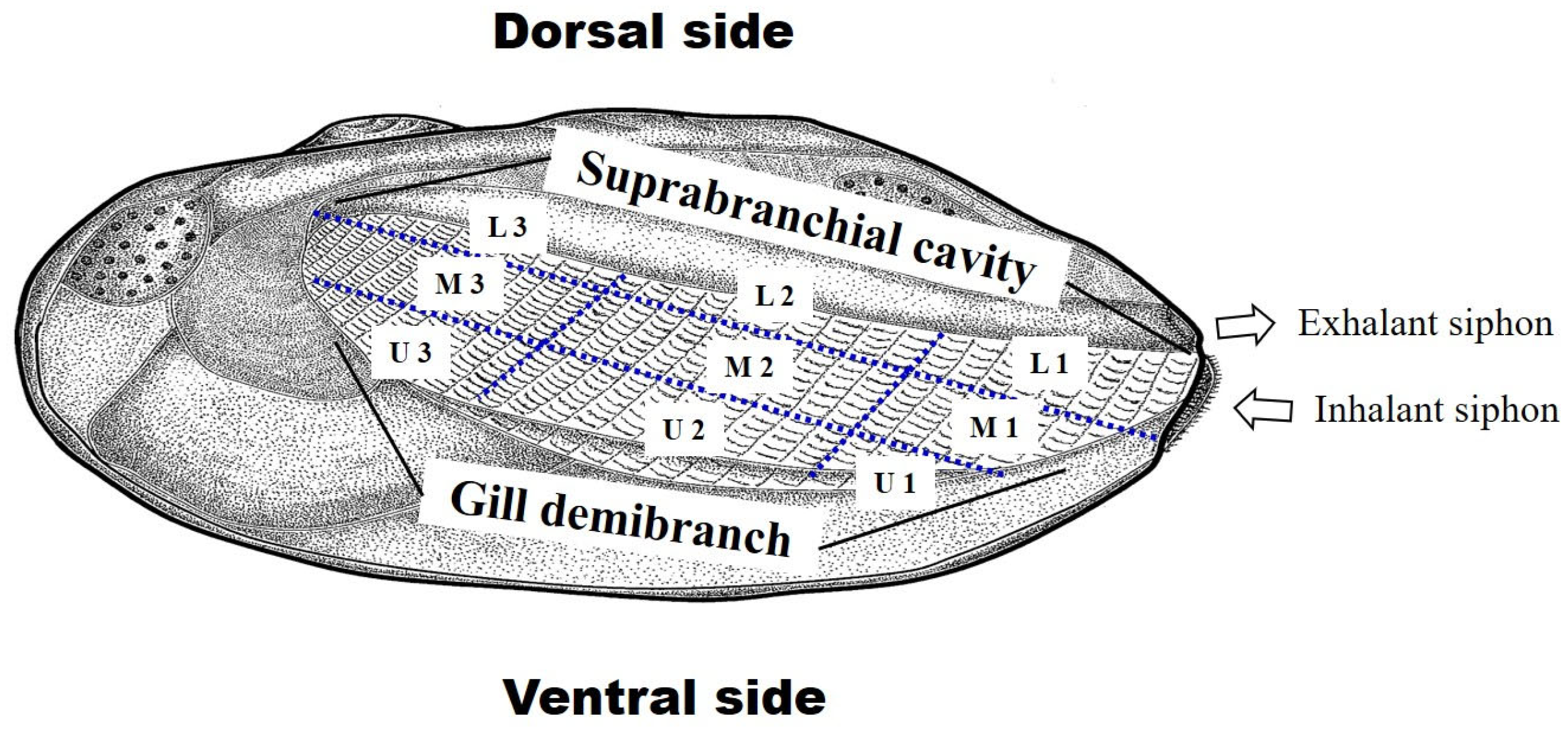
2.5. Utilization of Host Mussels
2.6. Identification of the Species of Bitterling Embryos
2.7. Statistical Analysis
3. Results
3.1. Spawning Period
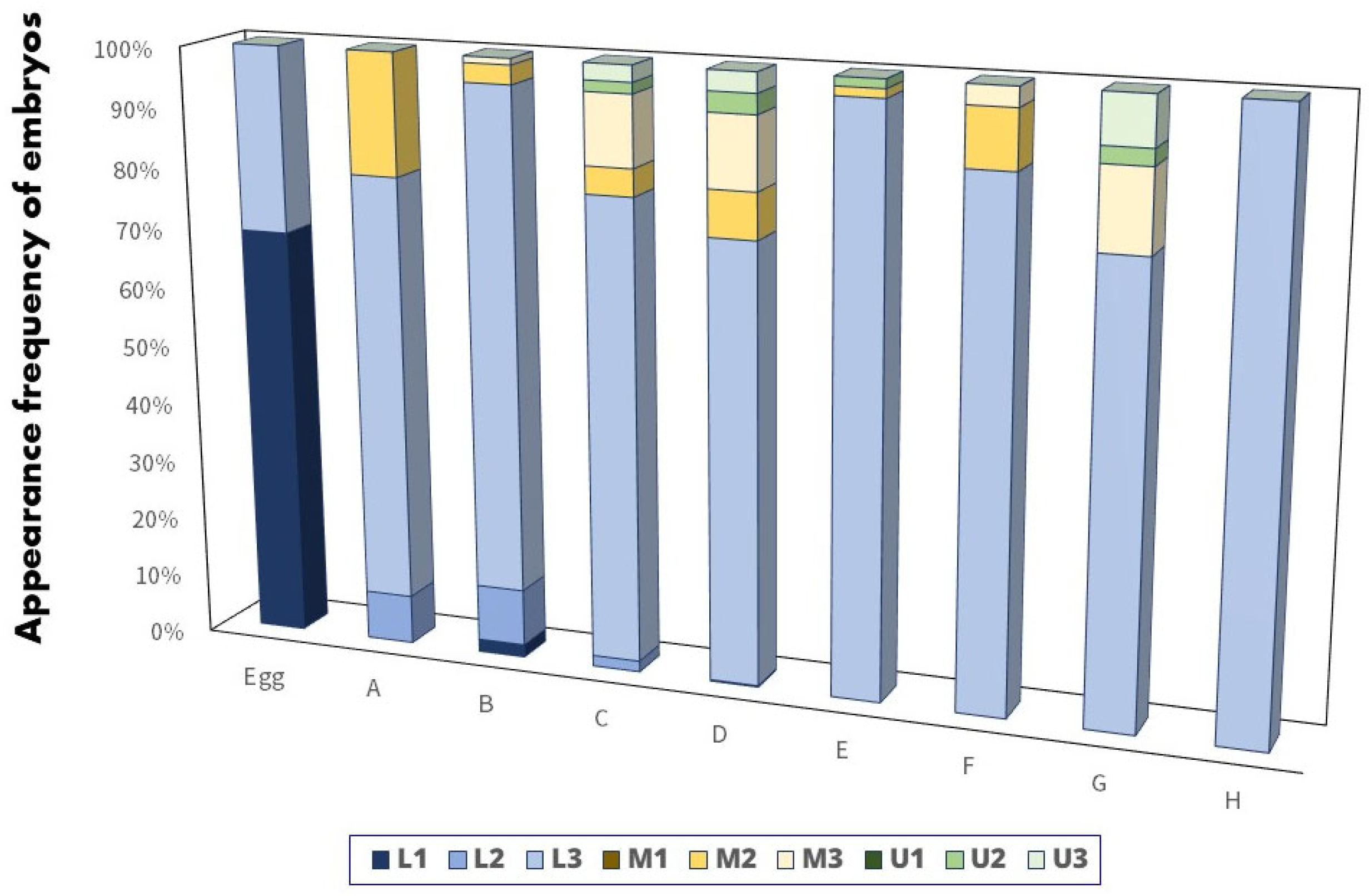
3.2. Developmental Stages of the Embryos inside Mussels
3.3. Observation of the Minute Tubercles on the Embryos
3.4. Utilization of Host Mussels
4. Discussion
4.1. Spawning Characteristics and Embryonic Development inside Mussels
4.2. Changes in the Minute Tubercles on Embryos
4.3. Distribution and Position of the Embryos in Mussels
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, J.N. The Coevolutionary Process; The University of Chicago Press: Chicago, IL, USA, 1994. [Google Scholar]
- Thompson, J.N. Coevolution. In Encyclopaedia of Evolution; Pagel, M., Ed.; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- Liu, H.; Zhu, Y.; Smith, C.; Reichard, M. Evidence of host specificity and congruence between phylogenies of bitterling and freshwater mussels. Zool. Stud. 2006, 45, 428–434. [Google Scholar]
- Thompson, J.N.; Burdon, J.J. Gene-for-gene coevolution between plants and parasites. Nature 1992, 360, 121–125. [Google Scholar] [CrossRef]
- Reynolds, J.D.; Debuse, V.J.; Aldridge, D.C. Host Specialisation in an Unusual Symbiosis: European Bitterlings Spawning in Freshwater Mussels. Oikos 1997, 78, 539–545. [Google Scholar] [CrossRef]
- Smith, C.; Wootton, R.J. The costs of parental care in teleost fishes. Rev. Fish Biol. Fish. 1995, 5, 7–22. [Google Scholar] [CrossRef]
- Kitamura, J. Factors affecting seasonal mortality of rosy bitterling (Rhodeus ocellatus kurumeus) embryos on the gills of their host mussel. Popul. Ecol. 2005, 47, 41–51. [Google Scholar] [CrossRef]
- Mills, S.C.; Reynolds, J.D. Mussel ventilation rates as a proximate cue for host selection by bitterling, Rhodeus sericeus. Oecologia 2002, 131, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Refsnider, J.M.; Janzen, F.J. Putting Eggs in One Basket: Ecological and Evolutionary Hypotheses for Variation in Oviposition-Site Choice. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 39–57. [Google Scholar] [CrossRef]
- Smith, C.; Reynolds, J.D.; Sutherland, W.J. Population consequences of reproductive decisions. Proc. Biol. Sci. 2000, 267, 1327–1334. [Google Scholar] [CrossRef]
