Impact of Iron Oxide on Anaerobic Digestion of Frass in Biogas and Methanogenic Archaeal Communities’ Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Iron Oxide Nanoparticles
2.2. Feedstock and Its Characteristics
2.3. Experimental Setup
2.4. Methods of Analysis and Calculation
2.5. Microbial Community Analysis
2.6. Statistical Analysis
3. Results and Discussion
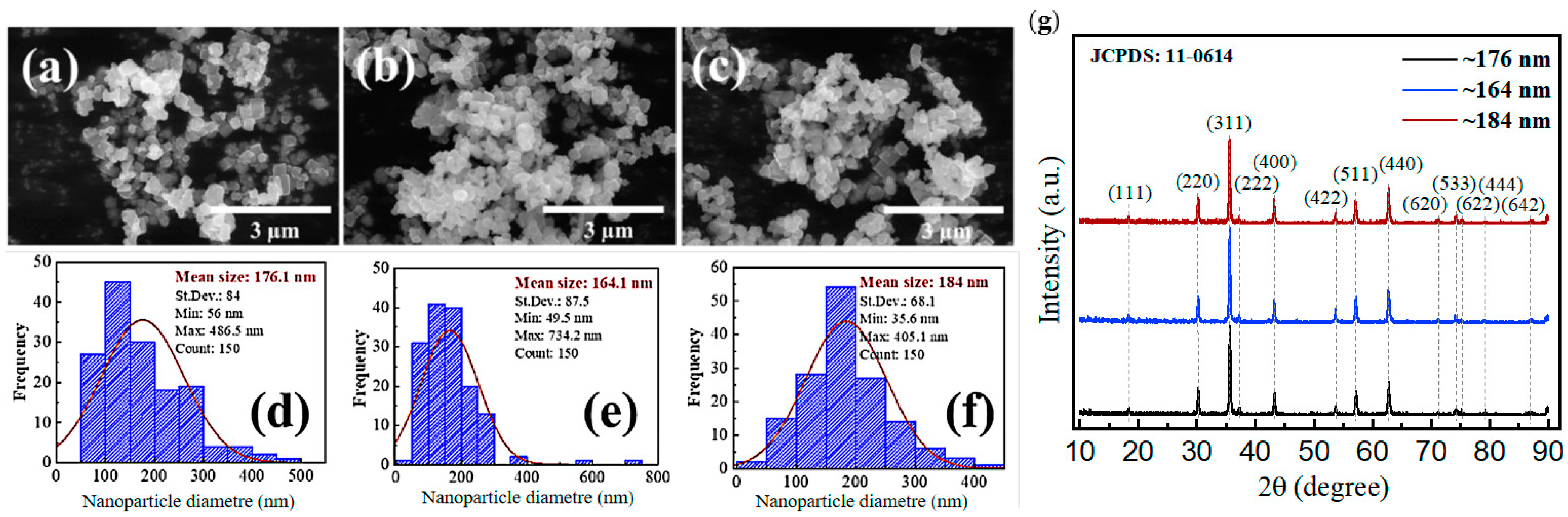
3.1. The Characterization of the Iron Oxide Nanoparticles
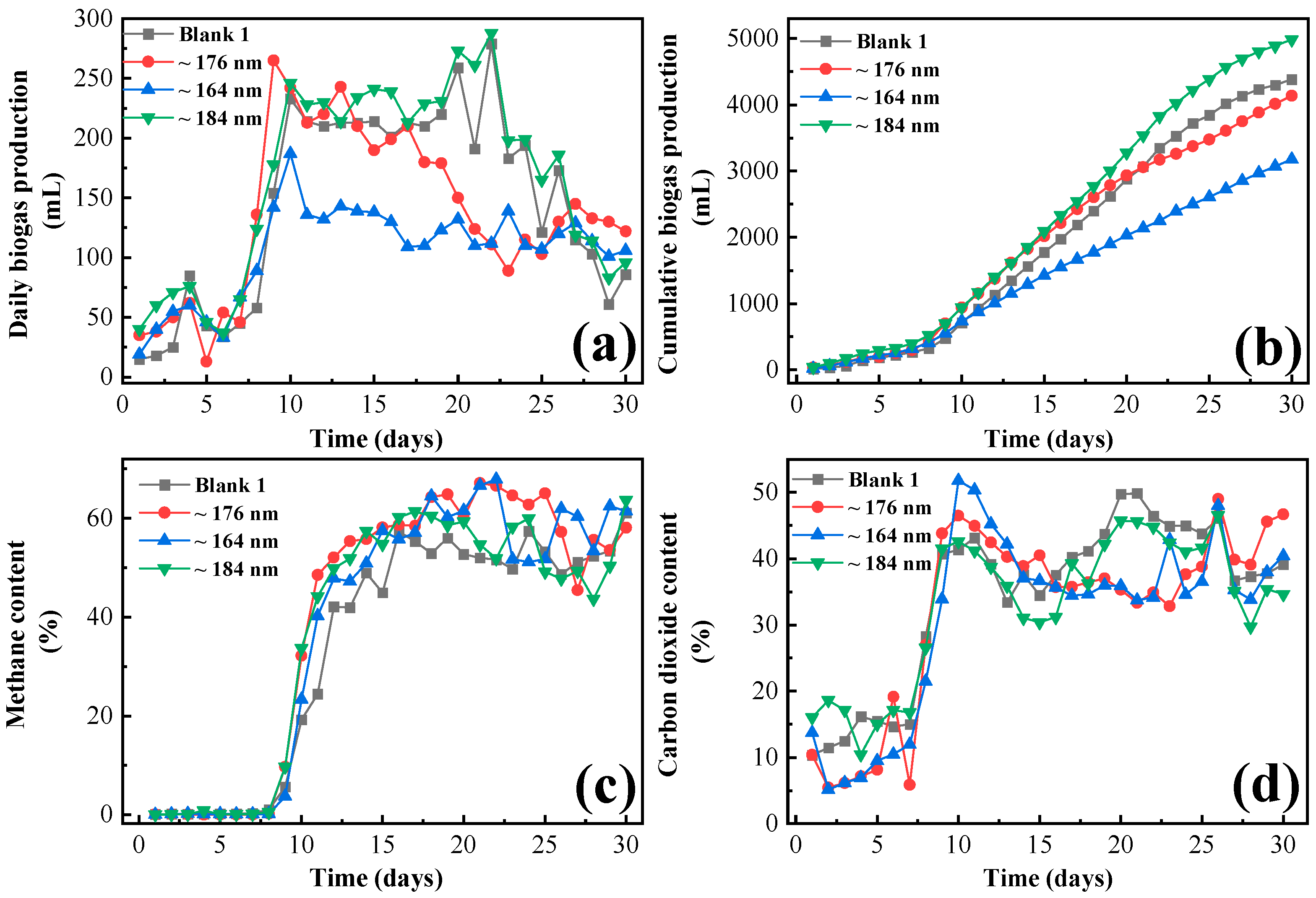
3.2. Influence of the Iron Oxide on Gas Production of Frass
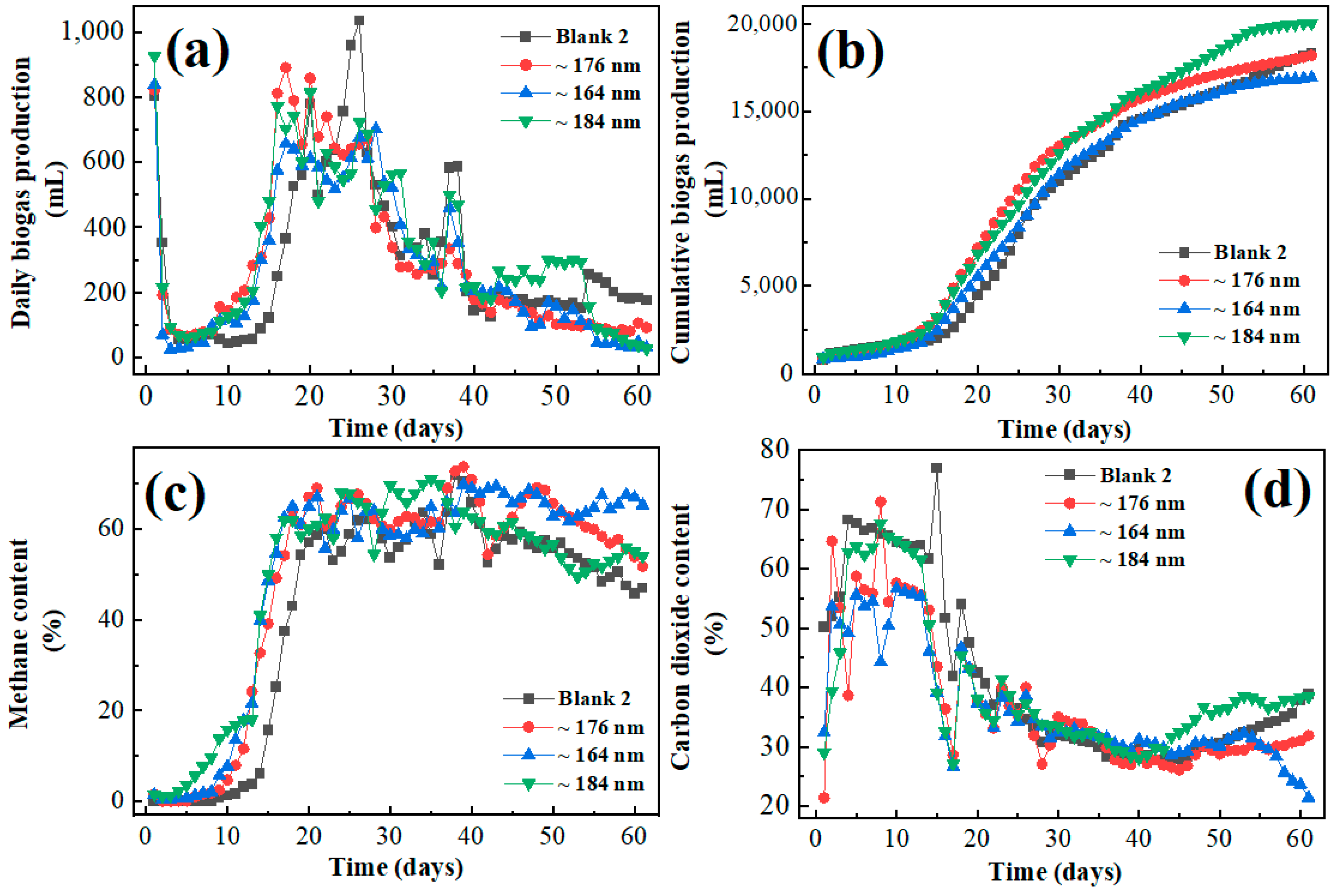
3.3. Influence of the Iron Oxide on Gas Production of Frass with Corn Straw
3.4. Methanogen Activity Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moustakas, K.; Loizidou, M.; Rehan, M.; Nizami, A. A review of recent developments in renewable and sustainable energy systems: Key challenges and future perspective. Renew. Sustain. Energy Rev. 2020, 119, 109418. [Google Scholar] [CrossRef]
- Archana, K.; Visckram, A.; Kumar, P.S.; Manikandan, S.; Saravanan, A.; Natrayan, L. A review on recent technological breakthroughs in anaerobic digestion of organic biowaste for biogas generation: Challenges towards sustainable development goals. Fuel 2024, 358, 130298. [Google Scholar] [CrossRef]
- Liu, W.-R.; Zeng, D.; She, L.; Su, W.-X.; He, D.-C.; Wu, G.-Y.; Ma, X.-R.; Jiang, S.; Jiang, C.-H.; Ying, G.-G. Comparisons of pollution characteristics, emission situations, and mass loads for heavy metals in the manures of different livestock and poultry in China. Sci. Total Environ. 2020, 734, 139023. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.-L.; Chen, F.; Liu, S.; Yang, Y.; Hou, C.; Wang, Y.-Z. Co-production of biogas and humic acid using rice straw and pig manure as substrates through solid-state anaerobic fermentation and subsequent aerobic composting. J. Environ. Manag. 2022, 320, 115860. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Guo, H.; Yi, X.; Gao, W.; Zhang, H.; Bai, Y.; Wang, T. Research on thermal insulation properties of plant fiber composite building material: A review. Int. J. Thermophys. 