Simple Summary
In the realm of modern medicine, the battle against cancer is a relentless pursuit that demands continuous innovation. Among the numerous elements in the complex world of cancer biology, TRAIL has emerged as a focal point for researchers and clinicians alike. Its diverse role in cancer, especially its influence on the tumor microenvironment (TME), has sparked extensive research. This article explores the intricacies of TRAIL’s function in cancer, revealing its dual nature—capable of both inhibiting and, conversely, promoting, cancer growth. TRAIL impacts a wide array of processes, from apoptosis to modulation of the immune response, and plays a crucial part in tumor initiation, progression, and invasion. Moving forward, the potential of TRAIL as a transformative element in cancer therapy is gaining recognition. New therapeutic approaches, including combination therapies, immunotherapies, and gene delivery methods, are being developed to leverage TRAIL’s potential for the benefit of cancer patients. Moreover, as our understanding of the TME and its effects on TRAIL’s efficacy grows, it is opening doors to more targeted and effective treatment strategies.
Abstract
Cancer continues to pose a significant threat to global health, with its status as a leading cause of death remaining unchallenged. Within the realm of cancer research, the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) stands out as a critical player, having been identified in the 1990s as the tenth member of the TNF family. This review examines the pivotal role of TRAIL in cancer biology, focusing on its ability to induce apoptosis in malignant cells through both endogenous and exogenous pathways. We provide an in-depth analysis of TRAIL’s intracellular signaling and intercellular communication, underscoring its potential as a selective anticancer agent. Additionally, the review explores TRAIL’s capacity to reshape the tumor microenvironment, thereby influencing cancer progression and response to therapy. With an eye towards future developments, we discuss the prospects of harnessing TRAIL’s capabilities for the creation of tailored, precision-based cancer treatments, aiming to enhance efficacy and improve patient survival rates.
1. Introduction
Cancer remains a major challenge in contemporary medical research, requiring research into groundbreaking strategies that can shed light on the multifaceted processes of tumorigenesis and provide effective therapeutic interventions. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) stands out among the large number of molecules being investigated for cancer therapy due to its ability to selectively induce cytotoxicity in malignant cells without harming healthy tissues [1]. As a member of the tumor necrosis factor (TNF) superfamily, TRAIL interacts with its death receptors DR4 and DR5 to trigger apoptosis, making it an attractive target for anticancer therapies [2].
However, despite its potential, barriers such as drug resistance and suboptimal pharmacokinetic profiles have hindered the widespread clinical use of TRAIL [3]. This review explores the critical role of TRAIL in tumorigenesis and progression. We focus on the dynamic interactions between TRAIL and the complex signaling networks that govern its apoptotic functions, as well as roles beyond these functions. In addition, we explore TRAIL-promoted intercellular communication between cancer cells and the tumor microenvironment, exploring extracellularly the current state of knowledge on the role of TRAIL and the mechanisms of resistance that have been identified.
In this review, we provide an overview of the status of ongoing clinical trials involving TRAIL and TRAIL-like drugs. It is worth pointing out that the utilization of the apoptotic pathway through gene delivery therapies targeting TRAIL offers a promising avenue for novel therapeutic approaches [4]. In this paper, we comprehensively review the role of TRAIL in carcinogenesis and evaluate the promise of targeting TRAIL signaling for cancer treatment. Our goal is to provide researchers with a clearer understanding of TRAIL therapeutic strategies for treating tumors and to inform clinical treatment.
2. TRAIL and Caspase-Dependent Apoptosis
2.1. TRAIL
The apoptotic ligand TRAIL (TNFSF10), the tenth member of the tumor necrosis factor (TNF) superfamily, is a type II transmembrane protein [2]. TRAIL plays a pivotal role in apoptosis induction (Figure 1) [5,6]. There are two main forms of TRAIL: membrane-bound TRAIL (mTRAIL) and soluble TRAIL (sTRAIL) [2]. mTRAIL, consisting of 281 amino acids, is expressed in various tissues and cell types, with a predominant presence on the cell surface of immune cells. The extracellular C-terminal region of TRAIL can be proteolytically cleaved from the cell surface by cysteine proteases, namely sTRAIL [7]. In healthy adult plasma, sTRAIL is typically found at a concentration of about 100 pg/mL, and at this concentration, sTRAIL could not induce apoptosis in most cell lines [8]. Moreover, a naturally occurring splice variant of TRAIL, called TRAILshort, has been identified as a membrane-bound short form of TRAIL, which lacks the apoptosis-inducing activity [9,10,11]. Monoclonal antibodies (mAbs) selectively targeting TRAILshort have been shown to enhance cancer susceptibility to TRAIL and improve the efficacy of autologous CD8+ T cells in isolated primary tumors [11].
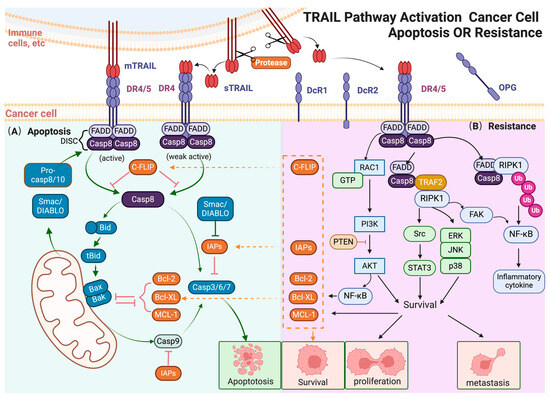
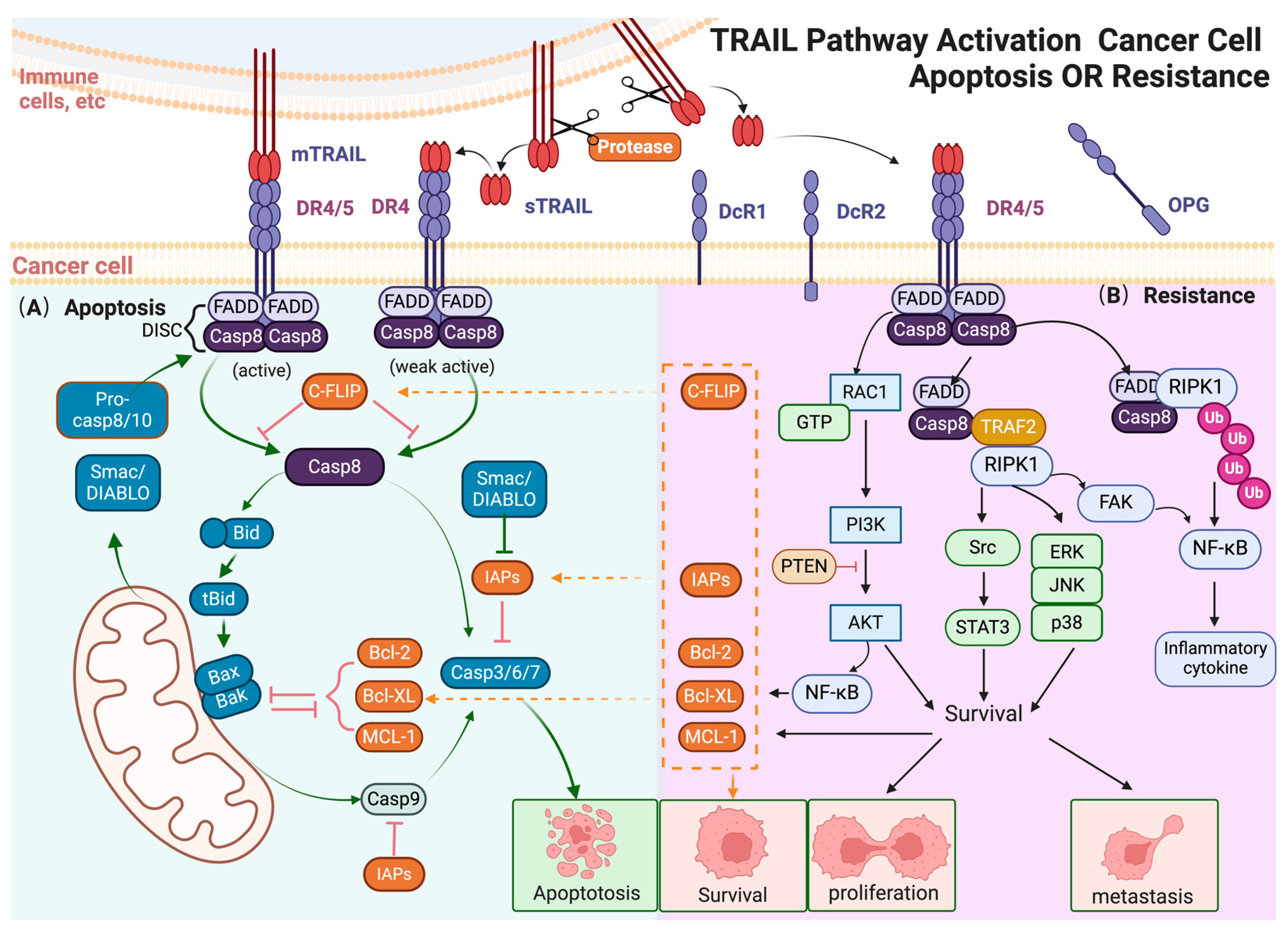
Figure 1.
TRAIL pathway activation cancer cell apoptosis or resistance. (A) TRAIL induces exogenous and endogenous apoptotic signals in sensitive cancer cells. In the exogenous pathway, mTRAIL binds to DR4/5, leading to the formation of the DISC, activation of caspases, and eventually cell apoptosis; in the endogenous pathway, TRAIL-activated caspase-8 cleaves Bid, triggering mitochondrial membrane disruption and cytochrome c release. This leads to apoptotic body formation, activating procaspase-9 and downstream caspases, resulting in cell apoptosis. (B) Non-apoptotic signals induced by TRAIL in apoptosis-resistant cancer cells. In TRAIL-insensitive cells, TRAIL can activate various protein kinases, including NF-κB, PI3K/AKT, JNK, p38, ERK, and STAT3, leading to pro-tumorigenic responses. These pathways promote cell proliferation, invasion, and metastasis. NF-κB activation, mediated by the FADD-RIPK1 “FADDosome” complex, leads to the transcription of anti-apoptotic genes. The MAPK family, particularly ERK, JNK, and p38, contributes to TRAIL-induced non-apoptotic effects, supporting apoptotic resistance and promoting cell survival. AKT activation upregulates anti-apoptotic factors and stimulates NF-κB, contributing to resistance. Other signaling molecules like RIPK1, Src, and STAT3 also enhance pro-survival, migration, and invasion outcomes. The arrow and t-bar represent activated and inhibitory interactions respectively. (Figure was created with Biorender.com, accessed on 1 June 2024).
2.2. Receptors of TRAIL
Human TRAIL can interact with five receptors. Among these, death receptor 4 (DR4/TRAIL-R1, encoded by TNFRSF10A) and death receptor 5 (DR5/TRAIL-R2, encoded by TNFRSF10B) are regarded as pro-apoptotic receptors [12]. They both feature the same death domain (DD) motif, which enables the recruitment of apoptotic signaling molecules to initiate cell death. Both DR4/5 could be activated by mTRAIL, while sTRAIL is only able to induce the trimerization of DR4 [12]. The molecular aggregation capacity of mTRAIL is 100–1000 times stronger than that of sTRAIL, which may explain why sTRAIL can only activate DR4 and exhibits significantly lower cytotoxic potential [12]. Because of the lack of DD motif, decoy receptor 1 (DcR1/TRAIL-R3, encoded by TNFRSF10C), decoy receptor 2 (DcR2/TRAIL-R4, encoded by TNFRSF10D) and osteoprotegerin receptor (OPG) cannot induce apoptosis upon TRAIL binding [2,13]. In contrast, they may compete with the pro-apoptotic receptors DR4 and DR5 for TRAIL binding, and thereby inhibit TRAIL-mediated apoptosis [13]. It is worth noting that OPG is a soluble receptor, which acts as the decoy receptor for TRAIL [14,15]. In a physiological environment, the affinity of TRAIL for OPG is significantly weaker than its affinity for membrane-bound receptors, suggesting that OPG’s role in influencing TRAIL signaling is relatively minor [13].
TRAIL receptors are most often found on the cell membrane surface, but are also found in the cytoplasm and nucleus. The subcellular localization affects the function of TRAIL receptors. Studies have shown that nuclear expression of DR5 inhibits the maturation of let-7 and increases tumor cell proliferation, which is independent of the interaction between DR5 and TRAIL [16,17]. Moreover, post-translational modification is involved in the activation of TRAIL receptors [18]. Studies have indicated that O-glycosylation of DR5 can regulate the sensitivity of cells to TRAIL-induced apoptosis [19], while N-glycosylation of DR4 can enhance its pro-apoptotic effect [20].