- Renfree, M.B.; Shaw, G. Diapause. Annu. Rev. Physiol. 2000, 62, 353–375. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.F. The Insects: Structure and Function, 5th ed.; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Murphy, W.J.; Collier, G.E. A molecular phylogeny for aplocheiloid fishes (Atherinomorpha, Cyprinodontiformes): The role of vicariance and the origins of annualism. Mol. Biol. Evol. 1997, 14, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Hrbek, T.; Larson, A. The evolution of diapause in the killifish family Rivulidae (Atherinomorpha, Cyprinodontiformes): A molecular phylogenetic and biogeographic perspective. Evolution 1999, 53, 1200–1216. [Google Scholar] [CrossRef]
- Kawamura, K.; Uehara, K. Effects of temperature on free-embryonic diapause in the autumn-spawning bitterling Acheilognathus rhombeus(Teleostei: Cyprinidae). J. Fish Biol. 2005, 67, 684–695. [Google Scholar] [CrossRef]
- Uehara, K.; Kawabata, K.; Ohta, H. Low temperature requirement for embryonic development of Itasenpara bitterling Acheilognathus longipinnis. J. Exp. Zool. 2006, 305A, 823–829. [Google Scholar] [CrossRef]
- Furness, A.I. The evolution of an annual life cycle in killifish: Adaptation to ephemeral aquatic environments through embryonic diapause. Biol. Rev. Camb. Philos. Soc. 2016, 91, 796–812. [Google Scholar] [CrossRef]
- Bánárescu, P. Zoogeography of fresh waters. In General Distribution and Dispersal of Freshwater Animals; AULA-Verlag: Wiesbaden, Germany, 1990; Volume 1, pp. 49–218. [Google Scholar]
- Van Damme, D.; Bogutskaya, N.; Hoffmann, R.C.; Smith, C. The introduction of the European bitterling (Rhodeus amarus) to west and central Europe. Fish Fish. 2007, 8, 79–106. [Google Scholar] [CrossRef]
- Smith, C.; Reichard, M.; Jurajda, P.; Przybylski, M. The reproductive ecology of the European bitterling (Rhodeus sericeus). J. Zool. 2004, 262, 107–124. [Google Scholar] [CrossRef]
- Nelson, J.S. Fishes of the World, 5th ed.; John Wiley & Sons, Inc.: Toronto, ON, Canada, 2016. [Google Scholar]
- Aldridge, D.C. Development of European bitterling in the gills of freshwater mussels. J. Fish Biol. 1999, 54, 138–151. [Google Scholar] [CrossRef]
- Mills, S.C.; Reynolds, J.D. The bitterling–mussel interaction as a test case for co-evolution. J. Fish Biol. 2003, 63, 84–104. [Google Scholar] [CrossRef]
- Reichard, M.; Bryja, J.; Polačik, M.; Smith, C. No evidence for host specialization or host-race formation in the European bitterling (Rhodeus amarus), a fish that parasitizes freshwater mussels. Mol. Ecol. 2011, 20, 3631–3643. [Google Scholar] [CrossRef]
