The Role of TRAIL Signaling in Cancer: Searching for New Therapeutic Strategies
Abstract
Simple Summary
Abstract
1. Introduction
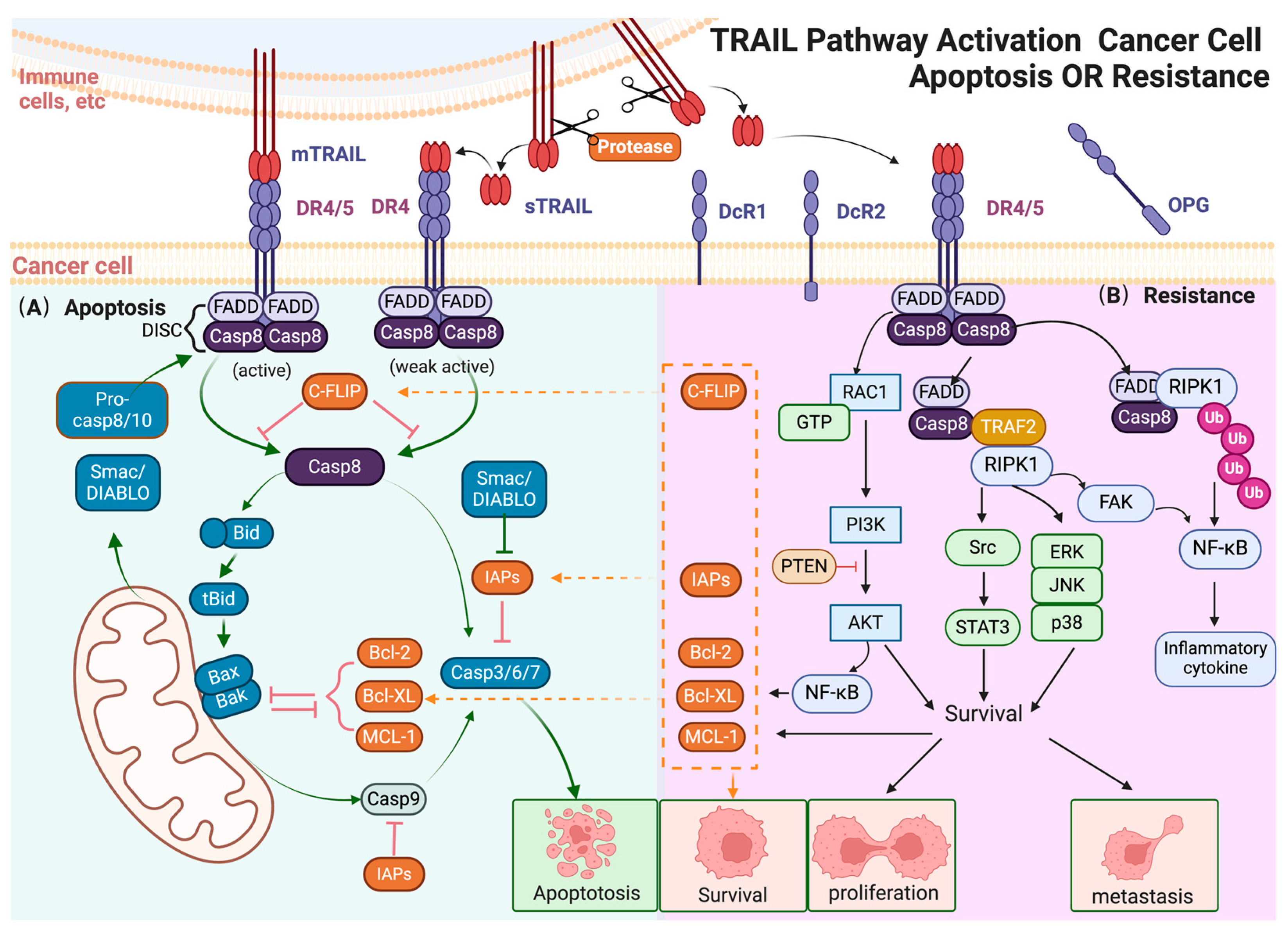
2. TRAIL and Caspase-Dependent Apoptosis
2.1. TRAIL
2.2. Receptors of TRAIL
2.3. Canonical Signaling Mediated by TRAIL
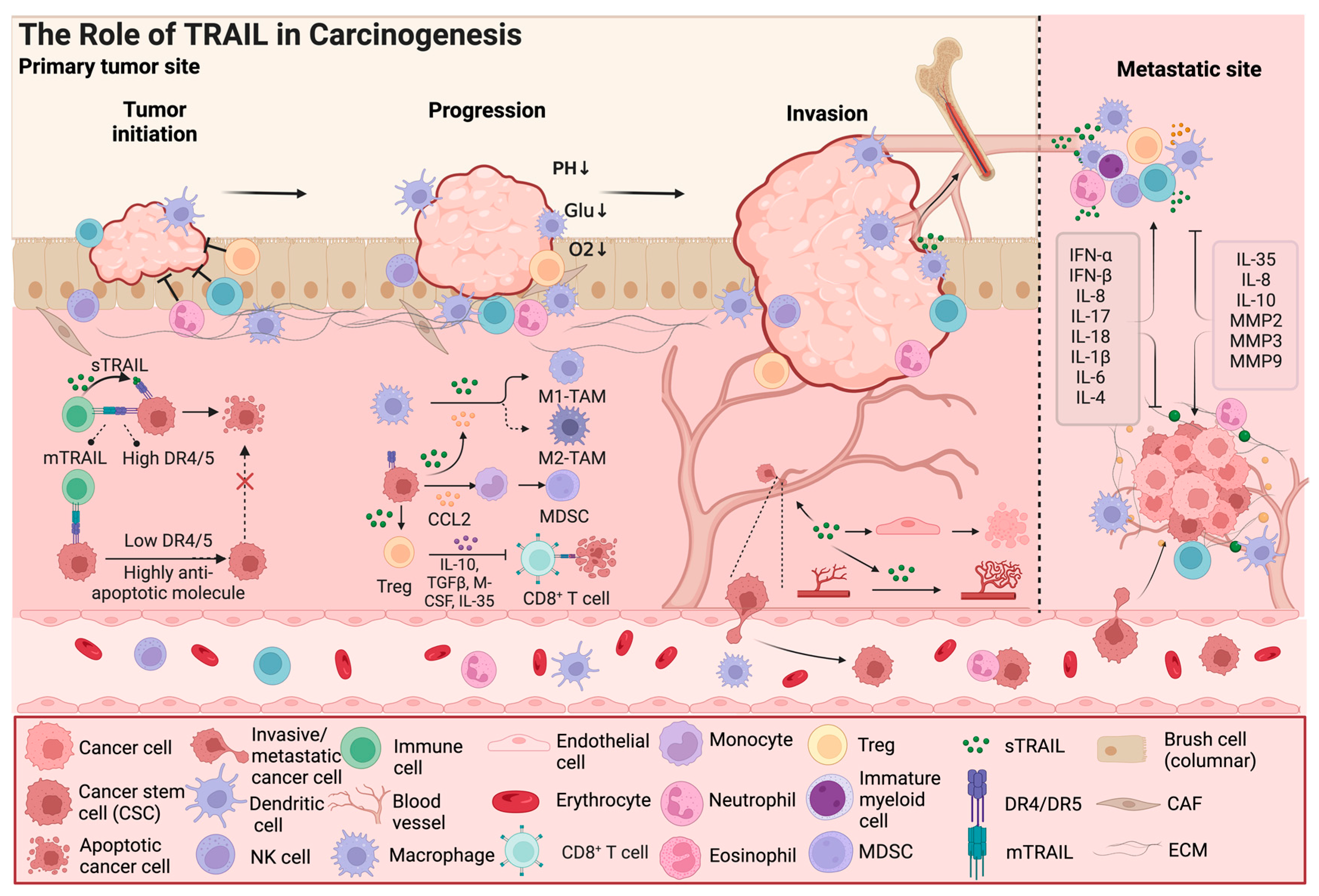
3. The Multiple Roles of TRAIL in the Tumor Microenvironment
3.1. Cancer Stem Cells Are TRAIL Resistant
3.2. TRAIL Has Dual Effects on Immune Cells
3.3. The Interaction of the TRAIL Signal Pathway and Other Cytokines Influencing the Tumor Microenvironment
3.4. TRAIL Modulates Cancer Angiogenesis
4. TRAIL as a Target for Cancer Gene Therapy
5. Novel Strategies for TRAIL-Based Therapy
5.1. TRAIL-Based Combination Therapy
5.1.1. Combination with Chemotherapy or Radiotherapy
5.1.2. Combination with Immunotherapy
5.1.3. Combination with Microenvironment-Modulating Agents
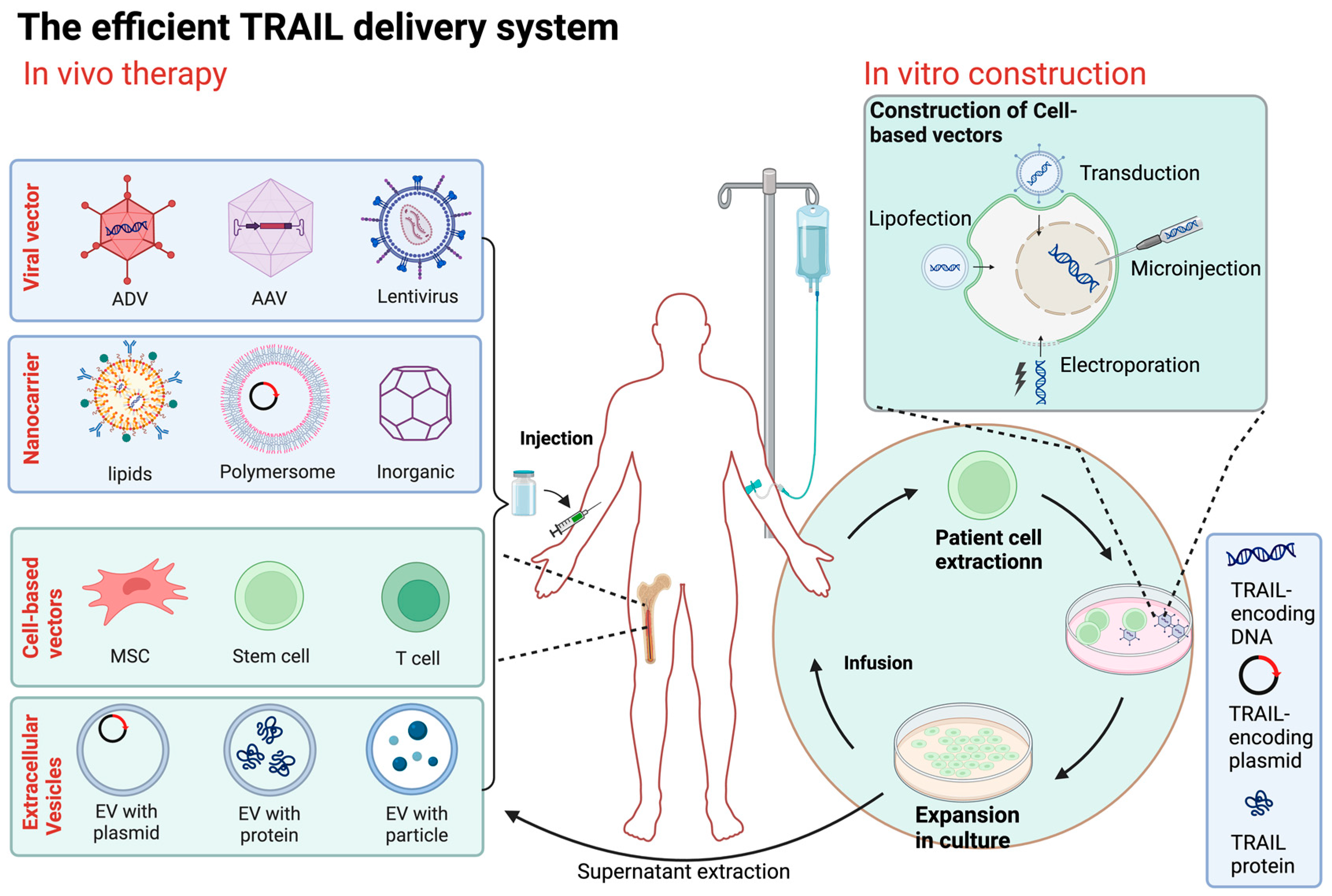
5.2. The Improved Efficient TRAIL Delivery System
5.2.1. Viral Vector-Based TRAIL Therapy
5.2.2. Nanodelivery Systems
5.2.3. Cell-Based Vectors
5.2.4. Cell Membrane/Extracellular Vesicle-Based Vectors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wajant, H. TRAIL- and TNF-induced signaling complexes-so similar yet so different. EMBO J. 2017, 36, 1117–1119. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, Y.; Wang, X.; Evers, B.M. Isolation and molecular characterization of the 5′-upstream region of the human TRAIL gene. Biochem. Biophys. Res. Commun. 2000, 276, 466–471. [Google Scholar] [CrossRef]
- Lemke, J.; von Karstedt, S.; Zinngrebe, J.; Walczak, H. Getting TRAIL back on track for cancer therapy. Cell Death Differ. 2014, 21, 1350–1364. [Google Scholar] [CrossRef]
- Zhong, H.-H.; Wang, H.-Y.; Li, J.; Huang, Y.-Z. TRAIL-based gene delivery and therapeutic strategies. Acta Pharmacol. Sin. 2019, 40, 1373–1385. [Google Scholar] [CrossRef]
- Cardoso Alves, L.; Corazza, N.; Micheau, O.; Krebs, P. The multifaceted role of TRAIL signaling in cancer and immunity. FEBS J. 2021, 288, 5530–5554. [Google Scholar] [CrossRef]
- Cartland, S.P.; Genner, S.W.; Martínez, G.J.; Robertson, S.; Kockx, M.; Lin, R.C.; O’Sullivan, J.F.; Koay, Y.C.; Manuneedhi Cholan, P.; Kebede, M.A.; et al. TRAIL-Expressing Monocyte/Macrophages Are Critical for Reducing Inflammation and Atherosclerosis. iScience 2019, 12, 41–52. [Google Scholar] [CrossRef]
- Wiley, S.R.; Schooley, K.; Smolak, P.J.; Din, W.S.; Huang, C.P.; Nicholl, J.K.; Sutherland, G.R.; Smith, T.D.; Rauch, C.; Smith, C.A. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995, 3, 673–682. [Google Scholar] [CrossRef]
- Gibellini, D.; Borderi, M.; De Crignis, E.; Cicola, R.; Vescini, F.; Caudarella, R.; Chiodo, F.; Re, M.C. RANKL/OPG/TRAIL plasma levels and bone mass loss evaluation in antiretroviral naive HIV-1-positive men. J. Med. Virol. 2007, 79, 1446–1454. [Google Scholar] [CrossRef]
- Nie, Z.; Aboulnasr, F.; Natesampillai, S.; Burke, S.P.; Krogman, A.; Bren, G.D.; Chung, T.D.Y.; Anderson, J.R.; Smart, M.K.; Katzmann, D.J.; et al. Correction: Both HIV-Infected and Uninfected Cells Express TRAILshort, Which Confers TRAIL Resistance upon Bystander Cells within the Microenvironment. J. Immunol. 2018, 201, 1599. [Google Scholar] [CrossRef]
- Aboulnasr, F.; Krogman, A.; Graham, R.P.; Cummins, N.W.; Misra, A.; Garcia-Rivera, E.; Anderson, J.R.; Natesampillai, S.; Kogan, N.; Aravamudan, M.; et al. Human Cancers Express TRAILshort, a Dominant Negative TRAIL Splice Variant, Which Impairs Immune Effector Cell Killing of Tumor Cells. Clin. Cancer Res. 2020, 26, 5759–5771. [Google Scholar] [CrossRef]
- de Miguel, D.; Pardo, J. TRAIL and Cancer Immunotherapy: Take a Walk on the Short Side. Clin. Cancer Res. 2020, 26, 5546–5548. [Google Scholar] [CrossRef]
- Wajant, H.; Moosmayer, D.; Wüest, T.; Bartke, T.; Gerlach, E.; Schönherr, U.; Peters, N.; Scheurich, P.; Pfizenmaier, K. Differential activation of TRAIL-R1 and -2 by soluble and membrane TRAIL allows selective surface antigen-directed activation of TRAIL-R2 by a soluble TRAIL derivative. Oncogene 2001, 20, 4101–4106. [Google Scholar] [CrossRef]
- Jong, K.X.J.; Mohamed, E.H.M.; Ibrahim, Z.A. Escaping cell death via TRAIL decoy receptors: A systematic review of their roles and expressions in colorectal cancer. Apoptosis 2022, 27, 787–799. [Google Scholar] [CrossRef]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The role of osteoprotegerin in the crosstalk between vessels and bone: Its potential utility as a marker of cardiometabolic diseases. Pharmacol. Ther. 2018, 182, 115–132. [Google Scholar] [CrossRef]
- Habibie, H.; Adhyatmika, A.; Schaafsma, D.; Melgert, B.N. The role of osteoprotegerin (OPG) in fibrosis: Its potential as a biomarker and/or biological target for the treatment of fibrotic diseases. Pharmacol. Ther. 2021, 228, 107941. [Google Scholar] [CrossRef]
- Haselmann, V.; Kurz, A.; Bertsch, U.; Hübner, S.; Olempska-Müller, M.; Fritsch, J.; Häsler, R.; Pickl, A.; Fritsche, H.; Annewanter, F.; et al. Nuclear death receptor TRAIL-R2 inhibits maturation of let-7 and promotes proliferation of pancreatic and other tumor cells. Gastroenterology 2014, 146, 278–290. [Google Scholar] [CrossRef]
- Beyer, K.; Baukloh, A.-K.; Stoyanova, A.; Kamphues, C.; Sattler, A.; Kotsch, K. Interactions of Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand (TRAIL) with the Immune System: Implications for Inflammation and Cancer. Cancers 2019, 11, 1161. [Google Scholar] [CrossRef]
- Micheau, O. Regulation of TNF-Related Apoptosis-Inducing Ligand Signaling by Glycosylation. Int. J. Mol. Sci. 2018, 19, 715. [Google Scholar] [CrossRef]