2.3. Canonical Signaling Mediated by TRAIL
TRAIL coordinates a complex series of molecular interactions in the classical exogenous and endogenous apoptotic pathways that ultimately lead to the programmed death of malignant cells (Figure 1A). TRAIL has the capability to initiate the exogenous apoptotic pathway. mTRAIL induces the trimerization of DR4/5 receptors on targeted cells, which is necessary for the recruitment of adaptor proteins, such as Fas-associated death domain (FADD). Procaspase-8 and pro-caspase-10 then combine with FADD to form the DISC complex [21]. The DISC complex integrates extracellular signals and triggers the apoptotic cascade, including the cleavage of caspase-8/10 and the activation of downstream effectors such as caspase-3/6/7 [22].
In addition, TRAIL also plays an important part in the endogenous apoptotic pathway. TRAIL-activated caspase-8 also cleaves BH3 interaction domain death agonist (Bid) into the carboxy-terminal fragment of Bid (tBid). tBid promotes the oligomeric polymerization of Bax and Bak on the mitochondrial outer membrane, which leads to the mitochondrial membrane potential depolarization and the release of cytochrome c [23]. Cytochrome c recruits apoptotic peptidase activator 1 (Apaf-1) and procaspase-9 to form apoptotic bodies, which activates caspase-9 and thereby induces endogenous apoptosis [24].
3. The Multiple Roles of TRAIL in the Tumor Microenvironment
Although TRAIL can induce apoptosis in various cancer cell lines, many other cell lines and most primary cancers are TRAIL resistant [3]. In TRAIL-resistant cancer cells, TRAIL can activate cell survival signal pathways, including IkB/nuclear factor κB (NF-κB), PI3K/AKT (phosphoinositide 3-kinase/protein kinase B), c-Jun N-terminal kinase (JNK), p38, extracellular signal-regulated kinase (ERK), and SRC-signal transduction and transcriptional activator 3 (STAT3) pathways (Figure 1B). In addition to cancer cells, TRAIL also exhibits dual effects on the components of the tumor microenvironment, such as cancer stem cells and immune cells [25]. In the following section, we mainly discuss how the TRAIL signal participates in cancer progression by regulating the tumor microenvironment (Figure 2).
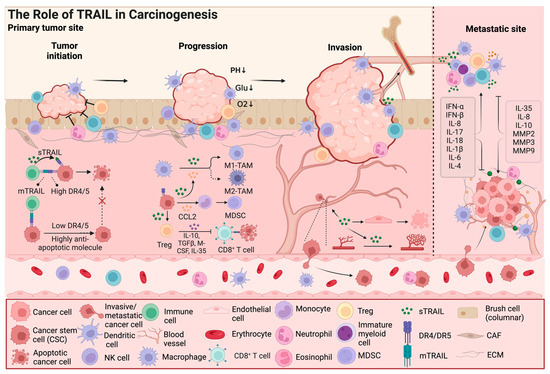
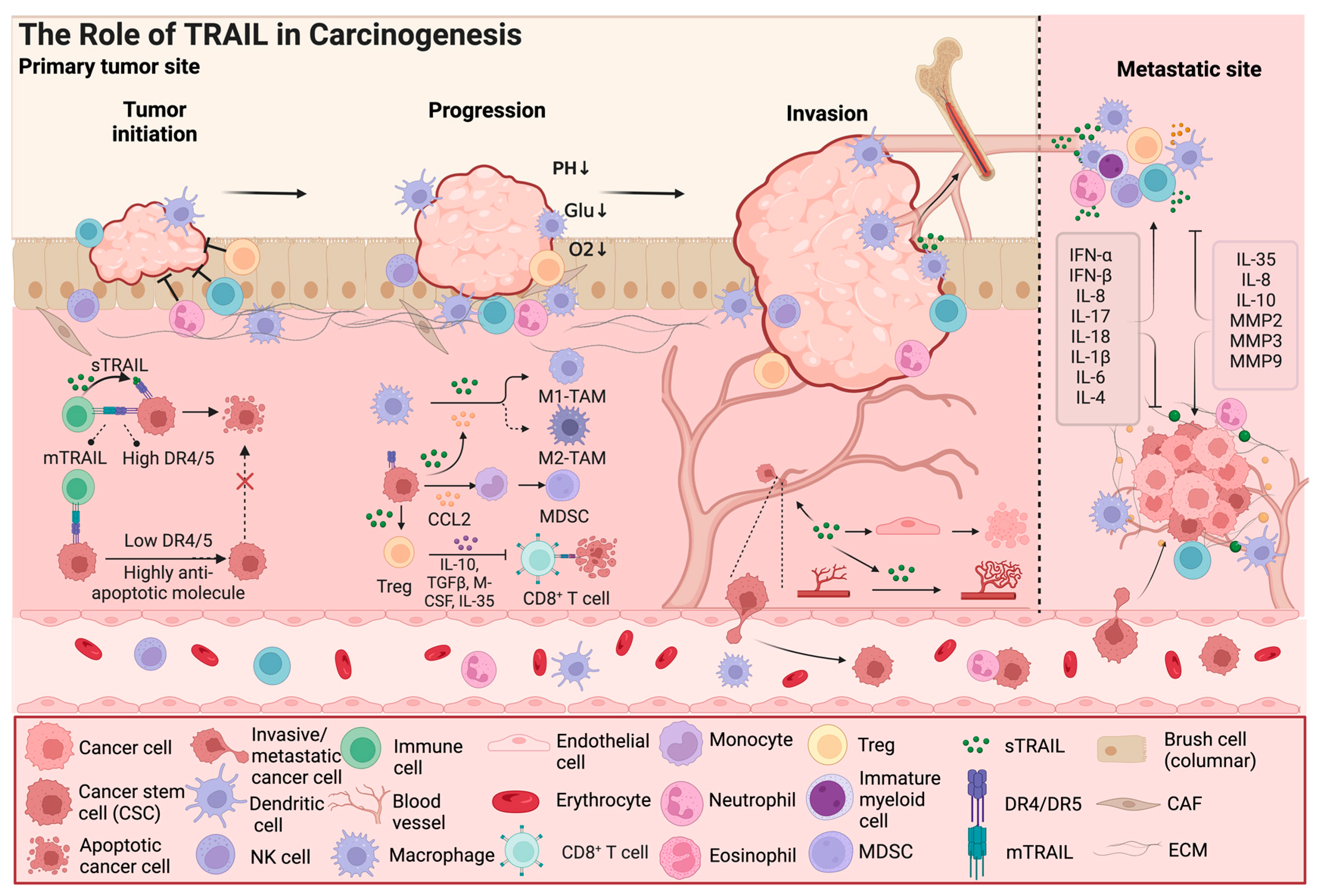
Figure 2.
The role of TRAIL in carcinogenesis. In addition to its effect on cancer cell apoptosis, TRAIL also exhibits dual effects on components of the tumor microenvironment, such as cancer stem cells and immune cells; Furthermore, TRAIL signaling is involved in cancer progression by regulating the tumor microenvironment, including remodeling of blood vessels and invasion and metastasis of cancer cells. TAMs, tumor-associated macrophages; MDSCs, myeloid-derived suppressor cells; CAFs, cancer-associated fibroblasts; CCL2, C-C Chemokine Ligand 2; IL, Interleukin; IFN, Interferon; TGF, tumor growth factor; M-CSF, macrophage colony stimulating factor; MMP, matrix metalloproteinase; ECM, extracellular matrix. The arrow and t-bar represent activated and inhibitory interactions, respectively. (Figure was created with Biorender.com, accessed on 1 June 2024).
3.1. Cancer Stem Cells Are TRAIL Resistant
Cancer stem cells (CSCs) are a subpopulation of cancer cells with self-renewal capacity and multi-lineage differentiation potential which can drive tumor initiation [26,27,28]. CSCs can be identified in most prevalent cancers, as they express various typical cellular markers, such as CD133, CD44, and ALDH [29,30]. Studies have shown that CSCs with high death receptor expression in breast and colon cancer respond effectively to TRAIL treatment [31,32]. Interestingly, an increasing body of evidence suggests that CSCs often exhibit low levels of DR4/5 and high levels of anti-apoptotic molecules, rendering them highly resistant to apoptosis [33]. These anti-apoptotic molecules (c-FLIP, IAPs, and Bcl-2, among others) act as blockers in the pathway of TRAIL-induced apoptosis (Figure 1B).
In addition, targeting TRAIL signals has also become a strategy to solve TRAIL-resistant CSCs. Studies have shown that the inhibitor of EZH2 sensitizes colon CSCs for TRAIL-induced apoptosis by upregulating DR4/5 expression [34]. The anti-apoptotic and pro-survival signaling pathways are also responsible for TRAIL resistance. The CSC surface marker CD133 can inhibit TRAIL-induced apoptosis by upregulating FLICE-like inhibitory protein (FLIP) expression and activating the PI3K-AKT signal [35]. The tumor suppressor PTEN, which inhibits the carcinogenic PI3K/AKT signaling pathway, can be negatively regulated by miR-25 overexpression, thus enhancing the presence of TRAIL-resistant CSCs [36]. Inhibition of JNK signaling can reduce DcR1 expression and increase DR4/5 expression through an IL-8-dependent autocrine process in pancreatic CSCs, which enhances the susceptibility to TRAIL treatment [37]. Overexpression of β-catenin and GLI3 in CSCs could impair TRAIL-induced apoptosis by activating the Wnt and Hedgehog signaling pathways [38,39].
3.2. TRAIL Has Dual Effects on Immune Cells
TRAIL is expressed in macrophages, cytotoxic T cells, neutrophils, natural killer (NK) cells, and dendritic cells, which plays a crucial role in antitumor immune responses [17]. TRAIL acts as a crucial element in inducing apoptosis of tumor cells particularly secreted by cytotoxic T cells and NK cells [40]. TRAIL can promote the polarization of human macrophages towards the pro-inflammatory/antitumor M1 phenotype, which reinforce the cytotoxic effect on malignant cells [41,42]. TRAIL also has the ability to induce the apoptosis of myeloid-derived suppressor cells (MDSCs), which enhances the antitumor activity of the immune system [43]. Additionally, TRAIL can trigger the production of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, which amplify the inflammatory response against tumors [41].
Conversely, TRAIL may contribute to an immunosuppressive microenvironment. IL-35 can induce N2 neutrophil polarization and promote neutrophil infiltration into the tumor microenvironment, which augments the proangiogenic and immunosuppressive function of neutrophils [44]. IL-10/IL-35 double-deficient Treg cells typically rely on the enhancement of TRAIL expression and the release of soluble TRAIL to provide functional plasticity [45].
Regulatory T (Treg) cells have a critical role in the prevention of autoimmunity, which also suppress robust antitumor immune responses. High doses of TRAIL have been demonstrated to boost the proliferation of Tregs, and also enhance the recruitment and polarization of immunosuppressive M2-like macrophages [46,47]. TRAIL-triggered CCL2 secretion from TRAIL-resistant cancer cells can drive monocyte polarization to MDSCs and M2-like macrophages, which promotes accumulation of tumor-supportive immune cells in the tumor microenvironment [48]. In a murine cholangiocarcinoma model, TRAIL from cancer cells is reported to facilitate MDSC proliferation though the noncanonical TRAIL signaling with consequent NF-κB activation [49]. TRAIL-induced immune tolerance may be one reason for immune escape of cancer cells which sustains tumor growth and metastasis.
3.3. The Interaction of the TRAIL Signal Pathway and Other Cytokines Influencing the Tumor Microenvironment
Cytokines regulate the TRAIL signal and TRAIL-induced apoptosis. Type I interferon (IFN) secreted by immune cells, such as DC and NK cells, can enhance TRAIL expression in neutrophils and NK cells [50,51]. Pro-inflammatory cytokines, such as IL-8, IL-17, and IL-18, have been shown to promote the release of TRAIL from human neutrophils and NK cells [52,53]. IL-1β can induce TRAIL expression in human umbilical cord mesenchymal stem cells (hUCMSCs), which induces the apoptosis of breast cancer cells [54]. Other studies revealed that IL-1β can also increase the expression of OPG, the decoy receptor of TRAIL, which protects cancer cells from TRAIL-induced apoptosis [55]. IL-6 is reported to enhance TRAIL-induced apoptosis through p53-dependent upregulation of DR4 and DR5 [56]. Similarly, IL-4 treatment can significantly upregulate DR4 expression [57]. IL-35, an immunosuppressive cytokine, has been shown to downregulate TRAIL expression in neutrophils [44].
The TRAIL signal may stimulate the release of cytokines. In non-small cell lung cancer and colon cancer cells, TRAIL receptor signaling could stimulate the secretion of IL-8 constitutively, which contributes to a pro-tumorigenic microenvironment [58,59]. TRAIL is reported to heighten the production of other pro-tumor inflammatory cytokines, such as IL-10, which inhibits the activity of NK cells and CD8+ T cells within the tumor microenvironment [47]. TRAIL can also enhance the secretion of matrix metalloproteinases (MMPs), including MMP2, MMP3, and MMP9, which promotes the epithelial-mesenchymal transition of esophageal squamous cell carcinoma cells [60]. Thus, cancer cells gain benefits from TRAIL signaling by secreting cytokines or chemokines, which may be a potential cause of TRAIL resistance.