- Spence, R.; Smith, C. Rose bitterling (Rhodeus ocellatus) embryos parasitize freshwater mussels by competing for nutrients and oxygen. Acta Zool. 2011, 94, 113–118. [Google Scholar] [CrossRef]
- Aldridge, D.C. Reproductive Ecology of Bitterling (Rhodeus sericeus Pallas) and Unionid Mussels. Ph.D. Dissertation, Cambridge University, Cambridge, UK, 1997. [Google Scholar]
- Kitamura, J.-I. Reproductive ecology of the striped bitterling Acheilognathus cyanostigma (Cyprinidae: Acheilognathinae). Ichthyol. Res. 2006, 53, 216–222. [Google Scholar] [CrossRef]
- Kitamura, J. Adaptive spatial utilization of host mussels by the Japanese rosy bitterling Rhodeus ocellatus kurumeus. J. Fish Biol. 2006, 69, 263–271. [Google Scholar] [CrossRef]
- Suzuki, N.; Akiyama, N.; Hibiya, T. Development of the bitterling Rhodeus uyekii (Cyprinidae), with a note on minute tubercles on the skin surface. Jpn. J. Ichthyol. 1985, 32, 28–34. [Google Scholar]
- Suzuki, N.; Hibiya, T. Minute tubercles on the skin surface of larvae of Rhodeus (Cyprinidae). Jpn. J. Ichthyol. 1984, 31, 198–202. [Google Scholar]
- Suzuki, N.; Hibiya, T. Minute tubercles on the skin surface of larvae ofAcheilognathus andPseudoperilampus (Cyprinidae). Jpn. J. Ichthyol. 1985, 32, 335–344. [Google Scholar] [CrossRef]
- Kim, H.S. Minute tubercles in bitterling larvae: Developmental dynamic structures to prevent premature ejection by host mussels. Ecol. Evol. 2020, 10, 5840–5851. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, H.T.; Park, J.S.; Im, J.H. Changes in the height of minute tubercles on the skin of Korean bitterling embryos (Acheilognathus signifer) and embryo movement in the host mussels. J. Fish Biol. 2022, 101, 676–685. [Google Scholar] [CrossRef]
- Suzuki, N.; Jeon, S.R. Development of eggs, larvae and juveniles of Rhodeus ocellatus from Ansung River, Korea (Pisces: Cyprinidae), with a note on minute tubercles on the skin surface. Korean J. Limnol. 1988, 21, 1–15. (In Korean) [Google Scholar]
- Suzuki, N.; Jeon, S.R. Development of the bitterling, Acheilognathus limbata (Cyprinidae) from Korea and Japan, with a note on minute tubercles on the skin surface and on the genetic implication in hybrid embryos. Korean J. Limnol. 1988, 21, 211–229. [Google Scholar]
- Suzuki, N.; Jeon, S.R. Development of the bitterling, Acheilognathus suigensis (Cyprinidae) from Korea, with a note on minute tubercles on the skin surface. Korean J. Limnol. 1988, 21, 231–242. (In Korean) [Google Scholar]