- Dufour, F.; Rattier, T.; Shirley, S.; Picarda, G.; Constantinescu, A.A.; Morlé, A.; Zakaria, A.B.; Marcion, G.; Causse, S.; Szegezdi, E.; et al. N-glycosylation of mouse TRAIL-R and human TRAIL-R1 enhances TRAIL-induced death. Cell Death Differ. 2017, 24, 500–510. [Google Scholar] [CrossRef]
- Yoshida, T.; Shiraishi, T.; Horinaka, M.; Wakada, M.; Sakai, T. Glycosylation modulates TRAIL-R1/death receptor 4 protein: Different regulations of two pro-apoptotic receptors for TRAIL by tunicamycin. Oncol. Rep. 2007, 18, 1239–1242. [Google Scholar] [CrossRef]
- Ralff, M.D.; El-Deiry, W.S. TRAIL pathway targeting therapeutics. Expert Rev. Precis. Med. Drug Dev. 2018, 3, 197–204. [Google Scholar] [CrossRef]
- Green, D.R. Caspases and Their Substrates. Cold Spring Harb. Perspect. Biol. 2022, 14, a041012. [Google Scholar] [CrossRef]
- Yuan, S.; Akey, C.W. Apoptosome structure, assembly, and procaspase activation. Structure 2013, 21, 501–515. [Google Scholar] [CrossRef]
- Trivedi, R.; Mishra, D.P. Trailing TRAIL Resistance: Novel Targets for TRAIL Sensitization in Cancer Cells. Front. Oncol. 2015, 5, 69. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Chang, J.C. Cancer stem cells: Role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine 2016, 95, S20–S25. [Google Scholar] [CrossRef]
- Aponte, P.M.; Caicedo, A. Stemness in Cancer: Stem Cells, Cancer Stem Cells, and Their Microenvironment. Stem Cells Int. 2017, 2017, 5619472. [Google Scholar] [CrossRef]
- Osum, M.; Kalkan, R. Cancer Stem Cells and Their Therapeutic Usage. In Cell Biology and Translational Medicine, Volume 20: Organ Function, Maintenance, Repair in Health and Disease; Advances in Experimental Medicine and Biology; Springer Nature: Berlin, Germany, 2023. [Google Scholar]
- Chen, K.; Huang, Y.; Chen, J. Understanding and targeting cancer stem cells: Therapeutic implications and challenges. Acta Pharmacol. Sin. 2013, 34, 732–740. [Google Scholar] [CrossRef]
- Quiroz-Reyes, A.G.; Delgado-Gonzalez, P.; Islas, J.F.; Gallegos, J.L.D.; Martínez Garza, J.H.; Garza-Treviño, E.N. Behind the Adaptive and Resistance Mechanisms of Cancer Stem Cells to TRAIL. Pharmaceutics 2021, 13, 1062. [Google Scholar] [CrossRef]
- Piggott, L.; Omidvar, N.; Martí Pérez, S.; French, R.; Eberl, M.; Clarkson, R.W.E. Suppression of apoptosis inhibitor c-FLIP selectively eliminates breast cancer stem cell activity in response to the anti-cancer agent, TRAIL. Breast Cancer Res. 2011, 13, R88. [Google Scholar] [CrossRef]
- Akbari, M.; Shomali, N.; Faraji, A.; Shanehbandi, D.; Asadi, M.; Mokhtarzadeh, A.; Shabani, A.; Baradaran, B. CD133: An emerging prognostic factor and therapeutic target in colorectal cancer. Cell Biol. Int. 2020, 44, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.; Ali, S.; Ahmad, A.; Philip, P.A.; Sarkar, F.H. The Role of Cancer Stem Cells in Recurrent and Drug-Resistant Lung Cancer. Adv. Exp. Med. Biol. 2016, 890, 57–74. [Google Scholar] [PubMed]
- Singh, A.K.; Verma, A.; Singh, A.; Arya, R.K.; Maheshwari, S.; Chaturvedi, P.; Nengroo, M.A.; Saini, K.K.; Vishwakarma, A.L.; Singh, K.; et al. Salinomycin inhibits epigenetic modulator EZH2 to enhance death receptors in colon cancer stem cells. Epigenetics 2021, 16, 144–161. [Google Scholar] [CrossRef] [PubMed]
- Barzegar Behrooz, A.; Syahir, A.; Ahmad, S. CD133: Beyond a cancer stem cell biomarker. J. Drug Target. 2019, 27, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Jiang, J.; Shi, S.; Xie, H.; Zhou, L.; Zheng, S. Knockdown of miR-25 increases the sensitivity of liver cancer stem cells to TRAIL-induced apoptosis via PTEN/PI3K/Akt/Bad signaling pathway. Int. J. Oncol. 2016, 49, 2600–2610. [Google Scholar] [CrossRef] [PubMed]
- Recio-Boiles, A.; Ilmer, M.; Rhea, P.R.; Kettlun, C.; Heinemann, M.L.; Ruetering, J.; Vykoukal, J.; Alt, E. JNK pathway inhibition selectively primes pancreatic cancer stem cells to TRAIL-induced apoptosis without affecting the physiology of normal tissue resident stem cells. Oncotarget 2016, 7, 9890–9906. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Xu, L.; Bandyopadhyay, S.; Sethi, S.; Reddy, K.B. Cisplatin and TRAIL enhance breast cancer stem cell death. Int. J. Oncol. 2011, 39, 891–898. [Google Scholar]
- Kurita, S.; Mott, J.L.; Almada, L.L.; Bronk, S.F.; Werneburg, N.W.; Sun, S.-Y.; Roberts, L.R.; Fernandez-Zapico, M.E.; Gores, G.J. GLI3-dependent repression of DR4 mediates hedgehog antagonism of TRAIL-induced apoptosis. Oncogene 2010, 29, 4848–4858. [Google Scholar] [CrossRef]
- Cardoso Alves, L.; Berger, M.D.; Koutsandreas, T.; Kirschke, N.; Lauer, C.; Spörri, R.; Chatziioannou, A.; Corazza, N.; Krebs, P. Non-apoptotic TRAIL function modulates NK cell activity during viral infection. EMBO Rep. 2020, 21, e48789. [Google Scholar] [CrossRef]
- Gao, J.; Wang, D.; Liu, D.; Liu, M.; Ge, Y.; Jiang, M.; Liu, Y.; Zheng, D. Tumor necrosis factor-related apoptosis-inducing ligand induces the expression of proinflammatory cytokines in macrophages and re-educates tumor-associated macrophages to an antitumor phenotype. Mol. Biol. Cell 2015, 26, 3178–3189. [Google Scholar] [CrossRef]
- Gunalp, S.; Helvaci, D.G.; Oner, A.; Bursalı, A.; Conforte, A.; Güner, H.; Karakülah, G.; Szegezdi, E.; Sag, D. TRAIL promotes the polarization of human macrophages toward a proinflammatory M1 phenotype and is associated with increased survival in cancer patients with high tumor macrophage content. Front. Immunol. 2023, 14, 1209249. [Google Scholar] [CrossRef] [PubMed]
- Condamine, T.; Kumar, V.; Ramachandran, I.R.; Youn, J.-I.; Celis, E.; Finnberg, N.; El-Deiry, W.S.; Winograd, R.; Vonderheide, R.H.; English, N.R.; et al. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. J. Clin. Investg. 2014, 124, 2626–2639. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.-M.; Qin, J.; Li, Y.-C.; Wang, Y.; Li, D.; Shu, Y.; Luo, C.; Wang, S.-S.; Chi, G.; Guo, F.; et al. IL-35 induces N2 phenotype of neutrophils to promote tumor growth. Oncotarget 2017, 8, 33501–33514. [Google Scholar] [CrossRef] [PubMed]
