Converging Mechanisms of Vascular and Cartilaginous Calcification
Abstract
Simple Summary
Abstract
1. Introduction
2. Types and Characteristics of Ectopic Calcifications: Correspondence with Osteogenesis
- -
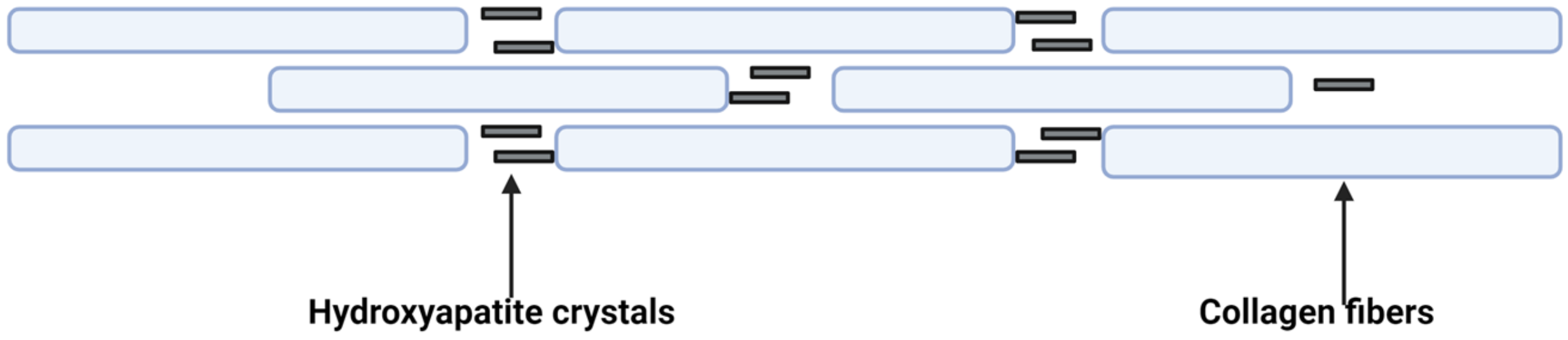
- Intimal calcification is an endochondral ossification process in which type II collagen is mineralized by calcium deposits.
- -
- Intramembranous ossification is responsible for media calcifications
- -
- Dystrophic calcification appears in necrotic tissues or as a reaction to tissue destruction leading to valvular calcification.
- -
- Vascular calciphylaxis or calcific uremic arteriolopathy is a systemic process characterized by diffuse calcification in the media of small and medium arteries or arterioles and intimal proliferation resulting in tissue necrosis.
3. Mechanisms of Vascular and Cartilaginous Calcifications