2020, 41, 87. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Zhu, X.; Blanco, E.; Bhatti, M.; Borrion, A. Impact of metallic nanoparticles on anaerobic digestion: A systematic review. Sci. Total Environ. 2021, 757, 143747. [Google Scholar] [CrossRef] [PubMed]
- Arif, S.; Liaquat, R.; Adil, M. Applications of materials as additives in anaerobic digestion technology. Renew. Sustain. Energy Rev. 2018, 97, 354–366. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, S.; Wang, X.; Zhang, S.; Hu, T.; Wang, X.; Wang, C.; Wu, J.; Xu, L.; Xu, G. Enhanced anaerobic digestion of kitchen waste at different solids content by alkali pretreatment and bentonite addition: Methane production enhancement and microbial mechanism. Bioresour. Technol. 2023, 369, 128369. [Google Scholar] [CrossRef]
- Faisal, S.; Salama, E.-S.; Malik, K.; Lee, S.-h.; Li, X. Anaerobic digestion of cabbage and cauliflower biowaste: Impact of iron oxide nanoparticles (IONPs) on biomethane and microbial communities alteration. Bioresour. Technol. Rep. 2020, 12, 100567. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Tabatabaei, M.; Aghbashlo, M.; Panahi, H.K.S.; Nizami, A.-S. A state-of-the-art review on the application of nanomaterials for enhancing biogas production. J. Environ. Manag. 2019, 251, 109597. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, E.; Samer, M.; Attia, Y.; Abdel-Hadi, M.; Hassan, H.; Badr, Y. Comparison of nanoparticles effects on biogas and methane production from anaerobic digestion of cattle dung slurry. Renew. Energy 2016, 87, 592–598. [Google Scholar] [CrossRef]
- Barrena, R.; del Carmen Vargas-García, M.; Catacora-Padilla, P.; Gea, T.; Markeb, A.A.; Moral-Vico, J.; Sánchez, A.; Font, X.; Aspray, T.J. Magnetite-based nanoparticles and nanocomposites for recovery of overloaded anaerobic digesters. Bioresour. Technol. 2023, 372, 128632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, Y. Conductive Fe3O4 nanoparticles accelerate syntrophic methane production from butyrate oxidation in two different lake sediments. Front. Microbiol. 