- Pillai, M.R.; Collison, L.W.; Wang, X.; Finkelstein, D.; Rehg, J.E.; Boyd, K.; Szymczak-Workman, A.L.; Doggett, T.; Griffith, T.S.; Ferguson, T.A.; et al. The plasticity of regulatory T cell function. J. Immunol. 2011, 187, 4987–4997. [Google Scholar] [CrossRef]
- de Miguel, D.; Lemke, J.; Anel, A.; Walczak, H.; Martinez-Lostao, L. Onto better TRAILs for cancer treatment. Cell Death Differ. 2016, 23, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Liu, W.; Liu, X.; Jia, X.; Feng, X.; Li, F.; Zhu, R.; Yu, J.; Zhang, H.; et al. Dose-related immunomodulatory effects of recombinant TRAIL in the tumor immune microenvironment. J. Exp. Clin. Cancer Res. 2023, 42, 216. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, T.; Montinaro, A.; von Karstedt, S.; Sevko, A.; Surinova, S.; Chakravarthy, A.; Taraborrelli, L.; Draber, P.; Lafont, E.; Arce Vargas, F.; et al. The TRAIL-Induced Cancer Secretome Promotes a Tumor-Supportive Immune Microenvironment via CCR2. Mol. Cell 2017, 65, 730–742.e5. [Google Scholar] [CrossRef] [PubMed]
- Loeuillard, E.; Li, B.; Stumpf, H.E.; Yang, J.; Willhite, J.; Tomlinson, J.L.; Wang, J.; Rohakhtar, F.R.; Simon, V.A.; Graham, R.P.; et al. Noncanonical TRAIL Signaling Promotes Myeloid-Derived Suppressor Cell Abundance and Tumor Growth in Cholangiocarcinoma. Cell Mol Gastroenterol Hepatol 2024, 17, 853–876. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, D.; D’Arcy, P.; Wennerberg, E.; Lidén, M.; Hu, J.; Winqvist, O.; Rolny, C.; Lundqvist, A. Activated monocytes augment TRAIL-mediated cytotoxicity by human NK cells through release of IFN-γ. Eur. J. Immunol. 2013, 43, 249–257. [Google Scholar] [CrossRef]
- Achard, C.; Guillerme, J.-B.; Bruni, D.; Boisgerault, N.; Combredet, C.; Tangy, F.; Jouvenet, N.; Grégoire, M.; Fonteneau, J.-F. Oncolytic measles virus induces tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated cytotoxicity by human myeloid and plasmacytoid dendritic cells. Oncoimmunology 2017, 6, e1261240. [Google Scholar] [CrossRef]
- Tu, Z.; Hamalainen-Laanaya, H.K.; Crispe, I.N.; Orloff, M.S. Synergy between TLR3 and IL-18 promotes IFN-γ dependent TRAIL expression in human liver NK cells. Cell. Immunol. 2011, 271, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Wang, Y.; Huang, C.-Y.; Zhou, Z.-Q.; Zhao, J.-J.; Zhang, X.-F.; Pan, Q.-Z.; Wu, J.-X.; Weng, D.-S.; Tang, Y.; et al. IL-17 induces antitumor immunity by promoting beneficial neutrophil recruitment and activation in esophageal squamous cell carcinoma. Oncoimmunology 2017, 7, e1373234. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.-W.; Hung, E.; Wu, J.-M.; Liang, Y.-H.; Chiu, Y.-H.; Tyan, Y.-S.; Wang, H.-S. Anti-tumor Effects of IL-1β Induced TRAIL-Expressing hUCMSCs on Embelin Treated Breast Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2023, 24, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- de Looff, M.; de Jong, S.; Kruyt, F.A.E. Multiple Interactions Between Cancer Cells and the Tumor Microenvironment Modulate TRAIL Signaling: Implications for TRAIL Receptor Targeted Therapy. Front. Immunol. 2019, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Sano, E.; Kazaana, A.; Tadakuma, H.; Takei, T.; Yoshimura, S.; Hanashima, Y.; Ozawa, Y.; Yoshino, A.; Suzuki, Y.; Ueda, T. Interleukin-6 sensitizes TNF-α and TRAIL/Apo2L dependent cell death through upregulation of death receptors in human cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Z.; Huang, W. Interleukin-4 Enhances the Sensitivity of Human Monocytes to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Through Upregulation of Death Receptor 4. J. Interferon Cytokine Res. 2018, 38, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Willms, A.; Schittek, H.; Rahn, S.; Sosna, J.; Mert, U.; Adam, D.; Trauzold, A. Impact of p53 status on TRAIL-mediated apoptotic and non-apoptotic signaling in cancer cells. PLoS ONE 2019, 14, e0214847. [Google Scholar] [CrossRef]
- Favaro, F.; Luciano-Mateo, F.; Moreno-Caceres, J.; Hernández-Madrigal, M.; Both, D.; Montironi, C.; Püschel, F.; Nadal, E.; Eldering, E.; Muñoz-Pinedo, C. TRAIL receptors promote constitutive and inducible IL-8 secretion in non-small cell lung carcinoma. Cell Death Dis. 2022, 13, 1046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qin, G.; Zhang, C.; Yang, H.; Liu, J.; Hu, H.; Wu, P.; Liu, S.; Yang, L.; Chen, X.; et al. TRAIL promotes epithelial-to-mesenchymal transition by inducing PD-L1 expression in esophageal squamous cell carcinomas. J. Exp. Clin. Cancer Res. 2021, 40, 209. [Google Scholar] [CrossRef]
- Patil, M.S.; Cartland, S.P.; Kavurma, M.M. TRAIL signals, extracellular matrix and vessel remodelling. Vasc. Biol. 2020, 2, R73–R84. [Google Scholar] [CrossRef]
- Wilson, N.S.; Yang, A.; Yang, B.; Couto, S.; Stern, H.; Gogineni, A.; Pitti, R.; Marsters, S.; Weimer, R.M.; Singh, M.; et al. Proapoptotic activation of death receptor 5 on tumor endothelial cells disrupts the vasculature and reduces tumor growth. Cancer Cell 2012, 22, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Secchiero, P.; Gonelli, A.; Carnevale, E.; Corallini, F.; Rizzardi, C.; Zacchigna, S.; Melato, M.; Zauli, G. Evidence for a proangiogenic activity of TNF-related apoptosis-inducing ligand. Neoplasia 2004, 6, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Riera-Domingo, C.; Leite-Gomes, E.; Charatsidou, I.; Zhao, P.; Carrá, G.; Cappellesso, F.; Mourao, L.; De Schepper, M.; Liu, D.; Serneels, J.; et al. Breast tumors interfere with endothelial TRAIL at the premetastatic niche to promote cancer cell seeding. Sci. Adv. 2023, 9, eadd5028. [Google Scholar] [CrossRef] [PubMed]