3.4. TRAIL Modulates Cancer Angiogenesis
Angiogenesis, the formation of new blood vessels, occurs in normal physiological processes and in pathological conditions [61]. TRAIL could lead to vascular disruption by inducing apoptosis in DR5-expressing tumor endothelial cells and vascular smooth muscle cells, which effectively inhibits tumor growth [61,62]. However, TRAIL might also play an unexpected role in promoting angiogenesis. Studies have shown that soluble TRAIL could promote the migration of endothelial cells and the formation of new blood vessels to a degree comparable to vascular endothelial growth factor (VEGF) [63]. In breast cancer, endothelial cells at the premetastatic niche co-express TRAIL and DR5. The interaction between TRAIL and DR5 intracellularly stimulates endothelial cell proliferation, migration, and tubular formation, which potentially promotes angiogenesis and metastasis [64]. TRAIL could also promote angiogenesis by interacting with other factors, such as FGF-2 and NOX4 [65,66]. Hypoxia is a ubiquitous feature of the tumor microenvironment, and is able to inhibit TRAIL-induced apoptosis and stimulate angiogenesis [67]. Hypoxia triggers ROS production, which leads to increased oxidative stress. TRAIL has the ability to protect endothelial cells from oxidative stress by reducing ROS formation [68].
4. TRAIL as a Target for Cancer Gene Therapy
Since the discovery of TRAIL, its ability to specifically bind to trigger apoptosis in cancer cells has made TRAIL/DR a target for clinical tumor therapy [69]. Unlike conventional chemotherapy, which often leads to non-specific cytotoxic effects on healthy tissue, TRAIL’s selective action holds promise for reducing side effects and improving patient tolerance [70]. As a result, the development of TRAIL-based drugs has taken various directions and strategies.
Currently, TRAIL-based drugs are being evaluated in clinical trials for a variety of cancer types, including solid tumors like lung, breast, pancreatic, and colon cancers, as well as hematological malignancies such as lymphomas and advanced cancers (Table 1 and Table 2). However, none have advanced beyond phase III clinical trials. The development of TRAIL faces challenges related to its extremely short half-life, drug-resistant cancer cell populations, and inadequate in vivo delivery, which have hindered its clinical application [69,70]. Several labeled recombinant forms of TRAIL, despite their advantages in promoting ligand trimerization and enhancing agonist activity, have not been tested in clinical trials due to concerns about hepatotoxicity [3,71,72,73]. Subsequently, unlabeled recombinant soluble TRAIL (Dulanermin/AMG-951/APO2L) and Circularly permuted TRAIL (CPT) were introduced in clinical trials, but phase III clinical trials did not show improved overall survival (OS) in both experimental and placebo groups. In addition, severe responses were observed in phase II clinical trials [74,75]. Apo2L.XL, another human-engineered cross-linking form of Dulanermin, exhibits stronger pro-apoptotic activity, although its safety profile remains to be explored [76].

Table 1.
Clinical trials targeting TRAIL and its receptors.

Table 2.
Clinical trials targeting TRAIL signaling pathways.
In clinical practice, antibody agonists targeting specific DR4 or DR5, effectively overcome the obstruction of TRAIL signaling by decoy receptors. These antibodies offer specific binding, high affinity, and longer half-lives [77]. Among various DR4 targeting antibodies, only the HGS-ETR1 antibody (Mapatumumab) has advanced to phase 2 clinical trials and demonstrated a good safety profile in several tumor therapies. However, its effectiveness has not been confirmed in other phase II clinical trials, and combining it with other drugs did not improve its efficacy [78,79]. Several clinical trials have been conducted with DR5 agonist antibodies, including Tigatuzumab (CS-1008) and conatumumab (AMG 655), which are currently in phase II clinical trials. Nevertheless, in vivo targeted therapies for several cancers have not achieved the desired results [74,80].
Studies have indicated that different cancer cells exhibit varying sensitivities to antibody agonists specific to DR4 or DR5 [81]. It has been suggested that apoptosis is induced under endoplasmic reticulum (ER) stress, and cell autonomous movement and invasion are described as occurring through TRAIL-R2. This suggests that targeting TRAIL-R1 for anticancer therapy may be more appropriate as it lacks pro-movement signaling [82].
In clinical studies, all agonistic TRAIL-R antibodies have been well tolerated, but the bivalent nature of monoclonal antibodies does not align with the mechanism of TRAIL trimer activation. This limits their ability to effectively cross-link TRAIL receptors and initiate apoptotic signaling. Innovative approaches such as the development of novel polyvalent antibodies aim to address this limitation, although cross-reactivity concerns remain to be resolved [80]. Novel DR5 polyvalent antibodies GEN1029, INBRX-109, and IGM-8444 have entered clinical trials. MEDI3039 is a novel multivalent DR5 agonist capable of inducing tumor regression in situ and inhibiting the growth of metastatic triple-negative breast cancer [83].
Innovative TRAIL therapies, including the development of more targeted and specific TRAIL derivatives, TRAIL couplings, and fusion proteins to overcome TRAIL resistance, require further clinical validation [74,84,85,86,87,88,89].
5. Novel Strategies for TRAIL-Based Therapy
The journey of clinical development and application of TRAIL has been riddled with challenges and unexpected twists, particularly during the early stages. The primary objectives during this initial phase were to address the insufficient excitability of TRAIL/DR, its poor bioavailability, and the issue of TRAIL resistance. In recent years, a significant revelation has come to light—TRAIL exhibits a dual effect within the TME. This revelation necessitates a shift in focus towards enhancing the TME itself. In the subsequent discussion, we explore promising avenues for novel therapeutic strategies in the context of the TME. These strategies include combination therapy, specialized TRAIL delivery techniques, and other innovative approaches. These approaches take into account the pivotal role of the TME. Their primary aim is twofold: to enhance TRAIL’s bioavailability and its sustainability within the body, and to circumvent the development of drug resistance in tumor cells. Simultaneously, these strategies are designed to exert a regulatory influence on the TME, thereby creating a more conducive setting for TRAIL-based therapy (Figure 3).
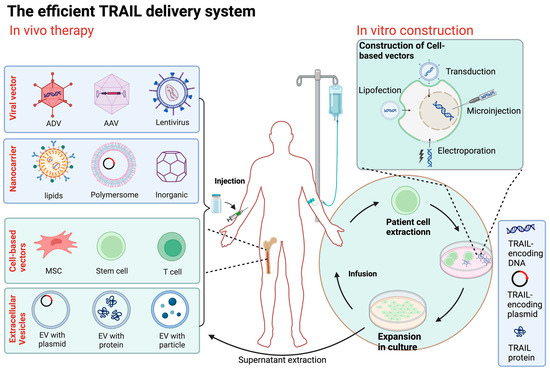
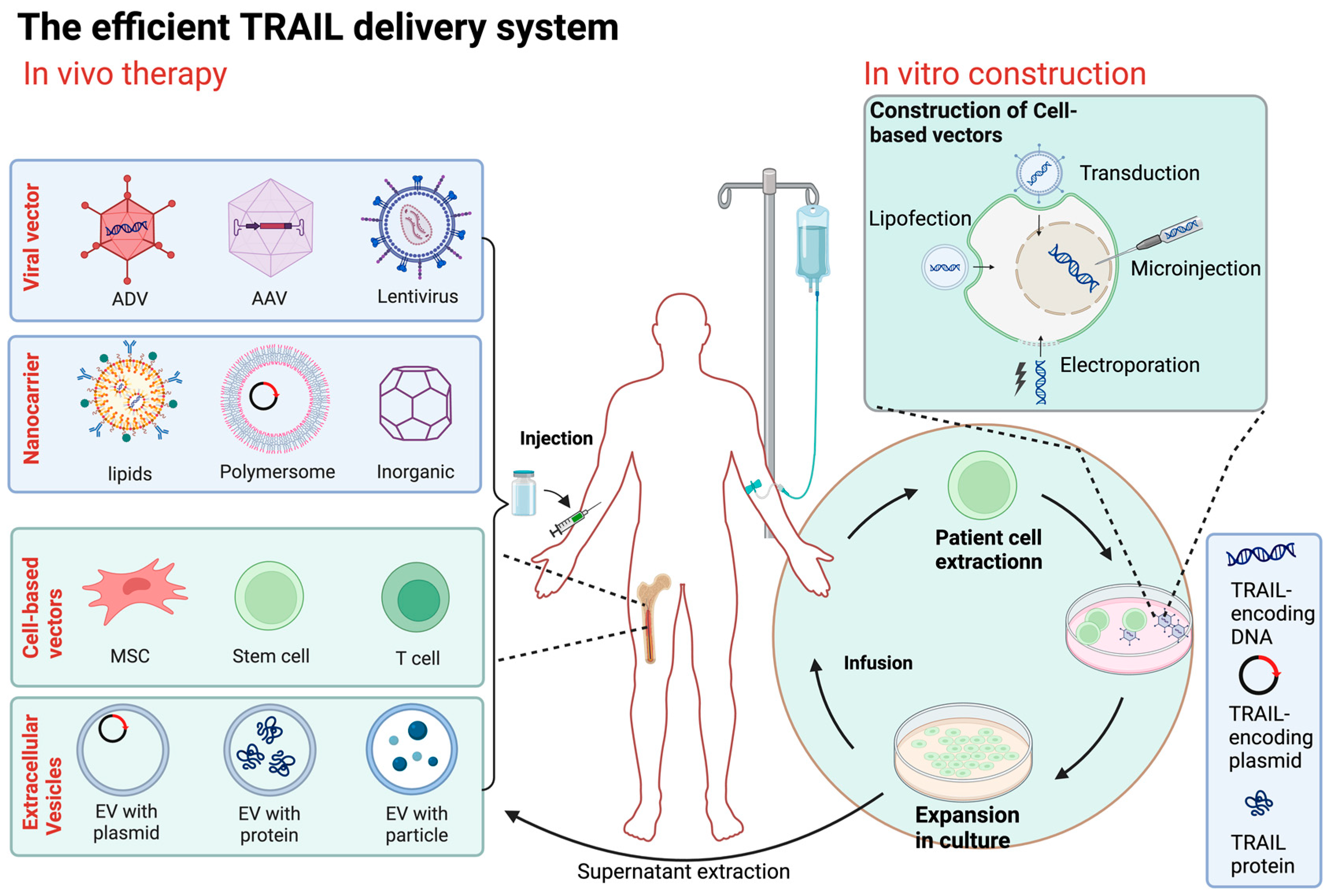
Figure 3.
Overview of TRAIL delivery systems. The method of direct delivery is to inject the constructed TRAIL transgene vector (including viral vector and nanocarrier) directly into the patient. Cell carrier gene therapy involves obtaining cells from allogeneic or autologous sources, genetically modifying them with a carrier carrying the TRAIL gene or other physical means, selecting and expanding them in culture, and then injecting the engineered cells into the patient. ADV, adenovirus; AAV, adeno-associated virus; MSC, mesenchymal stem cell; EV, extracellular vesicle. (Figure was created with Biorender.com, accessed on 1 June 2024).
In recent years, it has become clear that the TME plays a crucial role in the immunosuppression that shields tumor cells from immune cell attacks. To effectively control and treat cancer, it is essential to reverse this immunosuppressive environment. Several strategies aim to enhance TRAIL-induced apoptosis, focusing on targeting cells within the TME and improving its physical characteristics (Table 3).

Table 3.
Clinical trials targeting the TRAIL signal in the immune microenvironment.
5.1. TRAIL-Based Combination Therapy
5.1.1. Combination with Chemotherapy or Radiotherapy
TRAIL receptor agonists (TRAs) combined with chemotherapy or radiotherapy have demonstrated higher efficacy in both preclinical and clinical trials than when used alone. The combination can effectively target TRAIL-resistant tumor cells, including tumor stem cells. The combination of TRAIL and doxorubicin, a combination that increases the expression of TRAIL receptors DR4 and DR5, promotes apoptosis and anti-angiogenesis in soft tissue sarcoma cells [90]. Additionally, first-line chemotherapy drugs like cisplatin and gemcitabine can sensitize tumor cells when used alongside TRAIL [91,92]. Other chemotherapy agents, including irinotecan, camptothecin, 5-FU, and platinum-based agents, have also been studied in combination with TRAIL [93]. These agents can induce endoplasmic reticulum stress, upregulate DR4 and DR5, and enhance TRAIL-induced apoptosis.
Radiation therapy, which causes DNA damage and stabilizes p53 levels, synergistically amplifies the effects of TRAIL on tumor cells. The experimental results showed that the combination of TRAIL and radiotherapy had an excellent therapeutic effect on cervical cancer cell lines [94]. While TRAIL can induce apoptosis independently of p53, the status of p53 can affect the sensitivity of tumor cells to TRAIL [92]. P53 can function as a pro-apoptotic component, similar to Bax, and also acts as a transcription factor for DR4 and DR5 expression [58].