2016, 7, 204548. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, X.; Li, Y.; Su, Y.; Wu, D.; Xie, B. Enhanced anaerobic digestion performance of food waste by zero-valent iron and iron oxides nanoparticles: Comparative analyses of microbial community and metabolism. Bioresour. Technol. 2023, 371, 128633. [Google Scholar] [CrossRef] [PubMed]
- Orrantia, M.; Armenta, M.; Alvarez, L.H.; Burboa-Charis, V.A.; Meza-Escalante, E.R.; Olivas, A.; Arroyo, E.; Maytorena, V. Enhanced methane production via anaerobic digestion assisted with Fe3O4 nanoparticles supported on microporous granular activated carbon. Fuel 2024, 360, 130517. [Google Scholar] [CrossRef]
- Hassanein, A.; Kumar, A.N.; Lansing, S. Impact of electro-conductive nanoparticles additives on anaerobic digestion performance-A review. Bioresour. Technol. 2021, 342, 126023. [Google Scholar] [CrossRef] [PubMed]
- Boscaro, M.E.; Marin, D.F.C.; da Silva, D.C.; Maintinguer, S.I. Effect of Fe3O4 nanoparticles on microbial diversity and biogas production in anaerobic digestion of crude glycerol. Biomass Bioenergy 2022, 160, 106439. [Google Scholar] [CrossRef]
- Basri, N.E.A.; Azman, N.A.; Ahmad, I.K.; Suja, F.; Jalil, N.A.A.; Amrul, N.F. Potential applications of frass derived from black soldier fly larvae treatment of food waste: A review. Foods 2022, 11, 2664. [Google Scholar] [CrossRef]
- Van Huis, A. Insects as food and feed, a new emerging agricultural sector: A review. J. Insects Food Feed. 2020, 6, 27–44. [Google Scholar] [CrossRef]
- Čičková, H.; Newton, G.L.; Lacy, R.C.; Kozánek, M. The use of fly larvae for organic waste treatment. Waste Manag. 2015, 35, 68–80. [Google Scholar] [CrossRef] [PubMed]
- ur Rehman, K.; Cai, M.; Xiao, X.; Zheng, L.; Wang, H.; Soomro, A.A.; Zhou, Y.; Li, W.; Yu, Z.; Zhang, J. Cellulose decomposition and larval biomass production from the co-digestion of dairy manure and chicken manure by mini-livestock (Hermetia illucens L.). J. Environ. Manag. 2017, 196, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Pas, C.; Brodeur, D.; Deschamps, M.