- (A)
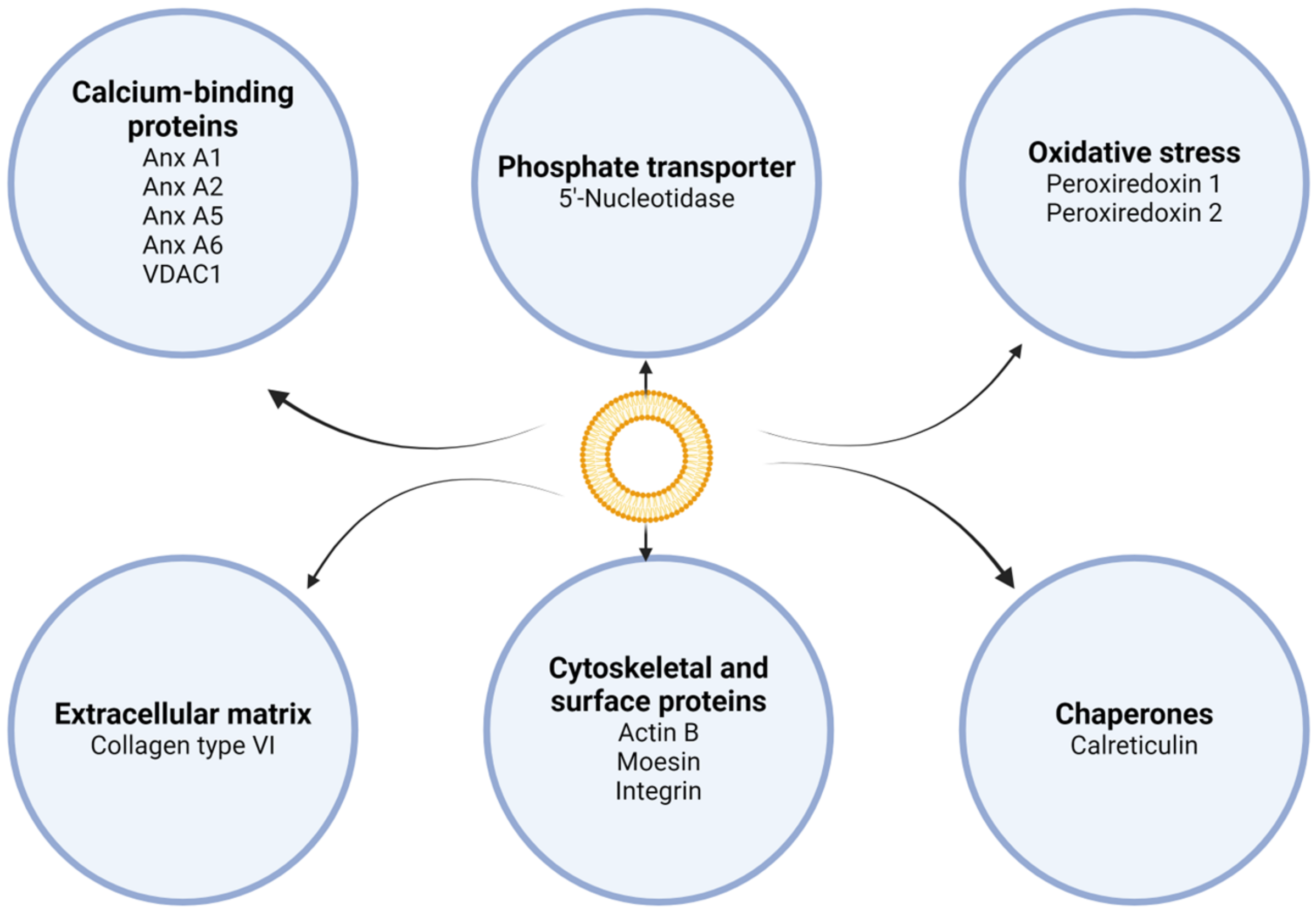
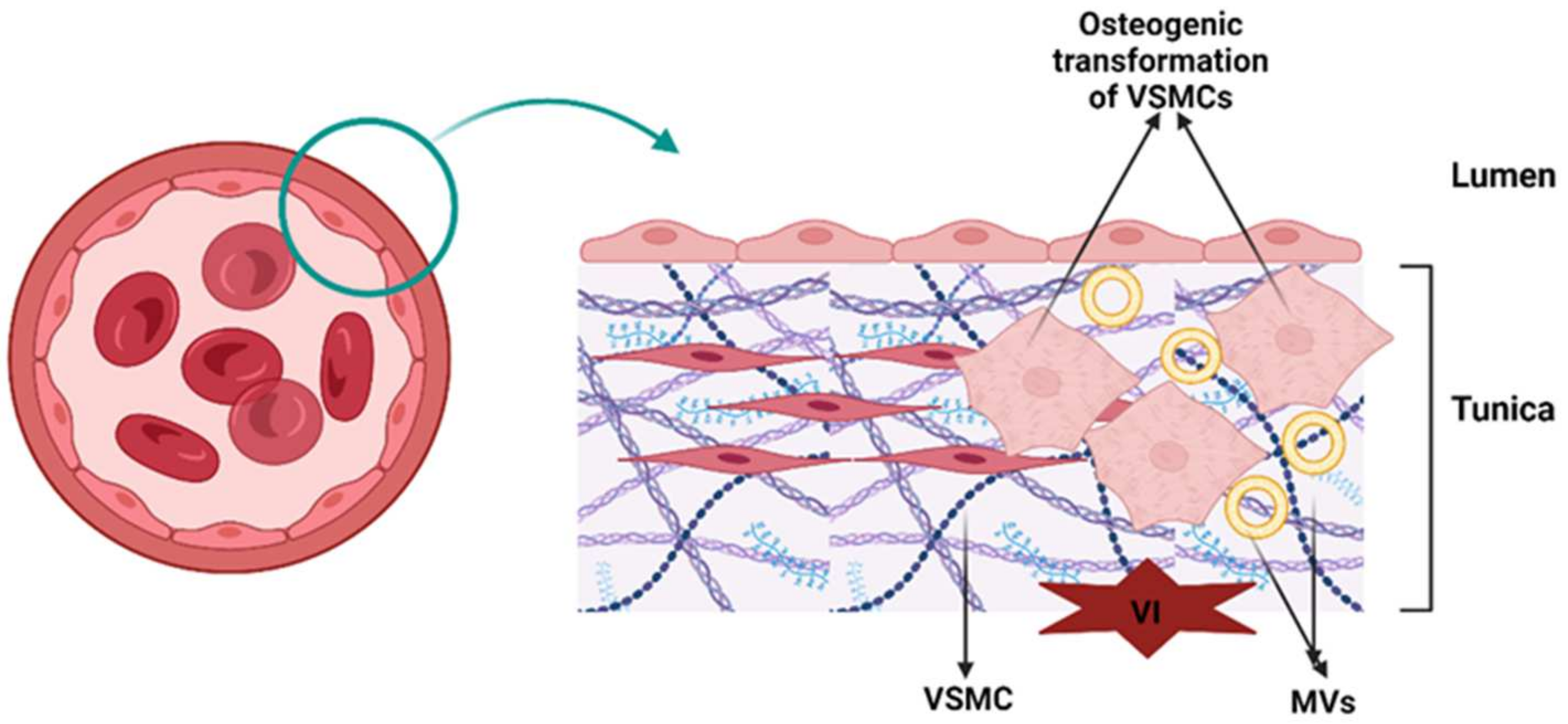
- Induction of osteoblastic–chondrogenic differentiation of VSMCs. In VSMCs of calcified arteries and atherosclerotic plaques, the mineralization process is as follows. VSMCs release MV-like structures, and under conditions of high concentrations of Ca2+ and Pi in the surrounding environment, VSMCs are stimulated to discharge competent MVs for mineralization [25,26]. VSMCs express ALP throughout chondrogenic differentiation, thereby increasing the local Pi level. ALP was also found in MVs released by VSMCs [26,42]. Moreover, in calcified arterial walls, as well as in VSMC culture, HCs had approximately the same pattern of composition during crystal growth as in the case of chondrocytes or bone [44,45]. Figure 3 summarizes the processes of vascular calcification.