Various drugs and biologics, such as proteasome inhibitors, Bcl-2 inhibitors, IAP antagonists, histone and deacetylase inhibitors, as well as natural compounds like resveratrol, quercetin, and Strophanthidin (SPTD), have been used in combination to sensitize tumor cells to TRAIL [95,96,97]. Overcoming drug resistance at different levels of the TRAIL signaling pathway presents challenges, and combination therapy emerges as a promising approach to address TRAIL resistance. However, it is crucial to carefully consider the choice of drug dosage and mode of administration to ensure the best synergies and effective antitumor effects. This includes precise dosing to the tumor site, co-dosing, and spatial dosing to enable more effective combination therapy.
5.1.2. Combination with Immunotherapy
In recent years, the combination of TRAIL and immunotherapy has become more common as the immune role of TRAIL in the tumor microenvironment has been gradually revealed. One such strategy involves combining shTRAIL with the macrophage-targeting drug tribetidine, which can reshape the tumor immune microenvironment, bolster antitumor immunity, and significantly enhance the antitumor effects [47]. Additionally, combining mAb13F3 with T-cell-activated V-domain immunoglobulin suppressor (VISTA) and Toll-like receptor 3 (TLR3) specific adjuvant can upregulate TRAIL, increase the CD8+ T cell/Treg ratio in the TME, and induce macrophage activation [98]. Furthermore, MDSCs hinder CAR-T cell function and persistence within the breast TME. However, CAR-T cells equipped with chimeric co-stimulatory receptors targeting tumor-associated MUC1 (MUC1) can target DR5 expressed on MDSCs, enhancing antitumor potential and promoting TME remodeling and T cell proliferation at the tumor site [99]. Combining TRAIL with PD1 inhibitors can effectively block the PD1/PDL1 checkpoint signals, reactivating impotent tumor-specific CD8+ T cells and initiating an immune response through immunogenic cancer cell death [100]. In fact, combining TRAIL with other immune-related inhibitors has shown promise, especially when used alongside cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors and anti-programmed cell death protein 1 (PD-1) drug preparations (ICIs), which have reduced recurrence rates and improved survival rates to a certain extent.
5.1.3. Combination with Microenvironment-Modulating Agents
Abnormalities in tumor blood vessels can result in inadequate blood supply and lead to a TME characterized by low glucose concentrations, low extracellular pH, and low oxygen levels. Improving the physical aspects of the TME can enhance TRAIL-induced apoptosis. Glucose deprivation, for instance, induces metabolic stress and promotes TRAIL-induced cytotoxicity. The combination of TRAIL and hypoglycemia has been shown to induce dose-dependent apoptosis by activating caspase (−8, −9, and −3). Glucose deprivation can also amplify BID-Bax-associated mitochondria-dependent pathways and enhance TRAIL-induced apoptosis [101]. Tumor cells upregulate various proteins involved in pH regulation, leading to an acidic extracellular TME that suppresses immune function and hinders the antitumor immune response. The use of pH-regulating drugs like Cariporide, Lansoprazole, and acetazolamide can partially alleviate this immune cell suppression. Cariporide treatment has the added benefit of reducing immunotherapy-associated CRC cell shedding DR5, DcR1, and PD-L1 [102]. For pancreatic ductal adenocarcinoma (PDAC), which has a rich desmosomal proliferative matrix, TRAIL delivery is hindered and tumor penetration is reduced. Nanotherapy of TRAIL not only removes the extracellular matrix barrier to increase TRAIL delivery to tumors, but also blocks anti-apoptotic mechanisms to overcome TRAIL resistance in PDAC. Co-delivery of TRAIL and NO via stroma-targeted nanogels can remodel the fibrotic TME and inhibit tumor growth, offering promising therapeutic potential for PDAC [103].
5.2. The Improved Efficient TRAIL Delivery System
Recombinant TRAIL proteins and antibodies targeting TRAIL receptors often face challenges in terms of rapid degradation and clearance from the systemic circulation due to their short half-life. This leads to insufficient exposure of tumors to TRAIL within the TME [46]. While increasing the frequency of TRAIL administration might seem like a solution to maintaining higher concentrations at the tumor site, this approach carries the risk of amplifying resistance and potential toxicity. Furthermore, many primary cancers exhibit intrinsic TRAIL resistance or acquire resistance after TRAIL treatment [3]. One of the fundamental hurdles in advancing TRAIL-based oncology therapy is the critical need for safe and effective delivery methods. Fortunately, recent advancements in viral vector, nanocarrier, and cellular vector delivery systems hold promise in overcoming the barriers that have hindered successful TRAIL administration in clinical trials. TRAIL-based delivery therapy offers the potential to enhance stability, prolong the plasma half-life of TRAIL, enable specific targeting, improve the TME, and overcome TRAIL resistance. These innovative delivery methods have the potential to revolutionize TRAIL-based cancer treatment (Figure 3).
5.2.1. Viral Vector-Based TRAIL Therapy
For the high delivery efficiency, viral vectors, such as adenoviruses, adeno-associated viruses (AAVs), and lentiviruses, are prominently used for tumor-targeted gene delivery. TRAIL-based gene therapy is a highly promising approach for cancer treatment [4]. These vectors can integrate TRAIL expression genes into the genome of cancer cells, which enables selective TRAIL expression and efficiently induces cell apoptosis [104,105]. TRAIL-based gene therapy could also create a “bystander” effect, by which cell apoptosis could be induced in not only TRAIL-integrated cancer cells, but also the surrounding cells by the secretion of TRAIL proteins [106]. This characteristic enhances the therapeutic effect of TRAIL gene therapy.
Oncolytic adenoviruses (OAs) could selectively replicate within and kill cancer cells, which also enhances the antitumor effect of TRAIL [107]. Oncolytic viruses can also induce the host antitumor immune response to further mediate the destruction of tumor cells [108]. OA carrying dual therapeutic genes ST13 and TRAIL has proved to be safe and efficient treatment of pancreatic ductal adenocarcinoma [109]. In addition, the combination of luteolin and the OA carrying the TRAIL gene exerts synergistic antitumor effects in vitro and in vivo [110]. Recent studies have explored plant virus-based platforms, such as Potato virus X (PVX), for TRAIL delivery, showing enhanced efficacy in activating apoptosis [111]. These plant virus-based vectors can be combined with other treatments, such as doxorubicin, to further enhance the eradication of cancer cells [112]. Viral vectors can also carry immunomodulatory genes to stimulate an antitumor immune response. This approach has shown promise in the treatment of various cancers, such as malignant pleural mesothelioma and bladder cancer [113,114].
While the promise of TRAIL gene therapy using viral vectors is substantial, challenges and caveats exist. Viral vectors may have limitations in their ability to target specific cell types within tumors and can infect normal cells. Immune responses to viral vectors in the TME can limit their effectiveness, and strategies to mitigate immunogenicity are crucial for success [115]. Additionally, there is a risk of integration site insertion mutations, which can lead to unexpected genetic changes. It is crucial to identify the least genotoxic viral vector.
5.2.2. Nanodelivery Systems
Nanoparticle materials used as nanocarriers have proven highly effective in transporting DNA encoding TRAIL into tumor cells, facilitating the local secretion of TRAIL proteins in the cell membrane or TME [116,117,118].
There are three main forms of TRAIL–nanoparticle assembly. The first is that TRAIL attaches to the surface of the NP, binding to TRAIL death receptors on tumor cells. The second is to encapsulate soluble TRAIL or TRAIL-encoding plasmids with nanoparticles (NPs). The last is a combination of the first two. Studies have shown that the pattern of TRAIL surface presentation has a better effect on TRAIL receptor aggregation and subsequent apoptosis induction than that of soluble TRAIL. For example, in sarcoma therapy, TRAIL-modified lipid nanoparticles have demonstrated greater cytotoxicity than soluble recombinant TRAIL [119]. Liposomes, polymers, and inorganic materials have been used to formulate TRAIL–nanoparticles (NPs) [117]. Liposome nanocarriers, in particular, serve as a common and important TRAIL delivery vector, significantly improving the TME and enhancing TRAIL’s apoptotic function. Research has shown that TRAIL-loaded liposomes effectively target and kill prostate circulating tumor cells (CTCs) in primary patient blood samples [120] and may systemically neutralize distant metastasis of a broad range of tumor types. Targeted delivery of TRAIL and the chemotherapy drug paclitaxel through nanocarriers has demonstrated success in enhancing remission in cases of melanoma drug resistance [121]. Multifunctional nanocarriers capable of co-loading human TRAIL coding plasmid genes and doxorubicin have been developed, showing promise in cancer therapy [122].
Nanovesicles carrying plasmids encoding TRAIL and arginine-coupled tocopherol lipids (ATS) offer a potentially safe and effective therapeutic strategy for gene therapy in glioblastoma [123]. Nanocarriers also facilitate the delivery of receptor agonist antibodies, such as DR5 antibodies conjugated with solid lipid nanoparticles for the selective targeting of cancer cells in triple-negative breast cancer (TNBC) therapy [124].
In addition, polymer and inorganic materials have their own advantages in TRAIL delivery. Wrapping DNA in NPs is a particularly attractive way to both prevent endonuclease degradation and extend the circulating half-life of DNA. In the context of TRAIL gene therapy, several polymer-based approaches, including polyethylenimide (PEI) and cationic dendrimers, have been used for in vivo delivery. However, the toxicity of high molecular weight PEI and the non-degradability of dendritic macromolecules are the factors that must be considered in TRAIL therapy [117]. In recent years, the development of inorganic nanomaterials has optimized the treatment of TRAIL. Graphene nanocarriers, when combined with DR4 antibodies and AKT siRNA, synergistically promote death receptor-mediated apoptosis and improve the therapeutic effect of tumors in vivo [125]. High TRAIL loading anodized alumina nanotubes (AANT) have exhibited excellent biocompatibility in breast cancer cells and immune response cells, inducing significant cell death within a very short latency period [126].
The combination of nanocarriers and viruses shows promise in cancer treatment. This approach addresses the challenges associated with viral gene therapy, where embedding adenovirus in nanoparticles or using nanoparticle formulations can enhance virus-mediated gene delivery, mitigate immunogenicity, and improve the safety and efficacy of viral vectors. Nanoparticles are also being explored in combination therapies, such as combining chemotherapy drugs with nucleic acid nanocarriers, to provide comprehensive cancer treatment [127].
5.2.3. Cell-Based Vectors
Various cell vectors, including red blood cells (RBCs), white blood cells, macrophages, DC s, and stem cells, have been extensively employed as therapeutic drug carriers [128]. Engineered stem cells as TRAIL gene vectors show great therapeutic promise because of their role in improving the tumor microenvironment. Stem cell adipose-derived stem cells (ADSCs), modified with TRAIL, can migrate to hepatocellular carcinoma (HCC) cells, effectively inhibiting tumor growth and blocking the metastasis of transplanted HCC tumors [129].
However, it is mesenchymal stem cells (MSCs) that have been most extensively studied. Engineered MSCs have emerged as promising cancer therapy options due to their unique properties, such as low immunogenicity, high gene transduction capacity, and the ability to migrate to tumor sites [130]. Engineered MSCs, serving as gene carriers, can infiltrate tumor tissues and expand the potential scope of cancer treatment [131]. For pancreatic cancer treatment, TRAIL gene therapy based on bone marrow mesenchymal stem cells is a potential approach [132,133]. Additionally, photochemical internalization (PCI) has shown promising results in improving the transfection efficiency of TRAIL-secreting human mesenchymal stem cells (hMSCs) for pancreatic cancer treatment [133]. Mesenchymal stem cell-based cancer gene therapy can incorporate gene segments, such as Caspase9-mediated suicide genes, to enhance therapeutic potential [134]. In mouse lymphoma models, lentivirus-mediated expression of soluble TRAIL and IL-12 in transgenic MSCs effectively inhibits tumor growth and metastasis [135]. MSCs play various roles in the TME. TRAIL-expressing exosomes from MSCs exhibit potent antitumor activity, especially in mouse melanoma models. MSCs not only express TRAIL but also encapsulate a range of cytokines, chemokines, and growth factors in their secreted microvesicles and extracellular vesicles [136]. These biologically active entities are crucial for regulating cell growth, angiogenesis, and inflammation processes [137].
MSCs, in combination with various approaches, have demonstrated therapeutic efficacy, including combined radiotherapy, chemotherapy drugs, cytokines, histone deacetylase inhibitors (HDACi), and viral vectors [138,139]. For instance, in preclinical studies of pancreatic adenocarcinoma, nanocarriers carrying soluble TRAIL and paclitaxel delivered by mesenchymal stem cells have shown promising results [140]. The combined effect of umbilical cord-derived MSCs with IFN-γ and TNF-α enhances the treatment of acute myeloid leukemia [141]. The efficacy of MSC-based sTRAIL gene therapy combined with the histone deacetylase inhibitor panobinostat has been enhanced in the treatment of malignant glioma [142]. The identification of small molecule inhibitors that jointly target cancer stem cells (CSCs) and tumor-specific signaling pathways can enhance MSC-TRAIL-mediated tumor inhibition [143]. Using adenovirus-associated virus 2 (AAV2) as a vector, mesenchymal stem cells (MSCs) can be engineered to express CRC-specific sTRAIL [138]
5.2.4. Cell Membrane/Extracellular Vesicle-Based Vectors
TRAIL binds to the surface of immune cells and uses its innate ability to transport throughout the cycle, avoiding clearance and uptake by the immune system while performing specific functions, including delivering nutrients to tissues, removing pathogens, and immune system surveillance.