-H.; Lebeuf, Y.; Adjalle, K.; Barnabé, S.; Eeckhout, M.; Vandenberg, G.; Vaneeckhaute, C. Valorization of pretreated biogas digestate with black soldier fly (Hermetia illucens, L.; Diptera: Stratiomyidae) larvae. J. Environ. Manag. 2022, 319, 115529. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Nie, E.; Lü, F.; Peng, W.; Zhang, H.; He, P. Screening of early warning indicators for full-scale dry anaerobic digestion of household kitchen waste. Environ. Res. 2022, 214, 114136. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Kawasaki, T.; Hirayasu, H.; Matsumoto, Y.; Fujitani, Y. Evaluation of fertilizer value of residues obtained after processing household organic waste with black soldier fly larvae (Hermetia illucens). Sustainability 2020, 12, 4920. [Google Scholar] [CrossRef]
- Pang, W.; Hou, D.; Chen, J.; Nowar, E.E.; Li, Z.; Hu, R.; Tomberlin, J.K.; Yu, Z.; Li, Q.; Wang, S. Reducing greenhouse gas emissions and enhancing carbon and nitrogen conversion in food wastes by the black soldier fly. J. Environ. Manag. 2020, 260, 110066. [Google Scholar] [CrossRef]
- Amrul, N.F.; Kabir Ahmad, I.; Ahmad Basri, N.E.; Suja, F.; Abdul Jalil, N.A.; Azman, N.A. A review of organic waste treatment using black soldier fly (Hermetia illucens). Sustainability 2022, 14, 4565. [Google Scholar] [CrossRef]
- Xiao, X.; Mazza, L.; Yu, Y.; Cai, M.; Zheng, L.; Tomberlin, J.K.; Yu, J.; van Huis, A.; Yu, Z.; Fasulo, S. Efficient co-conversion process of chicken manure into protein feed and organic fertilizer by Hermetia illucens L. (Diptera: Stratiomyidae) larvae and functional bacteria. J. Environ. Manag. 2018, 217, 668–676. [Google Scholar] [CrossRef]
- Wong, C.Y.; Lim, J.W.; Chong, F.K.; Lam, M.K.; Uemura, Y.; Tan, W.N.; Bashir, M.J.; Lam, S.M.; Sin, J.C.; Lam, S.S. Valorization of exo-microbial fermented coconut endosperm waste by black soldier fly larvae for simultaneous biodiesel and protein productions. Environ. Res. 2020, 185, 109458. [Google Scholar] [CrossRef]