- Cartland, S.P.; Genner, S.W.; Zahoor, A.; Kavurma, M.M. Comparative Evaluation of TRAIL, FGF-2 and VEGF-A-Induced Angiogenesis In Vitro and In Vivo. Int. J. Mol. Sci. 2016, 17, 2025. [Google Scholar] [CrossRef]
- Di Bartolo, B.A.; Cartland, S.P.; Prado-Lourenco, L.; Griffith, T.S.; Gentile, C.; Ravindran, J.; Azahri, N.S.M.; Thai, T.; Yeung, A.W.S.; Thomas, S.R.; et al. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) Promotes Angiogenesis and Ischemia-Induced Neovascularization Via NADPH Oxidase 4 (NOX4) and Nitric Oxide-Dependent Mechanisms. J. Am. Heart Assoc. 2015, 4, e002527. [Google Scholar] [CrossRef] [PubMed]
- Schito, L. Hypoxia-Dependent Angiogenesis and Lymphangiogenesis in Cancer. Adv. Exp. Med. Biol. 2019, 1136, 71–85. [Google Scholar] [PubMed]
- Forde, H.; Harper, E.; Rochfort, K.D.; Wallace, R.G.; Davenport, C.; Smith, D.; Cummins, P.M. TRAIL inhibits oxidative stress in human aortic endothelial cells exposed to pro-inflammatory stimuli. Physiol. Rep. 2020, 8, e14612. [Google Scholar] [CrossRef] [PubMed]
- Walczak, H.; Miller, R.E.; Ariail, K.; Gliniak, B.; Griffith, T.S.; Kubin, M.; Chin, W.; Jones, J.; Woodward, A.; Le, T.; et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat. Med. 1999, 5, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Pai, R.C.; Fong, S.; Leung, S.; Lawrence, D.A.; Marsters, S.A.; Blackie, C.; Chang, L.; McMurtrey, A.E.; Hebert, A.; et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Investg. 1999, 104, 155–162. [Google Scholar] [CrossRef]
- Ion, G.N.D.; Nitulescu, G.M.; Popescu, C.I. Targeting TRAIL. Bioorg. Med. Chem. Lett. 2019, 29, 2527–2534. [Google Scholar] [CrossRef]
- Jo, M.; Kim, T.H.; Seol, D.W.; Esplen, J.E.; Dorko, K.; Billiar, T.R.; Strom, S.C. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat. Med. 2000, 6, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Ganten, T.M.; Koschny, R.; Sykora, J.; Schulze-Bergkamen, H.; Büchler, P.; Haas, T.L.; Schader, M.B.; Untergasser, A.; Stremmel, W.; Walczak, H. Preclinical differentiation between apparently safe and potentially hepatotoxic applications of TRAIL either alone or in combination with chemotherapeutic drugs. Clin. Cancer Res. 2006, 12, 2640–2646. [Google Scholar] [CrossRef]
- Snajdauf, M.; Havlova, K.; Vachtenheim, J.; Ozaniak, A.; Lischke, R.; Bartunkova, J.; Smrz, D.; Strizova, Z. The TRAIL in the Treatment of Human Cancer: An Update on Clinical Trials. Front. Mol. Biosci. 2021, 8, 628332. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, T.; Liu, J.; Cui, Y.; Yang, S.; Zhao, R. Synergistic antitumor effects of circularly permuted TRAIL with doxorubicin in triple-negative breast cancer. Acta Biochim. Biophys. Sin. 2023, 55, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Naval, J.; de Miguel, D.; Gallego-Lleyda, A.; Anel, A.; Martinez-Lostao, L. Importance of TRAIL Molecular Anatomy in Receptor Oligomerization and Signaling. Implications for Cancer Therapy. Cancers 2019, 11, 444. [Google Scholar] [CrossRef]
- Wajant, H. Molecular Mode of Action of TRAIL Receptor Agonists—Common Principles and Their Translational Exploitation. Cancers 2019, 11, 954. [Google Scholar] [CrossRef]
- Medler, J.; Nelke, J.; Weisenberger, D.; Steinfatt, T.; Rothaug, M.; Berr, S.; Hünig, T.; Beilhack, A.; Wajant, H. TNFRSF receptor-specific antibody fusion proteins with targeting controlled FcγR-independent agonistic activity. Cell Death Dis. 2019, 10, 224. [Google Scholar] [CrossRef]
- Chuntharapai, A.; Dodge, K.; Grimmer, K.; Schroeder, K.; Marsters, S.A.; Koeppen, H.; Ashkenazi, A.; Kim, K.J. Isotype-dependent inhibition of tumor growth in vivo by monoclonal antibodies to death receptor 4. J. Immunol. 2001, 166, 4891–4898. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, A.; Micheau, O. Antibodies and Derivatives Targeting DR4 and DR5 for Cancer Therapy. Antibodies 2017, 6, 16. [Google Scholar] [CrossRef]
- Lemke, J.; Noack, A.; Adam, D.; Tchikov, V.; Bertsch, U.; Röder, C.; Schütze, S.; Wajant, H.; Kalthoff, H.; Trauzold, A. TRAIL signaling is mediated by DR4 in pancreatic tumor cells despite the expression of functional DR5. J. Mol. Med. 2010, 88, 729–740. [Google Scholar] [CrossRef]
- Dufour, F.; Rattier, T.; Constantinescu, A.A.; Zischler, L.; Morlé, A.; Ben Mabrouk, H.; Humblin, E.; Jacquemin, G.; Szegezdi, E.; Delacote, F.; et al. TRAIL receptor gene editing unveils TRAIL-R1 as a master player of apoptosis induced by TRAIL and ER stress. Oncotarget 2017, 8, 9974–9985. [Google Scholar] [CrossRef] [PubMed]
- Greer, Y.E.; Gilbert, S.F.; Gril, B.; Narwal, R.; Peacock Brooks, D.L.; Tice, D.A.; Steeg, P.S.; Lipkowitz, S. MEDI3039, a novel highly potent tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) receptor 2 agonist, causes regression of orthotopic tumors and inhibits outgrowth of metastatic triple-negative breast cancer. Breast Cancer Res. 2019, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xia, X.; Xia, X.-X.; Sun, Z.; Yan, D. Improving Targeted Delivery and Antitumor Efficacy with Engineered Tumor Necrosis Factor-Related Apoptosis Ligand-Affibody Fusion Protein. Mol. Pharm. 2021, 18, 3854–3861. [Google Scholar] [CrossRef] [PubMed]
- Prigozhina, T.B.; Szafer, F.; Aronin, A.; Tzdaka, K.; Amsili, S.; Makdasi, E.; Shani, N.; Dranitzki Elhalel, M. Fn14·TRAIL fusion protein is oligomerized by TWEAK into a superefficient TRAIL analog. Cancer Lett. 2017, 400, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Brin, E.; Wu, K.; Dagostino, E.; Kuo, M.M.-C.; He, Y.; Shia, W.-J.; Chen, L.-C.; Stempniak, M.; Hickey, R.; Almassy, R.; et al. TRAIL stabilization and cancer cell sensitization to its pro-apoptotic activity achieved through genetic fusion with arginine deiminase. Oncotarget 2018, 9, 36914–36928. [Google Scholar] [CrossRef] [PubMed]