Immune cells presenting TRAIL and the adhesion receptor E-selectin on their surface are employed to target and eliminate circulating colon and prostate cancer cells [144]. Functionalized natural killer (NK) cells with TRAIL and anti-NK1.1 combined liposomes target tumor metastasis in tumor-draining lymph nodes [145].
During the development of stem cell-based TRAIL cell vectors, extracellular vesicle-encapsulated TRAIL was also found to play an unexpected role in tumor therapy. Membrane vesicles (CIMVs) isolated from genetically modified human adipose tissue-derived mesenchymal stem cells (hADSCs) overexpressing TRAIL, PTEN, and IFN-β1 genes have immunomodulatory and antitumor properties. They can activate human immune cells in vitro and induce apoptosis in various types of cancer cells [146]. Induction of cytorelaxin B in MSCs leads to increased expression of TRAIL, PTEN, and IFN-β1 in vesicles, effectively eradicating malignant tumor cells [146]. Extracellular vesicles derived from mesenchymal stem cells protect against DISC (death-inducing signaling complex) downregulation through a microRNA-217-dependent mechanism [137]. Continuous expression and release of soluble TRAIL by umbilical cord mesenchymal stem cells (MSC-sTRAIL) effectively inhibit the proliferation and induce apoptosis of B-ALL (B-cell acute lymphoblastic leukemia) cells [147].
Exosomes from γdelta-T cells (γdelta-t-exos) maintain their tumor-killing and T-cell-promoting activity in the immunosuppressed nasopharyngeal carcinoma (NPC) microenvironment via the DR5/TRAIL pathway. γdelta-t-exos, in collaboration with radiotherapy, overcome the radiation resistance of nasopharyngeal carcinoma csc, effectively controlling nasopharyngeal carcinoma [148].
6. Conclusions
In the realm of modern medicine, the fight against cancer is an ongoing, formidable challenge that continually calls for innovative approaches. Among the many players in the intricate landscape of cancer biology, TRAIL has captured the attention of researchers and clinicians. TRAIL’s multifaceted role in cancer, particularly its involvement in shaping the TME, has become a focus of intense investigation. This article has delved into the complexities of TRAIL in cancer, shedding light on its diverse functions. TRAIL, we have discovered, is a double-edged sword, capable of both thwarting and, paradoxically, advancing cancer. It influences processes ranging from apoptosis to immune response modulation, playing a pivotal role in tumor initiation, progression, and invasion. Looking forward, the potential of TRAIL as a game-changer in cancer treatment is becoming increasingly evident. Innovative therapeutic strategies are emerging as promising avenues. Combination therapies, immunotherapies, and gene delivery techniques are being explored to harness the capabilities of TRAIL for the benefit of cancer patients. Furthermore, an ever-deepening understanding of the TME and its impact on TRAIL’s efficacy is paving the way for more precise and effective treatments. Finally, clinical differences in patient sensitivity to TRAIL have been found, and molecular typing of cancers is an aspect of TRAIL application that still needs to be explored, which may provide an important foundation for the future development of personalized TRAIL pathway-targeted therapies.
Author Contributions
Conceptualization, C.L., S.H. and F.S.; investigation, C.L.; resources, F.S.; writing—original draft preparation, C.L. and S.H.; writing—review and editing, F.S., J.Z. and L.S.; supervision, L.S.; funding acquisition, F.S. and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (82073030 (F.S.), 81602402 (F.S.) and 81502109 (L.S.)), Hunan Provincial Natural Science Foundation of China (2022JJ20083 (F.S.)) and Program of Education and Teaching Reform in Central South University (2023ALK032 (L.S.) and 2024JY035 (L.S.)).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wajant, H. TRAIL- and TNF-induced signaling complexes-so similar yet so different. EMBO J. 2017, 36, 1117–1119. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, Y.; Wang, X.; Evers, B.M. Isolation and molecular characterization of the 5′-upstream region of the human TRAIL gene. Biochem. Biophys. Res. Commun. 2000, 276, 466–471. [Google Scholar] [CrossRef]
- Lemke, J.; von Karstedt, S.; Zinngrebe, J.; Walczak, H. Getting TRAIL back on track for cancer therapy. Cell Death Differ. 2014, 21, 1350–1364. [Google Scholar] [CrossRef]
- Zhong, H.-H.; Wang, H.-Y.; Li, J.; Huang, Y.-Z. TRAIL-based gene delivery and therapeutic strategies. Acta Pharmacol. Sin. 2019, 40, 1373–1385. [Google Scholar] [CrossRef]
- Cardoso Alves, L.; Corazza, N.; Micheau, O.; Krebs, P. The multifaceted role of TRAIL signaling in cancer and immunity. FEBS J. 2021, 288, 5530–5554. [Google Scholar] [CrossRef]
- Cartland, S.P.; Genner, S.W.; Martínez, G.J.; Robertson, S.; Kockx, M.; Lin, R.C.; O’Sullivan, J.F.; Koay, Y.C.; Manuneedhi Cholan, P.; Kebede, M.A.; et al. TRAIL-Expressing Monocyte/Macrophages Are Critical for Reducing Inflammation and Atherosclerosis. iScience 2019, 12, 41–52. [Google Scholar] [CrossRef]
- Wiley, S.R.; Schooley, K.; Smolak, P.J.; Din, W.S.; Huang, C.P.; Nicholl, J.K.; Sutherland, G.R.; Smith, T.D.; Rauch, C.; Smith, C.A. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995, 3, 673–682. [Google Scholar] [CrossRef]
- Gibellini, D.; Borderi, M.; De Crignis, E.; Cicola, R.; Vescini, F.; Caudarella, R.; Chiodo, F.; Re, M.C. RANKL/OPG/TRAIL plasma levels and bone mass loss evaluation in antiretroviral naive HIV-1-positive men. J. Med. Virol. 2007, 79, 1446–1454. [Google Scholar] [CrossRef]
- Nie, Z.; Aboulnasr, F.; Natesampillai, S.; Burke, S.P.; Krogman, A.; Bren, G.D.; Chung, T.D.Y.; Anderson, J.R.; Smart, M.K.; Katzmann, D.J.; et al. Correction: Both HIV-Infected and Uninfected Cells Express TRAILshort, Which Confers TRAIL Resistance upon Bystander Cells within the Microenvironment. J. Immunol. 2018, 201, 1599. [Google Scholar] [CrossRef]
- Aboulnasr, F.; Krogman, A.; Graham, R.P.; Cummins, N.W.; Misra, A.; Garcia-Rivera, E.; Anderson, J.R.; Natesampillai, S.; Kogan, N.; Aravamudan, M.; et al. Human Cancers Express TRAILshort, a Dominant Negative TRAIL Splice Variant, Which Impairs Immune Effector Cell Killing of Tumor Cells. Clin. Cancer Res. 2020, 26, 5759–5771. [Google Scholar] [CrossRef]
- de Miguel, D.; Pardo, J. TRAIL and Cancer Immunotherapy: Take a Walk on the Short Side. Clin. Cancer Res. 2020, 26, 5546–5548. [Google Scholar] [CrossRef]
- Wajant, H.; Moosmayer, D.; Wüest, T.; Bartke, T.; Gerlach, E.; Schönherr, U.; Peters, N.; Scheurich, P.; Pfizenmaier, K. Differential activation of TRAIL-R1 and -2 by soluble and membrane TRAIL allows selective surface antigen-directed activation of TRAIL-R2 by a soluble TRAIL derivative. Oncogene 2001, 20, 4101–4106. [Google Scholar] [CrossRef]
- Jong, K.X.J.; Mohamed, E.H.M.; Ibrahim, Z.A. Escaping cell death via TRAIL decoy receptors: A systematic review of their roles and expressions in colorectal cancer. Apoptosis 2022, 27, 787–799. [Google Scholar] [CrossRef]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The role of osteoprotegerin in the crosstalk between vessels and bone: Its potential utility as a marker of cardiometabolic diseases. Pharmacol. Ther. 2018, 182, 115–132. [Google Scholar] [CrossRef]
- Habibie, H.; Adhyatmika, A.; Schaafsma, D.; Melgert, B.N. The role of osteoprotegerin (OPG) in fibrosis: Its potential as a biomarker and/or biological target for the treatment of fibrotic diseases. Pharmacol. Ther. 2021, 228, 107941. [Google Scholar] [CrossRef]
- Haselmann, V.; Kurz, A.; Bertsch, U.; Hübner, S.; Olempska-Müller, M.; Fritsch, J.; Häsler, R.; Pickl, A.; Fritsche, H.; Annewanter, F.; et al. Nuclear death receptor TRAIL-R2 inhibits maturation of let-7 and promotes proliferation of pancreatic and other tumor cells. Gastroenterology 2014, 146, 278–290. [Google Scholar] [CrossRef]
- Beyer, K.; Baukloh, A.-K.; Stoyanova, A.; Kamphues, C.; Sattler, A.; Kotsch, K. Interactions of Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand (TRAIL) with the Immune System: Implications for Inflammation and Cancer. Cancers 2019, 11, 1161. [Google Scholar] [CrossRef]
- Micheau, O. Regulation of TNF-Related Apoptosis-Inducing Ligand Signaling by Glycosylation. Int. J. Mol. Sci. 2018, 19, 715. [Google Scholar] [CrossRef]
- Dufour, F.; Rattier, T.; Shirley, S.; Picarda, G.; Constantinescu, A.A.; Morlé, A.; Zakaria, A.B.; Marcion, G.; Causse, S.; Szegezdi, E.; et al. N-glycosylation of mouse TRAIL-R and human TRAIL-R1 enhances TRAIL-induced death. Cell Death Differ. 2017, 24, 500–510. [Google Scholar] [CrossRef]
- Yoshida, T.; Shiraishi, T.; Horinaka, M.; Wakada, M.; Sakai, T. Glycosylation modulates TRAIL-R1/death receptor 4 protein: Different regulations of two pro-apoptotic receptors for TRAIL by tunicamycin. Oncol. Rep. 2007, 18, 1239–1242. [Google Scholar] [CrossRef]
- Ralff, M.D.; El-Deiry, W.S. TRAIL pathway targeting therapeutics. Expert Rev. Precis. Med. Drug Dev. 2018, 3, 197–204. [Google Scholar] [CrossRef]
- Green, D.R. Caspases and Their Substrates. Cold Spring Harb. Perspect. Biol. 2022, 14, a041012. [Google Scholar] [CrossRef]
- Yuan, S.; Akey, C.W. Apoptosome structure, assembly, and procaspase activation. Structure 2013, 21, 501–515. [Google Scholar] [CrossRef]
- Trivedi, R.; Mishra, D.P. Trailing TRAIL Resistance: Novel Targets for TRAIL Sensitization in Cancer Cells. Front. Oncol. 2015, 5, 69. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Chang, J.C. Cancer stem cells: Role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine 2016, 95, S20–S25. [Google Scholar] [CrossRef]
- Aponte, P.M.; Caicedo, A. Stemness in Cancer: Stem Cells, Cancer Stem Cells, and Their Microenvironment. Stem Cells Int. 2017, 2017, 5619472. [Google Scholar] [CrossRef]
- Osum, M.; Kalkan, R. Cancer Stem Cells and Their Therapeutic Usage. In Cell Biology and Translational Medicine, Volume 20: Organ Function, Maintenance, Repair in Health and Disease; Advances in Experimental Medicine and Biology; Springer Nature: Berlin, Germany, 2023. [Google Scholar]
- Chen, K.; Huang, Y.; Chen, J. Understanding and targeting cancer stem cells: Therapeutic implications and challenges. Acta Pharmacol. Sin. 2013, 34, 732–740. [Google Scholar] [CrossRef]
- Quiroz-Reyes, A.G.; Delgado-Gonzalez, P.; Islas, J.F.; Gallegos, J.L.D.; Martínez Garza, J.H.; Garza-Treviño, E.N. Behind the Adaptive and Resistance Mechanisms of Cancer Stem Cells to TRAIL. Pharmaceutics 2021, 13, 1062. [Google Scholar] [CrossRef]