- Fu, S.-F.; Wang, D.-H.; Xie, Z.; Zou, H.; Zheng, Y. Producing insect protein from food waste digestate via black soldier fly larvae cultivation: A promising choice for digestate disposal. Sci. Total Environ. 2022, 830, 154654. [Google Scholar] [CrossRef]
- Raman, S.S.; Stringer, L.C.; Bruce, N.C.; Chong, C.S. Opportunities, challenges and solutions for black soldier fly larvae-based animal feed production. J. Clean. Prod. 2022, 373, 133802. [Google Scholar] [CrossRef]
- Association, A.P.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1926; Volume 6. [Google Scholar]
- Abdelsalam, E.; Samer, M.; Attia, Y.; Abdel-Hadi, M.; Hassan, H.; Badr, Y. Influence of zero valent iron nanoparticles and magnetic iron oxide nanoparticles on biogas and methane production from anaerobic digestion of manure. Energy 2017, 120, 842–853. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, L.; Wang, Y.; Zhao, Y.; She, Z.; Gao, M.; Guo, Y. Application of iron oxide (Fe3O4) nanoparticles during the two-stage anaerobic digestion with waste sludge: Impact on the biogas production and the substrate metabolism. Renew. Energy 2020, 146, 2724–2735. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Xu, R.; Xiang, Y.; Jia, M.; Hu, J.; Zheng, Y.; Xiong, W.; Cao, J. Enhanced mesophilic anaerobic digestion of waste sludge with the iron nanoparticles addition and kinetic analysis. Sci. Total Environ. 2019, 683, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Papa, G.; Scaglia, B.; D’Imporzano, G.; Savoldelli, S.; Jucker, C.; Colombini, S.; Toschi, I.; Adani, F. Valorizing the organic fraction of municipal solid waste by producing black soldier fly larvae and biomethane in a biorefinery approach. J. Clean. Prod. 2022, 379, 134422. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, X.; Sun, Y.; Zhao, J.; Awasthi, M.K.; Liu, T.; Li, R.; Zhang, Z. Improvement of the composition and humification of different animal manures by black soldier fly bioconversion. J. Clean. Prod. 2021, 278, 123397. [Google Scholar] [CrossRef]
- Alkhrissat, T.; Kassab, G.; Abdel-Jaber, M.T. Impact of Iron Oxide Nanoparticles on Anaerobic Co-Digestion of Cow Manure and Sewage Sludge. Energies 2023, 16, 5844. [Google Scholar] [CrossRef]