- Tur, V.; van der Sloot, A.M.; Reis, C.R.; Szegezdi, E.; Cool, R.H.; Samali, A.; Serrano, L.; Quax, W.J. DR4-selective tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) variants obtained by structure-based design. J. Biol. Chem. 2008, 283, 20560–20568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, W.; Ren, Y.; Wei, D. SAC-TRAIL, a novel anticancer fusion protein: Expression, purification, and functional characterization. Appl. Microbiol. Biotechnol. 2022, 106, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Han, Z.; Stenzel, M.H.; Chapman, R. A High Throughput Approach for Designing Polymers That Mimic the TRAIL Protein. Nano Lett. 2022, 22, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, W.; Liu, J.; Lahat, G.; Torres, K.; Lopez, G.; Lazar, A.J.; Hayes-Jordan, A.; Liu, K.; Bankson, J.; et al. TRAIL and doxorubicin combination induces proapoptotic and antiangiogenic effects in soft tissue sarcoma in vivo. Clin. Cancer Res. 2010, 16, 2591–2604. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Guo, Y.; Han, L.; Duan, Y.; Fang, F.; Niu, S.; Ba, Q.; Zhu, H.; Kong, F.; Lin, C.; et al. In vitro and in vivo growth inhibition of drug-resistant ovarian carcinoma cells using a combination of cisplatin and a TRAIL-encoding retrovirus. Oncol. Lett. 2012, 4, 1254–1258. [Google Scholar] [CrossRef]
- Kretz, A.-L.; Trauzold, A.; Hillenbrand, A.; Knippschild, U.; Henne-Bruns, D.; von Karstedt, S.; Lemke, J. TRAILblazing Strategies for Cancer Treatment. Cancers 2019, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.-O.; Asami, Y.; Long, H.; Jang, J.H.; Bae, E.Y.; Kim, B.Y.; Choi, Y.H.; Kang, C.-H.; Ahn, J.S.; Kim, G.-Y. Verrucarin A sensitizes TRAIL-induced apoptosis via the upregulation of DR5 in an eIF2α/CHOP-dependent manner. Toxicol. Vitr. 2013, 27, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Huang, G.; Pan, S.; Chen, X.; Liu, T.; Yang, Z.; Chen, T.; Zhu, X. TRAIL-driven targeting and reversing cervical cancer radioresistance by seleno-nanotherapeutics through regulating cell metabolism. Drug Resist. Updates 2024, 72, 101033. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Li, F.; Shi, C.-W.; Du, J.-L.; Xue, Y.-J.; Liu, X.-Y.; Cao, X.; Wei, N. Mechanism and therapeutic prospect of resveratrol combined with TRAIL in the treatment of renal cell carcinoma. Cancer Gene Ther. 2020, 27, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Shabestari, F.A.; Vaezi, S.; Abak, A.; Shoorei, H.; Karimi, A.; Taheri, M.; Basiri, A. Emerging impact of quercetin in the treatment of prostate cancer. Biomed. Pharmacother. 2021, 138, 111548. [Google Scholar] [CrossRef]
- Tian, X.; Gu, L.; Zeng, F.; Liu, X.; Zhou, Y.; Dou, Y.; Han, J.; Zhao, Y.; Zhang, Y.; Luo, Q.; et al. Strophanthidin Induces Apoptosis of Human Lung Adenocarcinoma Cells by Promoting TRAIL-DR5 Signaling. Molecules 2024, 29, 877. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ou, Z.; Zhong, W.; Huang, L.; Liao, W.; Sheng, Y.; Guo, Z.; Chen, J.; Yang, W.; Chen, K.; et al. Effective Antitumor Immunity Can be Triggered by Targeting VISTA in Combination with a TLR3-specific Adjuvant. Cancer Immunol. Res. 2023, 11, 1656–1670. [Google Scholar] [CrossRef]
- Nalawade, S.A.; Shafer, P.; Bajgain, P.; McKenna, M.K.; Ali, A.; Kelly, L.; Joubert, J.; Gottschalk, S.; Watanabe, N.; Leen, A.; et al. Selectively targeting myeloid-derived suppressor cells through TRAIL receptor 2 to enhance the efficacy of CAR T cell therapy for treatment of breast cancer. J. Immunother. Cancer 2021, 9, e003237. [Google Scholar] [CrossRef]
- Yu, P.; Zheng, D.; Zhang, C.; Wu, M.; Liu, X. Protocol to prepare functional cellular nanovesicles with PD1 and TRAIL to boost antitumor response. STAR Protoc. 2021, 2, 100324. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, K.; Kim, J.H.; Park, Y.S.; Luo, X.; Zhang, L.; Ku, J.-L.; Choudry, M.H.A.; Lee, Y.J. Glucose deprivation-induced endoplasmic reticulum stress response plays a pivotal role in enhancement of TRAIL cytotoxicity. J. Cell. Physiol. 2021, 236, 6666–6677. [Google Scholar] [CrossRef]
- Huntington, K.E.; Louie, A.; Zhou, L.; Seyhan, A.A.; Maxwell, A.W.; El-Deiry, W.S. Colorectal cancer extracellular acidosis decreases immune cell killing and is partially ameliorated by pH-modulating agents that modify tumor cell cytokine profiles. Am. J. Cancer Res. 2022, 12, 138–151. [Google Scholar]
- Huang, H.-C.; Sung, Y.-C.; Li, C.-P.; Wan, D.; Chao, P.-H.; Tseng, Y.-T.; Liao, B.-W.; Cheng, H.-T.; Hsu, F.-F.; Huang, C.-C.; et al. Reversal of pancreatic desmoplasia by a tumour stroma-targeted nitric oxide nanogel overcomes TRAIL resistance in pancreatic tumours. Gut 2022, 71, 1843–1855. [Google Scholar] [CrossRef]
- Wu, X.; Wang, S.; Li, M.; Wang, A.; Zhou, Y.; Li, P.; Wang, Y. Nanocarriers for TRAIL delivery: Driving TRAIL back on track for cancer therapy. Nanoscale 2017, 9, 13879–13904. [Google Scholar] [CrossRef] [PubMed]
- Goklany, S.; Lu, P.; Godeshala, S.; Hall, A.; Garrett-Mayer, E.; Voelkel-Johnson, C.; Rege, K. Delivery of TRAIL-expressing plasmid DNA to cancer cells in vitro and in vivo using aminoglycoside-derived polymers. J. Mater. Chem. B 2019, 7, 7014–7025. [Google Scholar] [CrossRef]
- Kagawa, S.; He, C.; Gu, J.; Koch, P.; Rha, S.J.; Roth, J.A.; Curley, S.A.; Stephens, L.C.; Fang, B. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001, 61, 3330–3338. [Google Scholar]
- Ylösmäki, E.; Cerullo, V. Design and application of oncolytic viruses for cancer immunotherapy. Curr. Opin. Biotechnol. 2020, 65, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Xu, C.; Dong, J.; Wei, J. An oncolytic vaccinia virus encoding hyaluronidase reshapes the extracellular matrix to enhance cancer chemotherapy and immunotherapy. J. Immunother. Cancer 2024, 12, e008431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, M.; Huang, F.; Wang, S.; Wang, H.; Mou, X.; Wang, Y. Oncolytic Adenovirus Expressing ST13 Increases Antitumor Effect of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Against Pancreatic Ductal Adenocarcinoma. Hum. Gene Ther. 2020, 31, 891–903. [Google Scholar] [CrossRef]