- Piggott, L.; Omidvar, N.; Martí Pérez, S.; French, R.; Eberl, M.; Clarkson, R.W.E. Suppression of apoptosis inhibitor c-FLIP selectively eliminates breast cancer stem cell activity in response to the anti-cancer agent, TRAIL. Breast Cancer Res. 2011, 13, R88. [Google Scholar] [CrossRef]
- Akbari, M.; Shomali, N.; Faraji, A.; Shanehbandi, D.; Asadi, M.; Mokhtarzadeh, A.; Shabani, A.; Baradaran, B. CD133: An emerging prognostic factor and therapeutic target in colorectal cancer. Cell Biol. Int. 2020, 44, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.; Ali, S.; Ahmad, A.; Philip, P.A.; Sarkar, F.H. The Role of Cancer Stem Cells in Recurrent and Drug-Resistant Lung Cancer. Adv. Exp. Med. Biol. 2016, 890, 57–74. [Google Scholar] [PubMed]
- Singh, A.K.; Verma, A.; Singh, A.; Arya, R.K.; Maheshwari, S.; Chaturvedi, P.; Nengroo, M.A.; Saini, K.K.; Vishwakarma, A.L.; Singh, K.; et al. Salinomycin inhibits epigenetic modulator EZH2 to enhance death receptors in colon cancer stem cells. Epigenetics 2021, 16, 144–161. [Google Scholar] [CrossRef] [PubMed]
- Barzegar Behrooz, A.; Syahir, A.; Ahmad, S. CD133: Beyond a cancer stem cell biomarker. J. Drug Target. 2019, 27, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Jiang, J.; Shi, S.; Xie, H.; Zhou, L.; Zheng, S. Knockdown of miR-25 increases the sensitivity of liver cancer stem cells to TRAIL-induced apoptosis via PTEN/PI3K/Akt/Bad signaling pathway. Int. J. Oncol. 2016, 49, 2600–2610. [Google Scholar] [CrossRef] [PubMed]
- Recio-Boiles, A.; Ilmer, M.; Rhea, P.R.; Kettlun, C.; Heinemann, M.L.; Ruetering, J.; Vykoukal, J.; Alt, E. JNK pathway inhibition selectively primes pancreatic cancer stem cells to TRAIL-induced apoptosis without affecting the physiology of normal tissue resident stem cells. Oncotarget 2016, 7, 9890–9906. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Xu, L.; Bandyopadhyay, S.; Sethi, S.; Reddy, K.B. Cisplatin and TRAIL enhance breast cancer stem cell death. Int. J. Oncol. 2011, 39, 891–898. [Google Scholar]
- Kurita, S.; Mott, J.L.; Almada, L.L.; Bronk, S.F.; Werneburg, N.W.; Sun, S.-Y.; Roberts, L.R.; Fernandez-Zapico, M.E.; Gores, G.J. GLI3-dependent repression of DR4 mediates hedgehog antagonism of TRAIL-induced apoptosis. Oncogene 2010, 29, 4848–4858. [Google Scholar] [CrossRef]
- Cardoso Alves, L.; Berger, M.D.; Koutsandreas, T.; Kirschke, N.; Lauer, C.; Spörri, R.; Chatziioannou, A.; Corazza, N.; Krebs, P. Non-apoptotic TRAIL function modulates NK cell activity during viral infection. EMBO Rep. 2020, 21, e48789. [Google Scholar] [CrossRef]
- Gao, J.; Wang, D.; Liu, D.; Liu, M.; Ge, Y.; Jiang, M.; Liu, Y.; Zheng, D. Tumor necrosis factor-related apoptosis-inducing ligand induces the expression of proinflammatory cytokines in macrophages and re-educates tumor-associated macrophages to an antitumor phenotype. Mol. Biol. Cell 2015, 26, 3178–3189. [Google Scholar] [CrossRef]
- Gunalp, S.; Helvaci, D.G.; Oner, A.; Bursalı, A.; Conforte, A.; Güner, H.; Karakülah, G.; Szegezdi, E.; Sag, D. TRAIL promotes the polarization of human macrophages toward a proinflammatory M1 phenotype and is associated with increased survival in cancer patients with high tumor macrophage content. Front. Immunol. 2023, 14, 1209249. [Google Scholar] [CrossRef] [PubMed]
- Condamine, T.; Kumar, V.; Ramachandran, I.R.; Youn, J.-I.; Celis, E.; Finnberg, N.; El-Deiry, W.S.; Winograd, R.; Vonderheide, R.H.; English, N.R.; et al. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. J. Clin. Investg. 2014, 124, 2626–2639. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.-M.; Qin, J.; Li, Y.-C.; Wang, Y.; Li, D.; Shu, Y.; Luo, C.; Wang, S.-S.; Chi, G.; Guo, F.; et al. IL-35 induces N2 phenotype of neutrophils to promote tumor growth. Oncotarget 2017, 8, 33501–33514. [Google Scholar] [CrossRef] [PubMed]
- Pillai, M.R.; Collison, L.W.; Wang, X.; Finkelstein, D.; Rehg, J.E.; Boyd, K.; Szymczak-Workman, A.L.; Doggett, T.; Griffith, T.S.; Ferguson, T.A.; et al. The plasticity of regulatory T cell function. J. Immunol. 2011, 187, 4987–4997. [Google Scholar] [CrossRef]
- de Miguel, D.; Lemke, J.; Anel, A.; Walczak, H.; Martinez-Lostao, L. Onto better TRAILs for cancer treatment. Cell Death Differ. 2016, 23, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Liu, W.; Liu, X.; Jia, X.; Feng, X.; Li, F.; Zhu, R.; Yu, J.; Zhang, H.; et al. Dose-related immunomodulatory effects of recombinant TRAIL in the tumor immune microenvironment. J. Exp. Clin. Cancer Res. 2023, 42, 216. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, T.; Montinaro, A.; von Karstedt, S.; Sevko, A.; Surinova, S.; Chakravarthy, A.; Taraborrelli, L.; Draber, P.; Lafont, E.; Arce Vargas, F.; et al. The TRAIL-Induced Cancer Secretome Promotes a Tumor-Supportive Immune Microenvironment via CCR2. Mol. Cell 2017, 65, 730–742.e5. [Google Scholar] [CrossRef] [PubMed]
- Loeuillard, E.; Li, B.; Stumpf, H.E.; Yang, J.; Willhite, J.; Tomlinson, J.L.; Wang, J.; Rohakhtar, F.R.; Simon, V.A.; Graham, R.P.; et al. Noncanonical TRAIL Signaling Promotes Myeloid-Derived Suppressor Cell Abundance and Tumor Growth in Cholangiocarcinoma. Cell Mol Gastroenterol Hepatol 2024, 17, 853–876. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, D.; D’Arcy, P.; Wennerberg, E.; Lidén, M.; Hu, J.; Winqvist, O.; Rolny, C.; Lundqvist, A. Activated monocytes augment TRAIL-mediated cytotoxicity by human NK cells through release of IFN-γ. Eur. J. Immunol. 2013, 43, 249–257. [Google Scholar] [CrossRef]
- Achard, C.; Guillerme, J.-B.; Bruni, D.; Boisgerault, N.; Combredet, C.; Tangy, F.; Jouvenet, N.; Grégoire, M.; Fonteneau, J.-F. Oncolytic measles virus induces tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated cytotoxicity by human myeloid and plasmacytoid dendritic cells. Oncoimmunology 2017, 6, e1261240. [Google Scholar] [CrossRef]
- Tu, Z.; Hamalainen-Laanaya, H.K.; Crispe, I.N.; Orloff, M.S. Synergy between TLR3 and IL-18 promotes IFN-γ dependent TRAIL expression in human liver NK cells. Cell. Immunol. 2011, 271, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Wang, Y.; Huang, C.-Y.; Zhou, Z.-Q.; Zhao, J.-J.; Zhang, X.-F.; Pan, Q.-Z.; Wu, J.-X.; Weng, D.-S.; Tang, Y.; et al. IL-17 induces antitumor immunity by promoting beneficial neutrophil recruitment and activation in esophageal squamous cell carcinoma. Oncoimmunology 2017, 7, e1373234. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.-W.; Hung, E.; Wu, J.-M.; Liang, Y.-H.; Chiu, Y.-H.; Tyan, Y.-S.; Wang, H.-S. Anti-tumor Effects of IL-1β Induced TRAIL-Expressing hUCMSCs on Embelin Treated Breast Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2023, 24, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- de Looff, M.; de Jong, S.; Kruyt, F.A.E. Multiple Interactions Between Cancer Cells and the Tumor Microenvironment Modulate TRAIL Signaling: Implications for TRAIL Receptor Targeted Therapy. Front. Immunol. 2019, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Sano, E.; Kazaana, A.; Tadakuma, H.; Takei, T.; Yoshimura, S.; Hanashima, Y.; Ozawa, Y.; Yoshino, A.; Suzuki, Y.; Ueda, T. Interleukin-6 sensitizes TNF-α and TRAIL/Apo2L dependent cell death through upregulation of death receptors in human cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Z.; Huang, W. Interleukin-4 Enhances the Sensitivity of Human Monocytes to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Through Upregulation of Death Receptor 4. J. Interferon Cytokine Res. 2018, 38, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Willms, A.; Schittek, H.; Rahn, S.; Sosna, J.; Mert, U.; Adam, D.; Trauzold, A. Impact of p53 status on TRAIL-mediated apoptotic and non-apoptotic signaling in cancer cells. PLoS ONE 2019, 14, e0214847. [Google Scholar] [CrossRef]
- Favaro, F.; Luciano-Mateo, F.; Moreno-Caceres, J.; Hernández-Madrigal, M.; Both, D.; Montironi, C.; Püschel, F.; Nadal, E.; Eldering, E.; Muñoz-Pinedo, C. TRAIL receptors promote constitutive and inducible IL-8 secretion in non-small cell lung carcinoma. Cell Death Dis. 2022, 13, 1046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qin, G.; Zhang, C.; Yang, H.; Liu, J.; Hu, H.; Wu, P.; Liu, S.; Yang, L.; Chen, X.; et al. TRAIL promotes epithelial-to-mesenchymal transition by inducing PD-L1 expression in esophageal squamous cell carcinomas. J. Exp. Clin. Cancer Res. 2021, 40, 209. [Google Scholar] [CrossRef]
- Patil, M.S.; Cartland, S.P.; Kavurma, M.M. TRAIL signals, extracellular matrix and vessel remodelling. Vasc. Biol. 2020, 2, R73–R84. [Google Scholar] [CrossRef]
- Wilson, N.S.; Yang, A.; Yang, B.; Couto, S.; Stern, H.; Gogineni, A.; Pitti, R.; Marsters, S.; Weimer, R.M.; Singh, M.; et al. Proapoptotic activation of death receptor 5 on tumor endothelial cells disrupts the vasculature and reduces tumor growth. Cancer Cell 2012, 22, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Secchiero, P.; Gonelli, A.; Carnevale, E.; Corallini, F.; Rizzardi, C.; Zacchigna, S.; Melato, M.; Zauli, G. Evidence for a proangiogenic activity of TNF-related apoptosis-inducing ligand. Neoplasia 2004, 6, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Riera-Domingo, C.; Leite-Gomes, E.; Charatsidou, I.; Zhao, P.; Carrá, G.; Cappellesso, F.; Mourao, L.; De Schepper, M.; Liu, D.; Serneels, J.; et al. Breast tumors interfere with endothelial TRAIL at the premetastatic niche to promote cancer cell seeding. Sci. Adv. 2023, 9, eadd5028. [Google Scholar] [CrossRef] [PubMed]
- Cartland, S.P.; Genner, S.W.; Zahoor, A.; Kavurma, M.M. Comparative Evaluation of TRAIL, FGF-2 and VEGF-A-Induced Angiogenesis In Vitro and In Vivo. Int. J. Mol. Sci. 2016, 17, 2025. [Google Scholar] [CrossRef]
- Di Bartolo, B.A.; Cartland, S.P.; Prado-Lourenco, L.; Griffith, T.S.; Gentile, C.; Ravindran, J.; Azahri, N.S.M.; Thai, T.; Yeung, A.W.S.; Thomas, S.R.; et al. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) Promotes Angiogenesis and Ischemia-Induced Neovascularization Via NADPH Oxidase 4 (NOX4) and Nitric Oxide-Dependent Mechanisms. J. Am. Heart Assoc. 2015, 4, e002527. [Google Scholar] [CrossRef] [PubMed]
- Schito, L. Hypoxia-Dependent Angiogenesis and Lymphangiogenesis in Cancer. Adv. Exp. Med. Biol. 2019, 1136, 71–85. [Google Scholar] [PubMed]
- Forde, H.; Harper, E.; Rochfort, K.D.; Wallace, R.G.; Davenport, C.; Smith, D.; Cummins, P.M. TRAIL inhibits oxidative stress in human aortic endothelial cells exposed to pro-inflammatory stimuli. Physiol. Rep. 2020, 8, e14612. [Google Scholar] [CrossRef] [PubMed]
- Walczak, H.; Miller, R.E.; Ariail, K.; Gliniak, B.; Griffith, T.S.; Kubin, M.; Chin, W.; Jones, J.; Woodward, A.; Le, T.; et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat. Med. 1999, 5, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Pai, R.C.; Fong, S.; Leung, S.; Lawrence, D.A.; Marsters, S.A.; Blackie, C.; Chang, L.; McMurtrey, A.E.; Hebert, A.; et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Investg. 1999, 104, 155–162. [Google Scholar] [CrossRef]