- Hassanpourmoghadam, L.; Goharrizi, B.A.; Torabian, A.; Bouteh, E.; Rittmann, B.E. Effect of Fe3O4 nanoparticles on anaerobic digestion of municipal wastewater sludge. Biomass Bioenergy 2023, 169, 106692. [Google Scholar] [CrossRef]
- Lu, T.; Su, T.; Liang, X.; Wei, Y.; Zhang, J.; He, T. Dual character of methane production improvement and antibiotic resistance genes reduction by nano-Fe2O3 addition during anaerobic digestion of swine manure. J. Clean. Prod. 2022, 376, 134240. [Google Scholar] [CrossRef]
- Wedwitschka, H.; Gallegos Ibanez, D.; Jáquez, D.R. Biogas production from residues of industrial insect protein production from black soldier fly larvae Hermetia illucens (L.): An evaluation of different insect frass samples. Processes 2023, 11, 362. [Google Scholar] [CrossRef]
- Hijazi, O.; Abdelsalam, E.; Samer, M.; Attia, Y.; Amer, B.; Amer, M.; Badr, M.; Bernhardt, H. Life cycle assessment of the use of nanomaterials in biogas production from anaerobic digestion of manure. Renew. Energy 2020, 148, 417–424. [Google Scholar] [CrossRef]
- Wang, L.; Lei, Z.; Zhang, Z.; Shimizu, K.; Yuan, T.; Li, S.; Liu, S. Insight into enhanced acetic acid production from food waste in anaerobic hydrolysis/acidification with Fe3O4 supplementation. Waste Manag. 2022, 150, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Ping, Q.; Fang, Q.; Chen, Y.; Ding, W.; Xiao, Y.; Wang, Z.; Zhou, W. Effect of Fe3O4 on propionic acid production by anaerobic fermentation of waste cooking oil and aerobic sludge. J. Water Process Eng. 2022, 49, 102910. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Q.; Ye, X.; Wang, C.; Jia, Z.; Du, J.; Kong, X.; Xi, Y. Effect of different charged Fe3O4 nanoparticles on methane production for anaerobic digestion of wheat straw. J. Clean. Prod. 2021, 328, 129655. [Google Scholar] [CrossRef]