- Factors that stimulate chondrogenic transdifferentiation (metaplasia) of VSMCs include mechanical factors (e.g., strain and elongation) [46,47], hypoxia [48,49], Pi (which stimulates the expression of the core binding factor alpha1, a transcription factor associated with osteoblast differentiation and EM mineralization of VSMCs) [50], cytokines, growth factors (e.g., transforming growth factor β (TGF-β), which stimulates the mineralization of VSMCs, and tumor necrosis factor α (TNF-α), which promotes the final differentiation of VSMCs into chondrocyte-like cells) [51,52] and bone morphogenic protein (BMP) 2, which stimulates osteogenic and chondrogenic differentiation [53].
- Factors that promote osteogenic metaplasia include BMP-2 and BMP-4 (while BMP-7 prevents this differentiation) [54], core binding factor alpha 1 [43], Ca2+ and Pi [19], and ALP (which are highly expressed by VSMCs of calcified media vessels) [55]. ALP uses inorganic pyrophosphate (PPi), which is an inhibitor of EM mineralization, as a substrate. Other factors that promote osteogenic transformation are reactive oxygen species (ROS) (i.e., oxidized low-density lipoprotein cholesterol (LDL-C) stimulates the expression of BMP-2 and core binding factor alpha 1] [56]; vitamin D (1,25 dihydroxy vitamin D stimulates ALP activity and exacerbates dystrophic calcifications) [57]; warfarin (a vitamin K antagonist promotes vascular calcification by inhibiting γ-carboxylation of matrix Gla protein (MGP)) [58]; glucocorticoids (e.g., dexamethasone) mediate osteoblast differentiation, thus promoting calcification by suppressing calcification inhibitory molecules) [59]; leptin (a hormone involved in appetite regulation that mediates ectopic calcification by binding and activating β-adrenergic receptors on osteoblasts and increasing the levels of oxidative stress in aortic endothelial cells) [60,61]; and apoptosis (apoptotic remnants of foamy cells, debris VSMCs and MVs increase the local concentration of calcium phosphate, thus promoting an environment suitable for mineral nucleation) [35,43].
- (B)
- Apoptosis. The association between apoptosis and vascular or soft tissue calcification has been widely documented. Vascular calcification is less common because the cellular debris of VSMCs is removed by phagocytic cells, and its clearance may be inhibited under certain conditions, leading to the accumulation of ABs [62,63]. Depending on local conditions, ABs undergo mineralization [64]. Kockx et al. [65] demonstrated that atherosclerotic plaques contain residues of VSMCs and Abs. During apoptosis, Ca2+ and Pi stored in mitochondria and sarcoplasm are incorporated into ABs and contribute to the formation of calcium phosphate crystals. It was found that VSMCs also release vesicles rich in Ca2+ [64]. Anchorage-dependent cells, such as endothelial cells and VSMCs, depend on intercellular and cell–ECM contacts for survival. Integrin has been shown to promote cell survival by inhibiting cell death pathways and activating pro-survival factors such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). The downregulation of vascular endothelial cadherins can induce apoptosis, while the interaction of VSMCs with tenascin C via integrins can prevent apoptosis by changing cell shape and inducing the clustering of epidermal growth factor receptors [66]. Cell–ECM interactions are crucial for apoptotic signaling pathways. Fibronectin signaling through focal adhesion kinase promotes cell survival. Several factors, including mechanical stimulation and growth factors, influence cell survival by activating pathways that prevent apoptosis. Different types of cell death, such as necrosis, apoptosis, and autophagy, affect disease progression in atherosclerosis. Endothelial cell apoptosis is common in the early stage, while VSMC and macrophage apoptosis are common in vulnerable lesions. Factors such as TRAIL (tumor necrosis factor-related apoptosis-inducing ligand), FGF21 (fibroblast growth factor 21) and antiapoptotic factors regulate apoptosis in vascular cells [67,68]. The regulation of VSMC turnover and apoptosis by miRNAs (e.g., miR-21, miR-26a, and miR-29b) and long noncoding RNAs (lncRNAs) (e.g., taurine upregulated gene 1) that target growth factor pathways and other factors may have therapeutic implications in the treatment of atherosclerosis [69,70]. Understanding the molecular mechanisms of cell survival and death in cardiovascular disease may help develop targeted therapies to prevent vascular death and related pathologies.