- Ion, G.N.D.; Nitulescu, G.M.; Popescu, C.I. Targeting TRAIL. Bioorg. Med. Chem. Lett. 2019, 29, 2527–2534. [Google Scholar] [CrossRef]
- Jo, M.; Kim, T.H.; Seol, D.W.; Esplen, J.E.; Dorko, K.; Billiar, T.R.; Strom, S.C. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat. Med. 2000, 6, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Ganten, T.M.; Koschny, R.; Sykora, J.; Schulze-Bergkamen, H.; Büchler, P.; Haas, T.L.; Schader, M.B.; Untergasser, A.; Stremmel, W.; Walczak, H. Preclinical differentiation between apparently safe and potentially hepatotoxic applications of TRAIL either alone or in combination with chemotherapeutic drugs. Clin. Cancer Res. 2006, 12, 2640–2646. [Google Scholar] [CrossRef]
- Snajdauf, M.; Havlova, K.; Vachtenheim, J.; Ozaniak, A.; Lischke, R.; Bartunkova, J.; Smrz, D.; Strizova, Z. The TRAIL in the Treatment of Human Cancer: An Update on Clinical Trials. Front. Mol. Biosci. 2021, 8, 628332. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, T.; Liu, J.; Cui, Y.; Yang, S.; Zhao, R. Synergistic antitumor effects of circularly permuted TRAIL with doxorubicin in triple-negative breast cancer. Acta Biochim. Biophys. Sin. 2023, 55, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Naval, J.; de Miguel, D.; Gallego-Lleyda, A.; Anel, A.; Martinez-Lostao, L. Importance of TRAIL Molecular Anatomy in Receptor Oligomerization and Signaling. Implications for Cancer Therapy. Cancers 2019, 11, 444. [Google Scholar] [CrossRef]
- Wajant, H. Molecular Mode of Action of TRAIL Receptor Agonists—Common Principles and Their Translational Exploitation. Cancers 2019, 11, 954. [Google Scholar] [CrossRef]
- Medler, J.; Nelke, J.; Weisenberger, D.; Steinfatt, T.; Rothaug, M.; Berr, S.; Hünig, T.; Beilhack, A.; Wajant, H. TNFRSF receptor-specific antibody fusion proteins with targeting controlled FcγR-independent agonistic activity. Cell Death Dis. 2019, 10, 224. [Google Scholar] [CrossRef]
- Chuntharapai, A.; Dodge, K.; Grimmer, K.; Schroeder, K.; Marsters, S.A.; Koeppen, H.; Ashkenazi, A.; Kim, K.J. Isotype-dependent inhibition of tumor growth in vivo by monoclonal antibodies to death receptor 4. J. Immunol. 2001, 166, 4891–4898. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, A.; Micheau, O. Antibodies and Derivatives Targeting DR4 and DR5 for Cancer Therapy. Antibodies 2017, 6, 16. [Google Scholar] [CrossRef]
- Lemke, J.; Noack, A.; Adam, D.; Tchikov, V.; Bertsch, U.; Röder, C.; Schütze, S.; Wajant, H.; Kalthoff, H.; Trauzold, A. TRAIL signaling is mediated by DR4 in pancreatic tumor cells despite the expression of functional DR5. J. Mol. Med. 2010, 88, 729–740. [Google Scholar] [CrossRef]
- Dufour, F.; Rattier, T.; Constantinescu, A.A.; Zischler, L.; Morlé, A.; Ben Mabrouk, H.; Humblin, E.; Jacquemin, G.; Szegezdi, E.; Delacote, F.; et al. TRAIL receptor gene editing unveils TRAIL-R1 as a master player of apoptosis induced by TRAIL and ER stress. Oncotarget 2017, 8, 9974–9985. [Google Scholar] [CrossRef] [PubMed]
- Greer, Y.E.; Gilbert, S.F.; Gril, B.; Narwal, R.; Peacock Brooks, D.L.; Tice, D.A.; Steeg, P.S.; Lipkowitz, S. MEDI3039, a novel highly potent tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) receptor 2 agonist, causes regression of orthotopic tumors and inhibits outgrowth of metastatic triple-negative breast cancer. Breast Cancer Res. 2019, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xia, X.; Xia, X.-X.; Sun, Z.; Yan, D. Improving Targeted Delivery and Antitumor Efficacy with Engineered Tumor Necrosis Factor-Related Apoptosis Ligand-Affibody Fusion Protein. Mol. Pharm. 2021, 18, 3854–3861. [Google Scholar] [CrossRef] [PubMed]
- Prigozhina, T.B.; Szafer, F.; Aronin, A.; Tzdaka, K.; Amsili, S.; Makdasi, E.; Shani, N.; Dranitzki Elhalel, M. Fn14·TRAIL fusion protein is oligomerized by TWEAK into a superefficient TRAIL analog. Cancer Lett. 2017, 400, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Brin, E.; Wu, K.; Dagostino, E.; Kuo, M.M.-C.; He, Y.; Shia, W.-J.; Chen, L.-C.; Stempniak, M.; Hickey, R.; Almassy, R.; et al. TRAIL stabilization and cancer cell sensitization to its pro-apoptotic activity achieved through genetic fusion with arginine deiminase. Oncotarget 2018, 9, 36914–36928. [Google Scholar] [CrossRef] [PubMed]
- Tur, V.; van der Sloot, A.M.; Reis, C.R.; Szegezdi, E.; Cool, R.H.; Samali, A.; Serrano, L.; Quax, W.J. DR4-selective tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) variants obtained by structure-based design. J. Biol. Chem. 2008, 283, 20560–20568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, W.; Ren, Y.; Wei, D. SAC-TRAIL, a novel anticancer fusion protein: Expression, purification, and functional characterization. Appl. Microbiol. Biotechnol. 2022, 106, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Han, Z.; Stenzel, M.H.; Chapman, R. A High Throughput Approach for Designing Polymers That Mimic the TRAIL Protein. Nano Lett. 2022, 22, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, W.; Liu, J.; Lahat, G.; Torres, K.; Lopez, G.; Lazar, A.J.; Hayes-Jordan, A.; Liu, K.; Bankson, J.; et al. TRAIL and doxorubicin combination induces proapoptotic and antiangiogenic effects in soft tissue sarcoma in vivo. Clin. Cancer Res. 2010, 16, 2591–2604. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Guo, Y.; Han, L.; Duan, Y.; Fang, F.; Niu, S.; Ba, Q.; Zhu, H.; Kong, F.; Lin, C.; et al. In vitro and in vivo growth inhibition of drug-resistant ovarian carcinoma cells using a combination of cisplatin and a TRAIL-encoding retrovirus. Oncol. Lett. 2012, 4, 1254–1258. [Google Scholar] [CrossRef]
- Kretz, A.-L.; Trauzold, A.; Hillenbrand, A.; Knippschild, U.; Henne-Bruns, D.; von Karstedt, S.; Lemke, J. TRAILblazing Strategies for Cancer Treatment. Cancers 2019, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.-O.; Asami, Y.; Long, H.; Jang, J.H.; Bae, E.Y.; Kim, B.Y.; Choi, Y.H.; Kang, C.-H.; Ahn, J.S.; Kim, G.-Y. Verrucarin A sensitizes TRAIL-induced apoptosis via the upregulation of DR5 in an eIF2α/CHOP-dependent manner. Toxicol. Vitr. 2013, 27, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Huang, G.; Pan, S.; Chen, X.; Liu, T.; Yang, Z.; Chen, T.; Zhu, X. TRAIL-driven targeting and reversing cervical cancer radioresistance by seleno-nanotherapeutics through regulating cell metabolism. Drug Resist. Updates 2024, 72, 101033. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Li, F.; Shi, C.-W.; Du, J.-L.; Xue, Y.-J.; Liu, X.-Y.; Cao, X.; Wei, N. Mechanism and therapeutic prospect of resveratrol combined with TRAIL in the treatment of renal cell carcinoma. Cancer Gene Ther. 2020, 27, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Shabestari, F.A.; Vaezi, S.; Abak, A.; Shoorei, H.; Karimi, A.; Taheri, M.; Basiri, A. Emerging impact of quercetin in the treatment of prostate cancer. Biomed. Pharmacother. 2021, 138, 111548. [Google Scholar] [CrossRef]
- Tian, X.; Gu, L.; Zeng, F.; Liu, X.; Zhou, Y.; Dou, Y.; Han, J.; Zhao, Y.; Zhang, Y.; Luo, Q.; et al. Strophanthidin Induces Apoptosis of Human Lung Adenocarcinoma Cells by Promoting TRAIL-DR5 Signaling. Molecules 2024, 29, 877. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ou, Z.; Zhong, W.; Huang, L.; Liao, W.; Sheng, Y.; Guo, Z.; Chen, J.; Yang, W.; Chen, K.; et al. Effective Antitumor Immunity Can be Triggered by Targeting VISTA in Combination with a TLR3-specific Adjuvant. Cancer Immunol. Res. 2023, 11, 1656–1670. [Google Scholar] [CrossRef]
- Nalawade, S.A.; Shafer, P.; Bajgain, P.; McKenna, M.K.; Ali, A.; Kelly, L.; Joubert, J.; Gottschalk, S.; Watanabe, N.; Leen, A.; et al. Selectively targeting myeloid-derived suppressor cells through TRAIL receptor 2 to enhance the efficacy of CAR T cell therapy for treatment of breast cancer. J. Immunother. Cancer 2021, 9, e003237. [Google Scholar] [CrossRef]
- Yu, P.; Zheng, D.; Zhang, C.; Wu, M.; Liu, X. Protocol to prepare functional cellular nanovesicles with PD1 and TRAIL to boost antitumor response. STAR Protoc. 2021, 2, 100324. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, K.; Kim, J.H.; Park, Y.S.; Luo, X.; Zhang, L.; Ku, J.-L.; Choudry, M.H.A.; Lee, Y.J. Glucose deprivation-induced endoplasmic reticulum stress response plays a pivotal role in enhancement of TRAIL cytotoxicity. J. Cell. Physiol. 2021, 236, 6666–6677. [Google Scholar] [CrossRef]
- Huntington, K.E.; Louie, A.; Zhou, L.; Seyhan, A.A.; Maxwell, A.W.; El-Deiry, W.S. Colorectal cancer extracellular acidosis decreases immune cell killing and is partially ameliorated by pH-modulating agents that modify tumor cell cytokine profiles. Am. J. Cancer Res. 2022, 12, 138–151. [Google Scholar]
- Huang, H.-C.; Sung, Y.-C.; Li, C.-P.; Wan, D.; Chao, P.-H.; Tseng, Y.-T.; Liao, B.-W.; Cheng, H.-T.; Hsu, F.-F.; Huang, C.-C.; et al. Reversal of pancreatic desmoplasia by a tumour stroma-targeted nitric oxide nanogel overcomes TRAIL resistance in pancreatic tumours. Gut 2022, 71, 1843–1855. [Google Scholar] [CrossRef]
- Wu, X.; Wang, S.; Li, M.; Wang, A.; Zhou, Y.; Li, P.; Wang, Y. Nanocarriers for TRAIL delivery: Driving TRAIL back on track for cancer therapy. Nanoscale 2017, 9, 13879–13904. [Google Scholar] [CrossRef] [PubMed]
- Goklany, S.; Lu, P.; Godeshala, S.; Hall, A.; Garrett-Mayer, E.; Voelkel-Johnson, C.; Rege, K. Delivery of TRAIL-expressing plasmid DNA to cancer cells in vitro and in vivo using aminoglycoside-derived polymers. J. Mater. Chem. B 2019, 7, 7014–7025. [Google Scholar] [CrossRef]
- Kagawa, S.; He, C.; Gu, J.; Koch, P.; Rha, S.J.; Roth, J.A.; Curley, S.A.; Stephens, L.C.; Fang, B. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001, 61, 3330–3338. [Google Scholar]
- Ylösmäki, E.; Cerullo, V. Design and application of oncolytic viruses for cancer immunotherapy. Curr. Opin. Biotechnol. 2020, 65, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Xu, C.; Dong, J.; Wei, J. An oncolytic vaccinia virus encoding hyaluronidase reshapes the extracellular matrix to enhance cancer chemotherapy and immunotherapy. J. Immunother. Cancer 2024, 12, e008431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, M.; Huang, F.; Wang, S.; Wang, H.; Mou, X.; Wang, Y. Oncolytic Adenovirus Expressing ST13 Increases Antitumor Effect of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Against Pancreatic Ductal Adenocarcinoma. Hum. Gene Ther. 2020, 31, 891–903. [Google Scholar] [CrossRef]
- Xiao, B.; Qin, Y.; Ying, C.; Ma, B.; Wang, B.; Long, F.; Wang, R.; Fang, L.; Wang, Y. Combination of oncolytic adenovirus and luteolin exerts synergistic antitumor effects in colorectal cancer cells and a mouse model. Mol. Med. Rep. 2017, 16, 9375–9382. [Google Scholar] [CrossRef]