- Suzuki, N.; Jeon, S.R. Development of the bitterling, Acanthorhodeus asmussi (Cyprinidae) with a note on minute tubercles on the skin surface. Korean J. Limnol. 1989, 1, 73–82. (In Korean) [Google Scholar]
- Suzuki, N.; Jeon, S.R. Development of the bitterling, Acanthorhodeus gracilis (Cyprinidae), with a note on minute tubercles on the skin surface. Korean J. Limnol. 1990, 2, 169–181. [Google Scholar]
- Kim, C.H.; Park, J.Y.; Park, M.K.; Kang, E.J.; Kim, J.H. Minute tubercles on the skin surface of larvae in the Korean endemic bitterling, Rhodeus pseudosericeus (Pisces, Cyprinidae). J. Appl. Ichthyol. 2008, 24, 269–275. [Google Scholar] [CrossRef]
- Park, J.; Oh, M.; Kim, C.; Kang, E.; Beon, M. Morphology and distribution of the minute tubercles on the skin surface of larvae in the Korean endemic bitterling, Acheilognathus somjinensis (pisces, cyprinidae), with its larval growth. Anim. Cells Syst. 2008, 12, 297–304. [Google Scholar] [CrossRef]
- Yi, W.; Reichard, M.; Rücklin, M.; Richardson, M.K. Parasitic fish embryos do a “front-flip” on the yolk to resist expulsion from the host. Proc. Natl. Acad. Sci. USA 2024, 121, e2310082121. [Google Scholar] [CrossRef]
- Nakamura, M. Cyprinid Fishes of Japan; Special Publications of the Research Institute for Natural Resources, No. 4; Research Institute for Natural Resources: Tokyo, Japan, 1969. [Google Scholar]
- Suzuki, N.; Jeon, S.R. Development of the bitterling, Acheilognathus rhombeus (Cyprinidae), from Korea. J. Basic Sci. 1991, 5, 53–62. [Google Scholar]
- Kim, H.S.; Choi, H.S.; Park, J.Y. Embryonic development characteristics and host mussel utilization of flat bitterling Acheilognathus rhombeus (Cyprinidae) during winter in Korea. Environ. Biol. Fishes 2018, 101, 55–66. [Google Scholar] [CrossRef]
- Pepin, P. Effect of Temperature and Size on Development, Mortality, and Survival Rates of the Pelagic Early Life History Stages of Marine Fish. Can. J. Fish. Aquat. Sci. 1991, 48, 503–518. [Google Scholar] [CrossRef]
- Polačik, M.; Vrtílek, M.; Reichard, M.; Žák, J.; Blažek, R.; Podrabsky, J. Embryo ecology: Developmental synchrony and asynchrony in the embryonic development of wild annual fish populations. Ecol. Evol. 2021, 11, 4945–4956. [Google Scholar] [CrossRef]