- (C)
- The presence of circulating nucleation complexes. Nucleation complexes represent a calcification mechanism that is mostly modulated by the OPG–RANK–RANKL system (osteoprotegerin, a receptor activator of NF-κB, a receptor activator of NF-κB ligand). RANKL is an osteoblastic transmembrane protein that binds to its specific receptor RANK, a member of the TNF superfamily present in preosteoclasts and osteoclasts. The RANKL–RANK system stimulates osteoclastogenesis, whereas OPG (a member of the same TNF superfamily that is synthesized by osteoblasts) opposes this process. Because knockout mice with a deletion of the OPG gene have developed osteoporosis accompanied by severe media calcification of the aorta and renal artery, OPG is considered the main performer in the OPG–RANK–RANKL system [77]. OPG inhibited the arterial calcification induced by warfarin and vitamin D in rats [78]. Thus, we can envisage some of the links between the osteoclastic activity of bone remodeling and vascular calcification. It is acknowledged that RANKL enhances calcification of VSMCs by interacting with RANK and promoting BMP-4 production via activation of the alternative NF-κB pathway. Due to the evidence that RANKL promotes calcification of VSMCs, specific agents such as denosumab, a fully human monoclonal antibody, have been studied for their potential to prevent vascular calcification [79]. Most likely, the major determinant of bone metabolism is the OPG/RANKL ratio. Various factors influence the regulation of the RANKL–RANK–OPG system, such as certain cytokines (TNF-a, IL-1, IL-6 and IL-17), hormones (estrogen, vitamin D, and glucocorticoids), and growth factors [80]. Price et al. [81] suggested that the mineralization of soft tissue was induced by nucleation crystals generated in areas of bone resorption, which circulate through the bloodstream until they attach to tissues where they initiate calcification. In rats, Price et al. [82] discovered a complex consisting of phosphates, Ca2+, fetuin A and MGP (insoluble), which was released into the bloodstream from bone, thus explaining the blood transport of MGP. This complex was not found in humans [83].
- (D)
- Imbalance/decline of inhibitors. An increasing number of ectopic calcification inhibitors are being identified using knockout mice or rat models. The presence of these inhibitors in body fluids and tissues saturated with calcium phosphate ions explains why soft tissues do not calcify instantly. Inhibitors of EM mineralization include OPG; osteopontin (a glycoprotein secreted by osteoblasts and involved in bone remodeling); Ank (a transporting transmembrane protein that controls extracellular PPi export); phosphodiesterase nucleotide pyrophosphatase (an extracellular PPi-generating ectoenzyme) [43]; MGP (involved in the clearance of calcium phosphate but also an inhibitor of osteogenic differentiation of VSMCs in different pathologies) [84,85,86]; fetuin A (a circulating inhibitor of calcification that inhibits de novo formation of HC, but has no effect on the already formed crystals) [87]; and Smad 6 (Smad 6 null mice developed extensive vascular wall calcification and cartilaginous metaplasia in the aorta due to suppression of the BMP signaling pathway) [88].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hunziker, E.B. Cartilage histomorphometry. Methods Mol. Med. 2007, 135, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S3), S131–S139. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Uitto, J. Mineralization/anti-mineralization networks in the skin and vascular connective tissues. Am. J. Pathol. 2013, 183, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Boraldi, F.; Lofaro, F.D.; Costa, S.; Moscarelli, P.; Quaglino, D. Rare Co-occurrence of Beta-Thalassemia and Pseudoxanthoma elasticum: Novel Biomolecular Findings. Front. Med. 2019, 6, 322. [Google Scholar] [CrossRef] [PubMed]
- Mackey, R.H.; Venkitachalam, L.; Sutton-Tyrrell, K. Calcifications, arterial stiffness and atherosclerosis. Adv. Cardiol. 2007, 44, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, Q.; Uitto, J. Ectopic mineralization disorders of the extracellular matrix of connective tissue: Molecular genetics and pathomechanisms of aberrant calcification. Matrix Biol. 2014, 33, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, D.; Boraldi, F.; Lofaro, F.D. The biology of vascular calcification. Int. Rev. Cell Mol. Biol. 2020, 354, 261–353. [Google Scholar] [CrossRef] [PubMed]
- Karwowski, W.; Naumnik, B.; Szczepański, M.; Myśliwiec, M. The mechanism of vascular calcification—A systematic review. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2012, 18, RA1–RA11. [Google Scholar] [CrossRef] [PubMed]
- Boraldi, F.; Bartolomeo, A.; De Biasi, S.; Orlando, S.; Costa, S.; Cossarizza, A.; Quaglino, D. Innovative Flow Cytometry Allows Accurate Identification of Rare Circulating Cells Involved in Endothelial Dysfunction. PLoS ONE 2016, 11, e0160153. [Google Scholar] [CrossRef] [PubMed]