- Le, D.H.T.; Commandeur, U.; Steinmetz, N.F. Presentation and Delivery of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand via Elongated Plant Viral Nanoparticle Enhances Antitumor Efficacy. ACS Nano 2019, 13, 2501–2510. [Google Scholar] [CrossRef]
- Lee, K.L.; Murray, A.A.; Le, D.H.T.; Sheen, M.R.; Shukla, S.; Commandeur, U.; Fiering, S.; Steinmetz, N.F. Combination of Plant Virus Nanoparticle-Based in Situ Vaccination with Chemotherapy Potentiates Antitumor Response. Nano Lett. 2017, 17, 4019–4028. [Google Scholar] [CrossRef]
- Garofalo, M.; Wieczorek, M.; Anders, I.; Staniszewska, M.; Lazniewski, M.; Prygiel, M.; Zasada, A.A.; Szczepińska, T.; Plewczynski, D.; Salmaso, S.; et al. Novel combinatorial therapy of oncolytic adenovirus AdV5/3-D24-ICOSL-CD40L with anti PD-1 exhibits enhanced anti-cancer efficacy through promotion of intratumoral T-cell infiltration and modulation of tumour microenvironment in mesothelioma mouse model. Front. Oncol. 2023, 13, 1259314. [Google Scholar] [CrossRef]
- Li, Z.; Guo, R.; Zhang, Z.; Yong, H.; Guo, L.; Chen, Z.; Huang, D.; Zhou, D. Enhancing gene transfection of poly(β-amino ester)s through modulation of amphiphilicity and chain sequence. J. Control. Release 2024, 368, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.B.; Chen, M.; Chen, C.-K.; Pfeifer, B.A.; Jones, C.H. Overcoming Gene-Delivery Hurdles: Physiological Considerations for Nonviral Vectors. Trends Biotechnol. 2016, 34, 91–105. [Google Scholar] [CrossRef]
- Yagolovich, A.V.; Gasparian, M.E.; Dolgikh, D.A. Recent Advances in the Development of Nanodelivery Systems Targeting the TRAIL Death Receptor Pathway. Pharmaceutics 2023, 15, 515. [Google Scholar] [CrossRef]
- Guimarães, P.P.G.; Gaglione, S.; Sewastianik, T.; Carrasco, R.D.; Langer, R.; Mitchell, M.J. Nanoparticles for Immune Cytokine TRAIL-Based Cancer Therapy. ACS Nano 2018, 12, 912–931. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Duan, N.; Zhang, C.; Mo, R.; Hua, Z. Improved antitumor activity of TRAIL fusion protein via formation of self-assembling nanoparticle. Sci. Rep. 2017, 7, 41904. [Google Scholar] [CrossRef]
- Gallego-Lleyda, A.; De Miguel, D.; Anel, A.; Martinez-Lostao, L. Lipid Nanoparticles Decorated with TNF-Related Aptosis-Inducing Ligand (TRAIL) Are More Cytotoxic than Soluble Recombinant TRAIL in Sarcoma. Int. J. Mol. Sci. 2018, 19, 1449. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Otero, N.; Marshall, J.R.; Glenn, A.; Matloubieh, J.; Joseph, J.; Sahasrabudhe, D.M.; Messing, E.M.; King, M.R. TRAIL-coated leukocytes to kill circulating tumor cells in the flowing blood from prostate cancer patients. BMC Cancer 2021, 21, 898. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Y.; Wang, L.; Liu, W.; Xiao, L.; Lin, Q.; Gong, T.; Sun, X.; He, Q.; Zhang, Z.; et al. Improved melanoma suppression with target-delivered TRAIL and Paclitaxel by a multifunctional nanocarrier. J. Control. Release 2020, 325, 10–24. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, M.; Wang, T.; Zhang, B.; Liu, C.; Zhang, N. Multifunctionalized polyethyleneimine-based nanocarriers for gene and chemotherapeutic drug combination therapy through one-step assembly strategy. Int. J. Nanomed. 2017, 12, 8681–8698. [Google Scholar] [CrossRef][Green Version]
- Ravula, V.; Lo, Y.-L.; Wu, Y.-T.; Chang, C.-W.; Patri, S.V.; Wang, L.-F. Arginine-tocopherol bioconjugated lipid vesicles for selective pTRAIL delivery and subsequent apoptosis induction in glioblastoma cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112189. [Google Scholar] [CrossRef]
- Pindiprolu, S.K.S.S.; Krishnamurthy, P.T.; Dev, C.; Chintamaneni, P.K. DR5 antibody conjugated lipid-based nanocarriers of gamma-secretase inhibitor for the treatment of triple negative breast cancer. Chem. Phys. Lipids 2021, 235, 105033. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Gong, S.; Li, Y.; Zhang, H.; Li, N.; Tang, B. A DR4 capturer with AKT siRNA for the synergetic enhancement of death receptor-mediated apoptosis. Chem. Commun. 2018, 54, 13439–13442. [Google Scholar] [CrossRef]
- Wang, Y.; Santos, A.; Kaur, G.; Evdokiou, A.; Losic, D. Structurally engineered anodic alumina nanotubes as nano-carriers for delivery of anticancer therapeutics. Biomaterials 2014, 35, 5517–5526. [Google Scholar] [CrossRef] [PubMed]
- Eljack, S.; David, S.; Faggad, A.; Chourpa, I.; Allard-Vannier, E. Nanoparticles design considerations to co-deliver nucleic acids and anti-cancer drugs for chemoresistance reversal. Int. J. Pharm. X 2022, 4, 100126. [Google Scholar] [CrossRef]
- Yoo, J.-W.; Irvine, D.J.; Discher, D.E.; Mitragotri, S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 2011, 10, 521–535. [Google Scholar] [CrossRef]
- Liu, Z.; Li, S.; Ma, T.; Zeng, J.; Zhou, X.; Li, H.; Tang, M.; Liu, X.; Li, F.; Jiang, B.; et al. Secreted TRAIL gene-modified adipose-derived stem cells exhibited potent tumor-suppressive effect in hepatocellular carcinoma cells. Immun. Inflamm. Dis. 2021, 9, 144–156. [Google Scholar] [CrossRef]
- Shirjang, S.; Mansoori, B.; Solali, S.; Hagh, M.F.; Shamsasenjan, K. Toll-like receptors as a key regulator of mesenchymal stem cell function: An up-to-date review. Cell. Immunol. 2017, 315, 1–10. [Google Scholar] [CrossRef]
- Guiho, R.; Biteau, K.; Grisendi, G.; Taurelle, J.; Chatelais, M.; Gantier, M.; Heymann, D.; Dominici, M.; Redini, F. TRAIL delivered by mesenchymal stromal/stem cells counteracts tumor development in orthotopic Ewing sarcoma models. Int. J. Cancer 2016, 139, 2802–2811. [Google Scholar] [CrossRef]
- Spano, C.; Grisendi, G.; Golinelli, G.; Rossignoli, F.; Prapa, M.; Bestagno, M.; Candini, O.; Petrachi, T.; Recchia, A.; Miselli, F.; et al. Soluble TRAIL Armed Human MSC As Gene Therapy For Pancreatic Cancer. Sci. Rep. 2019, 9, 1788. [Google Scholar] [CrossRef]
- Han, J.; Hwang, H.S.; Na, K. TRAIL-secreting human mesenchymal stem cells engineered by a non-viral vector and photochemical internalization for pancreatic cancer gene therapy. Biomaterials 2018, 182, 259–268. [Google Scholar] [CrossRef]
- Rossignoli, F.; Grisendi, G.; Spano, C.; Golinelli, G.; Recchia, A.; Rovesti, G.; Orsi, G.; Veronesi, E.; Horwitz, E.M.; Dominici, M. Inducible Caspase9-mediated suicide gene for MSC-based cancer gene therapy. Cancer Gene Ther. 2019, 26, 11–16. [Google Scholar] [CrossRef]
- Quiroz-Reyes, A.G.; Gonzalez-Villarreal, C.A.; Limon-Flores, A.Y.; Delgado-Gonzalez, P.; Martinez-Rodriguez, H.G.; Said-Fernandez, S.L.; Soto-Dominguez, A.; Rivas-Estilla, A.M.; Islas, J.F.; Molina-De la Garza, J.F.; et al. Mesenchymal Stem Cells Genetically Modified by Lentivirus-Express Soluble TRAIL and Interleukin-12 Inhibit Growth and Reduced Metastasis-Relate Changes in Lymphoma Mice Model. Biomedicines 2023, 11, 595. [Google Scholar] [CrossRef]
- Shamili, F.H.; Bayegi, H.R.; Salmasi, Z.; Sadri, K.; Mahmoudi, M.; Kalantari, M.; Ramezani, M.; Abnous, K. Exosomes derived from TRAIL-engineered mesenchymal stem cells with effective anti-tumor activity in a mouse melanoma model. Int. J. Pharm. 2018, 549, 218–229. [Google Scholar] [CrossRef]
- Hao, Y.; Zhu, G.; Yu, L.; Ren, Z.; Zhang, P.; Zhu, J.; Cao, S. Extracellular vesicles derived from mesenchymal stem cells confer protection against intervertebral disc degeneration through a microRNA-217-dependent mechanism. Osteoarthr. Cartil. 2022, 30, 1455–1467. [Google Scholar] [CrossRef]
- Huang, K.C.-Y.; Chiang, S.-F.; Chang, H.-Y.; Chen, W.T.-L.; Yang, P.-C.; Chen, T.-W.; Liang, J.-A.; Shiau, A.-C.; Ke, T.-W.; Clifford Chao, K.S. Engineered sTRAIL-armed MSCs overcome STING deficiency to enhance the therapeutic efficacy of radiotherapy for immune checkpoint blockade. Cell Death Dis. 2022, 13, 610. [Google Scholar] [CrossRef]
- Fakiruddin, K.S.; Lim, M.N.; Nordin, N.; Rosli, R.; Abdullah, S. Chemo-Sensitization of CD133+ Cancer Stem Cell Enhances the Effect of Mesenchymal Stem Cell Expressing TRAIL in Non-Small Cell Lung Cancer Cell Lines. Biology 2021, 10, 1103. [Google Scholar] [CrossRef]
- Rossignoli, F.; Spano, C.; Grisendi, G.; Foppiani, E.M.; Golinelli, G.; Mastrolia, I.; Bestagno, M.; Candini, O.; Petrachi, T.; Recchia, A.; et al. MSC-Delivered Soluble TRAIL and Paclitaxel as Novel Combinatory Treatment for Pancreatic Adenocarcinoma. Theranostics 2019, 9, 436–448. [Google Scholar] [CrossRef]
- Sun, L.; Wang, J.; Wang, Q.; He, Z.; Sun, T.; Yao, Y.; Wang, W.; Shen, P. Pretreatment of umbilical cord derived MSCs with IFN-γ and TNF-α enhances the tumor-suppressive effect on acute myeloid leukemia. Biochem. Pharmacol. 2022, 199, 115007. [Google Scholar] [CrossRef]
- Choi, S.A.; Lee, C.; Kwak, P.A.; Park, C.-K.; Wang, K.-C.; Phi, J.H.; Lee, J.Y.; Chong, S.; Kim, S.-K. Histone deacetylase inhibitor panobinostat potentiates the anti-cancer effects of mesenchymal stem cell-based sTRAIL gene therapy against malignant glioma. Cancer Lett. 2019, 442, 161–169. [Google Scholar] [CrossRef]
- Shaik Fakiruddin, K.; Ghazalli, N.; Lim, M.N.; Zakaria, Z.; Abdullah, S. Mesenchymal Stem Cell Expressing TRAIL as Targeted Therapy against Sensitised Tumour. Int. J. Mol. Sci. 2018, 19, 2188. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Wayne, E.; Rana, K.; Schaffer, C.B.; King, M.R. TRAIL-coated leukocytes that kill cancer cells in the circulation. Proc. Natl. Acad. Sci. USA 2014, 111, 930–935. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Chan, M.F.; Li, J.; King, M.R. Super natural killer cells that target metastases in the tumor draining lymph nodes. Biomaterials 2016, 77, 66–76. [Google Scholar] [CrossRef]
- Chulpanova, D.S.; Gilazieva, Z.E.; Akhmetzyanova, E.R.; Kletukhina, S.K.; Rizvanov, A.A.; Solovyeva, V.V. Cytochalasin B-induced membrane vesicles from human mesenchymal stem cells overexpressing TRAIL, PTEN and IFN-β1 can kill carcinoma cancer cells. Tissue Cell 2021, 73, 101664. [Google Scholar] [CrossRef]
- Chen, F.; Zhong, X.; Dai, Q.; Li, K.; Zhang, W.; Wang, J.; Zhao, Y.; Shen, J.; Xiao, Z.; Xing, H.; et al. Human Umbilical Cord MSC Delivered-Soluble TRAIL Inhibits the Proliferation and Promotes Apoptosis of B-ALL Cell In Vitro and In Vivo. Pharmaceuticals 2022, 15, 1391. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Mu, X.; Tu, C.R.; Chung, Y.; Tsao, S.W.; Chan, G.C.-F.; Leung, W.-H.; Lau, Y.-L.; Liu, Y.; et al. Exosomes derived from γδ-T cells synergize with radiotherapy and preserve antitumor activities against nasopharyngeal carcinoma in immunosuppressive microenvironment. J. Immunother. Cancer 2022, 10, e003832. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).