- Denlinger, D.L.; Armbruster, P.A. Mosquito Diapause. Annu. Rev. Ѐntomol. 2014, 59, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Gerisch, B.; Antebi, A. Hormonal signals produced by DAF-9/cytochrome P450 regulate C. elegans dauer diapause in response to environmental cues. Development 2004, 131, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Rücklin, M.; Poelmann, R.E.; Aldridge, D.C.; Richardson, M.K. Normal stages of embryonic development of a brood parasite, the rosy bitterling Rhodeus ocellatus (Teleostei: Cypriniformes). J. Morphol. 2021, 286, 783–819. [Google Scholar] [CrossRef]
- Wootton, R.J. Ecology of Teleost Fishes; Kluwer Academic Publishers: London, UK, 1998. [Google Scholar]
- Suzuki, N.; Hibiya, T. Development of eggs and larvae of two bitterlings, Rhodeus atremius and Rhodeus suigensis (Cyprinidae). Jpn. J. Ichthyol. 1984, 31, 287–296. [Google Scholar]
- Back, H.M.; Song, H.B. Spawning in mussel and adaptation strategy of Acheilonathus signifer (Cyprinidae: Acheilognathinae). Korean J. Ichthyol. 2005, 17, 105–111. (In Korean) [Google Scholar]
- Song, H.B.; Kwon, O.K. Spawning of the bitterling, Acheilognathus yamatsutae (Cyprinidae) into the mussel. Korean Journal of Ichthyology 1994, 6, 39–50. (In Korean) [Google Scholar]
- Kim, H.S.; Ko, J.G.; Choi, W.S.; Park, J.Y. Population ecology of Korean rose bitterling, Rhodeus uyekii (Pisces: Acheilognathinae) in the Bongseocheon, Mankyeonggang (River), Korea. Korean J. Ichthyol. 2015, 27, 78–85. (In Korean) [Google Scholar]
- Fukuhara, S.; Nagata, Y.; Maekawa, W. Miniature scaly tubercles on the yolk sac of rhodeine cyprinid fishes in prolarval stages. Jpn. J. Ichthyol. 1982, 29, 232–236. [Google Scholar]
- Tankersley, R.A.; Dimock, R.V. The Effect of Larval Brooding on the Respiratory Physiology of the Freshwater Unionid Mussel Pyganodon cataracta. Am. Midl. Nat. 1993, 130, 146–163. [Google Scholar] [CrossRef]
- Li, F.; Smith, C.; Kawamura, K.; Vetešník, L.; Arai, R.; Reichard, M. Unusual egg shape diversity in bitterling fishes. Ecology 2022, 103, e3816. [Google Scholar] [CrossRef]
- Methling, C.; Douda, K.; Liu, H.; Rouchet, R.; Bartáková, V.; Yu, D.; Smith, C.; Reichard, M. Energetic costs in the relationship between bitterling and mussels in East Asia. Biol. J. Linn. Soc. 2018, 125, 750–759. [Google Scholar] [CrossRef]
- Choi, H.; Lee, H.J. Host size matters for reproduction: Evolution of spawning preference and female reproductive phenotypes in mussel-symbiotic freshwater bitterling fishes. Ecol. Evol. 2024, 14, e11142. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Chiba, H.; Shindo, K.; Kitazima, J.; Iwata, M. Reproductive ecology and adaptive host choice correlated with body size in an autumn-spawning bitterling Acheilognathus typus. J. Fish Biol. 2022, 100, 1195–1204. [Google Scholar] [CrossRef] [PubMed]