- Opdebeeck, B.; Orriss, I.R.; Neven, E.; D’Haese, P.C.; Verhulst, A. Extracellular Nucleotides Regulate Arterial Calcification by Activating Both Independent and Dependent Purinergic Receptor Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 636. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.J.; Ashraf, A.; Chung, E.J. Extracellular Vesicles as Regulators of the Extracellular Matrix. Bioengineering 2023, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Iwayama, T.; Bhongsatiern, P.; Takedachi, M.; Murakami, S. Matrix Vesicle-Mediated Mineralization and Potential Applications. J. Dent. Res. 2022, 101, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Rubin, M.R.; Silverberg, S.J. Vascular calcification and osteoporosis—The nature of the nexus. J. Clin. Endocrinol. Metab. 2004, 89, 4243–4245. [Google Scholar] [CrossRef] [PubMed]
- Dalmeijer, G.W.; van der Schouw, Y.T.; Magdeleyns, E.J.; Vermeer, C.; Verschuren, W.M.M.; Boer, J.M.A.; Beulens, J.W.J. Matrix Gla protein species and risk of cardiovascular events in type 2 diabetic patients. Diabetes Care 2013, 36, 3766–3771. [Google Scholar] [CrossRef] [PubMed]
- van Oostrom, O.; Fledderus, J.O.; de Kleijn, D.; Pasterkamp, G.; Verhaar, M.C. Smooth muscle progenitor cells: Friend or foe in vascular disease? Curr. Stem Cell Res. Ther. 2009, 4, 131–140. [Google Scholar] [CrossRef]
- Dalmeijer, G.W.; van der Schouw, Y.T.; Vermeer, C.; Magdeleyns, E.J.; Schurgers, L.J.; Beulens, J.W.J. Circulating matrix Gla protein is associated with coronary artery calcification and vitamin K status in healthy women. J. Nutr. Biochem. 2013, 24, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Cranenburg, E.C.M.; Vermeer, C.; Koos, R.; Boumans, M.-L.; Hackeng, T.M.; Bouwman, F.G.; Kwaijtaal, M.; Brandenburg, V.M.; Ketteler, M.; Schurgers, L.J. The circulating inactive form of matrix Gla Protein (ucMGP) as a biomarker for cardiovascular calcification. J. Vasc. Res. 2008, 45, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, C.; Carino, A.; Carabetta, N.; Manica, M.; Sabatino, J.; Cianflone, E.; Leo, I.; Strangio, A.; Torella, D.; De Rosa, S. Mechanisms of Cardiovascular Calcification and Experimental Models: Impact of Vitamin K Antagonists. J. Clin. Med. 2024, 13, 1405. [Google Scholar] [CrossRef] [PubMed]
- Persy, V.; D’Haese, P. Vascular calcification and bone disease: The calcification paradox. Trends Mol. Med. 2009, 15, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Towler, D.A. Inorganic pyrophosphate: A paracrine regulator of vascular calcification and smooth muscle phenotype. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, I.-K.; Jeon, J.-H. Vascular Calcification-New Insights into Its Mechanism. Int. J. Mol. Sci. 2020, 21, 2685. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Ochs, R.L.; Rosen, F.; Quach, J.; McCabe, G.; Solan, J.; Seegmiller, J.E.; Terkeltaub, R.; Lotz, M. Chondrocyte-derived apoptotic bodies and calcification of articular cartilage. Proc. Natl. Acad. Sci. USA 1998, 95, 3094–3099. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, T.; Nah, H.D.; Shapiro, I.M.; Pacifici, M. Regulated production of mineralization-competent matrix vesicles in hypertrophic chondrocytes. J. Cell Biol. 1997, 137, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.L.; Joannides, A.J.; Skepper, J.N.; McNair, R.; Schurgers, L.J.; Proudfoot, D.; Jahnen-Dechent, W.; Weissberg, P.L.; Shanahan, C.M. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in ESRD. J. Am. Soc. Nephrol. 2004, 15, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, A.; McGregor, D.H.; Anderson, H.C. Calcification in atherosclerosis. I. Human studies. J. Exp. Pathol. 1986, 2, 261–273. [Google Scholar] [PubMed]
- Kapustin, A.N.; Chatrou, M.L.L.; Drozdov, I.; Zheng, Y.; Davidson, S.M.; Soong, D.; Furmanik, M.; Sanchis, P.; De Rosales, R.T.M.; Alvarez-Hernandez, D.; et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ. Res. 2015, 116, 1312–1323. [Google Scholar] [CrossRef] [PubMed]
- Azoidis, I.; Cox, S.C.; Davies, O.G. The role of extracellular vesicles in biomineralisation: Current perspective and application in regenerative medicine. J. Tissue Eng. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rilla, K.; Mustonen, A.-M.; Arasu, U.T.; Härkönen, K.; Matilainen, J.; Nieminen, P. Extracellular vesicles are integral and functional components of the extracellular matrix. Matrix Biol. 2019, 75–76, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Majeska, R.J.; Wuthier, R.E. Studies on matrix vesicles isolated from chick epiphyseal cartilage. Association of pyrophosphatase and ATPase activities with alkaline phosphatase. Biochim. Biophys. Acta 1975, 391, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Fedde, K.N. Human osteosarcoma cells spontaneously release matrix-vesicle-like structures with the capacity to mineralize. Bone Miner. 1992, 17, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 2003, 5, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Houston, D.A.; Farquharson, C.; MacRae, V.E. Characterisation of matrix vesicles in skeletal and soft tissue mineralisation. Bone 2016, 87, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.H.; Camacho, N.P. Isolation of calcifiable vesicles from human atherosclerotic aortas. Atherosclerosis 1999, 143, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.-S.; Cai, J.; Towler, D.A. Molecular mechanisms of vascular calcification: Lessons learned from the aorta. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.E. Biomineralization and matrix vesicles in biology and pathology. Semin. Immunopathol. 