- Xiao, B.; Qin, Y.; Ying, C.; Ma, B.; Wang, B.; Long, F.; Wang, R.; Fang, L.; Wang, Y. Combination of oncolytic adenovirus and luteolin exerts synergistic antitumor effects in colorectal cancer cells and a mouse model. Mol. Med. Rep. 2017, 16, 9375–9382. [Google Scholar] [CrossRef]
- Le, D.H.T.; Commandeur, U.; Steinmetz, N.F. Presentation and Delivery of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand via Elongated Plant Viral Nanoparticle Enhances Antitumor Efficacy. ACS Nano 2019, 13, 2501–2510. [Google Scholar] [CrossRef]
- Lee, K.L.; Murray, A.A.; Le, D.H.T.; Sheen, M.R.; Shukla, S.; Commandeur, U.; Fiering, S.; Steinmetz, N.F. Combination of Plant Virus Nanoparticle-Based in Situ Vaccination with Chemotherapy Potentiates Antitumor Response. Nano Lett. 2017, 17, 4019–4028. [Google Scholar] [CrossRef]
- Garofalo, M.; Wieczorek, M.; Anders, I.; Staniszewska, M.; Lazniewski, M.; Prygiel, M.; Zasada, A.A.; Szczepińska, T.; Plewczynski, D.; Salmaso, S.; et al. Novel combinatorial therapy of oncolytic adenovirus AdV5/3-D24-ICOSL-CD40L with anti PD-1 exhibits enhanced anti-cancer efficacy through promotion of intratumoral T-cell infiltration and modulation of tumour microenvironment in mesothelioma mouse model. Front. Oncol. 2023, 13, 1259314. [Google Scholar] [CrossRef]
- Li, Z.; Guo, R.; Zhang, Z.; Yong, H.; Guo, L.; Chen, Z.; Huang, D.; Zhou, D. Enhancing gene transfection of poly(β-amino ester)s through modulation of amphiphilicity and chain sequence. J. Control. Release 2024, 368, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.B.; Chen, M.; Chen, C.-K.; Pfeifer, B.A.; Jones, C.H. Overcoming Gene-Delivery Hurdles: Physiological Considerations for Nonviral Vectors. Trends Biotechnol. 2016, 34, 91–105. [Google Scholar] [CrossRef]
- Yagolovich, A.V.; Gasparian, M.E.; Dolgikh, D.A. Recent Advances in the Development of Nanodelivery Systems Targeting the TRAIL Death Receptor Pathway. Pharmaceutics 2023, 15, 515. [Google Scholar] [CrossRef]
- Guimarães, P.P.G.; Gaglione, S.; Sewastianik, T.; Carrasco, R.D.; Langer, R.; Mitchell, M.J. Nanoparticles for Immune Cytokine TRAIL-Based Cancer Therapy. ACS Nano 2018, 12, 912–931. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Duan, N.; Zhang, C.; Mo, R.; Hua, Z. Improved antitumor activity of TRAIL fusion protein via formation of self-assembling nanoparticle. Sci. Rep. 2017, 7, 41904. [Google Scholar] [CrossRef]
- Gallego-Lleyda, A.; De Miguel, D.; Anel, A.; Martinez-Lostao, L. Lipid Nanoparticles Decorated with TNF-Related Aptosis-Inducing Ligand (TRAIL) Are More Cytotoxic than Soluble Recombinant TRAIL in Sarcoma. Int. J. Mol. Sci. 2018, 19, 1449. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Otero, N.; Marshall, J.R.; Glenn, A.; Matloubieh, J.; Joseph, J.; Sahasrabudhe, D.M.; Messing, E.M.; King, M.R. TRAIL-coated leukocytes to kill circulating tumor cells in the flowing blood from prostate cancer patients. BMC Cancer 2021, 21, 898. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Y.; Wang, L.; Liu, W.; Xiao, L.; Lin, Q.; Gong, T.; Sun, X.; He, Q.; Zhang, Z.; et al. Improved melanoma suppression with target-delivered TRAIL and Paclitaxel by a multifunctional nanocarrier. J. Control. Release 2020, 325, 10–24. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, M.; Wang, T.; Zhang, B.; Liu, C.; Zhang, N. Multifunctionalized polyethyleneimine-based nanocarriers for gene and chemotherapeutic drug combination therapy through one-step assembly strategy. Int. J. Nanomed. 2017, 12, 8681–8698. [Google Scholar] [CrossRef][Green Version]
- Ravula, V.; Lo, Y.-L.; Wu, Y.-T.; Chang, C.-W.; Patri, S.V.; Wang, L.-F. Arginine-tocopherol bioconjugated lipid vesicles for selective pTRAIL delivery and subsequent apoptosis induction in glioblastoma cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112189. [Google Scholar] [CrossRef]
- Pindiprolu, S.K.S.S.; Krishnamurthy, P.T.; Dev, C.; Chintamaneni, P.K. DR5 antibody conjugated lipid-based nanocarriers of gamma-secretase inhibitor for the treatment of triple negative breast cancer. Chem. Phys. Lipids 2021, 235, 105033. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Gong, S.; Li, Y.; Zhang, H.; Li, N.; Tang, B. A DR4 capturer with AKT siRNA for the synergetic enhancement of death receptor-mediated apoptosis. Chem. Commun. 2018, 54, 13439–13442. [Google Scholar] [CrossRef]
- Wang, Y.; Santos, A.; Kaur, G.; Evdokiou, A.; Losic, D. Structurally engineered anodic alumina nanotubes as nano-carriers for delivery of anticancer therapeutics. Biomaterials 2014, 35, 5517–5526. [Google Scholar] [CrossRef] [PubMed]
- Eljack, S.; David, S.; Faggad, A.; Chourpa, I.; Allard-Vannier, E. Nanoparticles design considerations to co-deliver nucleic acids and anti-cancer drugs for chemoresistance reversal. Int. J. Pharm. X 2022, 4, 100126. [Google Scholar] [CrossRef]
- Yoo, J.-W.; Irvine, D.J.; Discher, D.E.; Mitragotri, S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 2011, 10, 521–535. [Google Scholar] [CrossRef]
- Liu, Z.; Li, S.; Ma, T.; Zeng, J.; Zhou, X.; Li, H.; Tang, M.; Liu, X.; Li, F.; Jiang, B.; et al. Secreted TRAIL gene-modified adipose-derived stem cells exhibited potent tumor-suppressive effect in hepatocellular carcinoma cells. Immun. Inflamm. Dis. 2021, 9, 144–156. [Google Scholar] [CrossRef]