| Embryonic Development | Stage | Characteristics of Embryos and Minute Tubercles |
|---|---|---|
| Eggs | Not hatching, nearly ovoid-shaped with yellow in color No minute tubercles on the skin surface | |
| A | Motionless and undeveloped body parts Slight minute tubercles were observed on the skin surface | |
| B | The larvae tail elongated backwards, and caudal portion appeared Minute tubercles developed over the entire body | |
| Retarding stage | C | Optic cups without lens were clearly observed and moved faster The growth of minute tubercles was observed on the head and body regions |
| Diapausing stage | D | The heart pulsated and notochord began to bend upwards The height of minute tubercles reached the maximum |
| Reactivation stage | E | Melanin pigments formed on the optic cups and moved in a wiggly pattern The height of minute tubercles on the skin surface were reduced |
| Eye pigmentation stage | F | Lenses were completely developed; melanin pigmentation increased The minute tubercles almost disappeared |
| G | Formation of all the caudal fin rays was complete, and the dorsal and anal fin rays were formed The minute tubercles disappeared | |
| H | The length of the upper and lower jaws was equal; larvae started to swim slightly | |
| Free-swimming stage | I | Larvae swam out from the mussels and began to feed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Choe, J.; Ko, M. Reproductive Strategies and Embryonic Development of Autumn-Spawning Bitterling (Acheilognathus rhombeus) within the Mussel Host. Biology 2024, 13, 664. https://doi.org/10.3390/biology13090664
Kim H, Choe J, Ko M. Reproductive Strategies and Embryonic Development of Autumn-Spawning Bitterling (Acheilognathus rhombeus) within the Mussel Host. Biology. 2024; 13(9):664. https://doi.org/10.3390/biology13090664
Chicago/Turabian StyleKim, Hyeongsu, Jongryeol Choe, and Myeonghun Ko. 2024. "Reproductive Strategies and Embryonic Development of Autumn-Spawning Bitterling (Acheilognathus rhombeus) within the Mussel Host" Biology 13, no. 9: 664. https://doi.org/10.3390/biology13090664
APA StyleKim, H., Choe, J., & Ko, M. (2024). Reproductive Strategies and Embryonic Development of Autumn-Spawning Bitterling (Acheilognathus rhombeus) within the Mussel Host. Biology, 13(9), 664. https://doi.org/10.3390/biology13090664