2011, 33, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Magne, D.; Julien, M.; Vinatier, C.; Merhi-Soussi, F.; Weiss, P.; Guicheux, J. Cartilage formation in growth plate and arteries: From physiology to pathology. Bioessays 2005, 27, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Guicheux, J.; Palmer, G.; Shukunami, C.; Hiraki, Y.; Bonjour, J.P.; Caverzasio, J. A novel in vitro culture system for analysis of functional role of phosphate transport in endochondral ossification. Bone 2000, 27, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.N.Y.; Guo, Y.; Genge, B.R.; Ishikawa, Y.; Wuthier, R.E. Transport of inorganic phosphate in primary cultures of chondrocytes isolated from the tibial growth plate of normal adolescent chickens. J. Cell. Biochem. 2002, 86, 475–489. [Google Scholar] [CrossRef]
- Gunter, T.E.; Zuscik, M.J.; Puzas, J.E.; Gunter, K.K.; Rosier, R.N. Cytosolic free calcium concentrations in avian growth plate chondrocytes. Cell Calcium 1990, 11, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.N.; Genge, B.R.; Dunkelberger, D.G.; LeGeros, R.Z.; Concannon, B.; Wuthier, R.E. Physicochemical characterization of the nucleational core of matrix vesicles. J. Biol. Chem. 1997, 272, 4404–4411. [Google Scholar] [CrossRef] [PubMed]
- Magne, D.; Bluteau, G.; Faucheux, C.; Palmer, G.; Vignes-Colombeix, C.; Pilet, P.; Rouillon, T.; Caverzasio, J.; Weiss, P.; Daculsi, G.; et al. Phosphate is a specific signal for ATDC5 chondrocyte maturation and apoptosis-associated mineralization: Possible implication of apoptosis in the regulation of endochondral ossification. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2003, 18, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Speer, M.Y.; Giachelli, C.M. Regulation of cardiovascular calcification. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2004, 13, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.C.; Leopold, J.A.; Loscalzo, J. Vascular calcification: Pathobiological mechanisms and clinical implications. Circ. Res. 2006, 99, 1044–1059. [Google Scholar] [CrossRef]
- Tomazic, B.B. Physiochemical principles of cardiovascular calcification. Z. Kardiol. 2001, 90 (Suppl. S3), 68–80. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; McKee, M.D.; Steitz, S.; Giachelli, C.M. Calcification of vascular smooth muscle cell cultures: Inhibition by osteopontin. Circ. Res. 1999, 84, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Reusch, P.; Wagdy, H.; Reusch, R.; Wilson, E.; Ives, H.E. Mechanical strain increases smooth muscle and decreases nonmuscle myosin expression in rat vascular smooth muscle cells. Circ. Res. 1996, 79, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.; Nordström, I.; Albinsson, S.; Malmqvist, U.; Swärd, K.; Hellstrand, P. Stretch-induced contractile differentiation of vascular smooth muscle: Sensitivity to actin polymerization inhibitors. Am. J. Physiol. Cell Physiol. 2003, 284, C1387–C1396. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Bauer, G.E.; Caldwell, M.P.; Santilli, S.M. Association of artery wall hypoxia and cellular proliferation at a vascular anastomosis. J. Surg. Res. 2000, 91, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, C.P.; Phadke, S.A.; Batlle, D.; Sahai, A. Hypoxia stimulates osteopontin expression and proliferation of cultured vascular smooth muscle cells: Potentiation by high glucose. Diabetes 2001, 50, 1482–1490. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jono, S.; McKee, M.D.; Murry, C.E.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H.; Giachelli, C.M. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 2000, 87, E10–E17. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.E.; Boström, K.; Ravindranath, R.; Lam, T.; Norton, B.; Demer, L.L. TGF-beta 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J. Clin. Investig. 1994, 93, 2106–2113. [Google Scholar] [CrossRef]
- Tintut, Y.; Patel, J.; Parhami, F.; Demer, L.L. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation 2000, 102, 2636–2642. [Google Scholar] [CrossRef] [PubMed]
- Shea, C.M.; Edgar, C.M.; Einhorn, T.A.; Gerstenfeld, L.C. BMP treatment of C3H10T1/2 mesenchymal stem cells induces both chondrogenesis and osteogenesis. J. Cell. Biochem. 2003, 90, 1112–1127. [Google Scholar] [CrossRef]
- Davies, M.R.; Lund, R.J.; Hruska, K.A. BMP-7 is an efficacious treatment of vascular calcification in a murine model of atherosclerosis and chronic renal failure. J. Am. Soc. Nephrol. 2003, 14, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, C.M.; Cary, N.R.; Salisbury, J.R.; Proudfoot, D.; Weissberg, P.L.; Edmonds, M.E. Medial localization of mineralization-regulating proteins in association with Mönckeberg’s sclerosis: Evidence for smooth muscle cell-mediated vascular calcification. Circulation 1999, 100, 2168–2176. [Google Scholar] [CrossRef] [PubMed]
- Cola, C.; Almeida, M.; Li, D.; Romeo, F.; Mehta, J.L. Regulatory role of endothelium in the expression of genes affecting arterial calcification. Biochem. Biophys. Res. Commun. 2004, 320, 424–427. [Google Scholar] [CrossRef]
- Shioi, A.; Mori, K.; Jono, S.; Wakikawa, T.; Hiura, Y.; Koyama, H.; Okuno, Y.; Nishizawa, Y.; Morii, H. Mechanism of atherosclerotic calcification. Z. Kardiol. 2000, 89 (Suppl. S2), 75–79. [Google Scholar] [CrossRef] [PubMed]