- Shirjang, S.; Mansoori, B.; Solali, S.; Hagh, M.F.; Shamsasenjan, K. Toll-like receptors as a key regulator of mesenchymal stem cell function: An up-to-date review. Cell. Immunol. 2017, 315, 1–10. [Google Scholar] [CrossRef]
- Guiho, R.; Biteau, K.; Grisendi, G.; Taurelle, J.; Chatelais, M.; Gantier, M.; Heymann, D.; Dominici, M.; Redini, F. TRAIL delivered by mesenchymal stromal/stem cells counteracts tumor development in orthotopic Ewing sarcoma models. Int. J. Cancer 2016, 139, 2802–2811. [Google Scholar] [CrossRef]
- Spano, C.; Grisendi, G.; Golinelli, G.; Rossignoli, F.; Prapa, M.; Bestagno, M.; Candini, O.; Petrachi, T.; Recchia, A.; Miselli, F.; et al. Soluble TRAIL Armed Human MSC As Gene Therapy For Pancreatic Cancer. Sci. Rep. 2019, 9, 1788. [Google Scholar] [CrossRef]
- Han, J.; Hwang, H.S.; Na, K. TRAIL-secreting human mesenchymal stem cells engineered by a non-viral vector and photochemical internalization for pancreatic cancer gene therapy. Biomaterials 2018, 182, 259–268. [Google Scholar] [CrossRef]
- Rossignoli, F.; Grisendi, G.; Spano, C.; Golinelli, G.; Recchia, A.; Rovesti, G.; Orsi, G.; Veronesi, E.; Horwitz, E.M.; Dominici, M. Inducible Caspase9-mediated suicide gene for MSC-based cancer gene therapy. Cancer Gene Ther. 2019, 26, 11–16. [Google Scholar] [CrossRef]
- Quiroz-Reyes, A.G.; Gonzalez-Villarreal, C.A.; Limon-Flores, A.Y.; Delgado-Gonzalez, P.; Martinez-Rodriguez, H.G.; Said-Fernandez, S.L.; Soto-Dominguez, A.; Rivas-Estilla, A.M.; Islas, J.F.; Molina-De la Garza, J.F.; et al. Mesenchymal Stem Cells Genetically Modified by Lentivirus-Express Soluble TRAIL and Interleukin-12 Inhibit Growth and Reduced Metastasis-Relate Changes in Lymphoma Mice Model. Biomedicines 2023, 11, 595. [Google Scholar] [CrossRef]
- Shamili, F.H.; Bayegi, H.R.; Salmasi, Z.; Sadri, K.; Mahmoudi, M.; Kalantari, M.; Ramezani, M.; Abnous, K. Exosomes derived from TRAIL-engineered mesenchymal stem cells with effective anti-tumor activity in a mouse melanoma model. Int. J. Pharm. 2018, 549, 218–229. [Google Scholar] [CrossRef]
- Hao, Y.; Zhu, G.; Yu, L.; Ren, Z.; Zhang, P.; Zhu, J.; Cao, S. Extracellular vesicles derived from mesenchymal stem cells confer protection against intervertebral disc degeneration through a microRNA-217-dependent mechanism. Osteoarthr. Cartil. 2022, 30, 1455–1467. [Google Scholar] [CrossRef]
- Huang, K.C.-Y.; Chiang, S.-F.; Chang, H.-Y.; Chen, W.T.-L.; Yang, P.-C.; Chen, T.-W.; Liang, J.-A.; Shiau, A.-C.; Ke, T.-W.; Clifford Chao, K.S. Engineered sTRAIL-armed MSCs overcome STING deficiency to enhance the therapeutic efficacy of radiotherapy for immune checkpoint blockade. Cell Death Dis. 2022, 13, 610. [Google Scholar] [CrossRef]
- Fakiruddin, K.S.; Lim, M.N.; Nordin, N.; Rosli, R.; Abdullah, S. Chemo-Sensitization of CD133+ Cancer Stem Cell Enhances the Effect of Mesenchymal Stem Cell Expressing TRAIL in Non-Small Cell Lung Cancer Cell Lines. Biology 2021, 10, 1103. [Google Scholar] [CrossRef]
- Rossignoli, F.; Spano, C.; Grisendi, G.; Foppiani, E.M.; Golinelli, G.; Mastrolia, I.; Bestagno, M.; Candini, O.; Petrachi, T.; Recchia, A.; et al. MSC-Delivered Soluble TRAIL and Paclitaxel as Novel Combinatory Treatment for Pancreatic Adenocarcinoma. Theranostics 2019, 9, 436–448. [Google Scholar] [CrossRef]
- Sun, L.; Wang, J.; Wang, Q.; He, Z.; Sun, T.; Yao, Y.; Wang, W.; Shen, P. Pretreatment of umbilical cord derived MSCs with IFN-γ and TNF-α enhances the tumor-suppressive effect on acute myeloid leukemia. Biochem. Pharmacol. 2022, 199, 115007. [Google Scholar] [CrossRef]
- Choi, S.A.; Lee, C.; Kwak, P.A.; Park, C.-K.; Wang, K.-C.; Phi, J.H.; Lee, J.Y.; Chong, S.; Kim, S.-K. Histone deacetylase inhibitor panobinostat potentiates the anti-cancer effects of mesenchymal stem cell-based sTRAIL gene therapy against malignant glioma. Cancer Lett. 2019, 442, 161–169. [Google Scholar] [CrossRef]
- Shaik Fakiruddin, K.; Ghazalli, N.; Lim, M.N.; Zakaria, Z.; Abdullah, S. Mesenchymal Stem Cell Expressing TRAIL as Targeted Therapy against Sensitised Tumour. Int. J. Mol. Sci. 2018, 19, 2188. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Wayne, E.; Rana, K.; Schaffer, C.B.; King, M.R. TRAIL-coated leukocytes that kill cancer cells in the circulation. Proc. Natl. Acad. Sci. USA 2014, 111, 930–935. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Chan, M.F.; Li, J.; King, M.R. Super natural killer cells that target metastases in the tumor draining lymph nodes. Biomaterials 2016, 77, 66–76. [Google Scholar] [CrossRef]
- Chulpanova, D.S.; Gilazieva, Z.E.; Akhmetzyanova, E.R.; Kletukhina, S.K.; Rizvanov, A.A.; Solovyeva, V.V. Cytochalasin B-induced membrane vesicles from human mesenchymal stem cells overexpressing TRAIL, PTEN and IFN-β1 can kill carcinoma cancer cells. Tissue Cell 2021, 73, 101664. [Google Scholar] [CrossRef]
- Chen, F.; Zhong, X.; Dai, Q.; Li, K.; Zhang, W.; Wang, J.; Zhao, Y.; Shen, J.; Xiao, Z.; Xing, H.; et al. Human Umbilical Cord MSC Delivered-Soluble TRAIL Inhibits the Proliferation and Promotes Apoptosis of B-ALL Cell In Vitro and In Vivo. Pharmaceuticals 2022, 15, 1391. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Mu, X.; Tu, C.R.; Chung, Y.; Tsao, S.W.; Chan, G.C.-F.; Leung, W.-H.; Lau, Y.-L.; Liu, Y.; et al. Exosomes derived from γδ-T cells synergize with radiotherapy and preserve antitumor activities against nasopharyngeal carcinoma in immunosuppressive microenvironment. J. Immunother. Cancer 2022, 10, e003832. [Google Scholar] [CrossRef]
| Type | Drugs | Cancers | Phases | Clinical Trial ID |
|---|---|---|---|---|
| Rh TRAIL | Dulanermin (AMG 951) | NSCLC | II (2006–2011) | NCT00508625 |
| NSCLC | III (2016–2018) | NCT03083743 | ||
| Colorectal cancer | I (2009–2014) | NCT00873756 | ||
| NHL | I/II (2006–2010) | NCT00400764 | ||
| Colorectal cancer | I (2006–2012) | NCT00671372 | ||