- Ajayi-Banji, A.; Rahman, S. Efficacy of magnetite (Fe3O4) nanoparticles for enhancing solid-state anaerobic co-digestion: Focus on reactor performance and retention time. Bioresour. Technol. 2021, 324, 124670. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Wang, F.; Li, S.; Wang, H.; Zhang, D.; Yi, W.; Shen, X. Influence of nano-Fe3O4 biochar on the methanation pathway during anaerobic digestion of chicken manure. Bioresour. Technol. 2023, 377, 128979. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Li, L.; Dai, X.; Dai, L. A review on application of single and composite conductive additives for anaerobic digestion: Advances, challenges and prospects. Resour. Conserv. Recycl. 2021, 174, 105844. [Google Scholar] [CrossRef]
- Baniamerian, H.; Isfahani, P.G.; Tsapekos, P.; Alvarado-Morales, M.; Shahrokhi, M.; Vossoughi, M.; Angelidaki, I. Application of nano-structured materials in anaerobic digestion: Current status and perspectives. Chemosphere 2019, 229, 188–199. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, H.; Liu, J.; Gong, L.; Yang, X.; Zuo, T.; Zhou, Y.; Wang, J.; You, X.; Jia, Q. Effects of Fe3O4 nanoparticles on anaerobic digestion enzymes and microbial community of sludge. Environ. Technol. 2023, 44, 68–81. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Cheng, R.; Liu, R.; Liu, Z.; Yang, Q. Effects of nZVI and Fe3O4 NPs on anaerobic methanogenesis, microbial communities and metabolic pathways for treating domestic wastewater at ambient temperature. J. Water Process Eng. 2022, 48, 102845. [Google Scholar] [CrossRef]
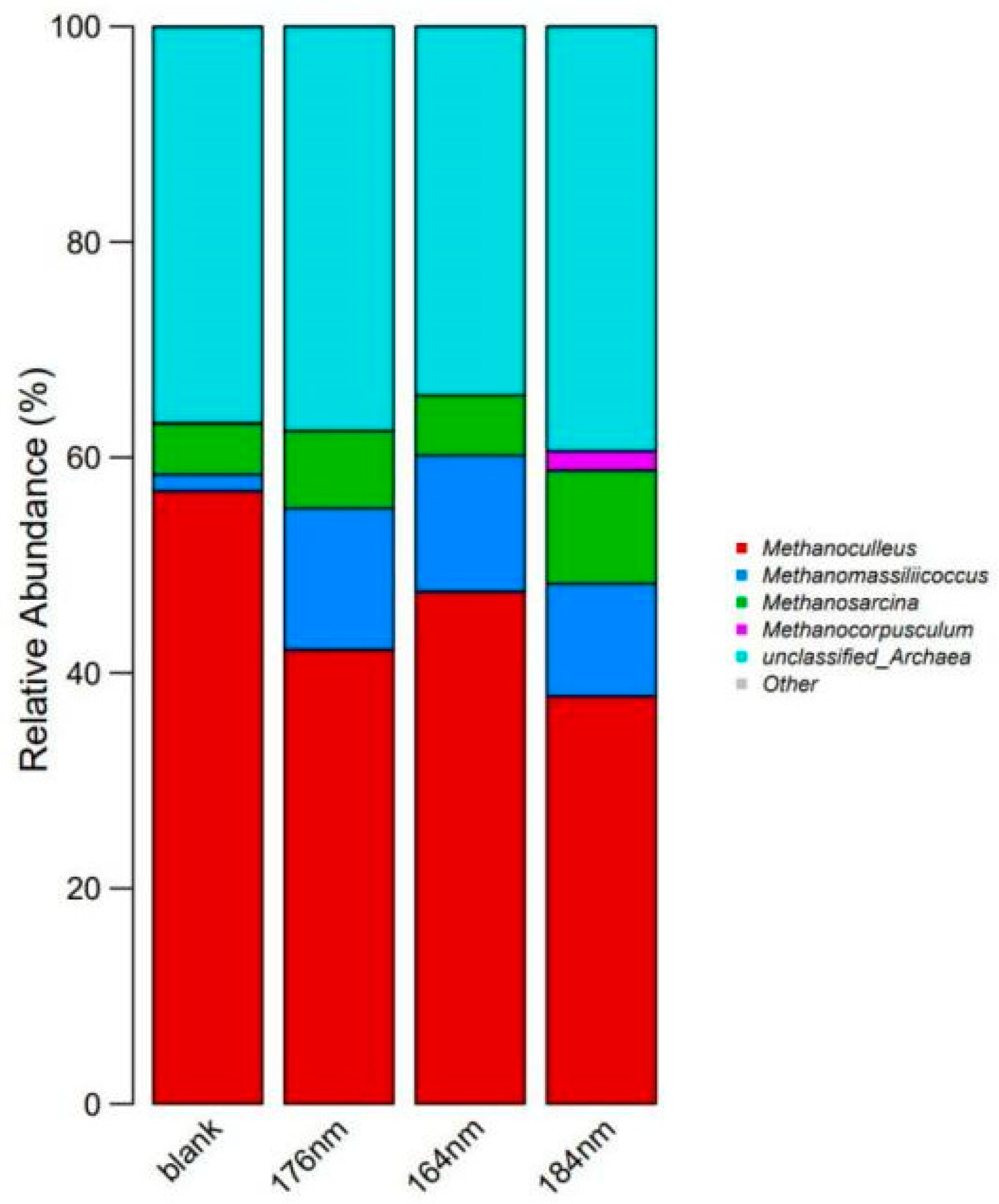

| Species | Blank 1 (/%) | ~176 nm (/%) | ~164 nm (/%) | ~184 nm (/%) |
|---|---|---|---|---|
| Methanoculleus | 56.88 | 42.15 | 47.54 | 37.83 |
| Methanomassiliicoccus | 1.53 | 13.13 | 12.64 | 10.46 |
| Methanosarcina | 4.70 | 7.19 | 5.58 | 10.51 |
| Methanocorpusculum | 0.08 | 0 | 0 | 1.78 |
| Unclassified Archaea | 36.76 | 37.53 | 34.22 | 39.42 |
| Other | 0.04 | 0 | 0.01 | 0.01 |
| Sample | Number | OTUs | Shannon | Chao | Ace | Simpson | Shannon Even | Coverage |
|---|---|---|---|---|---|---|---|---|
| Blank 1 | 21,181 | 18 | 1.36 | 18 | 18 | 0.37 | 0.47 | 1 |
| ~176 nm | 19,980 | 10 | 1.61 | 10 | 0 | 0.25 | 0.70 | 1 |