- Koos, R.; Mahnken, A.H.; Mühlenbruch, G.; Brandenburg, V.; Pflueger, B.; Wildberger, J.E.; Kühl, H.P. Relation of oral anticoagulation to cardiac valvular and coronary calcium assessed by multislice spiral computed tomography. Am. J. Cardiol. 2005, 96, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Kirton, J.P.; Wilkinson, F.L.; Canfield, A.E.; Alexander, M.Y. Dexamethasone downregulates calcification-inhibitor molecules and accelerates osteogenic differentiation of vascular pericytes: Implications for vascular calcification. Circ. Res. 2006, 98, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.I.; Edelstein, D.; Du, X.L.; Kaneda, Y.; Guzmán, M.; Brownlee, M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J. Biol. Chem. 2001, 276, 25096–25100. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Elefteriou, F.; Levasseur, R.; Liu, X.; Zhao, L.; Parker, K.L.; Armstrong, D.; Ducy, P.; Karsenty, G. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002, 111, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, D.; Davies, J.D.; Skepper, J.N.; Weissberg, P.L.; Shanahan, C.M. Acetylated low-density lipoprotein stimulates human vascular smooth muscle cell calcification by promoting osteoblastic differentiation and inhibiting phagocytosis. Circulation 2002, 106, 3044–3050. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M. Apoptosis and calcification. Scanning Microsc. 1995, 9, 1137–1138. [Google Scholar] [PubMed]
- Proudfoot, D.; Shanahan, C.M. Biology of calcification in vascular cells: Intima versus media. Herz 2001, 26, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Kockx, M.M.; De Meyer, G.R.; Muhring, J.; Jacob, W.; Bult, H.; Herman, A.G. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation 1998, 97, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Boraldi, F.; Lofaro, F.D.; Quaglino, D. Cells Apoptosis in the Extraosseous Calcification Process. Cells 2021, 10, 131. [Google Scholar] [CrossRef]
- Forde, H.; Harper, E.; Davenport, C.; Rochfort, K.D.; Wallace, R.; Murphy, R.P.; Smith, D.; Cummins, P.M. The beneficial pleiotropic effects of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) within the vasculature: A review of the evidence. Atherosclerosis 2016, 247, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Badrichani, A.Z.; Stroka, D.M.; Bilbao, G.; Curiel, D.T.; Bach, F.H.; Ferran, C. Bcl-2 and Bcl-XL serve an anti-inflammatory function in endothelial cells through inhibition of NF-kappaB. J. Clin. Investig. 1999, 103, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Leeper, N.J.; Maegdefessel, L. Non-coding RNAs: Key regulators of smooth muscle cell fate in vascular disease. Cardiovasc. Res. 2018, 114, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, H.; Yue, Y.; Li, S.; Zhang, D.; He, R. TUG1 knockdown ameliorates atherosclerosis via up-regulating the expression of miR-133a target gene FGF1. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2018, 33, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, J.P.; Brighton, C.T.; Stambough, J.E. Subcellular regulation of the ionized calcium pool in isolated growth-plate chondrocytes. Clin. Orthop. Relat. Res. 1989, 242, 285–293. [Google Scholar] [CrossRef]
- Vu, T.H.; Shipley, J.M.; Bergers, G.; Berger, J.E.; Helms, J.A.; Hanahan, D.; Shapiro, S.D.; Senior, R.M.; Werb, Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998, 93, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.N.; Wuthier, M.G.; Genge, B.R.; Wuthier, R.E. In situ levels of intracellular Ca2+ and pH in avian growth plate cartilage. Clin. Orthop. Relat. Res. 1997, 335, 310–324. [Google Scholar] [CrossRef]
- Heinegård, D.; Saxne, T. The role of the cartilage matrix in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Yammani, R.R.; Carlson, C.S.; Chen, H.; Cole, A.; Im, H.-J.; Bursch, L.S.; Yan, S. Du Articular chondrocytes express the receptor for advanced glycation end products: Potential role in osteoarthritis. Arthritis Rheum. 2005, 52, 2376–2385. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F. Integrins and chondrocyte-matrix interactions in articular cartilage. Matrix Biol. 2014, 39, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Bucay, N.; Sarosi, I.; Dunstan, C.R.; Morony, S.; Tarpley, J.; Capparelli, C.; Scully, S.; Tan, H.L.; Xu, W.; Lacey, D.L.; et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998, 12, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; June, H.H.; Buckley, J.R.; Williamson, M.K. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The Role of Osteoprotegerin and Its Ligands in Vascular Function. Int. J. Mol. Sci. 2019, 20, 705. [Google Scholar] [CrossRef] [PubMed]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. Biomed Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; June, H.H.; Buckley, J.R.; Williamson, M.K. SB 242784, a selective inhibitor of the osteoclastic V-H+ATPase, inhibits arterial calcification in the rat. Circ. Res. 2002, 91, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Caputo, J.M.; Williamson, M.K. Bone origin of the serum complex of calcium, phosphate, fetuin, and matrix Gla protein: Biochemical evidence for the cancellous bone-remodeling compartment. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2002, 17, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Wajih, N.; Borras, T.; Xue, W.; Hutson, S.M.; Wallin, R. Processing and Transport of Matrix-Carboxyglutamic Acid Protein and Bone Morphogenetic Protein-2 in Cultured Human Vascular Smooth Muscle Cells: Evidence for an Uptake Mechanism for Serum Fetuin. J. Biol. Chem. 2004, 279, 43052–43060. [Google Scholar] [CrossRef] [PubMed]