| SCB-313 | Peritoneal carcinomatosis | I (2019–2022) | NCT04047771 | |
| Peritoneal malignancy | I (2018–2021) | NCT03443674 | ||
| Malignant pleural effusion | I (2019–2021) | NCT03869697 | ||
| Malignant ascites | I (2019–2022) | NCT04051112 | ||
| Malignant pleural effusion | I (2020–2022) | NCT04123886 | ||
| TRAIL-R1 mAb | Mapatumumab (TRM-1/HGS-ETR1) | NHL | II (2004–2007) | NCT00094848 |
| NSCLC | II (2005) | NCT00092924 | ||
| Advanced cervical cancer | I/II (2010–2014) | NCT01088347 | ||
| HCC | I/II (2011–2017) | NCT01258608 | ||
| Multiple myeloma | II (2006–2010) | NCT00315757 | ||
| TRAIL-R2 mAb | Tigatuzumab (CS-1008) | Pancreatic cancer | II (2007–2010) | NCT00521404 |
| Solid malignancy and lymphoma | I (2007) | NCT00320827 | ||
| TNBC | II (2011–2017) | NCT01307891 | ||
| Conatumumab (AMG 655) | Lymphoma | Ⅰ (2008–2011) | NCT00791011 | |
| Colorectal carcinoma | II (2007–2011) | NCT00625651 | ||
| Colorectal carcinoma | II (2009–2012) | NCT00813605 | ||
| Unresectable soft tissue sarcoma | II (2007–2011) | NCT00626704 | ||
| Solid tumor | II (2009–2011) | NCT00819169 | ||
| Metastatic pancreatic cancer | I/II (2007–2012) | NCT00630552 | ||
| GEN1029 | Malignant solid tumor | II (2018–2021) | NCT03576131 | |
| INBRX-109 | Chondrosarcoma | II (2021–2025) | NCT04950075 | |
| Solid tumors including sarcoma | I (2018–2026) | NCT03715933 | ||
| IGM-8444 | All-comers solid tumors | I (2020–2027) | NCT04553692 | |
| Oba01(RC248-C001) | DR5 positive LA/mNSCLC | I (2023–2026) | NCT06083870 | |
| DS-8273a | Advanced colorectal cancer | I (2016–2017) | NCT02991196 | |
| TRA | ABBV-621 | Solid or hematologic malignancy | I (2017–2022) | NCT03082209 |
| Type | Drugs | Cancers | Phases | Clinical Trial ID |
|---|---|---|---|---|
| IAPs inhibitor | Xevinapant | SCCHN | III (2023–2030) | NCT05930938 |
| SCCHN | II (2024–2028) | NCT06084845 | ||
| Bcl-2 inhibitor | Venetoclax (ABT-199/GDC-0199) | CLL | I/II (2015–2024) | NCT02427451 |
| Solid malignancy | II (2018–2023) | NCT03552692 | ||
| Breast cancer | I (2019–2025) | NCT03900884 | ||
| ABT-263 (Navitoclax) | HGSC and TNBC | I (2022–2025) | NCT05358639 | |
| CLL | II (2010–2012) | NCT01087151 | ||
| AVALON | AML | I (2019–2020) | NCT04070807 | |
| BGB-11417 | Mature B-cell malignancy | I (2020–2027) | NCT04277637 | |
| BGB-21447 | Mature B-cell malignancy | I (2023–2026) | NCT05828589 | |
| TQB3909 | Breast cancer | I/II (2023) | NCT05775575 | |
| Malignancy | I (2022–2024) | NCT04975204 | ||
| LOXO-338 | Blood cancer | I (2021–2023) | NCT05024045 | |
| ZN-d5 | AML | I (2022–2026) | NCT05682170 | |
| APG-2575 | SCLC | I (2017–2024) | NCT03387332 | |
| FCN-338 | CLL | I (2021–2024) | NCT04682808 | |
| Bcl-2 DNAi | PNT2258 | Diffuse large B-cell lymphoma | II (2014–2016) | NCT02226965 |
| L-Bcl-2 | Oblimersen | WM | I/II (2003–2007) | NCT00062244 |
| Solid malignancy | I (2001–2010) | NCT00636545 | ||
| BP1002 | AML | I (2022–2024) | NCT05190471 | |
| G3139 | SCLC | I/II (2000–2001) | NCT00005032 | |
| Solid malignancy | I (2005–2006) | NCT00543231 | ||
| RCC | II (2003–2005) | NCT00059813 | ||
| Bcl-XL Inhibitor | Bcl-XL_42-CAF09b Vaccination | Prostate cancer with lymph node metastases | I (2018–2021) | NCT03412786 |
| AT-101 | Laryngeal cancer | II (2012–2021) | NCT01633541 | |
| Bcl-2 family Inhibitor | Pelcitoclax (APG-1252) | Neuroendocrine tumor | I (2022–2025) | NCT04893759 |
| SCLC | I (2017–2021) | NCT03387332 | ||
| Navitoclax | HGSC and TNBC | I (2022–2025) | NCT05358639 | |
| Bcl-2 and MCL-1 Inhibitor | VOB560-MIK665 | NHL, MM and AML | I (2021–2024) | NCT04702425 |
| MCL-1 Inhibitor | ABBV-467 | MM | I (2020–2021) | NCT04178902 |
| Murizatoclax (AMG 397) | Hematological malignancy | I (2018–2019) | NCT03465540 | |
| PRT1419 | Relapsed or refractory myeloid | I (2022–2024) | NCT05107856 | |
| MIK665 (S64315) | MM | I (2017–2019) | NCT02992483 | |
| AML and MDS | I (2017–2020) | NCT02979366 | ||
| AML | II (2021–2024) | NCT03672695 |
| Cancers | Drugs | Phases | Outcome | Clinical Trial ID |
|---|---|---|---|---|
| Metastatic Pancreatic Cancer | AMG 655 or AMG 479 targets DR5 on MDSCs | I/II (2007–2012) | Completed | NCT00630552 |
| Metastatic Renal Cancer | The α and β signaling chains of 2G-1 transduced into human lymphocytes by retroviral vectors | I (2009–2012) | Terminated | NCT00923390 |
| Unresectable Stage III or Stage IV melanoma | DS-8273a combined with Nivolumab (anti-PD-1 antibody) | I (2016–2021) | Completed | NCT02983006 |
| Solid Malignancies | Autologous CAR-T/TCR-T Cell combined with anti-DR5 antibody | I (2019–2021) | Completed | NCT03941626 |
| Metastatic NSCLC | Targeted stem cells expressing TRAIL combined with pemetrexed/cisplatin chemotherapy | I/II (2019–2025) | Ongoing, recruiting | NCT03298763 |
| Metastatic Breast Cancer | TRAIL-R2 and HER2 bi-specific CAR-T cells combined with IL-15 | I (2024–2033) | Ongoing, recruiting | NCT06251544 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, C.; He, S.; Shi, F.; Zhou, J.; Shang, L. The Role of TRAIL Signaling in Cancer: Searching for New Therapeutic Strategies. Biology 2024, 13, 521. https://doi.org/10.3390/biology13070521
Luo C, He S, Shi F, Zhou J, Shang L. The Role of TRAIL Signaling in Cancer: Searching for New Therapeutic Strategies. Biology. 2024; 13(7):521. https://doi.org/10.3390/biology13070521
Chicago/Turabian StyleLuo, Cheng, Shan He, Feng Shi, Jianhua Zhou, and Li Shang. 2024. "The Role of TRAIL Signaling in Cancer: Searching for New Therapeutic Strategies" Biology 13, no. 7: 521. https://doi.org/10.3390/biology13070521
APA StyleLuo, C., He, S., Shi, F., Zhou, J., & Shang, L. (2024). The Role of TRAIL Signaling in Cancer: Searching for New Therapeutic Strategies. Biology, 13(7), 521. https://doi.org/10.3390/biology13070521