| ~164 nm | 16,690 | 12 | 1.60 | 12 | 12 | 0.27 | 0.64 | 1 |
| ~184 nm | 25,551 | 17 | 1.81 | 17 | 17.75 | 0.22 | 0.64 | 1 |
| Group | Blank 1 | ~176 nm | ~164 nm | ~184 nm |
|---|---|---|---|---|
| Methanotrophy | 0 | 0 | 0 | 0 |
| Acetoclastic methanogenesis | 0 | 0 | 0 | 0 |
| Methanogenesis by disproportionation of methyl groups | 995 | 1436 | 931 | 2685 |
| Methanogenesis using formate | 0 | 0 | 0 | 0 |
| Methanogenesis by CO2 reduction with H2 | 13,061 | 9858 | 8866 | 12,805 |
| Methanogenesis by reduction of methyl compounds with H2 | 325 | 2623 | 2110 | 2673 |
| Hydrogenotrophic methanogenesis | 13,386 | 12,481 | 10,976 | 15,478 |
| Methanogenesis | 13,386 | 12,481 | 10,976 | 15,478 |
| Methanol oxidation | 0 | 0 | 0 | 0 |
| Methylotrophy | 325 | 2623 | 2110 | 2673 |
| Aerobic ammonia oxidation | 0 | 0 | 0 | 0 |
| Aerobic nitrite oxidation | 0 | 0 | 0 | 0 |
| Nitrification | 0 | 0 | 0 | 0 |
| Sulfate respiration | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Dong, A.; Liu, J.; Qadir, K.; Xu, T.; Fan, X.; Liu, H.; Ji, F.; Xu, W. Impact of Iron Oxide on Anaerobic Digestion of Frass in Biogas and Methanogenic Archaeal Communities’ Analysis. Biology 2024, 13, 536. https://doi.org/10.3390/biology13070536
Dong X, Dong A, Liu J, Qadir K, Xu T, Fan X, Liu H, Ji F, Xu W. Impact of Iron Oxide on Anaerobic Digestion of Frass in Biogas and Methanogenic Archaeal Communities’ Analysis. Biology. 2024; 13(7):536. https://doi.org/10.3390/biology13070536
Chicago/Turabian StyleDong, Xiaoying, Aoqi Dong, Juhao Liu, Kamran Qadir, Tianping Xu, Xiya Fan, Haiyan Liu, Fengyun Ji, and Weiping Xu. 2024. "Impact of Iron Oxide on Anaerobic Digestion of Frass in Biogas and Methanogenic Archaeal Communities’ Analysis" Biology 13, no. 7: 536. https://doi.org/10.3390/biology13070536
APA StyleDong, X., Dong, A., Liu, J., Qadir, K., Xu, T., Fan, X., Liu, H., Ji, F., & Xu, W. (2024). Impact of Iron Oxide on Anaerobic Digestion of Frass in Biogas and Methanogenic Archaeal Communities’ Analysis. Biology, 13(7), 536. https://doi.org/10.3390/biology13070536