- Silaghi, C.N.; Fodor, D.; Crăciun, A.M. Circulating matrix Gla protein: A potential tool to identify minor carotid stenosis with calcification in a risk population. Clin. Chem. Lab. Med. 2013, 51, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Spronk, H.M.H.; Soute, B.A.M.; Schurgers, L.J.; Cleutjens, J.P.M.; Thijssen, H.H.W.; De Mey, J.G.R.; Vermeer, C. Matrix Gla protein accumulates at the border of regions of calcification and normal tissue in the media of the arterial vessel wall. Biochem. Biophys. Res. Commun. 2001, 289, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Silaghi, C.N.; Fodor, D.; Gheorghe, S.R.; Crăciun, A.M. Serum total matrix Gla protein: Reference interval in healthy adults and variations in patients with vascular and osteoarticular diseases. Clin. Chim. Acta 2019, 490, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Heiss, A.; DuChesne, A.; Denecke, B.; Grötzinger, J.; Yamamoto, K.; Renné, T.; Jahnen-Dechent, W. Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J. Biol. Chem. 2003, 278, 13333–13341. [Google Scholar] [CrossRef] [PubMed]
- Galvin, K.M.; Donovan, M.J.; Lynch, C.A.; Meyer, R.I.; Paul, R.J.; Lorenz, J.N.; Fairchild-Huntress, V.; Dixon, K.L.; Dunmore, J.H.; Gimbrone, M.A.J.; et al. A role for smad6 in development and homeostasis of the cardiovascular system. Nat. Genet. 2000, 24, 171–174. [Google Scholar] [CrossRef]
- Jono, S.; Nishizawa, Y.; Shioi, A.; Morii, H. 1,25-Dihydroxyvitamin D3 increases in vitro vascular calcification by modulating secretion of endogenous parathyroid hormone-related peptide. Circulation 1998, 98, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.-A.U. Origin of the mediacalcosis in kidney failure. J. Mal. Vasc. 2009, 34, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Julien, M.; Khoshniat, S.; Lacreusette, A.; Gatius, M.; Bozec, A.; Wagner, E.F.; Wittrant, Y.; Masson, M.; Weiss, P.; Beck, L.; et al. Phosphate-dependent regulation of MGP in osteoblasts: Role of ERK1/2 and Fra-1. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2009, 24, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Watson, A.D.; Ji, S.; Boström, K.I. Heat shock protein 70 enhances vascular bone morphogenetic protein-4 signaling by binding matrix Gla protein. Circ. Res. 2009, 105, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Arking, D.E.; Krebsova, A.; Macek, M.S.; Macek, M.J.; Arking, A.; Mian, I.S.; Fried, L.; Hamosh, A.; Dey, S.; McIntosh, I.; et al. Association of human aging with a functional variant of klotho. Proc. Natl. Acad. Sci. USA 2002, 99, 856–861. [Google Scholar] [CrossRef] [PubMed]
| Bone Formation | Micro/Macrostructural Characteristics | Mechanism of Mineralization | Localization |
|---|---|---|---|
| Intramembranous ossification |
|
|
|
| Endochondral ossification |
|
|
|
| Vascular Calcification | Micro/Macrostructural Characteristics | Mechanism of Mineralization | Associated Pathology |
| Intima calcifications |
|
|
|
| Media calcifications (Mönckeberg’s sclerosis) |
|
|
|
| Calcifications of heart valves |
|
|
|
| Vascular calciphylaxis (Calcific uremic arteriolopathy) |
|
|
|
| Protective Factors of Calcification | Favoring Factors of Calcification |
|---|---|
| osteopontin | TNF-α |
| OPG | TGF-β |
| fetuin-A | oxidized and acetylated LDL-C |
| osteonectin | C-reactive protein |
| MGP | leptin |
| BMP-7 | BMP-2 |
| Mg2+ | advanced glycation end-products |
| PPi | Pi |
| ↓ Ca x PO4 | ↑ Ca x PO4 |
| vitamin K | interleukin-4 |
| HDL-C | interleukin-6 |
| growth arrest-specific protein 6 | glucocorticoids |
| albumin | ROS |
| parathyroid hormone | collagen tip I |
| parathyroid hormone related peptide | fibronectin |
| phosphonoformic acid | 25-OH colesterol |
| natriuretic peptide type C | 17β-estradiol |
| adrenomedulin | Ca2+ |
| uremic serum | |
| 1, 25-dihydroxycholecalciferol | |
| cyclic adenosine monophosphate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghe, S.R.; Crăciun, A.M.; Ilyés, T.; Tisa, I.B.; Sur, L.; Lupan, I.; Samasca, G.; Silaghi, C.N. Converging Mechanisms of Vascular and Cartilaginous Calcification. Biology 2024, 13, 565. https://doi.org/10.3390/biology13080565
Gheorghe SR, Crăciun AM, Ilyés T, Tisa IB, Sur L, Lupan I, Samasca G, Silaghi CN. Converging Mechanisms of Vascular and Cartilaginous Calcification. Biology. 2024; 13(8):565. https://doi.org/10.3390/biology13080565
Chicago/Turabian StyleGheorghe, Simona R., Alexandra M. Crăciun, Tamás Ilyés, Ioana Badiu Tisa, Lucia Sur, Iulia Lupan, Gabriel Samasca, and Ciprian N. Silaghi. 2024. "Converging Mechanisms of Vascular and Cartilaginous Calcification" Biology 13, no. 8: 565. https://doi.org/10.3390/biology13080565
APA StyleGheorghe, S. R., Crăciun, A. M., Ilyés, T., Tisa, I. B., Sur, L., Lupan, I., Samasca, G., & Silaghi, C. N. (2024). Converging Mechanisms of Vascular and Cartilaginous Calcification. Biology, 13(8), 565. https://doi.org/10.3390/biology13080565







